Abstract
IMPORTANCE
Early pregnancy loss (EPL) is the most common complication of pregnancy. A multicenter randomized clinical trial compared 2 strategies for medical management and found that mifepristone pretreatment is 25% more effective than the standard of care, misoprostol alone. The cost of mifepristone may be a barrier to implementation of the regimen.
OBJECTIVE
To assess the cost-effectiveness of medical management of EPL with mifepristone pretreatment plus misoprostol vs misoprostol alone in the United States.
DESIGN, SETTING, AND PARTICIPANTS
This preplanned. prospective economic evaluation was performed concurrently with a randomized clinical trial in 3 US sites from May 1, 2014, through April 30, 2017. Participants included 300 women with anembryonic gestation or embryonic or fetal demise. Cost-effectiveness was computed from the health care sector and societal perspectives, with a 30-day time horizon. Data were analyzed from July 1, 2018, to July 3, 2019.
INTERVENTIONS
Mifepristone pretreatment plus misoprostol administration vs misoprostol alone.
MAIN OUTCOMES AND MEASURES
Costs in 2018 US dollars, effectiveness in quality-adjusted life-years (QALYs), and treatment efficacy. Incremental cost-effectiveness ratios (ICERs) of mifepristone and misoprostol vs misoprostol alone were calculated, and cost-effectiveness acceptability curves were generated.
RESULTS
Among the 300 women included in the randomized clinical trial (mean [SD] age, 30.4 [6.2] years), mean costs were similar for groups receiving mifepristone pretreatment and misoprostol alone from the health care sector perspective ($696.75 [95% CI, $591.88-$801.62] vs $690.88 [95% CI, $562.38-$819.38]; P = .94) and the societal perspective ($3846.30 [95% CI, $2783.01-$4909.58] vs $4845.62 [95% CI, $3186.84-$6504.41]; P = .32). The mifepristone pretreatment group had higher QALYs (0.0820 [95% CI, 0.0815–0.0825] vs 0.0806 [95% CI, 0.0800–0.0812]; P = .001) and a higher completion rate after first treatment (83.8% vs 67.1%; P < .001) than the group receiving misoprostol alone. From the health care sector perspective, mifepristone pretreatment was cost-effective relative to misoprostol alone with an ICER of $4225.43 (95% CI, −$195 053.30 to $367 625.10) per QALY gained. From the societal perspective, mifepristone pretreatment dominated misoprostol alone (95% CI, −$5 111 629 to $1 801 384). The probabilities that mifepristone pretreatment was cost-effective compared with misoprostol alone at a willingnessto-pay of $150 000 per QALY gained from the health care sector and societal perspectives were approximately 90% and 80%, respectively.
CONCLUSIONS AND RELEVANCE
This study found that medical management of EPL with mifepristone pretreatment was cost-effective when compared with misoprostol alone.
TRIAL REGISTRATION
ClinicalTrials.gov Identifier: NCT02012491
Introduction
Early pregnancy loss (EPL) is the most common complication in pregnancy and affects approximately 1 million women in the United States annually.1,2 Women in the first trimester of pregnancy are often diagnosed with a nonviable pregnancy by means of ultrasonography and may prefer or require a clinical intervention to aid in completion of the miscarriage process.3 Current intervention options are classified as surgical (uterine aspiration) or medical (the use of medications to induce uterine contractions and tissue expulsion). These options differ in treatment efficacy, patient experience, and cost.4,5 A prostaglandin, specifically the prostaglandin E1 analogue misoprostol, is the most commonly used medical treatment option for EPL globally but has high failure rates in women with a closed cervical os. Misoprostol failures result in a prolonged treatment course with multiple doses of medication or ultimately in surgical management, all of which, despite the low cost of the drug itself, diminish the cost-effectiveness of medical management of EPL when compared with surgical management.5
Medical management remains an appealing option for patients, clinicians, and health systems.3 The multicenter randomized clinical Pregnancy Failure Regimens Trial6 compared the effectiveness of mifepristone pretreatment followed by misoprostol treatment with misoprostol alone for the management of EPL. Complete expulsion after 1 dose of misoprostol occurred in 124 of 148 women (83.8%) in the mifepristone-pretreatment group and in 100 of 149 women (67.1%) in the misoprostol-alone group a mean of 2 days after treatment. Clinicians in the United States may be hesitant to use mifepristone, however, owing to its mean cost, at the time of this analysis, of $90 per 200-mg pill. We therefore present data from a planned prospective economic evaluation within the multicenter trial to determine the relative cost-effectiveness of pretreatment with mifepristone followed by treatment with misoprostol compared with misoprostol use alone for the medical management of EPL within a 30-day time frame.
