Abstract
Purpose:
Although quality of life (QoL) improves over time for most breast cancer survivors (BCS), BCS may show different patterns of QoL. This study sought to identify distinct QoL trajectories among BCS and to examine characteristics associated with trajectory group membership.
Methods:
BCS (N = 653) completed baseline assessments within 8 months of diagnosis. QoL was assessed by the Functional Assessment of Cancer Therapy-Breast (FACT-B) at baseline and 6, 12, and 18 months later. Finite mixture modeling was used to determine QoL trajectories of the trial outcome index (TOI; a composite of physical well-being, functional well-being, and breast cancer-specific subscales) and emotional and social/family well-being subscales. Chi-square tests and F-tests were used to examine group differences in demographic, cancer-related, and psychosocial variables.
Results:
Unique trajectories were identified for all three subscales. Within each subscale, the majority of BCS had consistently medium or high QoL. The TOI analysis revealed only stable or improving groups, but the emotional and social/family subscales had groups that were stable, improved, or declined. Across all subscales, women in “consistently high” groups had the most favorable psychosocial characteristics. For the TOI and emotional subscales, psychosocial variables also differed significantly between women who started similarly but had differing trajectories.
Conclusions:
The majority of BCS report good QoL as they transition from treatment to survivorship. However, some women have persistently low QoL in each domain and some experience declines in emotional and/or social/family well-being. Psychosocial variables are consistently associated with improving and/or declining trajectories of physical/functional and emotional well-being.
Keywords: Quality of Life, Breast Cancer, Survivorship, Trajectories, Psychosocial
There were an estimated 3.56 million breast cancer survivors (BCS) in the U.S. in 2016, with an anticipated increase to 4.57 million by 2026 [1]. Quality of life (QoL) is a frequently measured patient-reported outcome and an important endpoint in clinical trials [2]. Though QoL is often studied, little is known about the heterogeneity of QoL among BCS from treatment to survivorship, a critical transition period [3]. Longitudinal studies have included only two time points and/or have examined only average changes in QoL, ignoring possible heterogeneity [4–6]. These studies suggest that although BCS may experience a disruption in QoL after diagnosis, on average, improvements occur over time. However, there is likely heterogeneity among BCS and some BCS may even fail to improve, or worsen, over time.
Finite mixture modeling is a statistical method to examine heterogeneity among groups. Studies using this method among BCS have primarily focused on outcomes of depressive symptoms or distress [7–9]. However, QoL is a broader, multidimensional construct that generally includes physical, functional, emotional, and social well-being [10]. Two studies of QoL trajectories among BCS have used the SF-36 [11] or the EORTC-QLQ-C30 [12], which are considered general QoL measures. The Functional Assessment of Cancer Therapy-Breast (FACT-B [13]), a breast cancer-specific measure, is considered to be more sensitive to disease-related changes and has been strongly recommended for measuring QoL in BCS [14].
Beyond determining distinct QoL trajectories, identifying characteristics of women who have different trajectories can help identify BCS who may benefit from intervention. The present study examines characteristics hypothesized to be associated with QoL trajectories based on our previous analyses and/or the literature. We examined variables including sociodemographic and cancer-related factors. Additionally, we examined psychosocial factors previously found to be related to QoL such as social support [15], optimism [16], coping [17], illness intrusiveness [18], and spirituality [19].
The objectives of the present study were: 1) to determine trajectories of QoL among BCS using the FACT-B, and 2) to identity sociodemographic, cancer-related, and psychosocial factors associated with trajectory group membership.
Materials and Methods
Study Population and Procedure
This is a secondary analysis of a longitudinal study that examined age-related differences in adjustment to breast cancer. Study design has been previously described [20]. Briefly, women were recruited within 8 months of breast cancer diagnosis from Memorial Sloan Kettering Cancer Center or the University of Texas-Southwestern Center for Breast Care between 2002-2006 and followed until 2008. Eligibility criteria included a first-time diagnosis of stage I-III breast cancer, age ≥18 years, and ability to read and write English. Baseline questionnaires were mailed to BCS to complete and return to the coordinating center at Wake Forest School of Medicine. Follow-up questionnaires were administered at 6, 12, and 18 months after baseline. Medical information was obtained through chart reviews after completion of primary treatment. All sites had approval from their Institutional Review Boards. Informed consent was provided by all study participants.
Measures
Primary Outcome.
QoL was assessed using the Functional Assessment of Cancer Therapy-Breast scale (FACT-B) [13]. The FACT-B consists of the FACT-General (FACT-G Version 2) [21] and the breast cancer-specific subscale [13]. The FACT-G is a cancer-specific QoL measure containing 26 items with four well-being subscales: physical, functional, emotional, and social/family. The breast cancer-specific subscale includes nine breast cancer-specific items. All items were rated on a 5-point Likert scale (0=not at all to 4=very) in terms of how true each statement had been in the past 7 days. The physical, functional, and breast cancer-specific sub scales were summed to create the trial outcome index (TOI) [22], an indicator of the physical/functional domain of QoL commonly used in clinical trials. The TOI ranges from 0-92 (α=0.91). The emotional well-being subscale measured the emotional domain of QoL and ranges from 0-20 (α=0.76). The social/family well-being subscale measured the social domain of QoL and ranges from 0-28 (α=0.74). Higher scores reflect better QoL.
Sociodemographic Factors obtained at baseline included: age at diagnosis (continuous), race (white/non-white), married/partnered (yes/no), college graduate (yes/no), presence of children <18 years in the home (yes/no), and ability to pay for basics (not very hard/very or somewhat hard).
Cancer-related factors obtained from chart reviews were: stage at diagnosis (I, II or III), mastectomy (vs. lumpectomy only), chemotherapy (none, without doxorubicin, or with doxorubicin), hormonal therapy (yes/no), and radiation (yes/no).
Psychosocial factors.
Optimism was assessed with the 8-item Life Orientation Test (possible range: 0-32; α=0.87) [23], Spiritual well-being was assessed with the Functional Assessment of Chronic Illness Therapy-Spiritual Well-Being scale, a 12-item scale with 2 subscales: meaning and peace (possible range: 0-32; α=0.81) and role of faith (possible range: 0-16; α=0.88) [24], The Illness Intrusiveness Rating Scale, a 13-item scale (possible range: 13-91; α=0.90), measured the impact of cancer on multiple life areas [25], Social support was assessed using the RAND Social Support Survey [26] which captures four aspects of perceived availability of support: emotional/informational support, tangible support, affectionate support, and positive social interaction. An overall support score is calculated using the mean of the 19 items (possible range: 1-5; α=0.97). The Beck Depression Inventory-IA (BDI-1 A), a 21-item scale (possible range: 0-63; α=0.87), measured depressive symptoms [27], The 28-item Brief COPE scale [28] was used to measure 14 types of coping strategies. Higher order exploratory factor analyses on our data [17] revealed two domains formed from seven strategies: active coping (active coping, use of emotional support, use of instrumental support, and positive reframing; α=0.81) and passive coping (self-blame, denial, and behavioral disengagement; α=0.51).
