Abstract
To remodel functional neuronal connectivity, neurons often alter dendrite arbors through elimination and subsequent regeneration of dendritic branches. However, the intrinsic mechanisms underlying this developmentally programmed dendrite regeneration and whether it shares common machinery with injury-induced regeneration remain largely unknown. Drosophila class IV dendrite arborization (C4da) sensory neurons regenerate adult-specific dendrites after eliminating larval dendrites during metamorphosis. Here we show that the microRNA miR-87 is a critical regulator of dendrite regeneration in Drosophila. miR-87 knockout impairs dendrite regeneration after developmentally-programmed pruning, whereas miR-87 overexpression in C4da neurons leads to precocious initiation of dendrite regeneration. Genetic analyses indicate that the transcriptional repressor Tramtrack69 (Ttk69) is a functional target for miR-87-mediated repression as ttk69 expression is increased in miR-87 knockout neurons and reducing ttk69 expression restores dendrite regeneration to mutants lacking miR-87 function. We further show that miR-87 is required for dendrite regeneration after acute injury in the larval stage, providing a mechanistic link between developmentally programmed and injury-induced dendrite regeneration. These findings thus indicate that miR-87 promotes dendrite regrowth during regeneration at least in part through suppressing Ttk69 in Drosophila sensory neurons and suggest that developmental and injury-induced dendrite regeneration share a common intrinsic mechanism to reactivate dendrite growth.
Author summary
Dendrites are the primary sites for synaptic and sensory inputs. To remodel or repair neuronal connectivity, dendrites often exhibit large-scale structural changes that can be triggered by developmental signals, alterations in sensory inputs, or injury. Despite the importance of dendritic remodeling to nervous system function, the molecular basis for this remodeling is largely unknown. Here we used an unbiased genetic screen and in vivo imaging in Drosophila sensory neurons to demonstrate that the microRNA miR-87 is a critical factor required in neurons to reactivate dendritic growth both in developmental remodeling and following injury. Our work supports the model that miR-87 promotes dendrite regeneration by blocking expression of the transcriptional repressor Tramtrack69 in neurons. This study thus establishes a role for miRNAs in temporal control of dendrite regeneration.
Introduction
During critical periods of brain development, neurons exhibit juvenile plasticity in which connectivity can be modified in response to sensory inputs. To achieve these changes in connectivity, neurons often remodel their dendrite shape by elimination and subsequent regeneration of dendritic branches. For instance, Purkinje cells in the mouse cerebellum initially eliminate all perisomatic dendrites followed by regenerating single stem dendritic branches to form mature dendritic trees during postnatal development [1]. Likewise, during early postnatal development, layer 4 neurons in the mouse barrel cortex refine their connectivity with thalamocortical axons by biased elimination and regeneration of preexisting dendritic branches [2,3]. Over time, many types of neurons progressively reduce dynamics and stabilize their dendritic arbors as they mature [4–6]. However, dendritic arbors of mature neurons can undergo dramatic regeneration under pathological conditions such as epilepsy and after injury [7–9]. Therefore, understanding the mechanisms that underlie dendrite regeneration has important implications for understanding normal development of functional dendrite arbors and functional repair of injured neural circuits.
Drosophila class IV dendrite arborization (C4da) neurons exhibit both developmentally programmed and damage-induced dendrite regeneration and therefore present a genetically tractable and optically accessible model to study cellular and molecular mechanisms underlying dendrite remodeling [10–12]. During metamorphosis, dendrites which elaborate during larval stages are completely pruned away and subsequently replaced with adult-specific dendritic arbors [13–18] (Fig 1A). This developmental dendrite regeneration after pruning requires intrinsic factors including transcriptional factors [19] as well as extrinsic mechanisms such as remodeling of the extracellular matrix [17, 18]. Recent studies indicate that, in addition to this developmental dendrite regeneration, removal of a part of dendritic branches during larval stages triggers robust dendrite regeneration in C4da neurons [20–23]. In the course of injury-induced dendrite regeneration, a new dendritic process initiates growth at the severed stump by ~24 hrs after injury and then further elongates and elaborates dendritic arbors by ~72 hrs after injury [20, 21]. This progression observed in the injury-induced dendrite regeneration is morphologically similar to what has been reported during developmental dendrite regeneration, but it is unknown whether these dendrite regrowth programs share common mechanisms.