Methods
Trial Design and Patients
The Pregnancy Failure Regimens Trial was a pragmatic comparative effectiveness trial conducted at 3 United States sites from May 1, 2014, to April 30, 2017, the details of which have been previously described.6 To summarize, 300 women with a confirmed anembryonic gestation or fetal demise before 12 completed gestational weeks and a closed cervical os were randomized to pretreatment with 200 mg of mifepristone, administered orally, followed by 800 μg of misoprostol, administered vaginally, or 800 μg of misoprostol alone, administered vaginally. Participants were scheduled to return at 24 to 96 hours after misoprostol use (day 3 visit) for assessment of treatment success at this early, patient-centered time point. If the gestational sac was not expelled, expectant management, a second dose of misoprostol, or uterine aspiration was offered. All participants were followed up for 30 days after randomization to verify the classification of the primary outcome of pregnancy expulsion and to assess adverse effects. The trial, including the present economic evaluation, was approved by the institutional review boards at the University of Pennsylvania, Philadelphia, the University of California, Davis, and the Albert Einstein College of Medicine, New York, New York, and written informed consent was obtained from the participants.
Economic Evaluation Design
We conducted a prospective, trial-based cost-effectiveness analysis from the health care sector and societal perspectives to compare the incremental cost per quality-adjusted life year (QALY) gained and the incremental cost per complete gestational sac expulsion between the 2 treatment regimens. This approach conforms with the recommendations of the Second Panel on Cost-Effectiveness in Health and Medicine,7,8 including reference case analysis from the health care sector and societal perspectives, and the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) reporting guideline for health economic evaluations.9 An intent-to-treat approach was used; all participants enrolled in the 2018 trial, regardless of treatment response, were included. The time horizon of the primary analysis was the 30 days of the trial enrollment.
Costs and Use of Resources
Costs from the health care sector perspective included those incurred by payers and participants for the therapies and for EPL-related health care costs. We estimated health care costs by combining health care data for use of services collected for all participants during the trial with national mean Medicare reimbursement rates or published prices. Costs from the societal perspective also included time costs of patients receiving care, time costs of unpaid caregivers, transportation costs, lost productivity, and other costs outside the formal health care sector. Costs related to time, lost productivity, and absenteeism were estimated using the lost wages method.10,11 Total daily compensation was based on mean hourly wages plus benefits for civilian workers in the United States in 2018.12,13 Resource use information was collected prospectively through case report forms completed at the scheduled study visits or telephone calls at study days 3, 8, and 30 and electronic medical record review. We captured EPL-related procedures performed (eg, uterine aspiration), adverse clinical events (eg, infections, additional office visits, emergency department visits, admissions), and other health care interventions for EPL (eg, medications used for pain and symptom management). Our prospective data collection also captured patient and caregiver time, days of school and work missed, and patient costs incurred, including transportation, medications, copayments, and other purchased comfort measures such as heating pads and sanitary supplies during the 30-day study period. Unit cost estimates were applied to calculate the total costs for each participant (details shown in eTable 1 in the Supplement). Results are expressed in 2018 US dollars.
Effectiveness Outcome
The primary effectiveness outcome was the 1-month QALY, which was based on a modified utility score from the EPL literature, with successful medical therapy defined as 1.00, and need for uterine aspiration or a second dosage after a failed medical or procedural treatment defined as 0.90.5 Quality-adjusted life years were calculated from utility scores, which were assumed to be constant during the 1-month trial. Other effectiveness outcomes included treatment success, defined as gestational sac expulsion with 1 dose of misoprostol at the first follow-up visit and no additional intervention within 30 days after treatment.
Statistical Analysis
Data were analyzed from July 1, 2018, to July 30, 2019. The primary cost-effectiveness measure was the incremental cost-effectiveness ratio (ICER), defined as the ratio of the difference in mean cost of mifepristone pretreatment vs misoprostol alone to the difference in mean QALYs for mifepristone pretreatment vs misoprostol alone for the 30-day trial.6 In addition, an ICER was calculated using treatment success as the effectiveness measure; the denominator was the difference in treatment success with 1 dose of misoprostol with mifepristone pretreatment vs misoprostol alone. The ICERs were calculated from the health care sector and societal perspectives.
