Abstract
Background:
The study was conducted to assess socio-behavioral and biological factors associated with unplanned pregnancy in the U.S. cohort of a microbicide trial.
Study design:
We conducted a mixed-method, nested case-control study of risk factors for pregnancy within the U.S. cohort of the microbicide trial HPTN 035. We developed an instrument to assess attitudes and beliefs about fertility control/contraceptive utilization among 122 women. Cases were HPTN 035 participants who became pregnant while enrolled, matched by time-on-study 1:4 with controls. Univariable and multivariable analysis were performed with pregnancy as the outcome of interest.
Results:
Contraceptive method change during the trial was associated with unplanned pregnancy (OR=1.76). Participant desire/partners’ desire for future children (OR=4.95) and young age (OR=0.88 annually above age 19 years) were independently associated with unintended pregnancy.
Conclusion:
Within a trial that enrolls heterosexually active women, there may be ways to identify those at highest risk of becoming pregnant a priori.
Keywords: Contraception, Microbicide, Clinical trials, Pregnancy
1. Introduction
The World Health Organization (WHO) estimates that in 2007, 15.5 million women worldwide were living with HIV [1]. Vaginal microbicides are in development to help women meet the urgent need to slow the heterosexual transmission of this disease. Like HIV, unplanned pregnancy is a major public health problem that affects women of reproductive age and their families [2]. Both HIV acquisition and unintended pregnancy occur most commonly in sexually active women under the age of 40 years [1, 3]. Women who are eligible to participate in microbicide trials may therefore be at risk for unplanned pregnancy. HIV prevention trial protocols are designed to enroll only women who are not seeking a pregnancy, and require that women who become pregnant during the trial discontinue product use for the duration of the pregnancy (if not for the duration of the trial) in order to protect the developing fetus from exposure to investigational drugs. Despite attempts to exclude women planning a pregnancy, data from the HIV Prevention Trials Network (HPTN) demonstrate that approximately 18% of trial participants become pregnant [4]. Because participants are discontinued from the study product, incident pregnancies can significantly affect trial conduct and outcomes, causing increases in interrupted use potential bias, and decreased ability to demonstrate a treatment effect in intention-to-treat analyses [5, 6]. From a public health perspective, the prevalence of such pregnancies also reveals the unmet family planning needs of the HIV-at-risk population worldwide.
Condom use reduces the risk of pregnancy and HIV. However, while male condom use is the only proven way to reduce the risk of HIV transmission from vaginal intercourse, condoms are not a highly effective method of contraception [7]. Many microbicide trial sites offer other contraceptive methods on site, or make referrals as necessary. Because of the rates of pregnancy despite these efforts, understanding what places women in HIV prevention trials at risk for unplanned pregnancy may inform protocol development methodology as well as preventive health policy for the non-clinical trial population.
One could argue that women who are at risk for HIV are by nature at risk for pregnancy. We hypothesize that even within the enrollment pool of at-risk women, there are characteristics that can help separate those who are at high risk of becoming pregnant during a clinical trial from those who are not at high risk of pregnancy. To test this hypothesis, we conducted a mixed-method (both quantitive and qualitative), nested case-control study of women in the U.S. cohort of a randomized, controlled microbicide trial, HPTN 035 [8]: A Phase II/IIb study of the vaginal microbicides BufferGel and 0.5% PRO 2000/5 Gel for the prevention of HIV infection in women. HPTN 035 was conducted between February 2005 and September 2008 among 3,099 HIV-negative women at seven clinical research sites in Malawi, South Africa, Zambia, Zimbabwe and the United States. As part of the inclusion criteria, all women enrolled in HPTN 035 had answered “no” to the required screening question, “Do you plan to become pregnant in the next 30 months (2 1/2 years)?” Our goal in this ancillary Pregnancy Risk Study was to measure the associations between relevant demographics, specific reproductive practices and beliefs, and the incidence of pregnancy among women enrolled in HPTN 035 at the University of Pennsylvania study site. By doing so, we hope to begin to refine the way we think about improving fertility control during clinical trials and the HIV-at-risk population in general.
2. Materials and methods
A total of 200 women were enrolled at the Philadelphia site for HPTN 035. HPTN 035 was designed to follow women for a minimum of 12 months and a maximum of 30 months. Philadelphia participants spent from 18 months to 30 months in this parent study, depending on the date at which they were enrolled. Condoms were available on-site, and other contraceptive services were available by referral at city and state funded family planning service providers. Our Pregnancy Risk Study ancillary was approved by the 035 protocol team, the Microbicides Trials Network1 (MTN), and the University of Pennsylvania Institutional Review Board. We interviewed women for the Pregnancy Risk Study from March through August 2008. We invited women of reproductive age only to participate either concurrent with or after their HPTN 035 participation, depending upon on their exit date from HPTN 035. Only pregnancies conceived during enrollment were counted. The case-control study design allowed us to match participants by time-on-study and decrease external factors that might affect pregnancy rates. Women who had become pregnant before January 2008 were eligible to enroll, and these participants were matched 1:4 by time-on-study with women who had not become pregnant by this date. With the exception of 3 women, all women had completed their pregnancy by the time of their Pregnancy Risk Study interview (so no longer visibly gravid).
