Abstract
Daodi medicinal materials (DMMs), with unique characteristics and specific ecological growing environments, are recognized as high-quality medicinal products of Chinese medicinal materials (CMMs). The quality evaluation of CMMs is fundamental for standardization. The concept and application of DMMs have a long history as described in records in ancient books and rooted in practice and experience over generations. DMM is the specific term for pure, superior medicinal herbs with the following characteristics: optimum harvest season (reflecting the appropriate developmental stage of the plant), scrupulous processing, traditional preparation technology, etc. As DMM and high-quality medicinal products are traditionally thought to be closely related, modern scientific studies that confirm the association of these products are described. This article aims to clarify the scientific elucidation of DMMs.
Keywords: Daodi medicinal materials, Chinese medicinal materials, Ecological environment, Chemical components, Pharmacological functions
Background
In recent years, as the use of Chinese medicinal materials (CMMs) has increased, the international attention paid to the safety, stability and efficacy of CMMs has increased. Some authentic and superior CMMs that are grown in specific regions and widely recognized as having better therapeutic effects are called daodi medicinal materials (DMMs) [1]. DMMs, based on the theory, origin, processing, and prominent curative effect of CMMs, are the essence of Chinese cultural heritage [2]. DMMs were first recorded in Zhen Zhu Nang Yao Xing Fu (Precious Drus in Rhyme), a book written 700 years ago. The term “daodi medicinal material” is widely found in Ben Cao Pin Hui Jing Yao (Essentials of Materia Medica Distinctions), a book compiled by the Imperial Hospital during the Ming Dynasty (1368–1644 A.D.), in which 268 medicinal herbals are listed. The entry “original source” was formally listed under each medicinal herb heading, specifying daodi production regions. It has been suggested that the quality of CMMs is highly correlated with their geographical origins. In ancient times, the identification of DMMs was commonly carried out based on the characteristics of the superficies, and this approach depended to a certain extent on empirical experiences and assumptions. Currently, modern scientific analytical techniques may be applied to confirm the validity of associations between high-quality medicinal products and DMM to ensure their utility, clarifying the scientific understanding of daodi medicinal materials.
The word “dao” (in “daodi”) is an ancient Chinese unit of measurement used to divide administrative districts, and this term can be retraced to the Eastern Han Dynasty (25–220 A.D.) as described in Hou Han Shu (Book of Later Han, 432–445 A.D.) [3]. In the Tang Dynasty (618–907 A.D.), the nation was divided into 10 “dao” according to landscapes in the Zhenguan Period, and then the number was increased to 15 “dao” in the Kaiyuan Period. Currently, “dao” is conceptually similar to the modern organizational system of provinces. The word “di” (in “daodi”) refers to regions and geography. Nowadays, “daodi medicinal materials” refers to the distinctively higher quality of the medicinal materials that grow in a certain area.
The establishment of DMMs is related to resources, agricultural technology and CMM development. In ancient China, due to agriculture production, rich experience along with advanced technology in growing and processing medicinal herbals was accumulated, which resulted in the exchange of resources around the world [4]. In addition, the vast territory of China, including plains, hills, mountains, lakes, rivers and seas with different climates, sunshine, soils and ecological environments, provides favorable conditions for the growth of medicinal herbs. Through meticulous selection over the course of production and continuous clinical tests, DMMs have been proven to be definitely curative in medical treatments and therefore have been handed down from generation to generation [5].
DMM can be very effective if proper consideration is given to the characteristics of the original sources, growth and seasonal changes of the material; however, the same medicinal herb grown in different areas does not have the same effectiveness even if they are the same plant. In China, traditional Chinese medicine doctors usually select a superior populations or variety to prescribe based on geographical features. In addition, different harvest times or processing methods can greatly influence the quality of CMMs in terms of chemical components and pharmacological functions. Thus, DMM, as an authentic and superior medicinal product, needs to be validated by modern analytical methods for each CMM. In this paper, the scientific elucidation of DMMs will be introduced, as well as a putative production chain for processing traditional, experience-based CMMs.
Daodi medicinal materials are the essence of Chinese cultural heritage
Over the long course of clinical selection, by discarding the inferior and retaining the superior, DMMs have been acquired. DMMs represent the influence of populations, together with optimum harvest season, habitat and growing conditions such as sunshine, soil, and water. Generally, the identification of DMMs is a useful tool for the quality evaluation of CMMs.
Populations
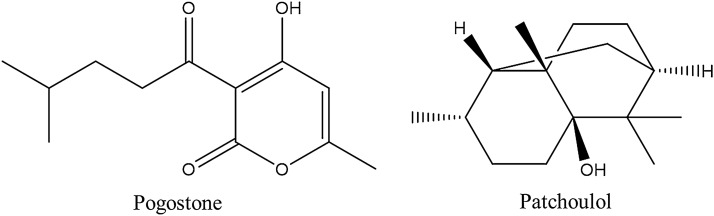
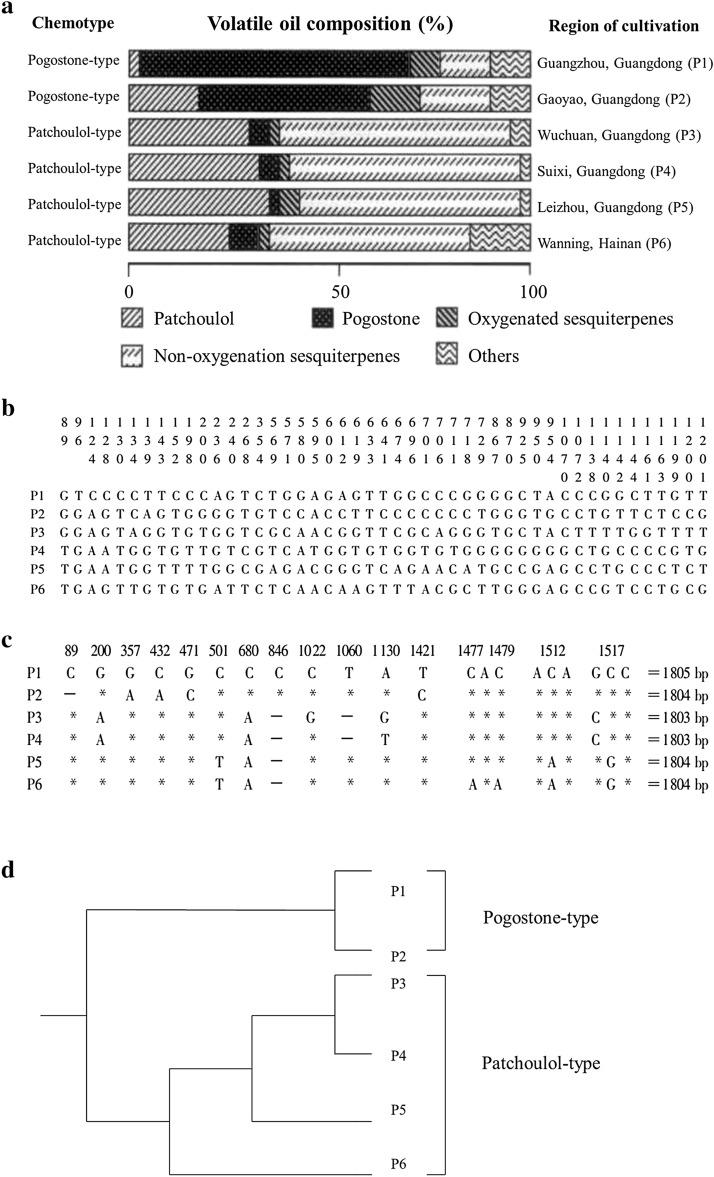