Univariate cost and effectiveness measures were compared using unpaired t tests to assess differences between the treatment arms. We directly calculated the ICERs and generated 95% CIs from 5000 bootstrapped replications with replacement.14–17 Cost-effectiveness acceptability curves were also generated.16–19
We conducted sensitivity analysis to identify the effect of key variables, including the cost of mifepristone, frequency of treatment success, and utility score differential between treatment success and failure, on cost-effectiveness results. All analyses were conducted using Stata, version 14.2 (StataCorp LLC). P < .05 indicated significance. All analyses were 2 sided.
Results
Participants
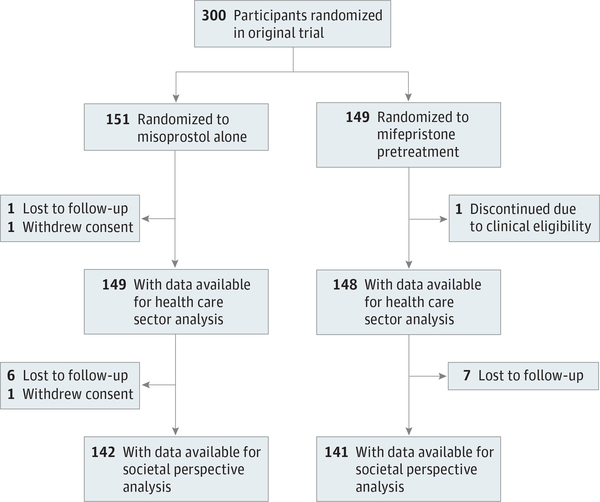
From May 1, 2014, through April 30, 2017, 303 women consented to participation. Three hundred women (mean [SD] age, 30.4 [6.2] years) underwent randomization to the mifepristone-pretreatment group (n = 149) or the misoprostol-alone group (n = 151) (Figure 1). Treatment success, QALY, and health care sector perspective cost data were evaluable for 297 participants; societal perspective costs, for 283 participants. Patient characteristics have been previously reported6 and were similar among groups (eTable 2 in the Supplement). Our study population was racially and ethnically diverse (132 black [44.0%]; 109 white [36.3%]; 78 Hispanic [26.0%]; 20 Asian [6.7%]; and 39 other [13.0%]), and most were college educated (170 [56.7%]). One hundred five participants (35.0%) had experienced miscarriage previously.
Figure 1.
Consort Diagram of Study Participants Included in Cost-effectiveness Analysis
Resources and Costs
From the health care sector perspective, the mean per-person costs were similar between groups: $696.75 (95% CI, $591.88-$801.62) for those receiving mifepristone pretreatment and $690.88 (95% CI, $562.38-$819.38) for those receiving misoprostol alone (P = .94). From the societal perspective, the mean per-person costs were $3846.30 (95% CI, $2783.01-$4909.58) for mifepristone pretreatment and $4845.62 (95% CI, $3186.84-$6504.41) for misoprostol alone (P = .32) (Table 1).
Table 1.
Costs and Outcomes of Medical Management of Early Pregnancy Loss With Mifepristone Pretreatment Plus Misoprostol vs Misoprostol Alone
| Characteristic | Treatment groupa | |||
|---|---|---|---|---|
| Mifepristone pretreatment | Misoprostol alone | P value | ||
| Mean per-person formal health care sector costs, $ | ||||
| Treatment | 555.34 (57.03) | 476.87 (68.15) | <.001 | |
| Uterine aspiration | 41.71 (134.87) | 111.54 (201.99) | <.001 | |
| Second misoprostol dose | 0.22 (0.80) | 0.34 (0.99) | .23 | |
| Unscheduled visitsb | 14.13 (52.68) | 15.44 (54.88) | .83 | |
| Other complicationsc | 84.03 (625.48) | 84.32 (720.62) | >.99 | |
| Pain control measuresd | 1.31 (1.47) | 2.36 (11.28) | .263 | |
| Total, mean (95% Cl) | 696.75 (591.88-801.62) | 690.88 (562.38-819.38) | .94 | |
| Indirect (informal health care and non-health care sector) costs, $ | ||||
| Work and school missed time | 1302.37 (3856.48) | 1683.75 (4464.58) | .42 | |
| Family/friend unpaid caregiver time | 1783.24 (4636.04) | 2382.96 (8010.09) | .43 | |
| Othere | 63.94 (166.23) | 78.61 (206.43) | .50 | |
| Total, mean (95% Cl) | 3149.55 (2089.65-4209.45) | 4154.75 (2515.15-5794.34) | .31 | |
| Total societal perspective costs, mean (95%CI)f | 3846.30 (2783.01–4909.58) | 4845.62 (3186.84–6504.41) | .32 | |
| Outcomes | ||||
| Treatment success after 1 dose of misoprostol, % | 83.8 (37.0) | 67.1 (47.1) | .001 | |
| QALYs per person, mean (95% Cl) | 0.0820 (0.0815–0.0825) | 0.0806 (0.0800–0.0812) | .001 |
Abbreviation: QALYs, quality-adjusted life-years.