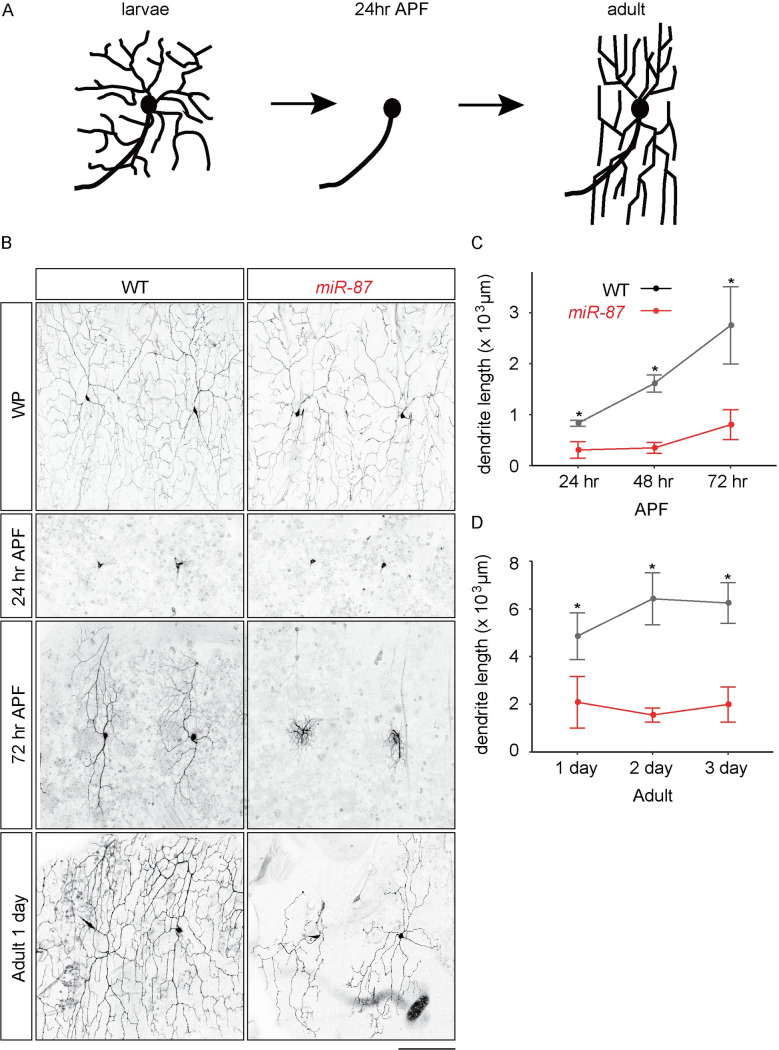
Fig 1. miR-87 is required for dendrite regeneration during metamorphosis.
(A) A schematic model of developmental dendrite regeneration in C4da neurons. (B) Dendrite regeneration in wild-type control (WT) and miR-87 knockout (miR-87) C4da neurons at the indicated time points. WP, white pupa; APF, after pupa formation; Adult 1 day, 1 day post-eclosion. Scale bar = 100 μm. (C-D) Quantitative comparison of total dendrite length in wild-type (WT) and miR-87 KO (miR-87) C4da neurons during pupal (C) and adult (D) stages. Points depict mean values, error bars indicate standard deviation values. n = 30. *p<0.01, Student’s t-test. Genotypes: WT, ppk-GFP; miR-87, ppk-GFP; miR-87KO/miR-87KO.
One salient feature of developmental dendrite remodeling that provides possible insight into its control is the stereotyped timing of the process, and microRNAs (miRNAs) have recently emerged as key factors regulating developmental timing in the nervous system [24–27]. miRNAs are short non-coding RNAs that interact with and generally inhibit expression of target mRNAs, typically binding target sites in the 3’UTR via base-pairing interactions and silencing gene expression via effects on mRNA stability and translation [28, 29]. Recent reports suggest both positive and negative roles of miRNAs in axon regeneration after injury in both the central and peripheral nervous system [30–35]. For example, miR-133b is upregulated after spinal cord transection and facilitates axon regeneration probably through reducing protein level of RhoA GTPase [34]. Conversely, miR-138 suppresses mammalian axon regeneration after injury by targeting SIRT1 [35] In contrast to the accumulating evidence suggesting potential roles of miRNAs in axon regeneration, much less is known about the cell-intrinsic role of miRNAs in dendrite regeneration.
In this study, we report a crucial role of the Drosophila miRNA miR-87 in dendrite regeneration of C4da neurons. Loss of miR-87 function in C4da neurons significantly diminished dendrite regeneration, whereas miR-87 overexpression in C4da neurons accelerated dendrite regrowth. We found that miR-87 expression was elevated in C4da neurons during the larva-to-pupa transition, and that miR-87 appears to promote dendrite regrowth at least in part by targeting the transcriptional repressor Tramtrack69. Finally, we found that miR-87 is required for dendrite regeneration that occurs after injury. These data suggest that miR-87 is a critical factor to reactivate branch outgrowth in both developmental and injury-induced dendrite regeneration.