Different growing environments may greatly influence the quality and chemical components of closely related populations. Modern experimental research, especially molecular biological identification, has validated that the different production areas of CMMs are closely related to the quality and DNA sequence divergence of CMMs [6–8]. For example, as the dominant constituents, patchoulol and pogostone are the basis for the anti-inflammatory activity of Pogostemon cablin (Blanco) Benth. [9]. And it has been found that pogostone also exhibits potent anti-fungal [9], antiapoptotic [10], antioxidant [11], and immunosuppressive [12] properties. In our previous study, P. cablin produced in Shipai (in Guangzhou city, Guangdong province, SP) and Gaoyao (in Zhaoqing city, Guangdong province, GY) differed from P. cablin cultivated in Hainan province (HN) and Zhanjiang city (in Guangdong province, ZJ) not only in the total amount of volatile oil but also in genotype [13–15]. According to the composition of the volatile oil, P. cablin is divided into two chemotypes: SP and GY cultivars belong to the pogostone-type, while the HN and ZJ cultivars belong to the patchoulol-type (Fig. 1). Moreover, we have demonstrated that the sequence divergence of both the matK and 18S rRNA genes among 6 samples of P. cablin from different locations was well correlated with the regions of cultivation and intraspecific essential oil chemotypes (Fig. 2) [16]. The same is true for other medicinal herbs. The major pharmacological components in Cnidium monnieri (L.) Cuss. are coumarins [17]. Similarly, according to the coumarin chemotypes, C. monnieri has been classified into three chemotypes, the osthol-linear furanocoumarins-type (chemotype I), principally cultivated in regions of Jiangsu and Hunan provinces; the angular furanocoumarins-type (chemotype II), mainly produced in Heilongjiang province; and the transition-type (chemotype III), largely came from Henan and Hebei regions [18]. It was found that there were 12 variable sites in the matK gene sequence of C. monnieri from different populations. A phylogenetic tree constructed by the neighbor-joining (NJ) method showed that the phylogenetic relationship of 6 C. monnieri cultivars was well correlated with their geographical distribution and intraspecific coumarin chemotypes of (Fig. 3) [19]. Likewise, Laboratory research showed a clear correlation between the rDNA ITS sequence and the phenotype of Dendrobium officinale Kimura et Migo from different populations [20]. The phylogenetic relationship predicted by the 5S rRNA spacer region data correlated well with the essential oil chemotype of Acorus calamus L. collected from various locations [21]. However, the difference in the alkaloid content of Fritillaria thunbergii Miq. from various habitats did not result from variation in the 5S rRNA sequence but from the microenvironment [22]. It’s reported that 5S rRNA is highly-conserved arcoss all species [23], thus, different environments did not produce changes in 5S rRNA of different F. thunbergii populations, but produced differences in secondary metabolites. In these cases, it can be concluded that genetic diversity existed among different populations is relevant to the cultivation regions except some highly-conserved DNA markers.
Fig. 1.
Structures of the pogostone and patchoulol in Pogostemon cablin
Fig. 2.
Volatile oil composition in the leaves (a), comparison of the variable sites in the matK sequence (b), comparison of the variable sites in the 18S rRNA sequence (c) and cluster trees of both the matK and 18S rRNA gene sequences (d) of Pogostemon cablin (Blanco) Benth. from 6 different locations. The top number indicates the nucleotide position upstream of the matK (b) and the 18S rRNA (c) sequence, an asterisk (*) indicates the same nucleotide as the P1 sequence, and a hyphen (—) indicates an alignment gap (c)
Fig. 3.
Phylogenetic tree of the matK gene sequence (E) of Cnidium monnieri (L.) Cuss. from six locations (C1–C6)
Harvest time
CMMs harvested during different growing periods contain different plant metabolites. For instance, the content of essential oil increased as the age of P. cablin increased: patchoulol, α-bulnesene and other sesquiterpenes accumulated to high concentrations at 210 days after maturation [24]. According to the report from Jin et al. [25], Chaenomeles speciosa (Sweet) Nakai produced the highest quality yields when harvested in early July. In the case of Desmodium styracifolium (Osb.) Merr., the best collection season was early October, when the highest concentrations of polysaccharides, flavonoids and schaftoside were measured [26].
Sunshine
Sunshine is an important factor for the formation of DMMs. For example, the content of volatile components in Houttuynia cordata Thunb. was closely related to light intensity: monoterpenoids and nonterpenoids were positively and negatively associated with light intensity, respectively [27]. In Viola yedoensis Makino, the content of flavonoids and coumarins was positively correlated with light intensity [28]. Although it has been demonstrated that higher concentrations of total patchoulol corresponded to lower light intensity in P. cablin, there was no statistically significant correlation between patchoulol content and shade [29]. In recent years, circadian clocks that temporally organize many aspects of growth and metabolism have even been found in numerous plant species [30–32]. For example, in Antirrhinum majus L., monoterpene synthase mRNA levels and corresponding monoterpene emission, which followed diurnal rhythms, were controlled by a circadian clock [33]. A similar daily fluctuation was found in the endogenous level of geranyl acetate and in the expression of its biosynthetic gene, alcohol acetyl transferase in Rosa rugosa Thunb. [31].
Soil
Soil is also important for the identification of DMMs and for the evaluation of quality. Modern pharmacologic studies have proven that the specific composition of soil has a large influence on the quality and quantity of the chemicals in medicinal herbs. For example, soil available iron (Fe) could promote the accumulation of flavonoids, while soil available manganese (Mn), total potassium (K), and available K had an inhibitory effect on flavonoid content in Spatholobus suberectus Dunn [34]. In Citrus grandis ‘Tomentosa’, the content of soil available copper (Cu), zinc (Zn), Mn, boron (B), and molybdenum (Mo) was positively associated with flavonoid concentrations and naringin concentrations [35]. There was a positive correlation between soil total nitrogen (N), available K and emodin in Polygonum cuspidatum Sieb. et Zucc. [36]. Soil Mn was a favorable factor for accumulating schisantherin A in Schisandra sphenanthera Rehd. et Wils., as a significant correlation was also found between these factors [37].