Unless otherwise indicated, data are expressed as mean (SD). Costs are given in 2018 US dollars.
Categorized as visits requiring a visit to a clinician and transvaginal ultrasonography.
Included visits to the emergency department related to the miscarriage, such as for pelvic inflammatory disease or need for a transfusion due to hemorrhage.
All patients received a prescription for ibuprofen and codeine to aid in pain management; costs were calculated based on patient self-reports of doses taken of each.
Included self-reported costs such as transportation and parking, sanitary product costs, and heating pads.
Derived by combining the calculated health care sector and indirect costs (indirect costs include informal health care and non-health care sectors).
Clinical Effectiveness and QALYs Outcomes
Treatment success after 1 dose of misoprostol occurred in 124 of 148 women (83.8% [SD, 37.0%]) in the mifepristone-pretreatment group and in 100 of 149 women (67.1% [SD, 47.1%]) in the misoprostol-alone group (relative risk, 1.25; 95% CI, 1.09–1.43) (Table 1). Uterine aspiration was performed less frequently in the mifepristone-pretreatment group than in the misoprostol-alone group (8.8% vs 23.5%; relative risk, 0.37; 95% CI, 0.21–0.68).
The QALYs differed between treatment groups. The mifepristone pretreatment group had a QALY of 0.0820 (95% CI, 0.0815–0.0825) vs 0.0806 (95% CI, 0.0800–0.0812) for the misoprostol-alone group (P = .001) (Table 1).
Cost-effectiveness
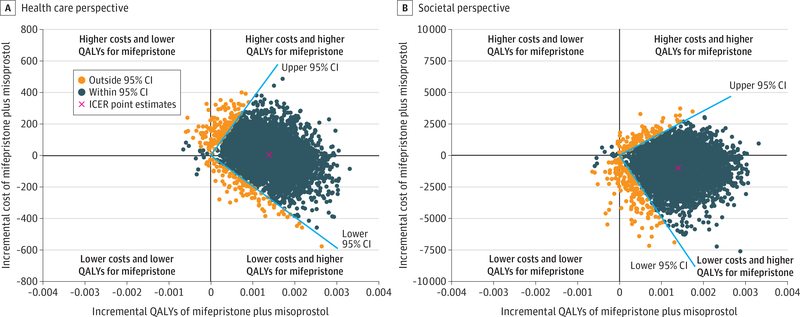
From the health care sector perspective, mifepristone pretreatment was cost-effective in comparison with misoprostol alone, with an ICER of $4225.43 (95% CI, −$195 053.30 to $367 625.10) per QALY gained. This ICER is below the commonly used cost-effectiveness thresholds of $100 000 to $150 000 per QALY.20 From the societal perspective, because incremental costs per QALY gained were negative, results suggest that mifepristone pretreatment dominated misoprostol alone (Table 2). The 95% CI of the ICER (−$5 111 629.02 to $1 801 384.19) (Table 2 and Figure 2) indicates that either (1) mifepristone pretreatment is more costly and more effective than misoprostol alone (upper right quadrant), in which case we are confident that mifepristone pretreatment is a good value if willingness to pay for a QALY gain exceeds the 95% CI upper limit of $367 625.10 (health care sector perspective) or $1 801 384.19 (societal perspective), both above the generally accepted maximum willingness-to-pay threshold $150 000 per QALY20; or (2) mifepristone pretreatment is less costly and more effective (lower right quadrant) and thus dominates misoprostol alone.
Table 2.