Since age was likely to be associated with pregnancy risk even within this entirely reproductive-aged cohort, we did not match participants by age. All participants gave written informed consent, and were compensated for their time with a $60 check.
2.1. Instrument development
Our 65-item questionnaire was developed using selections from the National Survey for Family Growth (NSFG) [9], the literature regarding unintended pregnancy risk [10–15], as well as a qualitative analysis that describes the contraceptive attitudes and behaviors of women who participated in an HIV prevention trial [16]. The NSFG survey, administered by the National Center for Health Statistics (NCHS), includes validated questions concerning marriage, relationships, pregnancy, fertility/infertility, and use of contraception. We incorporated relevant items from this questionnaire because of their established validity and widespread use in a variety of subpopulations in the United States. In order to more specifically answer our study question, additional items were developed in consultation with two survey experts from the Outcomes Measurement Methods Core of the University of Pennsylvania School of Medicine Center for Clinical Epidemiology and Biostatistics. All questions were tested for face validity by two separate readers as well the study coordinator (SW) prior to finalization.
The questionnaire was divided into two sections: the first section (61 questions) was read by one of two highly trained and experienced interviewers to each participant. The interviewers were masked to case/control status. At the end of this section, the study coordinator unmasked the interviewer, and those women who had had a pregnancy during their participation in HPTN 035 were asked 4 open-ended questions that focused on the meaning of pregnancy planning and avoidance, the women’s perception of her control over her fertility, and what, if any, efforts the participant made to prevent pregnancy during the parent trial.
2.2. Sample size and inclusion criteria
At the time of protocol development, 20 incident pregnancies had occurred at the University of Pennsylvania 035 study site. Our power calculation assumed a total of 20 women with a pregnancy, matched with 80 women who did not become pregnant. We estimated that 10% of the cases and 60% of the controls would have used a method of contraception in addition to the condoms they were using primarily for protection from HIV. Using these numbers, we had 99% power (at a significance level of 0.05) to detect a difference in contraceptive use of this magnitude or greater. We planned to include all women who became pregnant during the study period who met inclusion criteria and who were willing and able to participate.
2.3. Statistical analysis
Data were entered into SPSS version 16.0 and subsequently converted to a SAS file in order to perform univariate and multivariate analysis. The dependent variable was pregnancy status; independent variables included demographic information collected as a part of HPTN 035 as well as the items in our study instrument. Descriptive statistics were used to compare demographic characteristics and risk factors between case and control groups. We computed the mean and standard deviations for continuous variables, and the frequencies for dichotomous variables. Differences between groups for dichotomous variables were tested with chi-square test or Fisher’s exact test, with Student’s t-test for continuous variables.
Conditional logistic regression was performed to identify variables independently associated with pregnancy. We selected these variables based on both clinical importance and p value of ≤0.05 in the univariate analyses. Patients reporting tubal ligation were excluded from the regression analysis because the risk of pregnancy is so low in such women, and they are likely to have very different attitudes and beliefs about pregnancy risk when compared to nonsterilized women. We included age as a continuous variable in our model. Given the 26 cases in our final sample, we included three covariates in our final model based on well-validated recommendations [17] about the number of independent variables to include per number of cases. All statistical analyses were carried out using SAS version 9.1 (SAS Institute, Cary, NC
We tested the collinearity among the covariates using multicollinearity diagnostic statistics to examine Tolerance and Variance Inflation Factor for each variable. Generally values of VIF exceeding 10 are often regarded as indicating multicollinearity, but in weaker models, which is often the case in logistic regression, values above 2.5 may be a cause for concern. None of the covariates in our model had values of VIF greater than 1.1, indicating that multicollinearity is unlikely.
For the analysis of the qualitative portion of our instrument, two team members reviewed the transcripts and identified themes within the participants’ narratives. The results of the theme classifications were discussed, and discrepancies were resolved by consensus.
3. Results
The final pregnancy incidence rate was 10.1 per 100 women-years (95% CI 6.6, 13.6) for all of the Philadelphia participants in HPTN 035, with 33 women becoming pregnant. Of the 200 women participating in 035, 122 women were enrolled in the Pregnancy Risk Study: 26 cases and 96 controls. All of the eligible participants who met the case definition during our study period were included. Forty-three possible controls were ineligible for participation due to age (over 45 years and thus not at significant risk of pregnancy based on age alone). Other participants were not included for the following reasons: incarceration (4 participants); lost to follow-up/unable to locate (15 participants); medical reasons - hospital or rehabilitation (5 participants); out of state (2 participants); actively seeking pregnancy and therefore terminated from HPTN 035 (1 participant); unwilling to participate.