Water
Another matter regarding DMMs that warrants attention is water. As shown in Aconitum carmichaelii Debx., the heavy metals cadmium (Cd), arsenic (As), mercury (Hg), and lead (Pb) concentrations in it were positively associated with the water in Fujiang River (p < 0.05) [38]. This result indicates that the quality of CMMs can also be affected by water. To meet the growing demand of DMMs, additional detailed studies should be undertaken in this field.
Comprehensive ecological factors
Comprehensive research on the relationship between different ecological environment factors and the quality of CMMs has also been recorded. For example, in Scutellaria baicalensis Georgi, most of the chemical constituents were negatively correlated with latitude and positively correlated with temperature. Generally, the contents of 21 chemical constituents were higher at low latitudes than at high latitudes. By gradual regression analysis, it was found that the content of baicalin in S. baicalensis was negatively correlated with latitude. Similarly, the content of inorganic elements in soil was excessively high (magnesium (Mg) and calcium (Ca) excluded), which had a negative effect on the accumulation of chemical constituents in S. baicalensis [39, 40]. Taking the well-known antioxidant herb Panax ginseng C. A. Mey. as another example, low temperature was a favorable factor for the accumulation of ginsenosides, as a negative correlation was found between temperature and ginsenoside contents within a certain temperature range, while the levels of soil available B, effective Fe and available N were positively correlated to ginsenoside contents [41]. In recent years, due to overexploitation, the destruction of the ecological environment and the lack of proper cultivation practices, the geographical distributions of most DMMs may undergo large changes. For example, although the Changzhi region of Shanxi province and provinces of north-east China was P. ginseng’s original production center, the present production center of it is in Xiaoxinganling region (in Heilongjiang province) and Changbaishan region (in Jilin province) [42]. Similarly, Panax notoginseng (Burk.) F. H. Chen historically came from the Tianzhou region (in Guangxi province), but now the dominant medicinal material comes from Wenshan region (in Yunnan province) [43]. In these cases, predicting the geographical distribution of CMMs is important for resource conservation and regional management. Therefore, a geographic information system based on a computer program (TCMGIS) was developed to predict the distribution of CMMs. By integrating geographic location, climate and soil type databases, TCMGIS was able to determine the impacts of environmental components and predict the large-scale distribution of target medicinal herbs such as P. cablin [44], Artemisia annua L. [45], Polygonum multiflorum Thunb. [46], Morinda officinalis How [47], Aquilaria sinensis (Lour.) Gilg [48], Rheum tanguticum Maxim. ex Balf. [49], Amomum villosum Lour. [50], etc.
Traditional descriptions of daodi medicinal materials by famous physicians in ancient China
In the use of CMMs, a large emphasis has been placed on the identification of DMMs since ancient times. As recorded in Shen Nong Ben Cao Jing (The Divine Shennong’s Classic of Materia Medica, 25–220 A.D.) [51], “each medicinal material has laws for its production region, authenticity, and freshness.” In various chapters of that book, locations in ancient kingdoms and regions, such as mountain valley, river valley or marshes, were mentioned for the first time as medicinal herbs sources. This record indicates that different CMMs come from certain specific areas. In his immense book Ben Cao Jing Ji Zhu (Collection of Commentaries on the Classic of the Materia Medica, 480–498 A.D.) [52], Tao Hongjing, a well-known physician in the Northern and Southern Dynasties (420–589 A.D.), used such terms “good”, “quite good”, “fairly good”, “excellent” and “best” to describe the effects of over 40 medicinal herbals commonly used in medical treatment. Moreover, correlations between the sources, developmental stages and efficacy of these medicinal herbs were described. Sun Simiao, a famous physician and pharmacologist of the Tang Dynasty (618–907 A.D.), stated the following in his book Qian Jin Yi Fang (Formulas Worth a Thousand Gold Pieces, 682 A.D.) [53]: “Medicinal herbs used by ancient physicians were always from designated original sources, which accounted for their great effectiveness in medical treatment”. In that book, he comprehensively sorted 519 DMMs and systematically stipulated 133 regions of production. According to Kou Zongshi, a famous physician of the Song Dynasty (960–1279 A.D.), in his book Ben Cao Yan Yi (Extension of the Materia Medica, 1116 A.D.), “in prescribing medicinal herbs, care should always be taken to select those from proper sources to ensure their effectiveness”, greatly emphasizing the designated original sources of medicinal herbs [54]. During the Jin and Yuan Dynasties (1115–1368 A.D.), the text Yong Yao Fa Xiang (Medication Method, 1249 A.D.) also suggested that one could achieve excellent treatment results only by using DMMs with proper production regions and harvest time. Then, the Ming Dynasty (1368-1644 A.D.) document Ben Cao Meng Quan (Materia Medica Companion, 1565 A.D.) stated that “the effect will be definitely different if medicinal materials are produced in a different environment” [55]. The record in Yi Xue Yuan Liu Shi (Origins of Medicine, 1767 A.D.) attached similar importance to the use of DMMs collected from certain original sources [56]. All of this historical literature showed that the use of DMMs has been a practice since ancient times.
The scientific elucidation of daodi medicinal materials
DMMs are the subset of CMMs that meet the highest quality criteria. DMMs are not only associated with specific geographic regions (Fig. 4) but also linked to the chemical components and pharmacological function of CMMs.
Fig. 4.