Results
miR-87 is required for dendrite regeneration during metamorphosis
To understand molecular mechanisms underlying the temporal control of dendrite regeneration, we focused on microRNAs (miRNAs) as miRNAs are implicated in regulation of developmental timing in the nervous system [24–27]. To identify miRNAs that might be involved in C4da dendrite regeneration, we systematically assayed effects of miRNA knockout using the miRNA knockout (KO) collection [36] in combination with the C4da-specific pickpocket (ppk)-GFP reporter, which is expressed in C4da neurons from late embryonic stages through adulthood [15, 37]. Among 69 autosomal miRNA KO lines we tested, covering ~80% of expressed miRNAs (S1 Table), we identified a single miRNA required for dendrite regrowth. In larvae homozygous mutant for a miR-87 KO allele, C4da neurons showed a significant defect in dendrite degeneration during metamorphosis (Fig 1B). miR-87 KO neurons pruned their larval dendrites normally by 24 hr APF, but failed to properly regrow dendritic trees, resulting an inappropriate coverage of the receptive fields in newly eclosed adults (Fig 1A–1C). Although wild-type C4da neurons further extended terminal branches for the first 2 days post-eclosion to completely cover the body wall, we observed no significant dendrite growth in miR-87 KO neurons over the same period (Fig 1D, S1 Fig), suggesting that the dendrite regeneration defects in miR-87 KO neurons are due to a reduced ability in branch regrowth, rather than a developmental delay in dendrite growth.
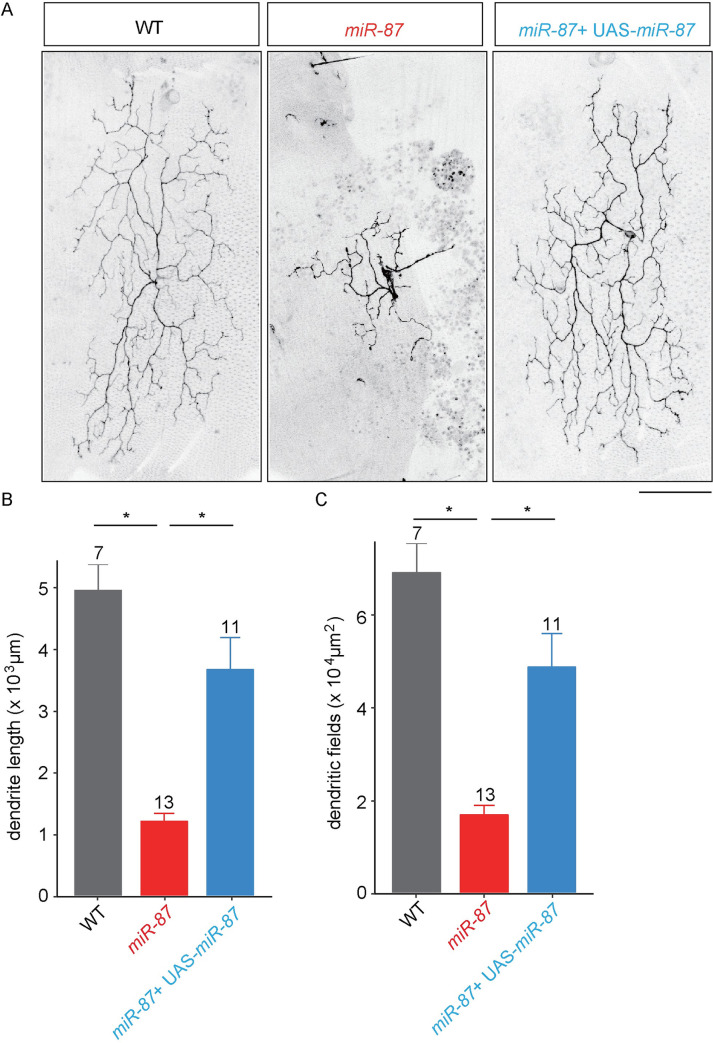
Similar to larval C4da dendrites, the regenerated adult C4da dendrites grow between the epidermis and the underlying musculature, largely confined to a 2-dimensonal territory [16–18]. It is thus possible that miR-87 could function in sensory neurons, the muscle, and/or epidermal cells to control dendrite regeneration; recent studies of the miRNA bantam provide precedent for the latter scenario [20, 38]. To distinguish between these possibilities, we performed MARCM (mosaic analysis with a repressible cell marker) to generate single neuron clones homozygous for miR-87 KO in a heterozygous background [39]. Compared to wild-type controls, we found that miR-87 MARCM clones exhibited severe dendrite regeneration defects which were comparable to the regeneration defects we observed in homozygous miR-87 mutants (Fig 2A–2C). These defects were largely rescued by expressing UAS-miR-87 in the miR-87 KO clones, demonstrating that the dendrite regeneration defects were associated with loss of miR-87 function in C4da neurons. We note that expressing UAS-miR-87 in the miR-87 KO clones did not fully restore dendrite length to the levels of wild-type clones; this difference may reflect differences in the timing/levels of miR-87 expression in the rescue clones or may reflect minor cell non-autonomous contributions of miR-87 in other tissues which are heterozygous for miR-87 mutation. Taken together, these results demonstrate that miR-87 is cell-autonomously required in C4da neurons for dendrite regeneration during metamorphosis.