The median age for cases was 26.7 years (SD=5.6), and the median age for controls was 34.8 years (SD = 8.1) (p-value = <0.01). Fig. 1 plots the distribution of pregnancy by age. The demographics and reproductive histories of our participants are shown in Table 1. At the time of enrollment into HPTN 035, 32% of participants reported that they were using hormonal contraceptives (oral contraceptive pills, hormonal patch, and depomedroxyprogesterone acetate injections) and 83% cited using condoms. None of our participants reported using long-acting reversible methods such as intrauterine devices or implants. Twenty-three percent reported having had a tubal ligation, and none of these women became pregnant during the study period. Details of the contraceptive practices, beliefs and attitudes as captured by our questionnaire are found in Table 2. Ninety-three percent of the women randomized to a gel group believed the gel itself would prevent pregnancy, but this belief was not associated with becoming pregnant. Forty percent believed that the study product could be harmful to a pregnancy. Contraceptive method change during the trial was associated with unplanned pregnancy (OR=1.76).
Figure 1:
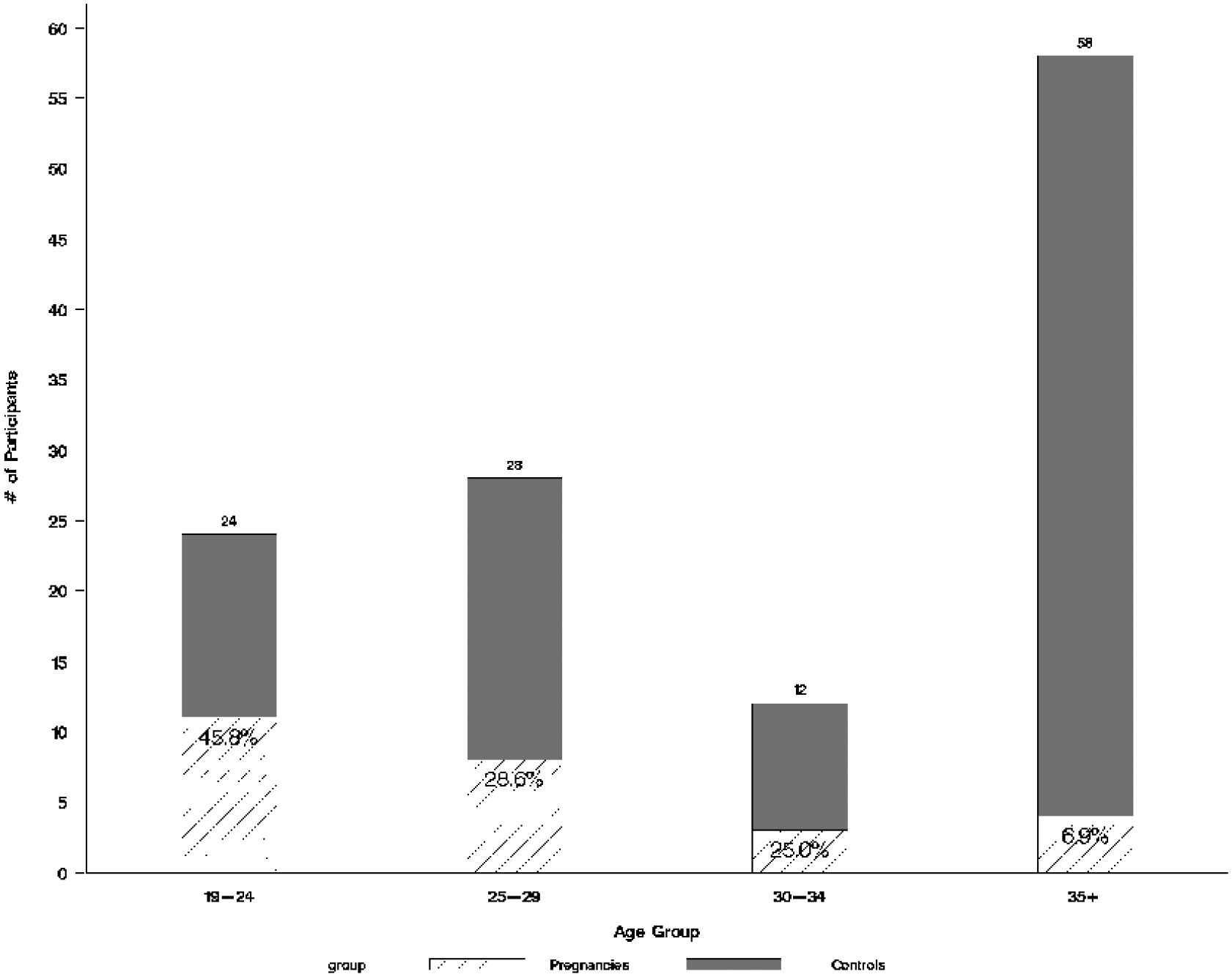
Distribution of Pregnancies by Age Group
Table 1.