Statistical Analyses
SAS PROC TRAJ was used to identify distinct subgroups of women who followed similar trajectories in subscale values over time [29], The procedure assumes that missing data are missing completely at random [30], Models were tested examining two to seven trajectory groups each for the TOI, emotional, and social/family subscales. We used a combination of a statistical criterion (the Bayesian Information Criterion, or BIC; higher BIC indicates better model fit) and subjective judgment (distinctiveness of trajectories) to select the optimal number of groups [30], We modeled trajectories of subscale scores as a function of months since diagnosis and included linear and quadratic terms for months since diagnosis.
The TRAJ procedure assigns posterior probabilities, which are estimates of a specific individual’s probabilities of belonging to each of the model’s trajectory groups. Women were assigned to the group for which they had the maximum posterior probability.
After group assignment, associations between group membership and the sociodemographic, cancer-related, and psychosocial variables previously described were assessed using chi-square tests for categorical variables and F-tests for continuous variables. For the psychosocial measures with repeated measures over each of the surveys, we modeled estimated values of these variables at 4 months after diagnosis (the median time since diagnosis), and then assessed trajectory group differences in these estimated values. Such modeling enabled us to use a common critical referent time point (i.e., diagnosis) for all women, rather than using the arbitrary time axis of survey administration. PROC MIXED was used to model the repeated longitudinal measures of these variables using time (months since diagnosis), time squared, and trajectory group membership as independent variables. Estimated means of the psychosocial variables at 4 months after diagnosis were obtained for each trajectory group using the “Estimate” statement.
After determining the number of groups, post-hoc comparisons of selected groups were conducted on variables that were significant in the global F-tests or chi-square tests. We were particularly interested in comparing groups that started at similar levels but had differing trajectories.
All analyses were performed in SAS 9.4. We used a two-tailed alpha level of 0.05 to denote statistical significance throughout.
Results
Participants
Of 740 surveys mailed to eligible women, 653 completed baseline surveys (response rate = 88%), and 565 (86.5%) remained until study completion. Women were predominantly Caucasian (90%), married/partnered (72%), and college-educated (63%; Table 1). The mean age was 55 years (SD=12.6; range=25-96). At baseline, the mean time since diagnosis was 4.5 months (SD=1.3; range=2 days–7.4 months).
Table 1.
Sample characteristics (N=653)
| N (%) | |
|---|---|
| Sociodemographics | |
| Age, M(SD) | 54.9 (12.6) |
| Race (White) | 585 (90) |
| College-educated | 409 (63) |
| Married/partnered | 468 (72) |
| Children <18 years at home | 171 (26) |
| Ability to pay for basics | |
| Very hard/Somewhat hard | 121 (19) |
| Not hard | 532 (81) |
| Cancer-related | |
| Stage | |
| I | 338 (52) |
| II | 262 (40) |
| III | 53 (8) |
| Mastectomy | 237 (36) |
| Chemotherapy | |
| No chemotherapy | 216 (33) |
| Chemotherapy, no doxorubicin | 110 (17) |
| Chemotherapy, with doxorubicin | 327 (50) |
| Radiation | 472 (72) |
| Hormonal Therapy | 476 (73) |
| Time since diagnosis (in months) at baseline, M(SD) | 4.5 (1.3) |
Model Selection
Table 2 provides the BIC for each trajectory model, with number of groups ranging from two to seven. The highest BIC for the TOI was associated with the 7-group model, though the 7-group solution added little substantive information. We therefore chose the 6-group solution. For the emotional subscale, the highest BIC was associated with the 5-group model and all groups provided clinically meaningful distinctions. The highest BIC for the social/family subscale was associated with the 7-group model, but the BIC change from the 6 to 7-group model was very small (1.3 points) and the seventh group added little substantive information. We therefore selected the 6 group model.
Table 2.
Bayesian Information Criterion (BIC) for model selection
| Groups | Trial Outcome Index | Emotional Well-Being | Social Well-Being |
|---|---|---|---|
| 2 | −9106.41 | −5719.85 | −6799.34 |
| 3 | −8970.06 | −5634.77 | −6712.01 |
| 4 | −8927.78 | −5627.90 | −6702.98 |
| 5 | −8899.55 | −5601.87 | −6675.64 |
| 6 | −8881.25 | −5603.30 | −6667.38 |
| 7 | −8871.84 | −5632.92 | −6666.05 |
Description of QoL Trajectories
In naming groups, we use the term “medium” to correspond to scores close to the average found in a FACT-B validation sample of BCS [13] with “low” and “high” used relative to this “medium.”
Over half the sample (62.5%) had consistently medium or high scores on the TOI (Figure 1), while 10.3% had “consistently low” scores. Three groups [“consistently very low” (4.0%), “recovery to medium” (13.0%) and “recovery to high” (10.3%)] showed clinically meaningful improvements [22] ranging from approximately 15 to 24 points, though the “consistently very low” remained the lowest.
Fig. 1.