Incremental Cost-effectiveness Ratio of Medical Management of Early Pregnancy Loss With Mifepristone Pretreatment Plus Misoprostol vs Misoprostol Alone by QALY and Percentage Treatment Completion
| Characteristic | Mifepristone pretreatment vs misoprostol alonea | P value |
|---|---|---|
| Costs per QALYb | ||
| Health care sector perspective | ||
| Difference in mean costs per person | 5.87 | .94 |
| Difference in mean QALYs per person | 0.0014 | .001 |
| Incremental cost per QALY gained (95% Cl), $ | 4225.43 (−195 053.30 to 367 625.10) | NA |
| Societal perspective | ||
| Difference in mean costs per person | −999.33 | .32 |
| Difference in mean QALYs per person | 0.0014 | .001 |
| Incremental cost per QALY gained (95% Cl), $ | Dominated by mifepristone pretreatment (−5111629.02 to 1801 384.19) | NA |
| Costs per completion after initial treatmentb | ||
| Health care sector perspective | ||
| Difference in mean costs per person | 5.87 | .94 |
| Difference in completion rate, % | 16.7 | .001 |
| Incremental cost per 1% increase in completion rate (95% Cl), $ | 0.35 (−16.25 to 30.64) | NA |
| Societal perspective | ||
| Difference in mean costs per person | −999.33 | .32 |
| Difference in completion rate, % | 16.7 | .001 |
| Incremental cost per 1% increase in completion rate (95% Cl), $ | Dominated by mifepristone pretreatment (−425.97 to 150.12) | NA |
Abbreviations: NA, not applicable; QALY, quality-adjusted life-year.
Costs are given in 2018 US dollars.
The primary cost-effectiveness measure was the 30-day ICER ratio of the difference in mean cost of mifepristone pretreatment vs misoprostol alone to the difference in mean QALYs for mifepristone pretreatment vs misoprostol alone in 2018 US dollars. ICERs were calculated from the health care sector and societal perspectives. ICERs were also calculated using completion after initial treatment as the effectiveness measure. 95%CIs were determined by bootstrapped replications as described in the Methods section and depicted in Figure 2.
Figure 2.
Incremental Cost-effectiveness Ratio (ICER) Scatterplot for Mifepristone Pretreatment Plus Misoprostol vs Misoprostol Only
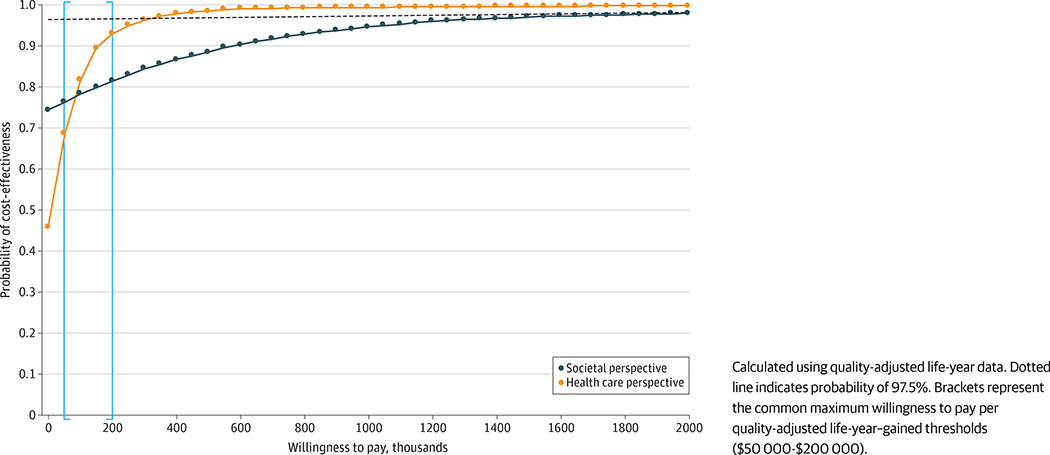
Cost-effectiveness acceptability curve analysis demonstrates that the probabilities that mifepristone pretreatment is cost-effective at the generally accepted maximum willingness-to-pay threshold of approximately $150 000 per QALY gained20 from the health care sector and societal perspectives are approximately 90% and 80%, respectively (Figure 3). When analyzing the cost-effectiveness by treatment success rates, mifepristone pretreatment was cost-effective in comparison with misoprostol alone, with an ICER of $0.35 per 1% in treatment success gained. From the societal perspective, incremental costs per 1% treatment success gained were negative (ICER 95% CI, −$425.97 to $150.12), suggesting that misoprostol alone was dominated by mifepristone pretreatment (Table 2). Uncertainty analysis for percentage completion showed a similar pattern on the cost-effectiveness plane to the results when using QALYs (eFigure 1 in the Supplement). Cost-effectiveness acceptability curve analysis demonstrated that the probability that mifepristone pretreatment is cost-effective relative to misoprostol alone is 97.5% from the health care and societal perspectives for all willingness-to-pay values greater than $30.64 and $150.12 per 1% in completion rate gained, respectively (eFigure 2 in the Supplement).