The distribution of some daodi medicinal materials in China
Chemical components
As we described above, ecological environments such as topography, sunshine, soil, and water directly influence the secondary metabolites (many of which are bioactive components) in medicinal herbs. The many names of DMMs reflect the connotations of production regions; for instance, “qin pi” (Fraxinus chinensis Roxb.), “fen qi” (Astragalus membranaceus (Fisch.) Bge.), “huai di huang” (Rehmannia glutinosa Libosch.) and “ba dou” (Croton tiglium L.), where “qin”, “fen”, “huai”, and “ba” refer to the names of regions used over the course of ancient Western Zhou Dynasty (1046–771 B.C.) [1]. Modern experimental research has validated that DMMs growing in a certain production region are often of high quality (Table 1). For example, the ancient Chinese medicine book Xin Xiu Ben Cao (Newly Revised Materia Medica, 659 A.D.) [57] said that “Fraxinus chinensis Roxb., which can change the color of water to a fluorescent color after soaking, is thought to be superior in quality” (Fig. 5). Currently, scientific evidence supporting the rational for such description is available. F. chinensis produced from Shaanxi province has a higher content of aesculin and aesculetin than that produced in Sichuan province and Liaoning province, and its stronger fluorescence reaction is consistent with the description written in ancient times [58, 59]. The same observation is true for Astragalus membranaceus (Fisch.) Bge. This herb is principally cultivated in a region in Shanxi province, and the cultivar produced in this region contains more astragaloside than do cultivars produced in Shandong, Inner Mongolia, Hebei and Jilin provinces [60]. It is generally recognized that Rehmannia glutinosa Libosch. cultivated in Henan province is of particularly high quality. Modern experimental studies have demonstrated that higher levels of the active constituent catalpol content are present in R. glutinosa grown in this region than in cultivars grown in areas of Xianyang (in Shaanxi province) and Dali (in Shaanxi province) [61]. Similarly, A. villosum cultivated in Yangchun (in Guangdong province) is believed to be superior in quality. The effective medicinal elements in A. villosum is bornyl acetate. Modern experimental research has validated that Yangchun-cultivated A. villosum has the highest effective component content among different populations [62–64]. In other example, P. cablin cultivated in Shipai (in Guangzhou city, Guangdong province) is of particularly high quality. It produced higher levels of the active constituent pogostone than that cultivated in Gaoyao (in Zhaoqing city, Guangdong province), Leizhou (in Zhanjiang city, Guangdong province), Wuchuan (in Zhanjiang city, Guangdong province) and Hainan province [65–70]. Interestingly, the same is true for toxicology. A. carmichaelii produced from plantation sites at Jiangyou county of Sichuan province is believed to be superior in quality. The proportions of the major bioactive constituents monoester alkaloids to toxic constituents diester alkaloids amount among 5 samples of A. carmichaelii from different localities were well correlative with their regions of cultivation. The highest proportion occurred in cultivar Jiangyou (in Sichuan province), followed by cultivars Hanzhong (in Shaaxi province), Butuo (in Sichuan province), Weishan (in Yunnan province), and Anxian (in Sichuan province) [71].
Table 1.
The contents (%) of active constituents in different Chinese medicinal materials in daodi production region and non-daodi production regions
| No. | CMM | Active constituents | Content (%) | References | ||||
|---|---|---|---|---|---|---|---|---|
| Daodi production region | Non-daodi production region | |||||||
| 1 | Fraxinus chinensis Roxb. | Aesculin, aesculetin | Shaanxi: aesculin (4.37%), aesculetin (1.92%) | Sichuan: aesculin (1.10%), aesculetin (0.21%) | Liaoning: aesculin (2.21%), aesculetin (0.19%) | [58, 59] | ||
| 2 | Astragalus membranaceus (Fisch.) Bge. | Astragaloside | Shanxi: 0.37% | Shandong: 0.12% | Inner Mongolia: 0.11% | Hebei: 0.29% | Jilin: 0.32% | [60] |
| 3 | Rehmannia glutinosa Libosch. | Catalpol | Henan: 0.76% | Xianyang, Shaanxi: 0.34% | Dali, Shaanxi: 0.33% | [61] | ||
| 4 | Amomum villosum Lour. | Bornyl acetate | Yangchun, Guangdong: 65.82% | Gaozhou, Guangdong: 55.95% | Guangxi: 61.75% | Yunnan: 50.92% | Burma: 60.71% | [62–64] |
| 5 | Pogostemon cablin (Blanco) Benth. | Pogostone | Shipai, Guangzhou, Guangdong: 68.43% | Gaoyao, Zhaoqing, Guangdong: 26.15% | Leizhou, Zhanjiang, Guangdong: 4.78% | Wuchuan, Zhanjiang, Guangdong: 5.20% | Hainan: 8.97% | [65–70] |
CMM Chinese medicinal material
Fig. 5.
The herbal classic Xin Xiu Ben Cao (Newly Revised Materia Medica) describes the metachromatism-based quality evaluation of “qin pi” (Fraxinus chinensis Roxb.)
Pharmacological functions
Pharmacological functions are actually the outside manifestations of CMMs. In the case of DMMs, the conditions in a certain region are thought to confer clinical superiority, and for this reason, DMMs are considered the most efficacious among CMMs [72]. Scientific evidence supporting the alleged clinical superiority of DMMs is the subject of ongoing research. For example, Dendrobium huoshanense C. Z. Tang et S. J. Cheng produced in Huoshan (in Anhui province) is considered to be superior in quality. Accordingly, the hepatoprotective effect is the best for the Huoshan cultivar, second for the Yunnan cultivar, and last for the other region cultivars [73]. P. cablin has been classified into two chemotypes, the patchoulol-type, including cultivars HN and ZJ, and the pogostone-type, including cultivars SP and GY. Accordingly, this cultivars produced in GY are more potent than those from ZJ in terms of promoting digestion [74] and antibacterial [75] effects. In addition, the toxicity of DMMs is often less potent than that of non-DMMs. For instance, P. multiflorum is principally cultivated in region Deqing county of Guangdong province, which has the largest output and the longest history of medicinal use. Accordingly, cultivar Deqing showed less potent cytotoxicity than cultivar Chongqing in HepG2 and LO2 cells [76]. These studies show considerable promise for explaining the scientific mechanism of DMM superiority.
Daodi medicinal materials are the basis of the medicinal industry and clinical practice
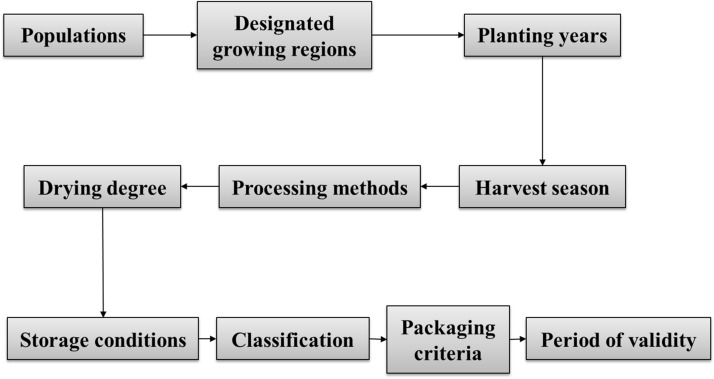
CMMs are the materials processed into decoction ingredients or used to produce proprietary drugs. The identification of DMMs is important in quality evaluation and disease treatment. In addition to the optimum harvest season, the processing and standard prescription of CMMs produce the unique characteristics of DMMs, and a plausible production chain of CMMs is hypothesized in Fig. 6. The production chain of CMMs is based on the content we described above, including populations, designated growing regions (daodi), and harvest season, as well as the extensive quality control knowledge accumulated for CMMs by Liu et al. [77–80]. Compared to previous production chain of CMMs [77–80], factors such as populations, designated growing regions and harvest time directly influenced the quality of CMMs were emphasized. Every procedure in the production chain should be standardized to guarantee the prominent curative effect of medicinal materials. For example, the manufacturing procedure for Pinellia ternata (Thunb.) Breit. was standardized by orthogonal design [81]. In addition, modern analytical methods have revealed the processing mechanism of many CMMs, including Coptis chinensis Franch. [82–84], Xanthium sibiricum Patr. [85–87], Siegesbeckia orientalis L. [88, 89], Descurainia sophia (L.) Webb. ex Prantl. [90, 91], Cassia obtusifolia L. [92, 93], etc. Additionally, cooperation among universities, research institutes and pharmaceutical manufacturers should be strengthened to communicate information regarding CMMs.
