Abstract
Background
Multiple myeloma (MM) patients with high cytogenetic risk have poor outcomes. In CASTOR, daratumumab plus bortezomib/dexamethasone (D-Vd) prolonged progression-free survival (PFS) versus bortezomib/dexamethasone (Vd) alone and exhibited tolerability in patients with relapsed or refractory MM (RRMM).
Methods
This subgroup analysis evaluated D-Vd versus Vd in CASTOR based on cytogenetic risk, determined using fluorescence in situ hybridization and/or karyotype testing performed locally. High-risk patients had t(4;14), t(14;16), and/or del17p abnormalities. Minimal residual disease (MRD; 10−5 sensitivity threshold) was assessed via the clonoSEQ® assay V2.0. Of the 498 patients randomized, 40 (16%) in the D-Vd group and 35 (14%) in the Vd group were categorized as high risk.
Results
After a median follow-up of 40.0 months, D-Vd prolonged median PFS versus Vd in patients with standard (16.6 vs 6.6 months; HR, 0.26; 95% CI, 0.19-0.37; P < 0.0001) and high (12.6 vs 6.2 months; HR, 0.41; 95% CI, 0.21–0.83; P = 0.0106) cytogenetic risk. D-Vd achieved deep responses, including higher rates of MRD negativity and sustained MRD negativity versus Vd, regardless of cytogenetic risk. The safety profile was consistent with the overall population of CASTOR.
Conclusion
These updated data reinforce the effectiveness and tolerability of daratumumab-based regimens for RRMM, regardless of cytogenetic risk status.
Trial registration
ClinicalTrials.gov, NCT02136134. Registered 12 May 2014
Keywords: Clinical trials, Multiple myeloma, Myeloma therapy
Background
Daratumumab is a human IgGκ monoclonal antibody targeting CD38 with a direct on-tumor [1–4] and immunomodulatory mechanism of action [5–7]. Intravenous daratumumab 16 mg/kg is approved as monotherapy in patients with heavily pre-treated relapsed or refractory multiple myeloma (RRMM) and in combination with bortezomib/dexamethasone (Vd) or lenalidomide/dexamethasone (Rd) in patients with multiple myeloma (MM) who received at least 1 prior line of therapy and in combination with pomalidomide/dexamethasone in patients with at least 2 prior therapies, including lenalidomide and a proteasome inhibitor [8]. Daratumumab is also approved in combination with bortezomib/melphalan/prednisone and in combination with Rd in patients with transplant-ineligible newly diagnosed MM, and in combination with bortezomib/thalidomide/dexamethasone in patients with transplant-eligible newly diagnosed MM [8].
In the primary analysis of the phase 3 CASTOR study of daratumumab plus Vd (D-Vd) versus Vd alone in patients with RRMM, at a median follow-up of 7.4 months, D-Vd significantly prolonged progression-free survival (PFS) and increased rates of minimal residual disease (MRD) negativity and demonstrated a tolerable safety profile [9, 10]. With more than 3 years of follow-up (median 40.0 months) and compared with patients receiving Vd only, patients receiving D-Vd demonstrated a 69% reduction in the risk of disease progression or death (median PFS, 16.7 months vs 7.1 months; hazard ratio [HR], 0.31; 95% confidence interval [CI], 0.25–0.40; P < 0.0001); showed significantly better overall response rates (85% vs 63%; P < 0.0001); and achieved better rates of complete response (CR) or better (30% vs 10%; P < 0.0001), very good partial response (VGPR) or better (63% vs 29%; P < 0.0001), and MRD negativity at the 10−5 sensitivity threshold (14% vs 2%; P < 0.000001) [11]. Patients who received 1 prior line of therapy demonstrated the greatest benefit with D-Vd, including a 78% reduction in the risk of disease progression or death versus Vd (median PFS, 27.0 months vs 7.9 months; HR, 0.22; 95% CI, 0.15–0.32; P < 0.0001) and a response of CR or better (43% vs 15%; P < 0.0001) and MRD negativity (10−5; 20% vs 3%; P = 0.000025). In CASTOR, no new safety concerns were observed with longer follow-up [11].
Patients with MM and specific cytogenetic markers are at higher risk for poor outcomes [12, 13]. The International Myeloma Working Group recommends defining high cytogenetic risk as testing positive for at least 1 of the following abnormalities: t(4;14), t(14;16), or del17p, determined by fluorescence in situ hybridization (FISH) [14]. This subgroup analysis of CASTOR presents updated efficacy and safety findings for D-Vd versus Vd treatment based on cytogenetic risk status after a median follow-up of 40.0 months.
Methods
Patients
Complete study methodology and primary results from CASTOR have been previously described [9, 15]. Briefly, eligible patients received at least 1 prior line of MM therapy, with at least a partial response to at least 1 prior MM therapy, and had documented progressive disease during or after their last regimen, as defined by the International Myeloma Working Group criteria [16, 17]. Key exclusion criteria included the following: creatinine clearance ≤ 20 mL/min/1.73 m2 body surface area, disease refractory or intolerant to bortezomib, disease refractory to a different proteasome inhibitor, or presence of grade ≥ 2 peripheral neuropathy or neuropathic pain.
Study design and treatment
CASTOR is a multicenter, randomized, open-label, active-controlled, phase 3 trial enrolling patients with RRMM. Randomization was stratified by the International Staging System (stage I, II, or III) at screening, the number of prior lines of therapy (1 vs 2 or 3 vs > 3), and previous bortezomib treatment (no vs yes). The study protocol was approved by an independent ethics committee or institutional review board at each study center and was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice guidelines. All patients provided written informed consent.
Patients were randomly assigned 1:1 to receive D-Vd or Vd. All patients received eight 21-day cycles of Vd. Bortezomib (1.3 mg/m2) was administered subcutaneously on days 1, 4, 8, and 11 during cycles 1 through 8. Dexamethasone (20 mg) was given orally or intravenously on days 1, 2, 4, 5, 8, 9, 11, and 12 during cycles 1 through 8. Daratumumab (16 mg/kg) was administered intravenously to patients in the D-Vd group once weekly during cycles 1 through 3, once every 3 weeks during cycles 4 through 8, and once every 4 weeks thereafter until disease progression. Patients in the Vd group were to receive a maximum of 8 cycles of Vd followed by observation until disease progression; following the primary analysis, patients whose disease progressed could choose to receive daratumumab monotherapy.
Cytogenetic risk
Cytogenetic risk was evaluated using local FISH or karyotyping. Determination of each abnormality and threshold of frequencies to consider a positive finding was determined locally and varied by site. Patients in the intent-to-treat (ITT) population who had at least 1 FISH or karyotyping assessment were included in the analysis. High-risk patients were defined as having 1 or more of the following cytogenetic abnormalities identified: t(4;14), t(14;16), or del17p.