Figure 3.
Cost-effectiveness Acceptability Curve for Mifepristone Pretreatment Plus Misoprostol vs Misoprostol Alone
Sensitivity Analysis
Even with an increase in the cost of mifepristone to $154 (increase of $64), $224 (increase of $134), or $293 (increase of $203) per dose, mifepristone pretreatment would remain cost-effective from the health care sector perspective at common willingness-to-pay thresholds of $50 000, $100 000, and $150 000 per QALY, respectively. With an increase of percentage completion rate for misoprostol alone from 67.1% to 83.2%, mifepristone pretreatment would remain cost-effective from the health care sector perspective at $50 000 per QALY. With a utility score differential of less than 0.01 between successful medical therapy and the need for uterine aspiration or second dosage after failed treatment, mifepristone pretreatment would remain cost-effective from the health care sector perspective at $50 000 per QALY.
Discussion
This cost-effectiveness comparison of mifepristone pretreatment before misoprostol administration vs misoprostol alone for the management of EPL using data within a randomized clinical trial demonstrates that mifepristone pretreatment is a good value from the health care sector and societal perspectives, with higher effectiveness and similar costs. This analysis demonstrates that the ICER for mifepristone pretreatment is well below the maximum willingness-to-pay threshold of approximately $150 000 per QALY gained. These findings are relevant to the range of stakeholders involved in managing EPL, including individuals who seek treatment, clinicians who offer treatment, and private and public payers of health care. Thus, mifepristone appears to be a valuable addition to the miscarriage treatment offerings.
Limitations
This study benefits from prospectively collected economic data of the cost of miscarriage care from the health care and societal perspectives from a pragmatic clinical trial. However, the clinical trial setting is accompanied by some limitations. At the time of the clinical trial and this analysis, the mean cost of mifepristone was $90 per dose. Since the completion of this analysis, the mifepristone price has decreased to a mean of $54 per dose, which only enhances the cost-effectiveness of mifepristone pretreatment. Generalizability may be limited, and costs may vary regionally owing to variable insurance coverage of mifepristone, resource availability, and reimbursement rates. We used 2018 National Medicaid reimbursement rates to help overcome these limitations and performed sensitivity analyses to demonstrate thresholds at which mifepristone would no longer be cost-effective. We found that if mifepristone’s cost increased from $90 to $293 per dose, it would remain cost-effective. In addition, we made assumptions when assigning utility scores for successful medical management and for failed management. The literature on assignment of utility preference score values in EPL is limited; we found only 1 study describing utility scores in this population.5 In this analysis, we chose utility scores based on the literature and performed sensitivity analysis to determine the smallest difference in utility scores between successful and failed medical management for which mifepristone pretreatment remained cost-effective. We found that if the utility score difference was reduced from 0.10 to less than 0.01, mifepristone pretreatment remained cost-effective. Future studies should strive to define standardized utility scores and QALYs for EPL management to establish generalizable measures that can be used across reproductive health research.
This study shows that mifepristone pretreatment is a cost-effective intervention for people seeking medical management for EPL care. Cost concerns, however, have not been the only barrier to access. Mifepristone is regulated by the Food and Drug Administration with a Risk Evaluation and Mitigation Strategy. This restriction precludes mifepristone’s availability in retail pharmacies, and because most clinics do not stock it,21 patients experiencing EPL have difficulty accessing this highly effective regimen.22 Although our findings do not address this access barrier directly, we believe the cost-effectiveness outcomes we provide herein should reduce access barriers driven by cost concerns.
Conclusions
This study’s results suggest that mifepristone pretreatment is an economically favorable strategy from the health care sector and societal perspectives compared with misoprostol alone. The demonstration of cost-effectiveness in this analysis provides support for mifepristone coverage by insurers, use by clinicians, and access for persons experiencing EPL.