Univariate analysis of baseline demographics and participant characteristics in a nested case-control study of U.S. microbicide trial participants (N=122)
| Baseline characteristics | Cases n=26 | Controls n=96 | p-value |
|---|---|---|---|
| Age, years | <0.01 | ||
| Mean ± std | 27 ± 6 | 35 ± 8 | |
| Range | 19–37 | 19–45 | |
| Age group | <0.01 | ||
| <30 | 19 (73.1) | 33 (34.4) | |
| >=30 | 7 (26.9) | 63 (65.6) | |
| Black | 0.19 | ||
| Yes | 23 (88.5) | 72 (75.0) | |
| No | 3 (11.5) | 24 (25.0) | |
| Earns own income | 0.49 | ||
| Yes | 16 (61.5) | 66 (68.8) | |
| No | 10 (38.5) | 30 (31.3) | |
| Married | 0.04 | ||
| Yes | 0 (0.0) | 15 (15.6) | |
| No | 26 (100.0) | 81 (84.4) | |
| Education | 0.53 | ||
| Primary complete | 0 (0.0) | 2 (2.1) | |
| Attended secondary | 6 (23.1) | 22 (22.9) | |
| Secondary complete | 12 (46.2) | 31 (32.3) | |
| College | 8 (30.8) | 41 (42.7) | |
| Pregnancy history | 0.28 | ||
| Never pregnant | 3 (11.5) | 22 (22.9) | |
| Ever pregnant | 23 (88.5) | 74 (77.1) | |
| Lifetime number of pregnancies prior to enrollment | 0.22 | ||
| 0 | 3 (11.5) | 22 (22.9) | |
| 1–2 | 12 (46.2) | 28 (29.2) | |
| 3–4 | 4 (15.4) | 26 (27.1) | |
| >4 | 7 (26.9) | 20 (20.8) | |
| Ever had live birth | 0.46 | ||
| Yes | 21 (80.8) | 68 (70.8) | |
| No | 5 (19.2) | 28 (29.2) | |
| Ever had miscarriage | 0.61 | ||
| Yes | 6 (23.1) | 27 (28.1) | |
| No | 20 (76.9) | 69 (71.9) | |
| Ever had induced abortion | 0.03 | ||
| Yes | 13 (50.0) | 26 (27.1) | |
| No | 13 (50.0) | 70 (72.9) | |
| Ever had stillbirth | >0.99 | ||
| Yes | 0 (0.0) | 2 (2.1) | |
| No | 26 (100.0) | 94 (97.9) | |
| Ever had ectopic pregnancy | 0.58 | ||
| Yes | 0 (0.0) | 4 (4.2) | |
| No | 11 (42.3) | 48 (50.0) | |
| Ever had a sexually transmitted infection | 0.14 | ||
| Yes | 19 (73.1) | 55 (57.3) | |
| No | 7 (26.9) | 41 (42.7) | |
| Douches after sex | 0.05 | ||
| Yes | 10 (38.5) | 19 (19.8) | |
| No | 16 (61.5) | 77 (80.2) | |
| Received a pregnancy test the12 months prior to HPTN 035 enrollment | 0.12 | ||
| Yes | 16 (61.5) | 41 (42.7) | |
| No | 26 (100.0) | 92 (95.8) |
Not all responses total 100% as participants were given the option not to answer any question.
Table 2.