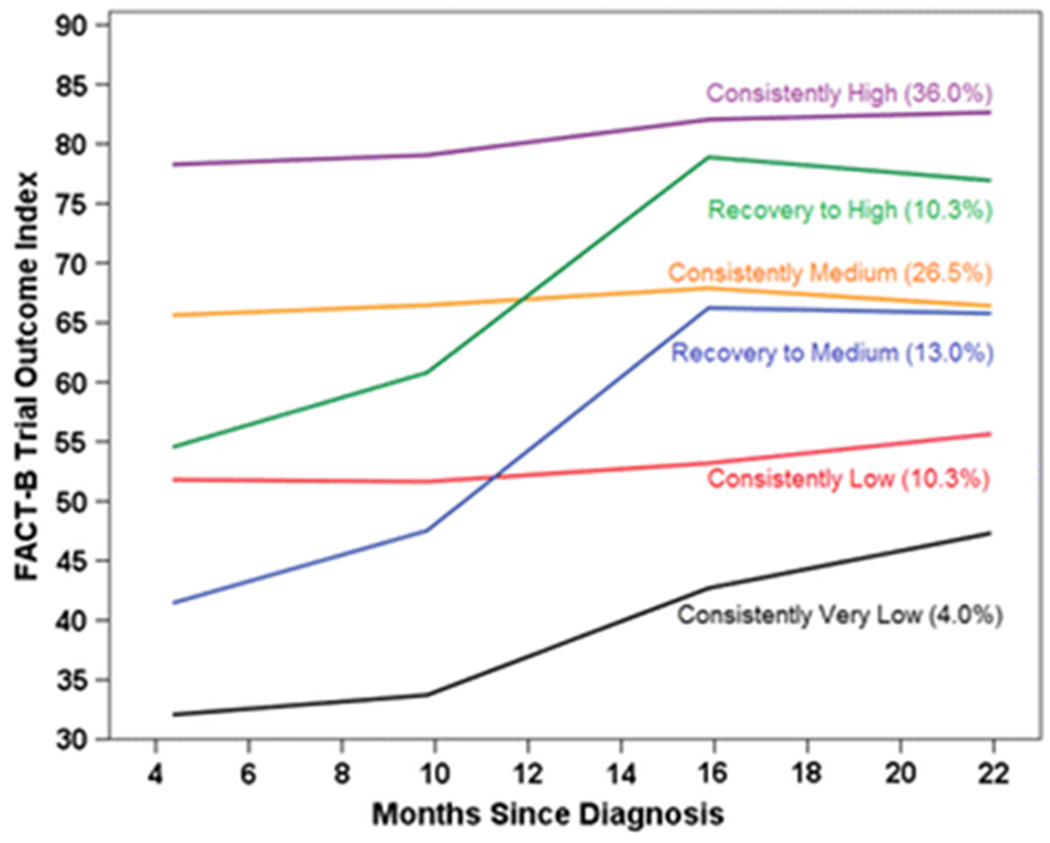
Predicted trial outcome index (TOI) scores for each trajectory group by months since diagnosis, with percentage of participants in each group
The majority of BCS (80.1%) had consistently medium or high scores on the emotional subscale (Figure 2). However, two groups started with low scores, with one remaining “consistently low” (4.1%) and one evincing “recovery to medium” (9.0%). In contrast to the TOI, a small group started with medium scores and declined over time (“declining to low”; 6.7%).
Fig. 2.
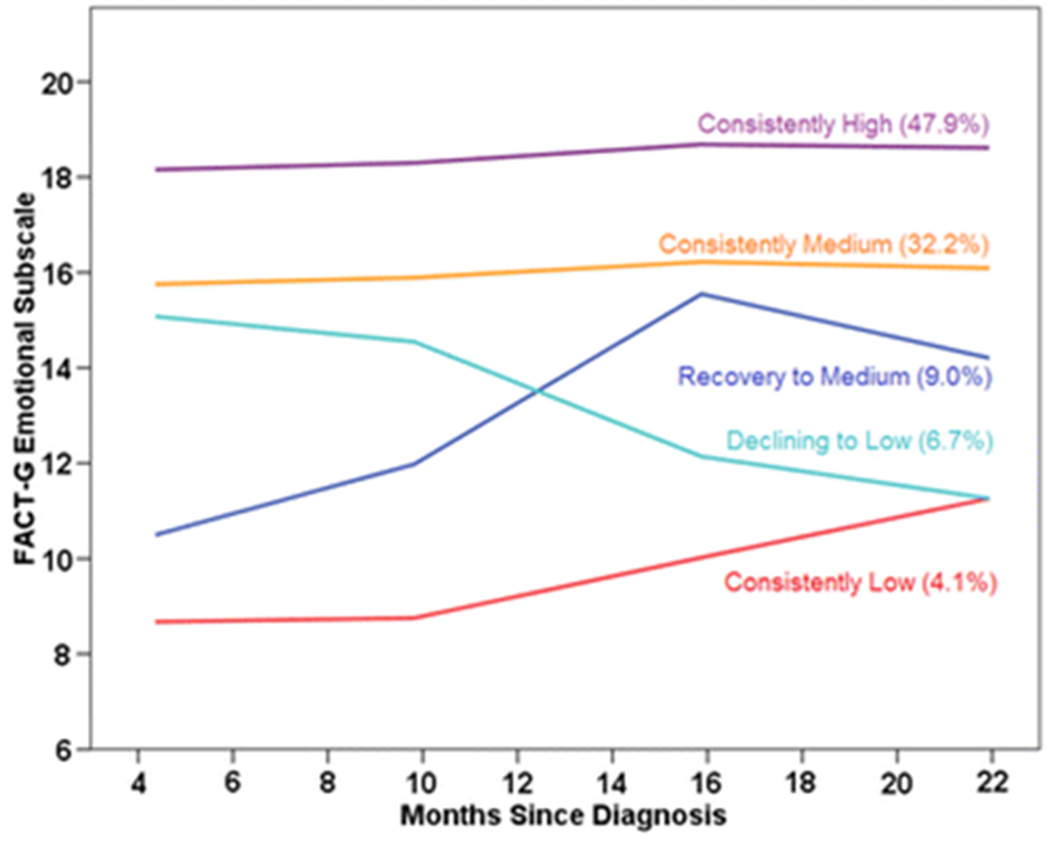
Predicted emotional well-being scores for each trajectory group by months since diagnosis, with percentage of participants in each group
Most BCS (72.1%) had consistently medium or high scores on the social/family subscale (Figure 3). Similar to the other subscales, one group started with low scores but showed a large improvement (“recovery to high”; 3.8%), and another stayed “consistently low” (13.5%). Similar to the emotional subscale, a group started with medium scores, but declined over time (“declining to low”; 9.3%). One group (1.2%) had “consistently very low” scores but was too small (n=8) to include in subsequent statistical analyses.
Fig. 3.