MRD evaluation
MRD was evaluated at the time of the suspected CR (including stringent CR; blinded to treatment group) and at 6 and 12 months after the first treatment dose (i.e., at the end of Vd therapy and 6 months later, respectively). Additional MRD evaluations were required every 12 months after CR. MRD was evaluated by next-generation sequencing using the clonoSEQ® assay V2.0 (Adaptive Biotechnologies, Seattle, WA, USA) at a sensitivity threshold of 10−5 (1 cancer cell per 100,000 nucleated cells). Patients were considered MRD positive if they had an MRD-positive or indeterminate test result or were not assessed. Sustained MRD negativity was defined as maintenance of MRD negativity at the 10−5 sensitivity threshold for at least 6 months or at least 12 months.
Statistical analyses and assessments
The primary endpoint of the study was PFS. Exploratory analyses were performed for subgroups of patients based on cytogenetic risk status. PFS was assessed in patients in the ITT population who met the biomarker criteria for risk assessment. The response-evaluable analysis set included patients who had measurable disease at the baseline or screening visit and who received at least 1 study treatment and had at least 1 post-baseline disease assessment. The safety population comprised individuals who received at least 1 administration of study treatment.
PFS and time to response were compared between the D-Vd and Vd groups using a stratified log-rank test. A Cox proportional hazards model was used to estimate HRs and 95% CIs, with treatment as the sole explanatory variable. The Kaplan-Meier method was used to estimate distributions. PFS on the subsequent line of therapy (PFS2) was defined as the time from randomization to progressive disease after the next line of subsequent therapy or death. Differences between treatment groups for overall response rates, VGPR or better rates, and CR or better rates were measured by a stratified Cochran-Mantel-Haenszel chi-square test.
Patients in the ITT population who met the biomarker criteria for risk assessment were evaluated for MRD and sustained MRD negativity to allow for stringent and unbiased evaluation. MRD-negativity rates were defined as the proportions of patients achieving MRD-negative status at any time after the first treatment dose and were compared between the D-Vd and Vd treatment groups using a Fisher’s exact test.
Results
Patients and treatments
A total of 498 patients were randomized, with 251 assigned to D-Vd and 247 assigned to Vd. A total of 356 (71%) patients underwent cytogenetic testing; 283 (57%) patients were evaluated using FISH, 217 (44%) patients were evaluated using karyotyping, and 144 (29%) were evaluated using both. Of these, 40 (16%) patients in the D-Vd group and 35 (14%) patients in the Vd group had high cytogenetic risk abnormalities. Forty of 158 patients in the D-Vd group and 35 of 173 patients in the Vd group who underwent FISH testing were defined as high risk. Two of 130 patients in the D-Vd group and 1 of 136 patients in the Vd group who underwent karyotype testing were defined as high risk. A total of 141 (56%) patients in the D-Vd group and 140 (57%) patients in the Vd group had standard cytogenetic risk. Patient demographics, baseline disease, and clinical characteristics stratified by cytogenetic risk status are shown in Table 1. Among patients achieving CR or better, MRD was not evaluated in 15 (16%) patients. Overall, 170 (62%) and 50 (68%) patients in the standard and high cytogenetic risk subgroups discontinued the treatment, respectively (Table 2). Among patients who received Vd and discontinued treatment due to progressive disease, 9 of 34 and 2 of 12 patients in the standard and high cytogenetic risk subgroups, respectively, received daratumumab monotherapy as subsequent therapy.
Table 1.
Patient demographics, baseline disease, and clinical characteristics
| Standard cytogenetic riska | High cytogenetic riska,b | |||
|---|---|---|---|---|
| Characteristic | D-Vd (n = 141) | Vd (n = 140) | D-Vd (n = 40) | Vd (n = 35) |
| Age, years | ||||
| Median (range) | 64 (40–88) | 64 (33–85) | 63 (37–79) | 59 (37–81) |
| ≥ 75 years, n (%) | 9 (6) | 20 (14) | 4 (10) | 5 (14) |
| Sex, n (%) | ||||
| Male | 79 (56) | 89 (64) | 22 (55) | 18 (51) |
| Race, n (%) | ||||
| White | 123 (87) | 123 (88) | 33 (83) | 31 (89) |
| Black or African American | 9 (6) | 2 (1) | 1 (3) | 1 (3) |
| Asian | 8 (6) | 8 (6) | 4 (10) | 2 (6) |
| Native Hawaiian or other Pacific Islander | 1 (1) | 0 | 0 | 0 |
| Other | 0 | 1 (1) | 0 | 0 |
| Unknown/not reported | 0 | 6 (4) | 2 (5) | 1 (3) |
| ISS stage, n (%)c | ||||
| I | 48 (34) | 55 (39) | 22 (55) | 14 (40) |
| II | 57 (40) | 56 (40) | 11 (28) | 16 (46) |
| III | 36 (26) | 29 (21) | 7 (18) | 5 (14) |
| ECOG performance status score, n (%) | ||||
| 0 | 54 (38) | 64 (46) | 16 (40) | 15 (43) |
| 1 | 78 (55) | 62 (44) | 22 (55) | 19 (54) |
| 2 | 9 (6) | 14 (10) | 2 (5) | 1 (3) |
| Cytogenetic profile, n (%)a,b | ||||
| t(4;14) | – | – | 13 (33) | 15 (43) |
| t(14;16) | – | – | 4 (10) | 5 (14) |
| del17p | – | – | 27 (68) | 20 (57) |
| ≥ 2 risk factorsd | – | – | 4 (10) | 4 (11) |
| Median (range) time from diagnosis, years | 4.3 (0.7–20.7) | 3.6 (0.6–18.6) | 3.3 (1.0–10.5) | 3.7 (1.0–14.8) |
| Prior lines of therapy, n (%) | ||||
| 1 | 70 (50) | 67 (48) | 21 (53) | 12 (34) |
| 2 | 32 (23) | 40 (29) | 11 (28) | 15 (43) |
| 3 | 25 (18) | 16 (11) | 5 (13) | 6 (17) |
| > 3 | 14 (10) | 17 (12) | 3 (8) | 2 (6) |
| Median (range) | 2 (1–9) | 2 (1–10) | 1 (1–6) | 2 (1–4) |
| Prior ASCT, n (%) | 90 (64) | 79 (56) | 27 (68) | 21 (60) |
| Prior PI, n (%) | 101 (72) | 94 (67) | 27 (68) | 28 (80) |
| Bortezomib | 98 (70) | 89 (64) | 25 (63) | 26 (74) |
| Prior IMiD, n (%) | 104 (74) | 110 (79) | 28 (70) | 29 (83) |
| Lenalidomide | 52 (37) | 66 (47) | 15 (38) | 17 (49) |
| Prior PI + IMiD, n (%) | 73 (52) | 67 (48) | 15 (38) | 22 (63) |
| Refractory to PI only, n (%) | 1 (1) | 2 (1) | 2 (5) | 1 (3) |
| Refractory to IMiD only, n (%) | 41 (29) | 51 (36) | 12 (30) | 11 (31) |
| Refractory to PI and IMiD, n (%) | 6 (4) | 2 (1) | 0 | 4 (11) |
| Refractory to last line of therapy, n (%) | 40 (28) | 48 (34) | 11 (28) | 14 (40) |
D-Vd daratumumab/bortezomib/dexamethasone, Vd bortezomib/dexamethasone, ISS International Staging System, ECOG Eastern Cooperative Oncology Group, ASCT autologous stem cell transplantation, PI proteasome inhibitor, IMiD immunomodulatory drug, FISH fluorescence in situ hybridization
Note: percentages may not equal 100% due to rounding
aBased on FISH/karyotype testing
bPatients with high cytogenetic risk had a t(4;14), t(14;16), or del17p abnormality
cISS stage is derived based on the combination of serum β2-microglobulin and albumin
dPatients with ≥ 2 of the t(4;14), t(14;16), or del17p risk factors
Table 2.