Fig. 6.
A putative production chain for CMMs
Conclusions
DMMs have long maintained, currently have, and will continue to maintain a good reputation on the basis of their excellent curative effects. In our review, DMM is the specific term for pure, superior medicinal herbs with the following characteristics: optimum harvest season (reflecting the appropriate developmental stage of the plant), scrupulous processing, traditional preparation technology, etc. Historical literature, modern phytochemical and pharmacological methods have provided additional scientific data and a theoretical basis to validate the mechanisms of DMMs. In addition, every procedure in the production chain of CMMs should be standardized to guarantee the prominent curative effect of medicinal materials. Effectively establishing a correlation among the active components, clinical efficacy and identity of DMMs is an important aspect in the quality evaluation of CMMs. The core scientific elucidation of DMMs should be continuously carried out, and multidisciplinary measures should be adopted to explore scientific and practical methodologies for the further research of DMMs.
Acknowledgements
Not applicable.
Abbreviations
- DMMs
daodi medicinal materials
- CMMs
Chinese medicinal materials
- SP
Shipai (in Guangzhou City, Guangdong province)
- GY
Gaoyao (in Zhaoqing City, Guangdong province)
- HN
Hainan province
- ZJ
Zhanjiang City (in Guangdong province)
- NJ
neighbor-joining
- Fe
iron
- Mn
manganese
- K
potassium
- Cu
copper
- Zn
zinc
- B
boron
- Mo
molybdenum
- N
nitrogen
- Cd
cadmium
- As
arsenic
- Hg
mercury
- Pb
lead
- Mg
magnesium
- Ca
calcium
- TCMGIS
geographic information system based on a computer program
Authors’ contributions
HC and XL conceived, designed and wrote the review; YZ, MW and ZM collected the data; YZ, ZH, FT, SD, SL, YZ, JZ, NL and XH revised the paper. All authors read and approved the final manuscript.
Funding
This work was supported by the 6th National Academic Experience Inheritance Program of Famous Chinese Medicine Experts (Prof. Hui Cao) (No. 176-2017-XMZC-0166-01).
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xindan Liu, Email: 627983246@qq.com.
Ying Zhang, Email: zhangying@jnu.edu.cn.
Menghua Wu, Email: zyfxwmh@163.com.
Zhiguo Ma, Email: mzg79@hotmail.com.
Zihan Huang, Email: 522395325@qq.com.
Fang Tian, Email: 1287882739@qq.com.
Sihan Dong, Email: 464953504@qq.com.
Simin Luo, Email: 1261908220@qq.com.
Yu Zhou, Email: 1102620734@qq.com.
Jinju Zhang, Email: 1549635053@qq.com.
Nanxin Li, Email: 1187021924@qq.com.
Xiaofang He, Email: 1343138782@qq.com.
Hui Cao, Email: kovhuicao@aliyun.com.
References
- 1.Zhao ZZ, Guo P, Brand E. The formation of daodi medicinal materials. J Ethnopharmacol. 2012;140(3):476–481. doi: 10.1016/j.jep.2012.01.048. [DOI] [PubMed] [Google Scholar]
- 2.Xiao XH, Chen SL, Huang LQ, Xiao PG. Survey of investigation on daodi Chinese medicinal materials in China sinces 1980s. China J Chin Mater Med. 2009;34(5):519–523. [PubMed] [Google Scholar]
- 3.Fan Y. Hou Han Shu (後漢書) Beijing: Zhonghua Book Company; 1965. [Google Scholar]
- 4.Cao H, Liao G. “One Belt, One Road” Chinese Medicine Cultural Relics Collection (“壹帶壹路”中醫藥文物圖譜集) Guangzhou: Jinan University Press; 2016. [Google Scholar]
- 5.Gao XM. Lectures on Chinese pharmacology-genuine and high-quality drugs. J Tradit Chin Med. 1994;14(2):147–151. [PubMed] [Google Scholar]
- 6.Cao H, Zhang Y. The latest progress in study of DNA molecular marker used in quality evaluation of Chinese medicinal materials. World Sci Technol Tradit Chin Med. 2003;5(1):39–47. [Google Scholar]
- 7.Cao H, Yang MS, Xiao PG. Recent advances in gene technology for identification of genuineness of Chinese herbal drugs. Acta Pharm Sin. 2005;40(7):577–584. [PubMed] [Google Scholar]
- 8.Cao H, Shaw PC, But PH. Molecular Identification of Chinese Medicinal Materials: Technology and Application (中藥分子鑒定技術與應用) Beijing: People’s Medical Publishing House; 2016. [Google Scholar]
- 9.Xu W, Wu YQ, Ding HR, Xiu YF. Research progress on pharmacological effects and mechanism of Herba Pogostemonis. Shanghai J Tradit Chin Med. 2017;51:103–106. [Google Scholar]
- 10.Chen XY, Chen HM, Liu YH, Zhang ZB, Zheng YF, Su ZQ, et al. The gastroprotective effect of pogostone from Pogostemonis Herba against indomethacin-induced gastric ulcer in rats. Exp Bio Med. 2016;241:193–204. doi: 10.1177/1535370215600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang XF, Huang YF, Wang L, Xu LQ, Yu XT, Liu YH, et al. Photo-protective activity of pogostone against UV-induced skin premature aging in mice. Exp Gerontol. 2016;77:76–86. doi: 10.1016/j.exger.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 12.Su JY, Luo X, Zhang XJ, Deng XL, Su ZR, Zhou L, et al. Immunosuppressive activity of pogostone on T cells: blocking proliferation via S phase arrest. Int Immunopharmacol. 2015;26:328–337. doi: 10.1016/j.intimp.2015.04.019. [DOI] [PubMed] [Google Scholar]
- 13.Luo JP, Liu YP, Feng YF, Cao H. Two chemotypes of Pogostemon cablin and influence of region of cultivation and harvesting time on volatile oil composition. Acta Pharm Sin. 2003;38(4):307–310. [PubMed] [Google Scholar]
- 14.Zhang Y, Chen Y, Zhang JC, Yang MS, Cao H, Xiao PG. Correlation between ITS genotype and geographical distribution of Pogostemon cablin. Acta Pharm Sin. 2007;42(1):93–97. [PubMed] [Google Scholar]
- 15.Zhang Y. Study on Guangdong authentic and superior medical material Pogostemon cablin by GC-MS fingerprinting and DNA molecular markers analysis. Beijing: Beijing University of Chinese Medicine; 2007. [Google Scholar]