Univariate analysis of contraceptive practices, beliefs, and attitudes among a U.S. cohort of microbicide trial participants
| Participant attribute | Cases n (%) | Control n (%) | p-value |
|---|---|---|---|
| Had family planning provider while enrolled in HPTN 035 | 0.36 | ||
| Yes | 20 (76.9) | 65 (67.7) | |
| No | 6 (23.1) | 31 (32.3) | |
| Had tubal sterilization prior to enrollment | <0.01 | ||
| Yes | 0 (0.0) | 28 (29.2) | |
| No | 26 (100.0) | 68 (70.8) | |
| Number of contraceptives concurrently used at enrollment in HPTN 035 | 0.70 | ||
| 0 | 0 (0.0) | 2 (2.1) | |
| 1 | 15 (57.7) | 45 (46.9) | |
| >1 | 11 (42.3) | 49 (51.0) | |
| Contraceptive method at study start* | |||
| Hormonal contraceptives | 0.20 | ||
| Yes | 11 (42.3) | 28 (29.2) | |
| No | 15 (57.7) | 68 (70.8) | |
| Condoms (including male and female) | 0.78 | ||
| Yes | 22 (84.6) | 79 (82.3) | |
| No | 4 (15.4) | 17 (17.7) | |
| Withdrawal | 0.78 | ||
| Yes | 4 (15.4) | 17 (17.7) | |
| No | 22 (84.6) | 79 (82.3) | |
| It is normal/acceptable to try to prevent pregnancy | 0.04 | ||
| Yes | 24 (92.3) | 96 (100.0) | |
| No | 2 (7.7) | 0 (0.0) | |
| Trying to prevent pregnancy while enrolled in HPTN 035 | 0.50 | ||
| Yes | 22 (84.6) | 85 (89.5) | |
| No | 4 (15.4) | 10 (10.5) | |
| Desires a baby in the future | <0.01 | ||
| Yes | 23 (88.5) | 28 (30.1) | |
| No | 3 (11.5) | 65 (69.9) | |
| Both participant and partner desire a baby in the future | <0.01 | ||
| Yes | 16 (66.7) | 19 (20.0) | |
| No | 8 (33.3) | 76 (80.0) | |
| Changed contraceptive method while in HPTN 035 | <0.01 | ||
| Yes | 12 (46.2) | 18 (18.8) | |
| No | 14 (53.8) | 78 (81.3) | |
| The change in method was switching methods | 0.01 | ||
| Yes | 6 (23.1) | 6 (6.3) | |
| No | 20 (76.9) | 90 (93.8) | |
| The change in method was adding a method | 0.45 | ||
| Yes | 1 (3.8) | 10 (10.4) | |
| No | 25 (96.2) | 86 (89.6) | |
| The change in method was stopping method | <0.01 | ||
| Yes | 5 (19.2) | 2 (2.1) | |
| No | 21 (80.8) | 94 (97.9) | |
| HPTN 035 study staff gave information about contraception | 0.01 | ||
| Yes | 12 (46.2) | 20 (21.1) | |
| No | 14 (53.8) | 75 (78.9) | |
| Believed the study gel would prevent pregnancy | 0.87 | ||
| Yes | 21 (80.8) | 69 (71.9) | |
| No | 1 (3.8) | 5 (5.2) | |
| Not sure | 0 (0.0) | 1 (10) | |
| N/A (condom only group) | 4 (15.4) | 21 (21.9) | |
| Believed the study gel could be harmful to a pregnancy | 0.41 | ||
| Yes | 9 (34.6) | 29 (30.5) | |
| No | 9 (34.6) | 20 (21.1) | |
| Not sure | 4 (15.4) | 24 (25.3) | |
| N/A (condom only group) | 4 (15.4) | 22 (23.2) | |
| Believed that not getting pregnant was better than getting pregnant while enrolled in HPTN 035 | 0.13 | ||
| Yes | 21 (80.8) | 89 (92.7) | |
| No | 5 (19.2) | 7 (7.3) | |
| Had knowledge of how to prevent pregnancy while enrolled in HPTN 035 | 0.04 | ||
| Yes | 24 (92.3) | 96 (100.0) | |
| No | 2 (7.7) | 0 (0.0) | |
| Had support to prevent pregnancy while enrolled in HPTN 035 | 0.20 | ||
| Yes | 24 (92.3) | 94 (97.9) | |
| No | 2 (7.7) | 2 (2.1) | |
| Used emergency contraception while enrolled in HPTN 035 | 0.06 | ||
| Yes | 21 (80.8) | 56 (59.6) | |
| No | 5 (19.2) | 38 (40.4) |
Note: p-values for cells greater than 5 are from chi-square tests; otherwise are from Fisher exact test.
Methods with the highest prevalence at study start.
Of those women who switched their contraceptive method during the study, 61 (50%) switched to condoms as their stated method, with almost equal proportions switching from a less effective method [withdrawal (2) or occasional emergency contraception use (1)] to a more effective method [injectable contraception or oral contraceptives (3)]. The other 50% switched to a method other than condoms including hormonal methods (4), vasectomy (1) and withdrawal (1). The factors most strongly associated with contraceptive method change were beliefs that the initial method decreased sexual pleasure (p<0.01) or changed menstrual cycle patterns (p<0.01).
Since there were no pregnancies among the women who had undergone a tubal ligation, and all of these patients were controls, we performed an analysis excluding these women (Table 3). Contraceptive method change during the study, young age, and couples’ desire to have children in the future remained associated with unplanned pregnancy during the study. Control women who were sterilized were on average 8 years older than whose who had not, which could have underestimated the age effect that was already so powerful in this study. Participant desire/partners’ desire for future children (OR=4.95) and young age (OR=0.88 annually above age 19) were independently associated with unintended pregnancy (Table 4) in this microbicide trial cohort.
Table 3.