Patient disposition based on cytogenetic risk status
| Standard riska | High riska,b | |||
|---|---|---|---|---|
| Treatment discontinuation, n (%)c | D-Vd (n = 137) | Vd (n = 136) | D-Vd (n = 40) | Vd (n = 34) |
| Patients who discontinued treatment | 108 (79) | 62 (46) | 33 (83) | 17 (50) |
| Reason for discontinuation | ||||
| Progressive disease | 86 (63) | 34 (25) | 27 (68) | 12 (35) |
| Adverse event | 11 (8) | 15 (11) | 5 (13) | 3 (9) |
| Noncompliance with study drugd | 5 (4) | 5 (4) | 0 | 1 (3) |
| Withdrawal by patient | 1 (1) | 6 (4) | 0 | 1 (3) |
| Death | 2 (1) | 2 (1) | 1 (3) | 0 |
| Physician decision | 3 (2) | 0 | 0 | 0 |
D-Vd daratumumab/bortezomib/dexamethasone, Vd bortezomib/dexamethasone, FISH fluorescence in situ hybridization
aBased on FISH/karyotyping
bPatients with high cytogenetic risk had a t(4;14), t(14;16), or del17p abnormality
cSafety population
dBased on reason “Patient refused to further study treatment” at “End of treatment”
Updated efficacy results
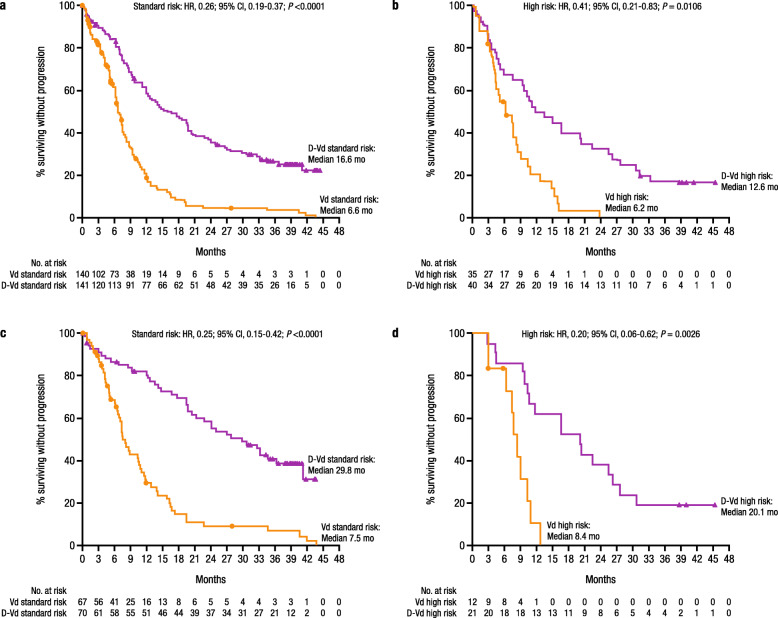
After a median follow-up of 40.0 months, treatment with D-Vd prolonged median PFS compared with Vd alone in patients with standard cytogenetic risk (16.6 months vs 6.6 months; HR, 0.26; 95% CI, 0.19–0.37; P < 0.0001; Fig. 1a) as well as high cytogenetic risk (12.6 months vs 6.2 months; HR, 0.41; 95% CI, 0.21–0.83; P = 0.0106; Fig. 1b) in the ITT population. Among a subset of patients who had received 1 prior line of therapy, treatment with D-Vd prolonged median PFS versus Vd in patients with standard cytogenetic risk (29.8 months vs 7.5 months; HR, 0.25; 95% CI, 0.15–0.42; P < 0.0001; Fig. 1c) and high cytogenetic risk (20.1 months vs 8.4 months; HR, 0.20; 95% CI, 0.06–0.62; P = 0.0026; Fig. 1d).
Fig. 1.
PFS based on cytogenetic risk status. PFS in the ITT/biomarker risk population (patients in the ITT population who met the biomarker criteria for risk assessment): a standard cytogenetic risk patients and b high cytogenetic risk patients. PFS in patients with 1 prior line of therapy: c standard cytogenetic risk patients and d high cytogenetic risk patients. CI, confidence interval; D-Vd, daratumumab plus bortezomib/dexamethasone; HR, hazard ratio; ITT intent-to-treat; PFS, progression-free survival; Vd, bortezomib/dexamethasone
Higher overall response rate was achieved with D-Vd versus Vd (standard risk, 84% vs 62%; P < 0.0001; high risk, 85% vs 56%; P = 0.0512), including deep responses of CR or better (standard risk, 28% vs 10%; high risk, 28% vs 6%) and VGPR or better (standard risk, 62% vs 28%; P < 0.0001; high risk, 59% vs 32%; P = 0.1259; Table 3). Median time to VGPR or better was decreased with D-Vd compared with Vd in patients with standard cytogenetic risk (3.5 months vs not estimable; HR, 2.16; 95% CI, 1.46–3.20; P < 0.0001) and high cytogenetic risk (3.5 months vs 6.2 months; HR, 1.96; 95% CI, 0.86–4.45; P = 0.1004).