- 16.Liu YP, Luo JP, Feng YF, Guo XL, Cao H. DNA profiling of Pogostemon cablin chemotypes differing in essential oil composition. Acta Pharm Sin. 2002;37(4):304–308. [PubMed] [Google Scholar]
- 17.Li YM, Jia M, Li HQ, Zhang ND, Wen X, Rahman K, et al. Cnidium monnieri: a review of traditional uses, phytochemical and ethnopharmacological properties. Am J Chinese Med. 2015;43(5):835–877. doi: 10.1142/S0192415X15500500. [DOI] [PubMed] [Google Scholar]
- 18.Cai JN, Zhang L, Wang ZT, Xu LS, Du F, Xu GJ. Variation and regularity of coumarin constituents in Fructus Cnidii collected from different regions of China. Acta Pharm Sin. 1999;34(10):767–771. [Google Scholar]
- 19.Cao H, Cai JN, Liu YP, Wang ZT, Xu LS. Correlative analysis between geographical distribution and nucleotide sequence of chloroplast matK gene of Cnidium monnieri fruit in China. Chin Pharm J. 2001;36(6):373–376. [Google Scholar]
- 20.Ding XY, Wang ZT, Xu LS, Xu H, Zhou KY, Shi GX. Study on sequence difference and SNP pheomenon of rDNA ITS region in F type and H type population of Dendrobium officinale. China J Chin Mater Med. 2002;27(2):85–89. [PubMed] [Google Scholar]
- 21.Sugimoto N, Kiuchi F, Mikage M, Mori M, Mizukami H, Tsuda Y. DNA profiling of Acorus calamus chemotypes differing in essential oil composition. Biol Pharm Bull. 1999;22(5):481–485. doi: 10.1248/bpb.22.481. [DOI] [PubMed] [Google Scholar]
- 22.Cai CH, Li P, Li SL, Dong TX, Zhan HQ. Sequences of 5S-rRNA gene spacer region and comparison of alkaloid content in Fritillaria thunbergii from different habitats. J Chin Med Mater. 2001;24(3):157–159. [PubMed] [Google Scholar]
- 23.Huang SJ, Aleksashin NA, Loveland AB, Klepacki D, Reier K, Kefi A, et al. Ribosome engineering reveals the importance of 5S rRNA autonomy for ribosome assembly. Nat Commun. 2020;11(1):2900. doi: 10.1038/s41467-020-16694-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Y, Wu YG, Xu Y, Zhang JF, Song XQ, Zhu GP, et al. Dynamic accumulation of sesquiterpenes in essential oil of Pogostemon cablin. Rev Bras Farmacogn. 2014;24(6):626–634. [Google Scholar]
- 25.Jin LN, Liu YM, Xiong YX, Chen KL. Study on the best harvesting period of Ziqiu Chaenomeles speciosa. J Chin Med Mater. 2014;37(6):963–966. [Google Scholar]
- 26.Chen DN, Tang XM, Zhang CR, Yang Q. Study on yield and quality of Desmodii Styracifolii Herba in different harvest seasons. Mod Chin Med. 2018;20(4):450–452. [Google Scholar]
- 27.Shi WT, Wu XJ, Wang X, Liang YZ. Analysis of effect of light intensity on the volatile components in Houttuynia cordata Thunb by gas chromatography-mass spectrometry combined with chemometric methods. J Instrum Anal. 2014;33(2):127–132. [Google Scholar]
- 28.Yan XL, Guo QS, Shi HZ, Xie XH. Effects of light intensities on growth, physiological characteristic and chemical composition of Viola yedoensis. China J Chin Mater Med. 2018;1(16):1–8. doi: 10.19540/j.cnki.cjcmm.20181226.008. [DOI] [PubMed] [Google Scholar]
- 29.Ji SG, Cai JL, Lu HJ, Zheng ZT. Effect of different light intensity on patchouli alcohol content of Pogostemonis cablin. Hubei Agricul Sci. 2016;55(2):406–409. [Google Scholar]
- 30.Ueda HR. Systems biology flowering in the plant clock field. Mol Syst Biol. 2006;2:60. doi: 10.1038/msb4100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hendel-Rahmanim K, Masci T, Vainstein A, Weiss D. Diurnal regulation of scent emission in rose flowers. Planta. 2007;226(6):1491–1499. doi: 10.1007/s00425-007-0582-3. [DOI] [PubMed] [Google Scholar]
- 32.Mcclung CR. Defence at dawn. Nature. 2011;470:44–45. doi: 10.1038/470044a. [DOI] [PubMed] [Google Scholar]
- 33.Dudareva N. (E)-beta-ocimene and myrcene synthase genes of floral scent biosynthesis in Snapdragon: function and expression of three terpene synthase genes of a new terpene synthase subfamily. Plant Cell. 2003;15(5):1227–1241. doi: 10.1105/tpc.011015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li MM, Liu JM, Luo DH, Zhan RT. Correlation of flavonoids content of Caulis Spatholobi with soil nutrients. Tradit Chin Drug Res Clin Pharmacol. 2017;28(2):238–243. [Google Scholar]
- 35.Lin LW, Zhong JH, Luo BS, Tan J, Liao GR. The distribution of mid- and micro- elements in soils in Citrus grandis growing area and its relationship with active officinal principles. Ecol Environ. 2008;17(3):1179–1183. [Google Scholar]
- 36.Ma YT, Wan DG, Huang QL. Correlation analysis between different soil factors and main components of Polygonum cuspidatum. Lishizhen Med Mater Med Res. 2009;20(6):1520–1522. [Google Scholar]
- 37.Zhang XD, Deng HS, Zhu SM, Cheng M. Effect of soil factors on schisantherin A in Schisandra sphenanthera Rehd. et Wils. J Henan Agri Sci. 2012;41(10):83–88. [Google Scholar]
- 38.Fang CX, Liu HM, Ye Q, Guo L. Effect of water quality on heavy metals in Aconti Lateralis Radix. China Sciencepaper. 2016;11(12):1403–1406. [Google Scholar]
- 39.Guo LP, Wang S, Zhang J, Yang G, Zhao MX, Ma WF, et al. Effects of ecological factors on secondary metabolites and inorganic elements of Scutellaria baicalensis and analysis of geoherblism. Sci China Life Sci. 2013;56(11):1047–1056. doi: 10.1007/s11427-013-4562-5. [DOI] [PubMed] [Google Scholar]
- 40.Guo LP, Wang S, Zhang J, Huang LQ. Effects of ecological factors on secondary metabolites and inorganic elements of Scutellaria baicalensis and analysis of geoherblism. Sci China. 2014;44(1):66–74. doi: 10.1007/s11427-013-4562-5. [DOI] [PubMed] [Google Scholar]
- 41.Xie CX, Suo FM, Jia GL, Song JY, Huang LF, Chen SL. Correlation between ecological factors and ginsenosides. Acta Ecol Sin. 2011;31(24):7551–7563. [Google Scholar]