Characteristics of participants who had not undergone surgical sterilization that are statically significantly associated with becoming pregnant during the trial period (n= 94*)
| Participant attribute | Cases (n=26) | Controls (n=68) | OR (95% CI) | p-value |
|---|---|---|---|---|
| Age, years | <0.01 | |||
| Mean ± std | 27 ± 6 | 35 ± 8 | ||
| Range | 19–37 | 19–45 | ||
| Age group | 0.31 (0.11,0.83) | 0.02 | ||
| <30 | 19 (73.1) | 31 (45.6) | ||
| >=30 | 7 (26.9) | 37 (54.4) | ||
| Douches after sex | 2.64 (0.98, 7.15) | 0.05 | ||
| Yes | 10 (38.5) | 13 (19.1) | ||
| No | 16 (61.5) | 55 (80.9) | ||
| Ever had an induced abortion | 2.78 (1.09, 7.10) | 0.03 | ||
| Yes | 13 (50.0) | 18 (26.5) | ||
| No | 13 (50.0) | 50 (73.5) | ||
| Self and partner desire a baby in the future | 5.44 (1.99, 14.89) | <0.01 | ||
| Yes | 16 (66.7) | 18 (26.9) | ||
| No | 8 (33.3) | 49 (73.1) | ||
| Change in contraceptive method while enrolled in HPTN 035 | 3.31 (1.25, 8.72) | 0.01 | ||
| Yes | 12 (46.2) | 14 (20.6) | ||
| No | 14 (53.8) | 54 (79.4) | ||
| HPTN 035 study staff gave information about contraception | 3.24 (1.23, 8.56) | 0.01 | ||
| Yes | 12 (46.2) | 14 (20.9) | ||
| No | 14 (53.8) | 53 (79.1) |
p-values for cells greater than 5 are from chi-square tests and otherwise are from Fisher exact test.
A total of 28 controls are excluded from this analysis due to prior surgical sterilization and exceedingly low risk of pregnancy. Three of these controls had been matched to a single case. Fourteen were matched 1:1 to a case and 13 were matched 2:1 to a case.
Table 4:
Adjusted odds ratios of possible explanatory variables for pregnancy risk in a U.S. microbicide cohort: conditional logistic regression (n=91*)
| Participant attribute | Odds ratio | 95% CI | p-value | |
|---|---|---|---|---|
| Age (continuous, referenced to age 19 years†) | 0.88 | 0.79 | 0.98 | 0.02 |
| Self and partner desire a baby in the future | 4.95 | 1.35 | 18.09 | 0.02 |
| Changed contraceptive while enrolled in HPTN035 | 1.57 | 0.41 | 6.04 | 0.52 |
Participants with prior tubal ligation were not included.
Given that the youngest age in our dataset is 19, we used this, instead of the usual age of zero, as our reference point. Therefore, with regard to the age variable, the odds of becoming pregnant decreased by a ratio of 0.871 for each a unit increase in age above 19 years.
One hundred percent of the women who became pregnant during this study stated that they were not attempting pregnancy at the time they became pregnant. Our open-ended interviews with the women who became pregnant revealed that women answered “no” to the required screening question, “Do you plan to become pregnant?” because they were either not seeking (passive) or avoiding (active) a pregnancy. When asked to define this further, three main themes were exposed: the majority of women stated that “not planning a pregnancy” meant that they were using a contraceptive method (specifically condoms, withdrawal or a hormonal method). The second most prevalent theme was that they were not actively seeking pregnancy. A third theme was not being currently ready for (more) children. A minority of women believed they had a prior diagnosis that meant they were infertile. Another minority expressed difficulty negotiating condom use with their partners when they explained why they had become pregnant. Many women expressed difficulty using contraceptives correctly or continuously for either financial or “control” reasons. None of the women discussed the relative efficacy of different modes of pregnancy prevention.
4. Discussion
Pregnancy prevention among sexually active women can be challenging, and in the clinical trial setting, the impact of that pregnancy reaches beyond the woman herself and can affect the integrity of the trial and its results. For this reason, the prevention of such pregnancies, especially if unplanned, is doubly important. The microbicide trial participants interviewed here clarified that that all of the incident pregnancies were indeed unplanned, and did not reflect a change in fertility intentions during the clinical trial period. We also showed that besides tubal sterilization, there was no contraceptive method used during the U.S. cohort of HPTN 035 that was clearly superior to the others at preventing pregnancy. However, none of our participants used the most effective methods such as intrauterine devices or implants. What we did show was that change in contraceptive method, regardless of the direction of the change, was associated with unplanned pregnancy. This finding in our univariate analyses is supported by the results of our conditional logistic regression even though the confidence interval was wide and not statistically significant. Our univariate analyses show that identifiable differences in knowledge about pregnancy prevention, attitudes about fertility and fertility control, and specific contraceptive behaviors were all associated with unplanned pregnancy during this study. If our data are confirmed in other populations, such information could inform the development of a better screening question to assess pregnancy risk, or interventions to help women avoid pregnancy during clinical trials.
The strongest associations with our subset of women becoming pregnant during HPTN 035 were the participant and her partner’s desire for a child in the future and young age. Interestingly, the women who answered that they and they partner desired a future child were more likely to state that their desire for children is influenced by their partner’s desire for children (p<0.01), and were also more likely to report that their partner was trying to get them pregnant during the study period (p<0.01). It appears that, at least in Philadelphia, a woman’s perception of her partner’s reproductive desires plays a significant role in her risk for becoming pregnant during a clinical trial.