Table 3.
Response and MRD-negativity rates in patients with standard and high cytogenetic risk
| Standard risk | High riska | |||||
|---|---|---|---|---|---|---|
| Response, n (%)b | D-Vd (n = 135) | Vd (n = 134) | P value | D-Vd (n = 39) | Vd (n = 34) | P value |
| ORR | 113 (84) | 83 (62) | < 0.0001 | 33 (85) | 19 (56) | 0.0512 |
| ≥ CRc | 38 (28) | 13 (10) | 11 (28) | 2 (6) | ||
| sCR | 12 (9) | 3 (2) | 4 (10) | 0 | ||
| CR | 26 (19) | 10 (8) | 7 (18) | 2 (6) | ||
| ≥ VGPRd | 83 (62) | 38 (28) | < 0.0001 | 23 (59) | 11 (32) | 0.1259 |
| VGPR | 45 (33) | 25 (19) | 12 (31) | 9 (27) | ||
| PR | 30 (22) | 45 (34) | 10 (26) | 8 (24) | ||
| MRD negative (10−5)e | ||||||
| n (%) | 16 (11) | 4 (3) | 0.0091 | 6 (15) | 0 | 0.0271 |
| Sustained MRD negativity (≥ 6 months), n (%) | 9 (6) | 3 (2) | 0.1374 | 5 (13) | 0 | 0.0569 |
| Sustained MRD negativity (≥ 12 months), n (%) | 2 (1) | 0 | 0.4982 | 3 (8) | 0 | 0.2432 |
CR complete response, D-Vd daratumumab plus bortezomib/dexamethasone, ITT intent-to-treat, MRD minimal residual disease, ORR overall response rate, PR partial response, sCR stringent complete response, Vd bortezomib/dexamethasone, VGPR very good partial response
aPatients with high cytogenetic risk had a t(4;14), t(14;16), or del17p abnormality
bResponse-evaluable population
c≥ CR = sCR + CR
d≥ VGPR = sCR + CR + VGPR
eITT population (standard risk: D-Vd, n = 141; Vd, n = 140; high risk: D-Vd, n = 40; Vd, n = 35)
Rates of MRD negativity at the 10−5 sensitivity threshold were higher with D-Vd compared with Vd in patients with standard cytogenetic risk (11% vs 3%; P = 0.0091) and high cytogenetic risk (15% vs 0%; P = 0.0271; Table 3). MRD negativity was sustained for at least 6 months in a greater number of patients treated with D-Vd versus Vd, regardless of cytogenetic risk status. MRD negativity was sustained for at least 12 months in 2 (1%) patients with standard cytogenetic risk and 3 (8%) patients with high cytogenetic risk in the D-Vd group compared with none in both cytogenetic risk categories in the Vd group.
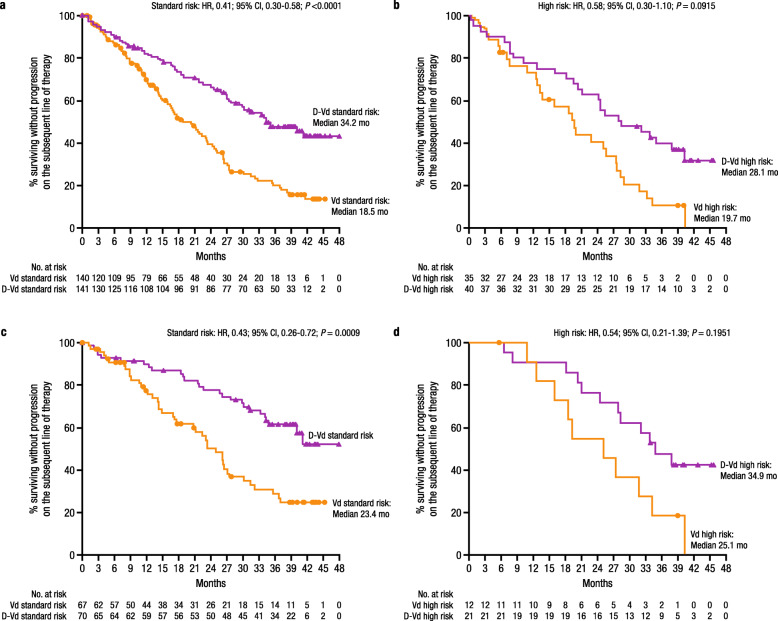
Median PFS2 was prolonged with D-Vd compared with Vd in the standard cytogenetic risk (34.2 months vs 18.5 months; HR, 0.41; 95% CI, 0.30–0.58; P < 0.0001; Fig. 2a) and high cytogenetic risk (28.1 months vs 19.7 months; HR, 0.58; 95% CI, 0.30–1.10; P = 0.0915; Fig. 2b) subgroups. Among the subset of patients with 1 prior line of therapy, median PFS2 was prolonged with D-Vd versus Vd in patients with standard cytogenetic risk (not estimable vs 23.4 months; HR, 0.43; 95% CI, 0.26–0.72; P = 0.0009; Fig. 2c). For patients with high cytogenetic risk, median PFS2 was prolonged with D-Vd versus Vd (34.9 months vs 25.1 months; HR, 0.54; 95% CI, 0.21–1.39; P = 0.1951; Fig 2d).
Fig. 2.
PFS2 based on cytogenetic risk status. PFS2 in the ITT/biomarker risk population (patients in the ITT population who met the biomarker criteria for risk assessment): a standard cytogenetic risk patients and b high cytogenetic risk patients. PFS2 in patients with 1 prior line of therapy: c standard cytogenetic risk patients and d high cytogenetic risk patients. CI, confidence interval; D-Vd, daratumumab plus bortezomib/dexamethasone; HR, hazard ratio; ITT intent-to-treat; PFS2, progression-free survival on the subsequent line of therapy; Vd, bortezomib/dexamethasone
At the time of the analysis, among patients with high cytogenetic risk, 21 deaths were observed in the D-Vd group versus 23 deaths in the Vd group. The overall survival data are immature and follow-up for overall survival is ongoing.
Updated safety results
The most frequent any grade treatment-emergent adverse events observed in at least 25% of patients and the most frequent grade 3/4 treatment-emergent adverse events observed in at least 5% of patients are summarized in Table 4. Treatment discontinuation rates due to treatment-emergent adverse events were similar between treatment groups for patients with standard cytogenetic risk (11 [8%] patients in the D-Vd group and 14 [10%] of patients in the Vd group) and among patients with high cytogenetic risk (4 [10%] patients in the D-Vd group and 3 [9%] patients in the Vd group).