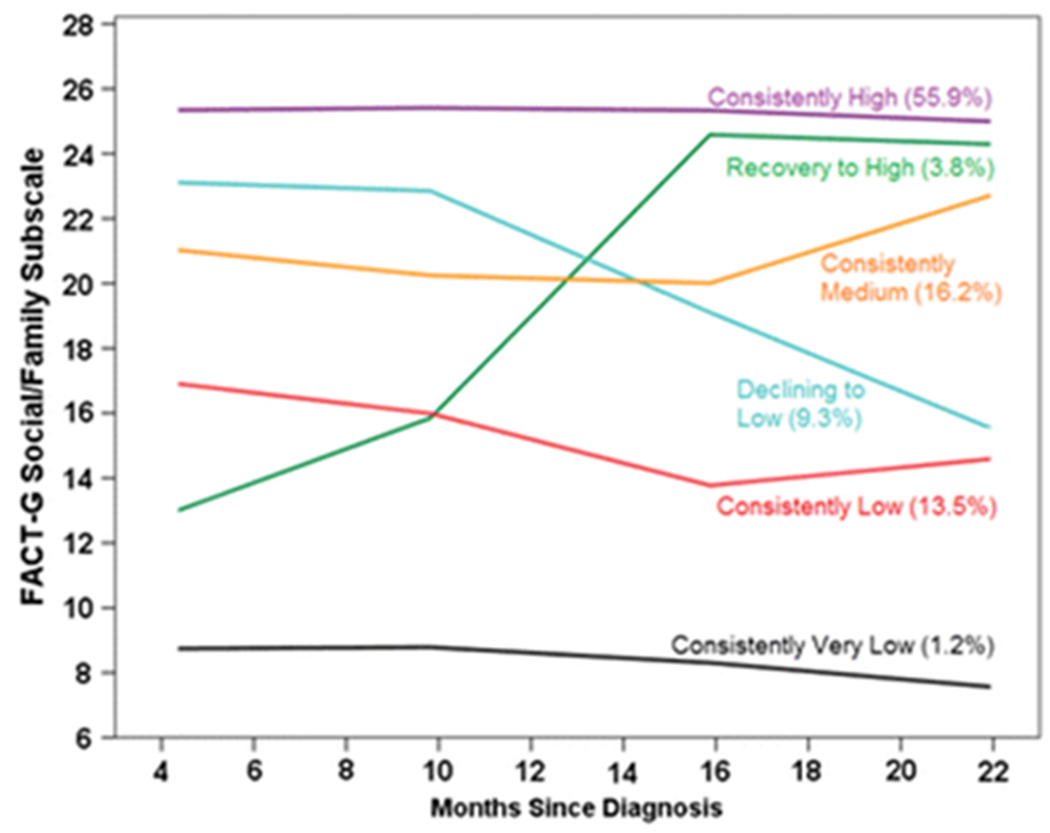
Predicted social well-being scores for each trajectory group by months since diagnosis, with percentage of participants in each group
Only 28.1% of the sample fell into a similar trajectory group across all three subscales. Twenty-three percent fell into the “consistently high” group on all three subscales, while only 2.5% always fell into the “consistently medium” group and 2% always fell into the “consistently low” or “very low” group. Only 0.6% were in a recovery group for all 3 subscales.
Associations with Trajectory Group Membership
Tables 3–5 present characteristics of each trajectory group, along with corresponding global significance test results and p-values. Significant differences across groups were found for most psychosocial measures. For all subscales, those in the “consistently high” group had the best psychosocial profiles compared to all other groups: highest scores on meaning/peace, role of faith, social support, and optimism, and lowest scores on passive coping, illness intrusiveness, and depressive symptoms. The “consistently high” groups for both the TOI and emotional subscales were also the oldest, had the least difficulty paying for basics, and were the least likely to have chemotherapy compared to the low groups. The social/family subscale groups showed a somewhat different pattern with both the “consistently high” and the “recovery to high” groups being the oldest, the least likely to have chemotherapy or mastectomy and the least likely to have difficulty paying for basics. Hormone therapy, radiation, and marital status were not significantly associated with trajectory group membership for any subscale.
Table 3.
Characteristics associated with each trial outcome index (TOI) group (N = 653), with chi-square and F-test results and post-hoc contrasts
| Trajectory group | Chi-square or F-test results | Contrast p-values | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Consistently Very Low (n=26) % or Mean(SE) | Recovery to Medium (n=85) % or Mean(SE) | Consistently Low (n=67) % or Mean(SE) | Recovery to High (n=67) % or Mean(SE) | Consistently Medium (n=173) % or Mean(SE) | Consistently High (n=235) % or Mean(SE) |
p-value |
Consistently Very Low vs. Recovery to Medium | Consistently Low vs. Recovery to High |
| Sociodemographics | |||||||||
| Age | 50.60 (2.41) | 50.26 (1.33) | 53.53 (1.50) | 51.45 (1.50) | 55.57 (0.93) | 58.06 (0.80) | <.001 | .90 | .33 |
| Very/somewhat hard to pay for basics | 62 | 27 | 39 | 10 | 21 | 6 | <.001 | .001 | <.001 |
| White race | 73 | 85 | 76 | 93 | 91 | 95 | <.001 | .18 | .01 |
| College-educated | 38 | 62 | 58 | 64 | 58 | 70 | .02 | .03 | .47 |
| Cancer-related | |||||||||
| Cancer stage | |||||||||
| I | 35 | 25 | 42 | 40 | 58 | 65 | <.001 | .60 | .42 |
| II | 54 | 64 | 46 | 54 | 35 | 28 | |||
| III | 12 | 12 | 12 | 6 | 7 | 7 | |||
| Chemotherapy | |||||||||
| None | 15 | 7 | 28 | 9 | 39 | 48 | <.001 | .27 | .01 |
| Chemotherapy, no doxorubicin | 19 | 13 | 13 | 13 | 20 | 18 | |||
| Chemotherapy, with doxorubicin | 65 | 80 | 58 | 78 | 41 | 34 | |||
| Mastectomy | 54 | 45 | 43 | 42 | 36 | 28 | .01 | .41 | .86 |
| Psychosociala | |||||||||
| Role of faith | 7.87 (0.96) | 8.09 (0.53) | 10.30 (0.59) | 8.80 (0.60) | 9.87 (0.37) | 10.46 (0.32) | <.001 | .84 | .07 |
| Meaning/peace | 16.01 (1.04) | 18.43 (0.58) | 20.38 (0.62) | 22.66 (0.63) | 23.77 (0.39) | 27.47 (0.34) | <.001 | .04 | .01 |
| Social support | 3.74 (0.14) | 4.05 (0.08) | 3.99 (0.08) | 4.45 (0.08) | 4.21 (0.05) | 4.55 (0.04) | <.001 | .05 | <.001 |
| Passive Coping | 1.85 (0.07) | 1.51 (0.04) | 1.54 (0.04) | 1.28 (0.04) | 1.32 (0.03) | 1.17 (0.02) | <.001 | <.001 | <.001 |
| Illness Intrusiveness | 55.40 (2.59) | 52.68 (1.43) | 44.55 (1.57) | 43.01 (1.58) | 33.89 (0.98) | 22.71 (0.85) | <.001 | .36 | .49 |
| Depressive Symptoms | 20.43 (0.97) | 15.92 (0.54) | 12.65 (0.58) | 10.48 (0.58) | 7.40 (0.36) | 3.76 (0.31) | <.001 | <.001 | .008 |
| Optimism | 15.19 (1.01) | 19.87 (0.56) | 18.90 (0.63) | 22.21 (0.63) | 21.71 (0.39) | 24.49 (0.34) | <.001 | <.001 | <.001 |
Values are estimated 4-month values, except for optimism.