While many nested case-control studies match the cases and controls by age, age was a variable under investigation in this setting. We acknowledge that it is well-known that fertility declines with age, but the risk of unintended pregnancy in the American population has two peaks, ages 15–19 years and age 40 and above [18]. Furthermore, within the reproductive-aged population, we found that even a year’s difference in age impacts pregnancy risk. Our finding that the majority of the unintended pregnancies occurred in women under 24 years is especially useful because no aged-based recommendations have been put forth with regard to adjusting how pregnancy risk is handled in the clinical trial setting.
There are important limitations of this study. We performed a great many tests for statistical significance and have reported nominal p-values, unadjusted for multiple comparisons. Thus, some of the positive associations we found could be due to chance. The case-control study design is subject to inherent biases. In particular, we were only able to assess risk factors by self-report, and the validity of our results may be affected by recall bias. For example, our finding that the provision of information about contraception from the HPTN 035 study staff was associated with an increased risk of pregnancy only reflects the participants’ perception of having received this information - we did not have an objective measure for this transfer of information. However, since the HPTN 035 protocol did require that all participants receive baseline and ongoing contraceptive counseling, it is possible that the association we found here is a result of differential recall bias. Our results may also be affected by social desirability bias: the tendency of respondents to reply in a manner that will be viewed favorably by others. This would result in ove-reporting “good” behavior and/or under-reporting “bad” behavior. Because our primary outcome (pregnancy) was assessed prospectively and within the context of a clinical trial, the likelihood of misclassification bias is extremely low. Since the gravid state can be obvious to the observer, it is possible that the masking of our interviewers was affected by this. However, only 3 of our participants were pregnant at the time of the interview, and our interviewers were not informed about whether the participants had completed their enrollment in HPTN 035 or were concurrently enrolled (so the pregnancy may have been conceived just before HPTN 035 study exit when the physical signs of pregnancy were not evident). Finally, this nested case-control study was performed in U.S. women only, and the majority of HIV prevention trial participants are from other parts of the globe. Until studies are done in other populations, the generalizability of our results is limited.
Despite these limitations, our nested case-control study was able to efficiently shed some light on risk factors for pregnancy within a U.S. microbicide cohort. In addition to our primary findings that age and desire for future child-bearing are independently associated with incident unplanned pregnancy, our univariate analyses show that stopping or switching a baseline method of contraception (regardless of the relative effectiveness of either method) is associated with becoming pregnant, which may inform future trials. The responses we received to our open-ended questions reveal that some women think of “not planning a pregnancy” as an active behavior (using contraception) while others interpret this as passive (not seeking pregnancy and/or not being ready for children). Women expressed difficulty using methods of contraception consistently or correctly for a variety of reasons that include finances (despite having access to federally-funded family planning services off-site), partner negotiation, and difficulty carrying out a plan. Our questionnaire also revealed that a woman’s partner plays a role in how and how well she prevents pregnancy, suggesting that male engagement may be an important way to improve study conduct when possible.
The ability to determine a question or group of questions that could reliably predict potential pregnancies could improve the power and precision of outcome estimation in HIV prevention trials. Since women are the target population in vaginal microbicide trials, the need to effectively manage the risk of pregnancy in this setting is clear. However, this concern affects clinical trials beyond the field of microbicides. Despite the U.S. government’s mandate to include women in clinical trials, there are few recommendations on how to modulate and/or account for the risk of pregnancy. We have shown that there may be efficient ways to curb the effect of pregnancy on clinical trial conduct through targeted screening. Future studies could focus on redesigning a screening question, or set of questions, that includes more valid ways of assessing parenthood desires and partners’ wishes. For the sake of efficiency, such a set of questions might be targeted towards women under 30 years of age, and, for those who screen positive, could prompt a referral for expert contraceptive counseling and dispensation.
Acknowledgements
This study was funded by the University of Pennsylvania Center for AIDS Research Pilot Grant Program.
The authors would like to thank:
Jessica Fishman, PhD - Co-Director and Abigail Cohen, PhD - Senior Project Manager of the Outcomes Measurement Methods Core of the University of Pennsylvania School of Medicine Center for Clinical Epidemiology and Biostatistics (CCEB) for their invaluable help with the development of the instrument used in this study. Dr. Cynthia Mollen of the CCEB for her critical help with the qualitative analyses. Dr. Susan Ellenberg for her guidance with the statistical analyses. HIV Prevention Trials Network and Microbicides Trials Network for their assistance with the obtaining the necessary data and guidance with this manuscript. We extend our gratitude to our participants.