Table 4.
Most common any-grade (≥ 25% of patients) and grade 3/4 (≥ 5% of patients) TEAEs
| Any grade | Grade 3/4 | |||||||
|---|---|---|---|---|---|---|---|---|
| Standard risk | High riska | Standard risk | High riska | |||||
| TEAE, n (%) | D-Vd (n = 137) | Vd (n = 136) | D-Vd (n = 40) | Vd (n = 34) | D-Vd (n = 137) | Vd (n = 136) | D-Vd (n = 40) | Vd (n = 34) |
| Hematologic | ||||||||
| Thrombocytopenia | 85 (62) | 58 (43) | 24 (60) | 16 (47) | 65 (47) | 45 (33) | 19 (48) | 12 (35) |
| Anemia | 45 (33) | 41 (30) | 7 (18) | 14 (41) | 25 (18) | 23 (17) | 4 (10) | 6 (18) |
| Neutropenia | 29 (21) | 16 (12) | 9 (23) | 3 (9) | 21 (15) | 6 (4) | 6 (15) | 2 (6) |
| Lymphopenia | 18 (13) | 5 (4) | 4 (10) | 4 (12) | 14 (10) | 3 (2) | 3 (8) | 3 (9) |
| Leukopenia | 15 (11) | 5 (4) | 3 (8) | 3 (9) | 5 (4) | 1 (1) | 1 (3) | 2 (6) |
| Nonhematologic | ||||||||
| Peripheral sensory neuropathy | 67 (49) | 50 (37) | 22 (55) | 13 (38) | 4 (3) | 8 (6) | 2 (5) | 4 (12) |
| Upper respiratory tract infection | 43 (31) | 20 (15) | 15 (38) | 8 (24) | 1 (1) | 0 | 3 (8) | 1 (3) |
| Diarrhea | 42 (31) | 35 (26) | 11 (28) | 6 (18) | 6 (4) | 2 (2) | 1 (3) | 0 |
| Cough | 40 (29) | 19 (14) | 9 (23) | 4 (12) | 0 | 0 | 0 | 0 |
| Fatigue | 25 (18) | 40 (29) | 17 (43) | 8 (24) | 6 (4) | 5 (4) | 2 (5) | 1 (3) |
| Back pain | 24 (18) | 15 (11) | 13 (33) | 1 (3) | 3 (2) | 0 | 1 (3) | 0 |
| Insomnia | 22 (16) | 20 (15) | 11 (28) | 5 (15) | 2 (2) | 0 | 0 | 1 (3) |
| Pneumonia | 22 (16) | 20 (15) | 5 (13) | 4 (12) | 15 (11) | 14 (10) | 2 (5) | 3 (9) |
| Asthenia | 15 (11) | 19 (14) | 4 (10) | 9 (27) | 1 (1) | 3 (2) | 0 | 1 (3) |
| Hypertension | 15 (11) | 5 (4) | 4 (10) | 1 (3) | 9 (7) | 1 (1) | 2 (5) | 0 |
| Decreased appetite | 14 (10) | 8 (6) | 10 (25) | 1 (3) | 0 | 1 (1) | 1 (3) | 0 |
| Spinal pain | 4 (3) | 3 (2) | 2 (5) | 0 | 1 (1) | 0 | 2 (5) | 0 |
| Gastroenteritis | 2 (2) | 3 (2) | 2 (5) | 1 (3) | 0 | 2 (2) | 2 (5) | 1 (3) |
| Squamous cell carcinoma of the skin | 0 | 0 | 2 (5) | 0 | 0 | 0 | 2 (5) | 0 |
TEAE treatment-emergent adverse event, D-Vd daratumumab/bortezomib/dexamethasone, Vd bortezomib/dexamethasone
aPatients with high cytogenetic risk had a t(4;14), t(14;16), or del17p abnormality
Discussion
After a median follow-up of more than 3 years, D-Vd continued to demonstrate substantially improved efficacy in terms of PFS compared with Vd alone in patients with RRMM, regardless of cytogenetic risk status. D-Vd reduced the risk of disease progression or death by 74% versus Vd alone in patients with standard cytogenetic risk and by 59% in patients with high cytogenetic risk. Among patients treated with D-Vd, median PFS was 16.6 months in patients with standard cytogenetic risk (vs 6.6 months with Vd; P < 0.0001) and 12.6 months in patients with high cytogenetic risk (vs 6.2 months with Vd; P = 0.0106). The PFS benefit of D-Vd over Vd was especially pronounced in the subset of patients who received 1 prior line of therapy, reducing the risk of disease progression or death by 75% and 80% in patients with standard and high cytogenetic risk, respectively. D-Vd achieved deep responses compared with Vd, with higher rates of VGPR or better and CR or better, regardless of cytogenetic risk status. Rates of MRD negativity (10−5 sensitivity threshold) were higher with D-Vd versus Vd in patients with standard cytogenetic risk (11% vs 3%; P = 0.0091) and high cytogenetic risk (15% vs 0%; P = 0.0271). Moreover, sustained MRD-negative responses were observed in more patients treated with D-Vd compared with Vd regardless of cytogenetic risk status. D-Vd prolonged median PFS2 versus Vd alone in both cytogenetic risk subgroups. Overall, improved outcomes were achieved by D-Vd versus Vd in patients with high cytogenetic risk, but clinical benefits were of lesser magnitude than D-Vd in patients with standard cytogenetic risk.
The safety profile of D-Vd in standard and high cytogenetic risk subgroups was consistent with the overall population of CASTOR. No new safety signals were identified.
The results reported here after extended follow-up further strengthen results reported after a median follow-up of 13.0 months [18]. In this earlier analysis in patients with high cytogenetic risk, PFS was prolonged with D-Vd versus Vd (median 11.2 months vs 7.2 months; HR, 0.49; 95% CI, 0.27–0.89; P = 0.0167). With a median follow-up of 40.0 months, the efficacy of D-Vd versus Vd in these high-risk patients was maintained, with prolonged median PFS (HR, 0.41) and higher MRD-negativity rates (15% vs 0%; P = 0.0271) in this difficult-to-treat patient population.