Note: Children<18 at home, married/partnered, radiation, hormonal therapy, and active coping were not significant
Table 5.
Characteristics associated with each social/family subscale group (N = 653), with chi-square and F-test results and post-hoc contrasts
| Trajectory groupa | Chi-square or F-test results | Contrast p-values | ||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Consistently Low (n=88) % or Mean(SE) | Recovery to High (n=25) % or Mean(SE) | Consistently Medium(n=106) % or Mean(SE) | Declining to Low (n=61) % or Mean(SE) | Consistently High (n=365) % or Mean(SE) | p | Consistently Low vs. Recovery to High | Consistently Medium vs. Declining to Low |
| Sociodemographics | ||||||||
| Age | 51.68 (1.33) | 57.44 (2.49) | 51.95 (1.21) | 53.47 (1.59) | 56.73 (0.65) | <.001 | .04 | .45 |
| Very/somewhat hard to pay for basics | 30 | 16 | 22 | 23 | 14 | .01 | .18 | .85 |
| White race | 89 | 84 | 82 | 90 | 93 | .03 | .53 | .16 |
| Children <18 at home | 42 | 28 | 37 | 21 | 20 | <.001 | .20 | .04 |
| Cancer-related | ||||||||
| Chemotherapy | ||||||||
| None | 34 | 40 | 25 | 28 | 36 | .03 | .64 | .13 |
| Chemotherapy, no doxorubicin | 8 | 12 | 15 | 26 | 18 | |||
| Chemotherapy, with doxorubicin | 58 | 48 | 60 | 46 | 46 | |||
| Mastectomy | 43 | 28 | 42 | 49 | 32 | .02 | .17 | .40 |
| Psychosocialb | ||||||||
| Role of faith | 7.72 (0.52) | 7.28 (0.98) | 9.60 (0.47) | 9.63 (0.63) | 10.40 (0.26) | <.001 | .69 | .97 |
| Meaning/peace | 18.78 (0.60) | 20.79 (1.15) | 22.19 (0.53) | 23.55 (0.75) | 25.70 (0.30) | <.001 | .12 | .14 |
| Active Coping | 2.62 (0.07) | 2.44 (0.13) | 2.84 (0.06) | 2.85 (0.09) | 2.95 (0.03) | <.001 | .24 | .96 |
| Passive Coping | 1.46 (0.04) | 1.41 (0.08) | 1.40 (0.04) | 1.34 (0.05) | 1.25 (0.02) | <.001 | .57 | .38 |
| Illness Intrusiveness | 45.09 (1.72) | 46.60 (3.27) | 40.11 (1.55) | 38.34 (2.10) | 29.89 (0.85) | <.001 | .68 | .50 |
| Depressive Symptoms | 12.80 (0.65) | 12.29 (1.24) | 10.50 (0.58) | 9.00 (0.80) | 6.32(0.32) | <.001 | .71 | .13 |
| Optimism | 18.30 (0.56) | 21.80 (1.06) | 20.70 (0.51) | 21.20 (0.68) | 23.51 (0.28) | <.001 | .004 | .56 |
“Excludes the “consistently very low” group (n=8)
Values are estimated 4-month values, except for optimism.
Note: College-educated, married/partnered, cancer stage, radiation, and hormonal therapy were not significant; Social support was removed due to conceptual overlap.
Post-hoc Comparisons
Post-hoc comparisons were performed between groups beginning at similar levels but with differing trajectory shapes to examine characteristics that differentiated women in the recovery or declining groups compared to women who started similarly but improved less or remained stable (shown in Tables 3–5). We selected these comparisons as the most clinically meaningful among the groups. Within the TOI, comparisons were made between “recovery to medium” and “consistently very low” and between “recovery to high” and “consistently low.” Within the emotional subscale, we compared “recovery to medium” with “consistently low” and “declining to low” to “consistently medium.” Within the social/family subscale, we compared “recovery to high” with “consistently low” and “declining to low” to “consistently medium.”
Higher meaning/peace and social support were seen among the recovery groups for both the TOI and emotional subscales. Recovery groups were also more likely to have chemotherapy with doxorubicin. Additionally, both TOI recovery groups were less likely to have difficulty paying for basics, reported less passive coping and depressive symptoms, and had greater optimism compared to their comparison groups. The “declining to low” group on the emotional subscale reported more passive coping and illness intrusiveness compared to the “consistently medium” group.
Few variables distinguished the recovery and declining groups on the social/family subscale. Older age and higher optimism differentiated the recovery group from the “consistently low” group and being less likely to have children <18 at home differentiated the declining group from the “consistently medium” group.
Discussion
Previous longitudinal studies have demonstrated that, on average, QoL improves over time for BCS [4,6]. The present study goes beyond this research by identifying trajectories in three separate QoL domains (physical/functional, emotional, and social). Our finding that less than one-third of BCS were in similar trajectory groups across domains reinforces the value of assessing domain-specific trajectories of QoL.
The majority of women reported consistently medium or high QoL, similar to other studies [11,12,31], or experienced recovery over time within each of the domains. Additionally, we found small percentages of women in each domain who had persistently low QoL or even declined over time, as in the emotional and social domains. Other studies have also found cancer survivors whose emotional [11,12,31] or social well-being declined with time [31]. We did not find a group that declined in the physical/functioning domain, which is in contrast to other studies of BCS [11,12]. This may be due to the longer follow-up in the other studies or the specific measure used. The FACT-B TOI captures a broader construct of physical well-being and functional and breast-cancer specific concerns compared to the physical functioning scales of the SF-36 and EORTC-QLQ-C30 [11,12].
An important finding from the present study is that a higher percentage of BCS were at risk for consistently low/very low or declining social well-being (24%) than in the physical/functional (14%) and emotional (11%) domains. This finding highlights that for cancer survivors transitioning from treatment to survivorship, there may be a discrepancy between the social needs of the cancer survivor and the perceived support provided by friends and family [32]. Social well-being is often an understudied aspect of QoL with few data on longitudinal changes. Recent findings that lower social well-being is related to more leukocyte pro-inflammatory and pro-metastatic gene expression [33] and increased risk of mortality [34] among BCS, emphasize the importance of this domain.
Psychosocial factors (i.e., social support, meaning/peace, role of faith, optimism, passive coping, illness intrusiveness) were consistently related to group membership in all domains, with those in the “consistently high” groups faring the best on these factors. The present analyses extend our previous work on the reciprocal relationship between coping and overall QoL [17], We previously reported that while passive coping was reciprocally related to QoL, QoL predicted subsequent active coping, but not vice versa. Our analyses of specific QoL domains found that passive coping was related to all three measured QoL domains, but active coping was only associated with group membership in the social domain. A likely explanation is that active coping includes seeking and using social support. In contrast to the psychosocial variables, few sociodemographic and cancer-related variables were related to group membership across all domains.