- 42.Guo HM, Cong W, Meng XC. History and prospects for Chinese medicine resource in northeast China. Mod Chin Med. 2017;19(9):1326–1330. [Google Scholar]
- 43.Cui XM, Xu LS, Wang Q, Chen ZJ. Analysis on the geologic background and physicochemical properties of soil for the cultivation of Panax notoginseng in Yunnan province. China J Chin Mater Med. 2005;30(5):332–335. [PubMed] [Google Scholar]
- 44.Wu ML, Li XW, Huang SJ, Wu J, Chen SL. A suitability evaluation of the global producing area of Pogostemon cablin based on GMPGIS. World Sci Technol Mod Tradit Chin Med. 2016;18(8):1251–1257. [Google Scholar]
- 45.Huang LF, Xie CX, Duan BZ, Chen SL. Research mapping the potential distribution of high artemisinin-yielding Artemisia annua L. (qinghao) in China with a geographic information system. Chin Med. 2010;5(1):18. doi: 10.1186/1749-8546-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang ZH, Xu W, Zhang J, Qiu XH. Global ecological suitability analysis of Polygonum multiflorum Thunb. World Chin Med. 2017;12(5):982–985. [Google Scholar]
- 47.Gong L, Wang P, Tan RX, Huang ZH. Global producing area of south Chinese medicine Morinda officinalis How. World Chin Med. 2017;12(5):986–988. [Google Scholar]
- 48.Xu J, Wang P, Li XW, Huang ZH. Global suitability analysis of Aquilaria sinensis (Lour.) Gilg base on GMPGIS. World Chin Med. 2017;12(5):989. [Google Scholar]
- 49.Yu H, Xie C, Song J, Zhou Y, Chen S. TCMGIS-II based prediction of medicinal plant distribution for conservation planning: a case study of Rheum tanguticum. Chin Med. 2010;5:31. doi: 10.1186/1749-8546-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gong L, Ye YS, Wu J, Huang ZH. Global production area prediction of Amomum villosum Lour. through GMPGIS. World Chin Med. 2017;12(5):989–991. [Google Scholar]
- 51.Wu P, Sun XY, Sun FJ. Shen Nong Ben Cao Jing (神農本草經) Beijing: People’s Medical Publishing House; 1984. [Google Scholar]
- 52.Tao HJ, Shang ZJ. Ben Cao Jing Ji Zhu (本草經集注) Beijing: People’s Medical Publishing House; 1994. p. 4. [Google Scholar]
- 53.Sun SM. Qian Jin Ji Fang (千金翼方) Shenyang: Liaoning Science and Technology Publishing House; 1997. p. 45. [Google Scholar]
- 54.Kou ZS. Ben Cao Yan Yi (本草衍義) Beijing: People’s Medical Publishing House; 1990. p. 144. [Google Scholar]
- 55.Chen JM, Zhang YS, Han XJ, Zhao HL. Ben Cao Meng Quan (本草蒙筌) Beijing: Ancient Books of Traditional Chinese Medicine Press; 2009. [Google Scholar]
- 56.Xu DC, Wan F. Yi Xue Yuan Liu Shi (醫學源流史) Beijing: People’s Medical Publishing House; 2007. [Google Scholar]
- 57.Su J. Xin Xiu Ben Cao (新修本草) Anhui: Anhui Science & Technology Publishing House; 2005. p. 188. [Google Scholar]
- 58.Zuo YM, Wang ZY, Cui HH, Liu LM. Determination of aesculin and aesculetin in periderm and leaves of Cortex Fraxini among different provenances. Chin Tradit Pat Med. 2003;25(7):552–554. [Google Scholar]
- 59.Liu LM, Li ML, Feng WH, Chen L, Wang RH, Wu P. Determination of coumarins in Fraxinus chinensis by HPLC. Chin Tradit Herb Drugs. 2004;35(7):819–822. [Google Scholar]
- 60.Wang ZQ, Jia JM, Pei CY. Determination of astragaloside IV in Radix Astragalus from different habitats by HPLC-ELSD. China Pharm. 2013;22(19):10–12. [Google Scholar]
- 61.Du JS, Li QA. Comparative study on quality of rehmannia rehmannii and rehmannia huaidi in shaanxi province. Neimonggu J Tradit Chin Med. 2013;32(12):126–127. [Google Scholar]
- 62.Ao H, Liu HM, Wang JR, Ye Q. Determination of volatile oil by GC-MS and evaluation of heavy metals residue in Fructus Amomi from different producing areas. Tradit Chin Drug Res Clin Pharmacol. 2016;27(2):250–254. [Google Scholar]
- 63.Zhao HN, Huang LF, Liu XL, Ji SG. Study on quality variation of Amomum villosum Lour. from different producing areas. Acad J Guangdong Coll Pharm. 2016;32(2):176–180. [Google Scholar]
- 64.Fu C, Zhou RS, Zhou GX. GC-MS analysis of chemical constituents of essential oil from fruits of Amomum villa Lour growing in different production regions. Lishizhen Med Mater Med Res. 2010;21(10):2534–2536. [Google Scholar]
- 65.Luo JP, Feng YF, He B, Guo XL, Chen XX, Cao H, et al. Authentic research of Pogostemon cablin. J Chin Med Mater. 2005;28(12):1121–1125. [Google Scholar]
- 66.Luo JP, Feng YF, Guo XL. Analysis of volatile oil of Pogostemon cablin. Chin Tradit Herb Drugs. 2001;32(4):299–302. [Google Scholar]
- 67.Luo JP, Feng YF, Guo XL, Li XQ. GC-MS analysis of volatile oil of Herba Pogostemonis collected from Gaoyao county. J Chin Med Mater. 1999;22(1):25–28. [PubMed] [Google Scholar]
- 68.Feng YF, Guo XL, Luo JP. GC-MS analysis of volatile oil of Herba Pogostemonis collected from Leizhou county. J Chin Med Mater. 1999;22(5):241–243. [PubMed] [Google Scholar]
- 69.Guo XL, Feng YF, Luo JP. GC-MS analysis of volatile oil of Herba Pogostemonis collected from Wuchuan county. J Chin Med Mater. 2002;25(4):262–263. [PubMed] [Google Scholar]
- 70.Luo JP, Guo XL, Feng YF. Constituents analysis on volatile oil of Pogostemon cablin from different collection time cultivated in Hainan. J Chin Med Mater. 2002;25(1):21–23. [PubMed] [Google Scholar]
- 71.Zhang DK, Wang JB, Yang M, Peng C, Xiao XH. Exploring in integrated quality evaluation of Chinese herbal medicines: the integrated quality index (IQI) for aconite. China J Chin Mater Med. 2015;40(13):2582–2588. [PubMed] [Google Scholar]