While cross-trial comparisons should be approached with caution, especially due to lack of consensus on thresholds for risk groups, the efficacy of D-Vd in patients with high cytogenetic risk appears favorable to that reported in other studies of proteasome inhibitor–containing regimens in RRMM (Table 5). In a pre-planned subgroup analysis of the ENDEAVOR study based on baseline cytogenetic risk, carfilzomib in combination with dexamethasone reduced the risk of disease progression or death by 35% versus Vd in patients with high-risk RRMM (defined as t[4;14] or t[14;16] in ≥ 10% of screened plasma cells or del17p in ≥ 20% of screened plasma cells assessed by FISH) [19]. Median PFS was 8.8 months with carfilzomib in combination with dexamethasone versus 6.0 months with Vd in patients with high cytogenetic risk (HR, 0.65; 95% CI, 0.45–0.92; P = 0.0075) and CR or better rates were 16% versus 4%, respectively. In a pre-planned subgroup analysis of the phase 3 ASPIRE study, among patients with high cytogenetic risk (t[4;14], t[14;16], or del17p in ≥ 60% of screened plasma cells assessed by FISH), median PFS was 23.1 months with carfilzomib plus Rd versus 13.9 months with Rd alone (HR, 0.70; 95% CI, 0.43–1.16; P = 0.0829) [20]. In the phase 3 PANORAMA-1 trial of panobinostat plus Vd versus Vd alone in RRMM, the HR for median PFS in high-risk patients (t[4;14], t[14;16], or del17p assessed by FISH) was 0.47 (95% CI, 0.18–1.25) in favor of panobinostat-Vd [21].
Table 5.
Summary of median PFS of high cytogenetic risk patients with RRMM in randomized, phase 3 trials
| Trial name | High cytogenetic risk definition | Arm 1 (n) | Arm 2 (n) | Arm 1 median PFS, months | Arm 2 median PFS, months | Hazard ratio (95% CI); P value |
|---|---|---|---|---|---|---|
| CASTOR | t(4;14), t(14;16), or del17p assessed by FISH or karyotyping | D-Vd (40) | Vd (35) | 12.6 | 6.2 | 0.41 (0.21–0.83); 0.0106 |
| ENDEAVOR [19] | t(4;14) or t(14;16) in ≥ 10% of screened plasma cells or del17p in ≥ 20% of screened plasma cells assessed by FISH | Kd (97) | Vd (113) | 8.8 | 6.0 | 0.65 (0.45–0.92); 0.0075 |
| ASPIRE [20] | t(4;14), t(14;16), or del17p (in ≥ 60% of screened plasma cells) assessed by FISH | KRd (48) | Rd (52) | 23.1 | 13.9 | 0.70 (0.43–1.16); 0.0829 |
| PANORAMA-1 [21] | t(4;14), t(14;16), or del17p assessed by FISH | Panobinostat plus Vd | Vd | – | – | 0.47 (0.18–1.25) |
CI confidence interval, D-Vd daratumumab plus bortezomib/dexamethasone, FISH fluorescence in situ hybridization, Kd carfilzomib/dexamethasone, KRd carfilzomib/lenalidomide/dexamethasone, PFS progression-free survival, Rd lenalidomide/dexamethasone, Vd bortezomib/dexamethasone
The efficacy of daratumumab plus standard of care, regardless of cytogenetic risk status, was also demonstrated in the phase 3 POLLUX study of daratumumab plus Rd versus Rd alone in RRMM [22]. At a median follow-up of more than 3 years, median PFS was prolonged with daratumumab plus Rd (D-Rd) versus Rd in patients with standard (not reached vs 19.9 months; HR, 0.41; 95% CI, 0.31–0.55; P < 0.0001) and high (26.8 vs 8.8 months; HR, 0.54; 95% CI, 0.32–0.91; P = 0.0175) cytogenetic risk, and deep responses were achieved with D-Rd in both cytogenetic risk subgroups. It is noteworthy that D-Vd and D-Rd, but not Vd nor Rd, achieved MRD negativity in patients with high cytogenetic risk, which suggests that targeting CD38 in combination with other standard of care regimens helps improve the historically poor outcomes observed in this patient population [23–27]. Looking ahead, there continues to be a gap in treatment options for high-risk patients with RRMM; potential treatment regimens that can be studied include daratumumab in combination with pomalidomide, carfilzomib, or bortezomib, lenalidomide, and dexamethasone.
This report is limited by incomplete cytogenetic abnormality data collected for patients enrolled in the CASTOR study; cytogenetic testing was not performed in 29% of patients in the study. Cytogenetic testing was performed locally and no per-protocol specific cut-off values were used for defining the presence of genetic abnormalities; cut-off values used at each site were not collected. Additionally, MRD was not assessed in patients with VGPR and in 16% of patients with CR or better. Of patients with available cytogenetic abnormality data, patients without MRD assessment were considered MRD positive, potentially underestimating the rate of MRD negativity. Lastly, small sample sizes in the cytogenetic risk subgroups precluded us from conducting a multivariate analysis to account for baseline differences.
Conclusions
In this subgroup analysis, D-Vd demonstrated a clear efficacy benefit compared with Vd in patients with RRMM and high cytogenetic risk in CASTOR. When combined with the recently updated POLLUX results, these findings reinforce the effectiveness and tolerability of daratumumab plus standard of care as a treatment for MM, regardless of cytogenetic risk status.
Acknowledgments
The authors would like to thank the patients who participated in this study and their families, as well as the study co-investigators, research nurses, and coordinators at each of the clinical sites. The authors thank Maria Krevvata, PhD, of Janssen Research & Development, LLC, for contributions to the study. Medical writing and editorial support were provided by Kristin Runkle, PhD, of MedErgy, and were funded by Janssen Global Services, LLC.
Abbreviations
- CI
Confidence interval
- CR
Complete response
- D-Rd
Daratumumab plus lenalidomide/dexamethasone
- D-Vd
Daratumumab plus bortezomib/dexamethasone
- FISH
Fluorescence in situ hybridization
- HR
Hazard ratio
- ITT
Intent-to-treat
- MRD
Minimal residual disease
- MM
Multiple myeloma
- PFS
Progression-free survival
- PFS2
Progression-free survival on the subsequent line of therapy
- Rd
Lenalidomide/dexamethasone
- RRMM
Relapsed or refractory multiple myeloma
- Vd
Bortezomib/dexamethasone
- VGPR
Very good partial response
Authors’ contributions
VH, WB, AAC-K, MB, HA, and M-VM designed the research study, acquired and analyzed the data, drafted the manuscript, and approved the final version. ND designed the research study, analyzed the data, drafted the manuscript, and approved the final version. KW and PS designed the research study, drafted the manuscript, and approved the final version. SL, TMM, IS, AB, HQ, MM, CL, MCavo, JU, and RK acquired and analyzed the data, drafted the manuscript, and approved the final version. TM, BL, M-DL, J-JL, C-KM, NH, RO, J-CJ, and H-JS acquired the data, drafted the manuscript, and approved the final version. AS, HA-L, MCapra, AN, PC, and TC analyzed the data, drafted the manuscript, and approved the final version.