Supplementary Material
Key Points.
Question
Is pretreatment with mifepristone a cost-effective regimen for the medical management of early pregnancy loss?
Findings
In this planned economic evaluation including 300 women from a randomized clinical trial, pretreatment with mifepristone plus misoprostol had fewer treatment failures and an incremental cost-effectiveness ratio of $4225.43 per quality-adjusted life year gained in the United States, compared with the standard regimen of misoprostol alone.
Meaning
These findings suggest that pretreatment with mifepristone is of clinical and economic value when compared with misoprostol alone for the medical management of early pregnancy loss.
Acknowledgments
Funding/Support: This study was supported by Eunice Kennedy Shriver award R01-HD0719-20 (Dr Schreiber) and Women’s Reproductive Health Research award K12-HD001265-18 (Dr Sonalkar) from the National Institute of Child Health and Human Development of the NIH and a Research Fund Midcareer Mentor Award from the Society of Family Planning (Dr Schreiber).
Footnotes
Conflict of Interest Disclosures: Dr Chen reported receiving grants from the National Institutes of Health (NIH) during the conduct of the study. Dr Schreiber reported receiving grants from NIH during the conduct of the study; personal fees from Danco Laboratories LLC outside the submitted work; and having US provisional patent application 62/777,369 pending. No other disclosures were reported.
Role of the Funder/Sponsor: The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Concept and design: Nagendra, Koelper, Atrio, Schreiber, Harvie.
Acquisition, analysis, or interpretation of data: Nagendra, Koelper, Loza-Avalos, Sonalkar, Chen, Schreiber, Harvie.
Drafting of the manuscript: Nagendra, Koelper, Loza-Avalos, Harvie.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Nagendra, Koelper, Harvie.
Obtained funding: Schreiber.
Administrative, technical, or material support: Nagendra, Loza-Avalos, Atrio, Schreiber, Harvie.
Supervision: Schreiber, Harvie.
+ Supplemental content
Author affiliations and article information are listed at the end of this article.
Contributor Information
Divyah Nagendra, Department of Obstetrics and Gynecology, University of Pennsylvania Perelman School of Medicine, Philadelphia.
Nathanael Koelper, Department of Obstetrics and Gynecology, University of Pennsylvania Perelman School of Medicine, Philadelphia.
Sandra E. Loza-Avalos, Department of Obstetrics and Gynecology, University of Pennsylvania Perelman School of Medicine, Philadelphia.
Sarita Sonalkar, Department of Obstetrics and Gynecology, University of Pennsylvania Perelman School of Medicine, Philadelphia.
Melissa Chen, Department of Obstetrics and Gynecology, University of California, Davis, Sacramento.
Jessica Atrio, Department of Obstetrics and Gynecology, Montefiore Hospital, Albert Einstein College of Medicine, Bronx, New York.
Courtney A. Schreiber, Department of Obstetrics and Gynecology, University of Pennsylvania Perelman School of Medicine, Philadelphia.
Heidi S. Harvie, Department of Obstetrics and Gynecology, University of Pennsylvania Perelman School of Medicine, Philadelphia.