Unique to the present study were comparisons between women who either recovered or declined and women who started out similarly but did not recover or decline. Higher levels of meaning/peace and perceived social support characterized the recoverers in both the emotional and physical/functional domains. These variables have been previously related to better mental and physical health in cancer survivors [11,15,35,36], but our findings suggest that they are also related to improved QoL over time. Personal resources such as social support and the ability to find meaning/peace in one’s life may be especially important for women who have aggressive chemotherapy with agents such as doxorubicin. For those in the recovery group, although QoL was likely negatively affected by treatment, personal resources may have facilitated recovery. The specific group comparisons also suggest that passive coping may have a negative impact on QoL. Those who declined in the emotional domain reported more use of passive coping and those who recovered in the physical/functional domain reported less use of passive coping compared to groups who started similarly but had a differing trajectory.
In contrast to the emotional and physical/functional domains, few measured factors differentiated the improvers and decliners on the social domain. This could be a function of the specific items on the social well-being subscale which address support from and communication with friends and family. Change in social well-being may be more a function of a person’s social network and less related to the personal characteristics and treatment variables we measured. Nonetheless, higher optimism, which was related to recovery in the physical/functional domain, was also reported by the recoverers compared to those who stayed persistently low in the social domain.
Our findings have several clinical implications. Cancer survivors with few social or personal resources may be particularly vulnerable to persistently low QoL and/or declines in QoL suggesting that such factors should be assessed at diagnosis. Additionally, findings that emotional and social well-being can decline for some BCS support recommendations that emotional distress should be regularly monitored post-diagnosis [37], but also suggest the need to monitor social well-being. Finally, early referral of patients at risk for poor outcomes to psychosocial or spiritual support may be potentially beneficial.
Our findings also suggest potential directions for future research. First, the finding that some areas of QoL may actually decline during the transition from active treatment to survivorship reinforces the need to continue developing interventions for this time period [38], Second, social support, sense of meaning/peace, passive coping and optimism were related to either recovery or decline across more than one domain. Although intervention studies often target coping and better utilizing one’s social support [39,40], little research has attempted to modify optimism or a sense of meaning and peace. There is some evidence that optimism and meaning and peace are modifiable through intervention, [40–42], but our results suggest these are worth further investigation. Finally, given the high percentage of BCS who were at risk for persistently low or declining social well-being, and that we identified few variables that differentiated these women, more research should seek to characterize factors that may put BCS at risk for poor social well-being over time.
There are several study limitations. First, the sample is racially and socioeconomically fairly homogeneous, which limits generalizability of findings. Second, though analyses assume data are missing completely at random, study drop-outs had poorer QoL on all three subscales at baseline than those who remained in the study. Consequently, the percentage of women in the low groups may be an underestimate. However, it is likely that the estimated differences between the low groups and other groups are conservative if we assume that those who dropped out were more "extreme" on a variety of variables than those who remained but who also had lower QoL values. We believe the impact of differential dropout on our findings is relatively small given the study’s overall high retention rate. The relatively low internal consistency for the passive coping factor may decrease the reliability of findings with this variable. Lastly, additional psychosocial factors that may be related to QoL, such as self-efficacy and perceived control [43] were not available in our study.
The present study has a number of strengths. First, this was a large sample followed longitudinally up to 26 months post-diagnosis with high retention. We were thus able to identify multiple trajectories in QoL over the critical transition period from active treatment to survivorship in women not receiving a planned intervention. Second, this study provides information on QoL trajectories in multiple domains, allowing for identification of predictors of trajectory group membership by specific domain. Third, we examined numerous predictors in addition to demographic and clinical variables to help identify factors differentiating BCS who started with similar levels of QoL, but had differing trajectories.
Overall, this study suggests that although many BCS do well in the transition from active treatment to survivorship, continued monitoring of domain-specific QoL overtime is warranted. Several potential risk factors for persistently low or declines in QoL were identified. Early intervention targeted to improve psychosocial resources may be beneficial, especially for those at risk for persistently low emotional or physical/functional well-being. Additional research on the longitudinal changes in social well-being and factors that may impact change in this outcome are needed.
Table 4.
Characteristics associated with each emotional subscale group (N = 653), with chi-square and F-test results and post-hoc contrasts
| Trajectory group | Chi-square or F-test results | Contrast p-values | ||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Consistently Low (n=27) % or Mean(SE) | Recovery to Medium (n=59) % or Mean(SE) | Consistently Medium (n=210) % or Mean(SE) | Declining to Low (n=44) % or Mean(SE) | Consistently High (n=313) % or Mean(SE) | p | Consistently Low vs. Recovery to Medium | Consistently Medium vs. Declining to Low |
| Sociodemographics | ||||||||
| Age | 53.09 (2.40) | 50.17 (1.62) | 54.59 (0.86) | 51.72 (1.88) | 56.70 (0.70) | .001 | .31 | .16 |
| Very/somewhat hard to pay for basics | 44 | 42 | 19 | 23 | 11 | <.001 | .86 | .53 |
| White race | 78 | 76 | 90 | 95 | 92 | .001 | .88 | .29 |
| Children <18 at home | 41 | 42 | 27 | 34 | 20 | .001 | .89 | .35 |
| Cancer-related | ||||||||
| Chemotherapy | ||||||||
| None | 26 | 24 | 35 | 27 | 35 | .03 | .04 | .50 |
| Chemotherapy, no doxorubicin | 22 | 5 | 15 | 21 | 20 | |||
| Chemotherapy, with doxorubicin | 52 | 72 | 50 | 52 | 45 | |||
| Psychosociala | ||||||||
| Role of faith | 6.53(0.92) | 8.74 (0.62) | 8.96 (0.33) | 9.38 (0.73) | 10.74(0.28) | <.001 | .048 | .60 |
| Meaning/peace | 15.57(1.00) | 18.44 (0.67) | 22.46 (0.37) | 21.29 (0.81) | 26.64(0.30) | <.001 | .02 | .19 |
| Social support | 3.68 (0.13) | 4.18 (0.09) | 4.19 (0.05) | 4.14 (0.11) | 4.47 (0.04) | <.001 | .002 | .63 |
| Passive Coping | 1.81 (0.07) | 1.68 (0.04) | 1.33 (0.02) | 1.53 (0.05) | 1.18 (0.02) | <.001 | .08 | <.001 |
| Illness Intrusiveness | 44.04 (3.05) | 47.85 (2.05) | 37.92 (1.11) | 43.64 (2.44) | 29.06 (0.91) | <.001 | .30 | .03 |
| Optimism | 15.63 (0.98) | 17.54 (0.66) | 21.04 (0.35) | 20.18 (0.77) | 24.23 (0.29) | <.001 | .11 | .30 |
Values are estimated 4-month values, except for optimism.