- 72.Yang X, Tian X, Zhou Y, Liu Y, He L. Evidence-based study to compare daodi traditional Chinese medicinal material and non-daodi traditional Chinese medicinal material. Evid Based Complement Alternat Med. 2018;2018:1–12. doi: 10.1155/2018/6763130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang K, Sui DJ, Wang CS, Wei Y. Protective effects of five different types of Dendrobium on CCl4-induced liver injury in mice. China J Chin Mater Med. 2017;42(10):1945–1950. doi: 10.19540/j.cnki.cjcmm.2017.0082. [DOI] [PubMed] [Google Scholar]
- 74.He B, Chen XX, Li XQ, Luo JP. Comparison of effects of Herba Pogostemonis from Gaoyao and Wuchuan on digestive system. J Chin Med Mater. 1999;22(4):201–203. [PubMed] [Google Scholar]
- 75.Liu HH, Luo JP, Lai PL. Studies on the anti-enteropathogenic bacteria action of Herba Pogostemonis extracts. J Chin Med Mater. 1999;22(8):408–411. [PubMed] [Google Scholar]
- 76.Rui W. Identification, activity evaluation and biosynthetic pathways analysis of the differential constituents in aqueous-methanol extracts of Polygonum multiflorum Thunb. Guangzhou: Southern Medical University; 2018. [Google Scholar]
- 77.Zhang L, Yan JB, Liu XM, Ye ZG, Yang XH, Meyboom R, et al. Pharmacovigilance practice and risk control of traditional Chinese medicine drugs in China: current status and future perspective. J Ethnopharmacol. 2012;140(3):519–525. doi: 10.1016/j.jep.2012.01.058. [DOI] [PubMed] [Google Scholar]
- 78.Liu Y, Nie Q, Chen J. Suggestions to strengthen quality management of herbal decoction pieces—based on production chain of herbal decoction pieces. China J Chin Mater Med. 2015;40(16):3319–3322. [PubMed] [Google Scholar]
- 79.Yu WK, Dong L, Pei WX, Wang Y. Development of whole process quality control and management system of traditional Chinese medicine decoction pieces based on traditional Chinese medicine quality tree. China J Chin Mater Med. 2017;42(23):4488–4493. doi: 10.19540/j.cnki.cjcmm.20171113.004. [DOI] [PubMed] [Google Scholar]
- 80.Zhang C, Liu Y, Xiao YQ. Strengthening production management of Chinese medicinal materials and stabilizing quality of traditional Chinese medicine products. Chin J Exp Tradit Med Form. 2017;23(15):1–4. [Google Scholar]
- 81.Su T, Zhang WW, Zhang YM, Cheng BCY, Fu XQ, Li T, et al. Standardization of the manufacturing procedure for Pinelliae Rhizoma Praeparatum cum Zingibere et Alumine. J Ethnopharmacol. 2016;193:663–669. doi: 10.1016/j.jep.2016.09.038. [DOI] [PubMed] [Google Scholar]
- 82.Huang P, Qian XC, Li JS, Cui XB, Chen LH, Cai BC, et al. Simultaneous determination of 11 alkaloids in crude and wine-processed Rhizoma Coptidis by HPLC-PAD. J Chromatogr Sci. 2015;53(1):73–78. doi: 10.1093/chromsci/bmu019. [DOI] [PubMed] [Google Scholar]
- 83.Qian XC, Zhang L, Tao Y, Huang P, Li JS, Chai C, et al. Simultaneous determination of ten alkaloids of crude and wine-processed Rhizoma Coptidis aqueous extracts in rat plasma by UHPLC-ESI-MS/MS and its application to a comparative pharmacokinetic study. J Pharmaceut Biomed. 2015;105:64–73. doi: 10.1016/j.jpba.2014.11.049. [DOI] [PubMed] [Google Scholar]
- 84.Yang CY, Guo FQ, Zang C, Li C, Cao H, Zhang BX. The effect of ginger juice processing on the chemical profiles of Rhizoma coptidis. Molecules. 2018;23(2):1–14. doi: 10.3390/molecules23020380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Su T, Cheng BCY, Fu XQ, Li T, Guo H, Cao HH, et al. Comparison of the toxicities, activities and chemical profiles of raw and processed Xanthii Fructus. Bmc Complem Altern M. 2016;16:1–8. doi: 10.1186/s12906-016-0994-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jiang H, Yang L, Xing XD, Yan ML, Guo XY, Hou AJ, et al. A UPLC-MS/MS application for comparisons of the hepatotoxicity of raw and processed Xanthii Fructus by energy metabolites. Rsc Adv. 2019;9(5):2756–2762. doi: 10.1039/c8ra08272c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jiang H, Yang L, Xing XD, Yan ML, Guo XY, Yang BY, et al. Chemometrics coupled with UPLC-MS/MS for simultaneous analysis of markers in the raw and processed Fructus Xanthii, and application to optimization of processing method by BBD design. Phytomedicine. 2019;57:191–202. doi: 10.1016/j.phymed.2018.12.020. [DOI] [PubMed] [Google Scholar]
- 88.Su T, Yu H, Kwan HY, Ma XQ, Cao HH, Cheng CY, et al. Comparisons of the chemical profiles, cytotoxicities and anti-inflammatory effects of raw and rice wine-processed Herba Siegesbeckiae. J Ethnopharmacol. 2014;156:365–369. doi: 10.1016/j.jep.2014.09.038. [DOI] [PubMed] [Google Scholar]
- 89.Li SJ, Liu DY, Liu P, Fu ZH, Sun ML, Zhang YH, et al. Comparison of attenuating renal ischemia/reperfusion injury effects of raw and honey wine-processed Herba Siegesbeckiae. Int J Clin Exp Med. 2017;10(1):524–531. [Google Scholar]
- 90.Luo HY, Yu XK, Zhang X, Wang ZJ. Determination of contents of five components in Descurainiae semen before and after being processed by HPLC. Chin J Exp Tradit Med Form. 2019;25(10):115–120. [Google Scholar]
- 91.Li HW, Shi YB, Tian LQ, Feng WS. Effects of five processing methods on compositions and contents of fatty oils in Descurainiae Semen. Chin Tradit Pat Med. 2017;39(8):1661–1665. [Google Scholar]
- 92.Guo RX, Wu HW, Yu XK, Xu MY, Zhang X, Tang LY, et al. Simultaneous determination of seven anthraquinone aglycones of crude and processed Semen Cassiae extracts in rat plasma by UPLC-MS/MS and its application to a comparative pharmacokinetic study. Molecules. 2017;22(11):1–14. doi: 10.3390/molecules22111803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Guo RX, Yu XK, Zhang X, Wang ZJ. Chemical study on Cassiae Semen processing procedure. China J Chin Mater Med. 2018;43(15):3145–3149. doi: 10.19540/j.cnki.cjcmm.20180611.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.