REFERENCES
- 1.Casterline JB. Collecting data on pregnancy loss: a review of evidence from the World Fertility Survey. Stud Fam Plann 1989;20(2):81–95. doi: 10.2307/1966462 [DOI] [PubMed] [Google Scholar]
- 2.Ventura SJ, Curtin SC, Abma JC, Henshaw SK. Estimated pregnancy rates and rates of pregnancy outcomes for the United States, 1990–2008. Natl Vital Stat Rep. 2012;60(7):1–21. [PubMed] [Google Scholar]
- 3.Schreiber CA, Chavez V, Whittaker PG, Ratcliffe SJ, Easley E, Barg FK. Treatment decisions at the time of miscarriage diagnosis. Obstet Gynecol. 2016;128(6):1347–1356. doi: 10.1097/AOG.0000000000001753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang J, Gilles JM, Barnhart K, Creinin MD, Westhoff C, Frederick MM; National Institute of Child Health Human Development (NICHD) Management of Early Pregnancy Failure Trial. A comparison of medical management with misoprostol and surgical management for early pregnancy failure. N Engl J Med. 2005;353(8):761–769. doi: 10.1056/NEJMoa044064 [DOI] [PubMed] [Google Scholar]
- 5.Rausch M, Lorch S, Chung K, Frederick M, Zhang J, Barnhart K. A cost-effectiveness analysis of surgical versus medical management of early pregnancy loss. Fertil Steril. 2012;97(2):355–360. doi: 10.1016/j.fertnstert.2011.11.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schreiber CA, Creinin MD, Atrio J, Sonalkar S, Ratcliffe SJ, Barnhart KT. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378(23):2161–2170. doi: 10.1056/NEJMoa1715726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: Second Panel on Cost-effectiveness in Health and Medicine. JAMA. 2016; 316(10):1093–1103. doi: 10.1001/jama.2016.12195 [DOI] [PubMed] [Google Scholar]
- 8.Sanders GD, Maciejewski ML, Basu A. Overview of cost-effectiveness analysis. JAMA. 2019;321(14):1400–1401. doi: 10.1001/jama.2019.1265 [DOI] [PubMed] [Google Scholar]
- 9.Husereau D, Drummond M, Petrou S, et al. ; ISPOR Health Economic Evaluation Publication Guidelines-CHEERS Good Reporting Practices Task Force. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)—explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health. 2013;16(2):231–250. doi: 10.1016/j.jval.2013.02.002 [DOI] [PubMed] [Google Scholar]
- 10.Mattke S, Balakrishnan A, Bergamo G, Newberry SJ. A review of methods to measure health-related productivity loss. Am J Manag Care. 2007;13(4):211–217. [PubMed] [Google Scholar]
- 11.Berger ML, Murray JF, Xu J, Pauly M. Alternative valuations of work loss and productivity. J Occup Environ Med. 2001;43(1):18–24. doi: 10.1097/00043764-200101000-00005 [DOI] [PubMed] [Google Scholar]
- 12.United States Department of Labor. Employer costs for employee compensation news release: December 2008. United States Department of Labor Bureau of Labor Statistics; Updated March 12, 2009 Accessed December 2, 2018 https://www.bls.gov/news.release/archives/ecec_03122009.htm [Google Scholar]
- 13.Nicholson S, Pauly MV, Polsky D, Sharda C, Szrek H, Berger ML. Measuring the effects of work loss on productivity with team production. Health Econ 2006;15(2):111–123. doi: 10.1002/hec.1052 [DOI] [PubMed] [Google Scholar]
- 14.Efron B, Tibshirani R. An Introduction to the Bootstrap. Chapman & Hall; 1994. [Google Scholar]
- 15.Barber JA, Thompson SG. Analysis of cost data in randomized trials: an application of the non-parametric bootstrap. Stat Med 2000;19(23):3219–3236. doi: [DOI] [PubMed] [Google Scholar]
- 16.Glick HA, Doshi JA, Sonnad SS, Polsky D. Economic Evaluation in Clinical Trials. 2nd ed Oxford University Press; 2013. [Google Scholar]
- 17.Ramsey SD, Willke RJ, Glick H, et al. Cost-effectiveness analysis alongside clinical trials II: an ISPOR Good Research Practices Task Force report. Value Health. 2015;18(2):161–172. doi: 10.1016/j.jval.2015.02.001 [DOI] [PubMed] [Google Scholar]
- 18.Fenwick E, O’Brien BJ, Briggs A. Cost-effectiveness acceptability curves: facts, fallacies and frequently asked questions. Health Econ. 2004;13(5):405–415. doi: 10.1002/hec.903 [DOI] [PubMed] [Google Scholar]
- 19.Fenwick E, Byford S. A guide to cost-effectiveness acceptability curves. Br J Psychiatry. 2005;187:106–108. doi: 10.1192/bjp.187.2.106 [DOI] [PubMed] [Google Scholar]
- 20.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness: the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796–797. doi: 10.1056/NEJMp1405158 [DOI] [PubMed] [Google Scholar]
- 21.Raymond EG, Blanchard K, Blumenthal PD, et al. ; Mifeprex REMS Study Group. Sixteen years of overregulation: time to unburden mifeprex. N Engl J Med. 2017;376(8):790–794. doi: 10.1056/NEJMsb1612526 [DOI] [PubMed] [Google Scholar]
- 22.Gordon M, McCammon S. A drug that eases miscarriages is difficult for women to get. NPR website. January 10, 2019. Accessed July 31, 2019 https://www.npr.org/sections/health-shots/2019/01/10/666957368/a-drug-that-eases-miscarriages-is-difficult-for-women-to-get
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.