HPTN 035 was funded by the US National Institutes of Health (NIH). The study was designed and implemented by the HIV Prevention Trials Network (HPTN) and the Microbicide Trials Network (MTN). The HPTN (U01AI46749) has been funded by the National Institute of Allergy and Infectious Diseases (NIAID), National Institute of Child Health and Development (NICHD), National Institute of Drug Abuse (NIDA), and National Institute of Mental Health (NIMH). The MTN (U01AI068633) has been funded by NIAID, NICHD, and NIMH. The study products were provided free of charge by Indevus Pharmaceuticals, Inc., and ReProtect, Inc. The US Agency for International Development (USAID) provided funding for manufacturing of BufferGel for this study.
This study was funded by the University of Pennsylvania Center for AIDS Research Pilot Grant
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
HPTN 035 was conducted by the Microbicide Trials Network (MTN). Prior to the establishment of the MTN, the study was led by the NIAID-funded HIV Prevention Trials Network (HPTN), from which the study gets its name
References
- [1].UNAIDS. AIDS epidemic update. http://data.unaids.org/pub/EPISlides/2007/2007_epiupdate
- [2]. http://www.healthypeople.gov/Data/midcourse/html/focusareas/FA09Introduction.htm.
- [3].Finer LB, Henshaw SK. Disparities in rates of unintended pregnancy in the United States, 1994 and 2001. Perspect Sex Reprod Health 2006;38:90–6. [DOI] [PubMed] [Google Scholar]
- [4].Ramjee G, Kapiga S, Weiss S, et al. HPTN 055 Study Team. The value of site preparedness studies for future implementation of phase 2/IIb/III HIV prevention trials: experience from the HPTN 055 study. J Acquir Immune Defic Syndr. 2008. January 1;47:93–100. [DOI] [PubMed] [Google Scholar]
- [5].Schreiber CA, Sammel M, Hillier SH, Barnhart KT. A little bit pregnant: Modeling how the accurate detection of pregnancy can improve HIV prevention trials. Am J Epidemiol 2009;4:515–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Vermund S Overview of methodological challenges in HIV prevention trials. IOM Committee on Methodological Challenges in HIV Prevention Trials. Public Meeting Washington, D.C., February 6–7, 2007. [Google Scholar]
- [7].Kost K, Singh S, Vaughan B, Trussell J, Bankole A. Estimates of contraceptive failure from the 2002 National Survey of Family Growth. Contraception. 2008;77:10–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Karim SA, Coletti A, Richardson B, et al. Safety and effectiveness of vaginal microbicides BufferGel and 0.5% PRO 2000/5 gel for the prevention of HIV Iinfection in women: Results of the HPTN 035 trial CROI 2009, Montreal, Canada, February 2009. [Google Scholar]
- [9].United States Department of Health and Human Services. National Center for Health Statistics. National Survey of Family Growth, Cycle VI, 2002. ICPSR04157-v1. Ann Arbor, MI: Institute for Social Research, 2004. Ann Arbor, MI: Inter-university Consortium for Political and Social Research, 2008-10-01. doi: 10.3886/ICPSR04157. [DOI] [Google Scholar]
- [10].Stanford JB, Hobbs R, Jameson P, DeWitt MJ, Fischer RC. Defining dimensions of pregnancy intendedness. Matern Child Health J 2000;4:183–9. [DOI] [PubMed] [Google Scholar]
- [11].Moos MK, Bartholomew NE, Lohr KN. Counseling in the clinical setting to prevent unintended pregnancy: an evidence-based research agenda. Contraception 2003;67:115–32. [DOI] [PubMed] [Google Scholar]
- [12].MacPhail C, Pettifor A, Pascoe S, Rees H. Predictors of dual method use for pregnancy and HIV prevention among adolescent South African women. Contraception 2007;75:383–9. [DOI] [PubMed] [Google Scholar]
- [13].Raine T, Minnis AM, Padian NS. Determinants of contraceptive method among young women at risk for unintended pregnancy and sexually transmitted infections. Contraception 2003; 68:19–25. [DOI] [PubMed] [Google Scholar]
- [14].Harvey SM, Henderson JT, Branch MR. Protecting against both pregnancy and disease: predictors of dual method use among a sample of women. Women’s Health 2004;39:25–43. [DOI] [PubMed] [Google Scholar]
- [15].Bearinger LH, Resnick MD. Dual method use in adolescents: a review and framework for research in use of STD and pregnancy protection J Adolesc Health 2003;32:340–9. [DOI] [PubMed] [Google Scholar]
- [16].MacQueen KM, Johnson L, Alleman P, et al. Pregnancy prevention practices among women with multiple partners in an HIV preventiont. J Acquir Immune Defic Syndr 2007:32–8. [DOI] [PubMed] [Google Scholar]
- [17].Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 1996:1373–9. [DOI] [PubMed] [Google Scholar]
- [18].Henshaw SK. Unintended Pregnancy in the United States. Fam Plann Perspec 1998;30:24–9. [PubMed] [Google Scholar]