Note: College-educated, married/partner, cancer stage, radiation, hormonal therapy, mastectomy, and active coping were not significant; Depressive symptoms was removed due to conceptual overlap.
Acknowledgments
Funding: National Cancer Institute R25 CA122061 and Department of Defense grant DAMD17-01-1-0447
Footnotes
Ethical approval: “All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.”
Conflict of Interest: The authors declare that they have no conflicts of interest to report.
References
- 1.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A (2016) Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 66 (4):271–289. doi: 10.3322/caac.21349 [DOI] [PubMed] [Google Scholar]
- 2.Lemieux J, Goodwin PJ, Bordeleau LJ, Lauzier S, Theberge V (2011) Quality-of-life measurement in randomized clinical trials in breast cancer: an updated systematic review (2001-2009). J Natl Cancer Inst 103 (3):178–231. doi: 10.1093/jnci/djq508 [DOI] [PubMed] [Google Scholar]
- 3.Institute of Medicine and National Research Council (2006) From Cancer Patient to Cancer Survivor: Lost in Transition. The National Academies Press, Washington, D.C. doi: 10.17226/11468 [DOI] [Google Scholar]
- 4.Ganz PA, Kwan L, Stanton AL, Bower JE, Belin TR (2011) Physical and psychosocial recovery in the year after primary treatment of breast cancer. J Clin Oncol 29 (9):1101–1109. doi: 10.1200/JCO.2010.28.8043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karlsen RV, Frederiksen K, Larsen MB, von Heymann-Horan AB, Appel CW, Christensen J, Tjonneland A, Ross L, Johansen C, Bidstrup PE (2016) The impact of a breast cancer diagnosis on health-related quality of life. A prospective comparison among middle-aged to elderly women with and without breast cancer. Acta Oncol 55 (6):720–727. doi: 10.3109/0284186X.2015.1127415 [DOI] [PubMed] [Google Scholar]
- 6.Taira N, Shimozuma K, Shiroiwa T, Ohsumi S, Kuroi K, Saji S, Saito M, Iha S, Watanabe T, Katsumata N (2011) Associations among baseline variables, treatment-related factors and health-related quality of life 2 years after breast cancer surgery. Breast Cancer Res Treat 128 (3):735–747. doi: 10.1007/s10549-011-1631-y [DOI] [PubMed] [Google Scholar]
- 7.Henselmans I, Helgeson VS, Seltman H, de Vries J, Sanderman R, Ranchor AV (2010) Identification and prediction of distress trajectories in the first year after a breast cancer diagnosis. Health Psychol 29 (2):160–168. doi: 10.1037/a0017806 [DOI] [PubMed] [Google Scholar]
- 8.Lam WW, Shing YT, Bonanno GA, Mancini AD, Fielding R (2012) Distress trajectories at the first year diagnosis of breast cancer in relation to 6 years survivorship. Psychooncology 21 (1):90–99. doi: 10.1002/pon.1876 [DOI] [PubMed] [Google Scholar]
- 9.Avis NE, Levine BJ, Case LD, Naftalis EZ, Van Zee KJ (2015) Trajectories of depressive symptoms following breast cancer diagnosis. Cancer Epidemiol Biomarkers Prev 24 (11):1789–1795. doi: 10.1158/1055-9965.EPI-15-0327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cella DF (1994) Quality of life: concepts and definition. J Pain Symptom Manage 9 (3):186–192 [DOI] [PubMed] [Google Scholar]
- 11.Helgeson VS, Snyder P, Seltman H (2004) Psychological and physical adjustment to breast cancer over 4 years: identifying distinct trajectories of change. Health Psychol 23 (1):3–15. doi: 10.1037/0278-6133.23.1.3 [DOI] [PubMed] [Google Scholar]
- 12.Dura-Ferrandis E, Mandelblatt JS, Clapp J, Luta G, Faul L, Kimmick G, Cohen HJ, Yung RL, Hurria A (2017) Personality, coping, and social support as predictors of long-term quality-of-life trajectories in older breast cancer survivors: CALGB protocol 369901 (Alliance). Psychooncology 26 (11):1914–1921. doi: 10.1002/pon.4404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brady MJ, Cella DF, Mo F, Bonomi AE, Tulsky DS, Lloyd SR, Deasy S, Cobleigh M, Shiomoto G (1997) Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. J Clin Oncol 15 (3):974–986. doi: 10.1200/jco.1997.15.3.974 [DOI] [PubMed] [Google Scholar]
- 14.Maratia S, Cedillo S, Rejas J (2016) Assessing health-related quality of life in patients with breast cancer: a systematic and standardized comparison of available instruments using the EMPRO tool. Qual Life Res 25 (10):2467–2480. doi: 10.1007/s11136-016-1284-8 [DOI] [PubMed] [Google Scholar]
- 15.Leung J, Pachana NA, McLaughlin D (2014) Social support and health-related quality of life in women with breast cancer: a longitudinal study. Psychooncology 23 (9):1014–1020. doi: 10.1002/pon.3523 [DOI] [PubMed] [Google Scholar]
- 16.Schou I, Ekeberg O, Sandvik L, Hjermstad MJ, Ruland CM (2005) Multiple predictors of health-related quality of life in early stage breast cancer. Data from a year follow-up study compared with the general population. Qual Life Res 14 (8):1813–1823. doi: 10.1007/s11136-005-4344-z [DOI] [PubMed] [Google Scholar]
- 17.Paek MS, Ip EH, Levine B, Avis NE (2016) Longitudinal Reciprocal Relationships Between Quality of Life and Coping Strategies Among Women with Breast Cancer. Ann Behav Med 50 (5):775–783. doi: 10.1007/s12160-016-9803-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bloom JR, Stewart SL, Johnston M, Banks P (1998) Intrusiveness of illness and quality of life in young women with breast cancer. Psychooncology 7 (2):89–100. doi: [DOI] [PubMed] [Google Scholar]
- 19.Bai M, Lazenby M (2015) A systematic review of associations between spiritual well-being and quality of life at the scale and factor levels in studies among patients with cancer. J Palliat Med 18 (3):286–298. doi: 10.1089/jpm.2014.0189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Avis NE, Levine B, Naughton MJ, Case LD, Naftalis E, Van Zee KJ (2013) Age-related longitudinal changes in depressive symptoms following breast cancer diagnosis and treatment. Breast Cancer Res Treat 139 (1):199–206. doi: 10.1007/s10549-013-2513-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, Silberman M, Yellen SB, Winicour P, Brannon J, et al. (1993) The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol 11 (3):570–579. doi: 10.1200/jco.1993.11.3.570 [DOI] [PubMed] [Google Scholar]