Funding
This CASTOR study was sponsored by Janssen Research & Development, LLC.
Availability of data and materials
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
Ethics approval and consent to participate
The study protocol was approved by an independent ethics committee or institutional review board at each study center and was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice guidelines. All patients provided written informed consent.
Consent for publication
Not applicable.
Competing interests
KW received honoraria from GlaxoSmithKline, Sanofi, Adaptive, Amgen, Bristol-Myers Squibb, Celgene, Janssen, and Takeda; served in a consulting or advisory role for GlaxoSmithKline, Amgen, Adaptive, Bristol-Myers Squibb, Celgene, Janssen, Takeda, Sanofi, and Juno; and received institutional research funding from Amgen, Celgene, Sanofi, and Janssen. AS received honoraria from Celgene, Janssen, Amgen, AbbVie, Servier, and Takeda; served in a consulting or advisory role for Celgene, Janssen, Servier, and AbbVie; and served on a speakers bureau for and received institutional funding from Janssen, Takeda, and Celgene. SL holds stock and patents, royalties, or other intellectual property from Caelum Biosciences; served in a consulting or advisory role for Caelum Biosciences, Takeda, Janssen, Celgene, Bristol-Myers Squibb, AbbVie, and Bayer; received research funding from Sanofi; declared another relationship with Clinical Care Options; and served on data safety monitoring boards for Sorrento and Bayer. TMM holds stock in AbbVie; received honoraria and research funding from Celgene; and served in a consulting or advisory role for Janssen, Takeda, and Adaptive. M-DL is a consultant for and receives honoraria and travel support from AbbVie, Celgene, Amgen, and Janssen. AB received honoraria from Amgen; served in a consulting or advisory role for Takeda; and had travel, accommodations, or other expenses paid or reimbursed by Celgene, Janssen, and Amgen. VH served in a consulting or advisory role and on a speakers bureau for AbbVie, Amgen, Celgene, Janssen, Takeda, and Bristol-Myers Squibb. MCavo received honoraria from Celgene, Janssen, Amgen, Bristol-Myers Squibb, AbbVie, and Takeda; served in a consulting or advisory role for Janssen, Celgene, Amgen, and AbbVie; and served on a speakers bureau for and had travel, accommodations, or other expenses paid or reimbursed by Janssen and Celgene. AN received honoraria from and served in a consulting or advisory role for Janssen, GlaxoSmithKline, Celgene, Amgen, Takeda, Spectrum, Bristol-Myers Squibb, Karyopharm, Oncopeptides, and Adaptive and received research funding from Janssen, GlaxoSmithKline, Celgene, Amgen, Takeda, Karyopharm, and Bristol-Myers Squibb. HQ served in a consulting or advisory role for Celgene, Amgen, Karyopharm, and GlaxoSmithKline and received research funding from Celgene and Amgen. MM received honoraria from Janssen, Bristol-Myers Squibb, Celgene, and Amgen; served in a consulting or advisory role for Janssen, Bristol-Myers Squibb, Takeda, Celgene, Amgen, and Heidelberg Pharma; received research funding from Incyte and Bristol-Myers Squibb; and had travel, accommodations, or other expenses paid or reimbursed by Janssen, Bristol-Myers Squibb, Takeda, Celgene, and Amgen. CL received honoraria from and consulted for Amgen, Janssen-Cilag, Takeda, and Celgene. PC received honoraria from Daiichi Sankyo, Kite, KiowaKirin, Celgene, Janssen, Novartis, Roche, Takeda, Sanofi, Amgen, Gilead, and AbbVie and had travel, accommodations, or other expenses paid or reimbursed by Novartis, Janssen, Celgene, Bristol-Myers Squibb, Takeda, Gilead, Amgen, and AbbVie. AAC-K received research funding from the Mayo Clinic. NH served on the board of directors or advisory committees for Janssen. MCapra received honoraria from AbbVie, GlaxoSmithKline, Bristol-Myers Squibb, Adaptive, Takeda, Janssen, and Celgene. MB served in a consulting or advisory role and on a speakers bureau for Amgen, Janssen, and Takeda. RO consulted for Janssen. PS received honoraria from and research funding from Amgen, Celgene, Janssen, SkylineDx, and Takeda. TC is an employee of Janssen and holds stock options from Johnson & Johnson. ND, HA, JU, and RK are employees of Janssen. M-VM received honoraria from and served in a consulting or advisory role for Janssen, Celgene, Amgen, Takeda, GlaxoSmithKline, AbbVie, Seattle Genetics, and Adaptive. HA-L, IS, TM, BL, J-JL, WB, C-KM, J-CJ, and H-JS have no conflicts of interest to disclose.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.de Weers M, Tai YT, van der Veer MS, Bakker JM, Vink T, Jacobs DC, et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol. 2011;186(3):1840–1848. doi: 10.4049/jimmunol.1003032. [DOI] [PubMed] [Google Scholar]
- 2.Lammerts van Bueren J, Jakobs D, Kaldenhoven N, Roza M, Hiddingh S, Meesters J, et al. Direct in vitro comparison of daratumumab with surrogate analogs of CD38 antibodies MOR03087, SAR650984 and Ab79. Blood. 2014;124(21):3474. doi: 10.1182/blood.V124.21.3474.3474. [DOI] [Google Scholar]
- 3.Overdijk MB, Verploegen S, Bogels M, van Egmond M, Lammerts van Bueren JJ, Mutis T, et al. Antibody-mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. MAbs. 2015;7(2):311–321. doi: 10.1080/19420862.2015.1007813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Overdijk MB, Jansen JH, Nederend M, Lammerts van Bueren JJ, Groen RW, Parren PW, et al. The therapeutic CD38 monoclonal antibody daratumumab induces programmed cell death via Fcgamma receptor-mediated cross-linking. J Immunol. 2016;197(3):807–813. doi: 10.4049/jimmunol.1501351. [DOI] [PubMed] [Google Scholar]
- 5.Krejcik J, Casneuf T, Nijhof IS, Verbist B, Bald J, Plesner T, et al. Daratumumab depletes CD38+ immune-regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood. 2016;128(3):384–394. doi: 10.1182/blood-2015-12-687749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiu C, Casneuf T, Axel A, Lysaght A, Bald J, Khokhar NZ, et al. Daratumumab in combination with lenalidomide plus dexamethasone induces clonality increase and T-cell expansion: results from a phase 3 randomized study (POLLUX) Blood. 2016;128:4531. doi: 10.1182/blood.V128.22.4531.4531. [DOI] [Google Scholar]
- 7.Adams HC, 3rd, Stevenaert F, Krejcik J, Van der Borght K, Smets T, Bald J, et al. High-parameter mass cytometry evaluation of relapsed/refractory multiple myeloma patients treated with daratumumab demonstrates immune modulation as a novel mechanism of action. Cytometry A. 2019;95(3):279–289. doi: 10.1002/cyto.a.23693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DARZALEX™ (daratumumab) injection, for intravenous use [package insert]. Horsham, PA: Janssen Biotech, Inc.; 2018.
- 9.Palumbo A, Chanan-Khan A, Weisel K, Nooka AK, Masszi T, Beksac M, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(8):754–766. doi: 10.1056/NEJMoa1606038. [DOI] [PubMed] [Google Scholar]
- 10.Avet-Loiseau H, Casneuf T, Chiu C, Laubach JP, Lee JJ, Moreau P, et al. Evaluation of minimal residual disease (MRD) in relapsed/refractory multiple myeloma (RRMM) patients treated with daratumumab in combination with lenalidomide plus dexamethasone or bortezomib plus dexamethasone. Blood. 2016;128:246. doi: 10.1182/blood.V128.22.246.246. [DOI] [Google Scholar]
- 11.Mateos M-V, Sonneveld P, Hungria VTM, Nooka AK, Estell J, Barreto WG, et al. Efficacy and safety of daratumumab, bortezomib, and dexamethasone (D-Vd) versus bortezomib and dexamethasone (Vd) in first relapse patients: two-year update of CASTOR. Poster presented at: 60th Annual Meeting of the American Society of Hematology; December 1-4, 2018; San Diego, CA.Abstract 3270.
- 12.Sonneveld P, Avet-Loiseau H, Lonial S, Usmani S, Siegel D, Anderson KC, et al. Treatment of multiple myeloma with high-risk cytogenetics: a consensus of the International Myeloma Working Group. Blood. 2016;127(24):2955–2962. doi: 10.1182/blood-2016-01-631200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rajan AM, Rajkumar SV. Interpretation of cytogenetic results in multiple myeloma for clinical practice. Blood Cancer J. 2015;5:e365. doi: 10.1038/bcj.2015.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fonseca R, Bergsagel PL, Drach J, Shaughnessy J, Gutierrez N, Stewart AK, et al. International Myeloma Working Group molecular classification of multiple myeloma: spotlight review. Leukemia. 2009;23(12):2210–2221. doi: 10.1038/leu.2009.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spencer A, Lentzsch S, Weisel K, Avet-Loiseau H, Mark TM, Spicka I, et al. Daratumumab plus bortezomib and dexamethasone versus bortezomib and dexamethasone in relapsed or refractory multiple myeloma: updated analysis of CASTOR. Haematologica. 2018;103(12):2079–2087. doi: 10.3324/haematol.2018.194118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Durie BGM, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20(9):1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 17.Rajkumar SV, Harousseau JL, Durie B, Anderson KC, Dimopoulos M, Kyle R, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011;117(18):4691–4695. doi: 10.1182/blood-2010-10-299487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mateos MV, Estell J, Barreto W, Corradini P, Min CK, Medvedova E, et al., editors. Efficacy of daratumumab, bortezomib, and dexamethasone versus bortezomib and dexamethasone in relapsed or refractory myeloma based on prior lines of therapy: updated analysis of CASTOR. Presented at the 58th Annual Meeting & Exposition of the American Society of Hematology (ASH); December 3-6, 2016; San Diego, CA Abstract 1150; 2016.
- 19.Chng WJ, Goldschmidt H, Dimopoulos MA, Moreau P, Joshua D, Palumbo A, et al. Carfilzomib-dexamethasone vs bortezomib-dexamethasone in relapsed or refractory multiple myeloma by cytogenetic risk in the phase 3 study ENDEAVOR. Leukemia. 2016;31(6):1368–1374. doi: 10.1038/leu.2016.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Avet-Loiseau H, Fonseca R, Siegel D, Dimopoulos MA, Spicka I, Masszi T, et al. Carfilzomib significantly improves the progression-free survival of high-risk patients in multiple myeloma. Blood. 2016;128(9):1174–1180. doi: 10.1182/blood-2016-03-707596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.San-Miguel JF, Hungria VT, Yoon SS, Beksac M, Dimopoulos MA, Elghandour A, et al. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: a multicentre, randomised, double-blind phase 3 trial. Lancet Oncol. 2014;15(11):1195–1206. doi: 10.1016/S1470-2045(14)70440-1. [DOI] [PubMed] [Google Scholar]
- 22.Kaufman JL, Dimopoulos MA, Leiba M, Morton J, Ho PJ, Kim K, et al. Efficacy and safety of daratumumab, lenalidomide, and dexamethasone (D-Rd) in relapsed or refractory multiple myeloma (RRMM): updated subgroup analysis of POLLUX based on cytogenetic risk. J Clin Oncol. 2019;37(suppl): Abstract 8038.
- 23.Dimopoulos MA, Weisel KC, Song KW, Delforge M, Karlin L, Goldschmidt H, et al. Cytogenetics and long-term survival of patients with refractory or relapsed and refractory multiple myeloma treated with pomalidomide and low-dose dexamethasone. Haematologica. 2015;100(10):1327–1333. doi: 10.3324/haematol.2014.117077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Avet-Loiseau H, Attal M, Moreau P, Charbonnel C, Garban F, Hulin C, et al. Genetic abnormalities and survival in multiple myeloma: the experience of the Intergroupe Francophone du Myelome. Blood. 2007;109(8):3489–3495. doi: 10.1182/blood-2006-08-040410. [DOI] [PubMed] [Google Scholar]
- 25.Avet-Loiseau H, Hulin C, Campion L, Rodon P, Marit G, Attal M, et al. Chromosomal abnormalities are major prognostic factors in elderly patients with multiple myeloma: the Intergroupe Francophone du Myelome experience. J Clin Oncol. 2013;31(22):2806–9. [DOI] [PMC free article] [PubMed]
- 26.Chng WJ, Dispenzieri A, Chim CS, Fonseca R, Goldschmidt H, Lentzsch S, et al. IMWG consensus on risk stratification in multiple myeloma. Leukemia. 2014;28(2):269–277. doi: 10.1038/leu.2013.247. [DOI] [PubMed] [Google Scholar]
- 27.Sawyer JR. The prognostic significance of cytogenetics and molecular profiling in multiple myeloma. Cancer Gene Ther. 2011;204(1):3–12. doi: 10.1016/j.cancergencyto.2010.11.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.