- 22.Eton DT, Cella D, Yost KJ, Yount SE, Peterman AH, Neuberg DS, Sledge GW, Wood WC (2004) A combination of distribution- and anchor-based approaches determined minimally important differences (MIDs) for four endpoints in a breast cancer scale. J Clin Epidemiol 57 (9):898–910. doi: 10.1016/j.jclinepi.2004.01.012 [DOI] [PubMed] [Google Scholar]
- 23.Scheier MF, Carver CS (1985) Optimism, coping, and health: assessment and implications of generalized outcome expectancies. Health Psychol 4 (3):219–247 [DOI] [PubMed] [Google Scholar]
- 24.Peterman AH, Fitchett G, Brady MJ, Hernandez L, Cella D (2002) Measuring spiritual well-being in people with cancer: the functional assessment of chronic illness therapy--Spiritual Well-being Scale (FACIT-Sp). Ann Behav Med 24 (1):49–58 [DOI] [PubMed] [Google Scholar]
- 25.Devins GM (2010) Using the illness intrusiveness ratings scale to understand health-related quality of life in chronic disease. J Psychosom Res 68 (6):591–602. doi: 10.1016/j.jpsychores.2009.05.006 [DOI] [PubMed] [Google Scholar]
- 26.Sherbourne CD, Stewart AL (1991) The MOS social support survey. Soc Sci Med 32 (6):705–714 [DOI] [PubMed] [Google Scholar]
- 27.Beck AT, Steer RA, Ball R, Ranieri W (1996) Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess 67 (3):588–597. doi: 10.1207/s15327752jpa6703_13 [DOI] [PubMed] [Google Scholar]
- 28.Carver CS (1997) You want to measure coping but your protocol’s too long: consider the brief COPE. Int J Behav Med 4 (1):92–100. doi: 10.1207/s15327558ijbm0401_6 [DOI] [PubMed] [Google Scholar]
- 29.Jones B Traj: group-based modeling of longitudinal data. https://www.andrew.cmu.edu/user/biones/index.htm. Accessed June 2017
- 30.Nagin D (2005) Group-based modeling of development. Harvard University Press, Cambridge, MA [Google Scholar]
- 31.Lam WW, Ye M, Fielding R (2012) Trajectories of quality of life among Chinese patients diagnosed with nasopharynegeal cancer. PLoS One 7 (9):e44022. doi : 10.1371/journal.pone.0044022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stanton AL, Ganz PA, Rowland JH, Meyerowitz BE, Krupnick JL, Sears SR (2005) Promoting adjustment after treatment for cancer. Cancer 104 (11 Suppl):2608–2613. doi : 10.1002/cncr.21246 [DOI] [PubMed] [Google Scholar]
- 33.Jutagir DR, Blomberg BB, Carver CS, Lechner SC, Timpano KR, Bouchard LC, Gudenkauf LM, Jacobs JM, Diaz A, Lutgendorf SK, Cole SW, Heller AS, Antoni MH (2017) Social well being is associated with less pro-inflammatory and pro-metastatic leukocyte gene expression in women after surgery for breast cancer. Breast Cancer Res Treat 165 (1):169–180. doi: 10.1007/s10549-017-4316-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Epplein M, Zheng Y, Zheng W, Chen Z, Gu K, Penson D, Lu W, Shu XO (2011) Quality of life after breast cancer diagnosis and survival. J Clin Oncol 29 (4):406–412. doi: 10.1200/JC0.2010.30.6951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jim HS, Pustejovsky JE, Park CL, Danhauer SC, Sherman AC, Fitchett G, Merluzzi TV, Munoz AR, George L, Snyder MA, Salsman JM (2015) Religion, spirituality, and physical health in cancer patients: A meta-analysis. Cancer 121 (21):3760–3768. doi: 10.1002/cncr.29353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salsman JM, Pustejovsky JE, Jim HS, Munoz AR, Merluzzi TV, George L, Park CL, Danhauer SC, Sherman AC, Snyder MA, Fitchett G (2015) A meta-analytic approach to examining the correlation between religion/spirituality and mental health in cancer. Cancer 121 (21):3769–3778. doi: 10.1002/cncr.29350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andersen BL, DeRubeis RJ, Berman BS, Gruman J, Champion VL, Massie MJ, Holland JC, Partridge AH, Bak K, Somerfield MR, Rowland JH, American Society of Clinical O (2014) Screening, assessment, and care of anxiety and depressive symptoms in adults with cancer: an American Society of Clinical Oncology guideline adaptation. J Clin Oncol 32 (15):1605–1619. doi: 10.1200/JCO.2013.52.4611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stanton AL (2012) What happens now? Psychosocial care for cancer survivors after medical treatment completion. J Clin Oncol 30 (11):1215–1220. doi: 10.1200/JCO.2011.39.7406 [DOI] [PubMed] [Google Scholar]
- 39.Andersen BL, Farrar WB, Golden-Kreutz DM, Glaser R, Emery CF, Crespin TR, Shapiro CL, Carson WE 3rd, (2004) Psychological, behavioral, and immune changes after a psychological intervention: a clinical trial. J Clin Oncol 22 (17):3570–3580. doi: 10.1200/JCO.2004.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Antoni MH, Lehman JM, Kilbourn KM, Boyers AE, Culver JL, Alferi SM, Yount SE, McGregor BA, Arena PL, Harris SD, Price AA, Carver CS (2001) Cognitive-behavioral stress management intervention decreases the prevalence of depression and enhances benefit finding among women under treatment for early-stage breast cancer. Health Psychol 20 (1):20–32 [DOI] [PubMed] [Google Scholar]
- 41.Smith SK, Herndon JE, Lyerly HK, Coan A, Wheeler JL, Staley T, Abernethy AP (2011) Correlates of quality of life-related outcomes in breast cancer patients participating in the Pathfinders pilot study. Psychooncology 20 (5):559–564. doi: 10.1002/pon.1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bower JE, Crosswell AD, Stanton AL, Crespi CM, Winston D, Arevalo J, Ma J, Cole SW, Ganz PA (2015) Mindfulness meditation for younger breast cancer survivors: a randomized controlled trial. Cancer 121 (8):1231–1240. doi: 10.1002/cncr.29194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stanton AL, Revenson TA, Tennen H (2007) Health psychology: psychological adjustment to chronic disease. Annu Rev Psychol 58:565–592. doi: 10.1146/annurev.psych.58.110405.085615 [DOI] [PubMed] [Google Scholar]