Fig 2. miR-87 functions cell-autonomously in C4da neurons to promote dendrite regeneration.
(A) MARCM analysis of wild-type control (WT), miR-87 KO (miR-87), and miR-87 KO with UAS-miR-87 expression (miR-87 + UAS-miR-87) C4da neurons at 96 hrs APF. Scale bar, 100 μm. (B, C) Quantitative analysis of the total dendrite length (B) and the dendritic field areas (C) of MARCM clones. The sample numbers examined are shown on the top of each column, Bars indicate mean ± standard deviation, *p<0.01 (ANOVA with a post hoc Bonferroni correction). Clone genotypes: (WT) hsFLP, ppk-Gal4, UAS-mCD8GFP/+; FRT40A, (miR-87) hsFLP, ppk-Gal4, UAS-mCD8GFP/+; miR-87, FRT40A, and (miR-87 + UAS-miR-87) hsFLP, ppk-Gal4, UAS-mCD8GFP/+; miR-87, FRT40A; UAS-miR-87/+.
miR-87 KO neurons are defective in the initial elongation of regenerating dendrites
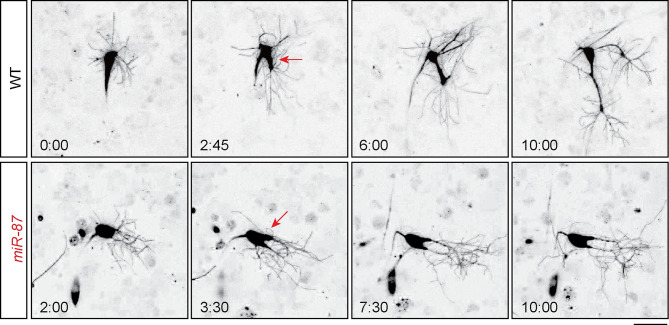
To gain insight into the cellular basis of miR-87 KO dendrite regeneration defects, we performed time-lapse imaging to monitor dendrite growth dynamics in wild-type and miR-87 KO mutant larvae. Consistent with prior reports [14, 17], we found that dendritic arbors of the larval v’ada neurons were completely pruned away by ~24 hr APF in wild-type and miR-87 KO neurons, while the soma and axonal processes remained intact (Fig 1A and 1B). We thus started our time-lapse analysis at 24 hr APF. During the first ~3 hr after the completion of dendrite arbor pruning, wild-type C4da neurons elaborated dynamic protrusions which extended and retracted from the soma. These fine branches were transient structures (S1 Movie), therefore C4da neurons exhibited very little net regrowth of dendritic branches during this time window which we refer to as the “pausing” stage of dendrite regrowth. Next, 1–2 branches among the many existing protrusions thickened at ~27 hr APF and subsequently elongated, eventually forming major dendrite branches by ~36 hr APF that would serve as the scaffold of adult C4da dendrite arbors (Fig 3, S1 Movie). C4da neurons in miR-87 KO mutants similarly elaborated fine, dynamic protrusions and exhibited thickening of select branches at ~27 hr APF. However, unlike wild-type controls, the nascent “thick” branches failed to elongate in miR-87 KO mutants. Consequently, dendrite regrowth was largely arrested (Fig 3, S2 Movie). We noted that the nascent dendritic branches of miR-87 neurons made frequent contact with one another during this window of dendrite regrowth, however contact events were not followed by dendrite retraction, suggesting that dendrite regeneration defects in miR-87 neurons are unrelated to contact-dependent retraction of dendritic branches. These observations suggest that miR-87 is required for efficient elongation of dendritic branches during regeneration in C4da neurons.
Fig 3. miR-87 is required for branch elongation in dendrite regeneration.
Time-lapse imaging of dendrite regeneration in wild-type control (WT) and miR-87 KO (miR-87) C4da neurons. Recording started at 24 hr APF and continued for ~12 hrs. Time stamps represent the intervals after 24 hr APF (hours: minutes). Red arrows indicate branches undergoing thickening. Scale bar = 25μm. Genotypes: WT, ppk-GFP; miR-87, ppk-GFP; miR-87KO/miR-87KO.
miR-87 activity is elevated in C4da neurons during the larva-to-pupa transition
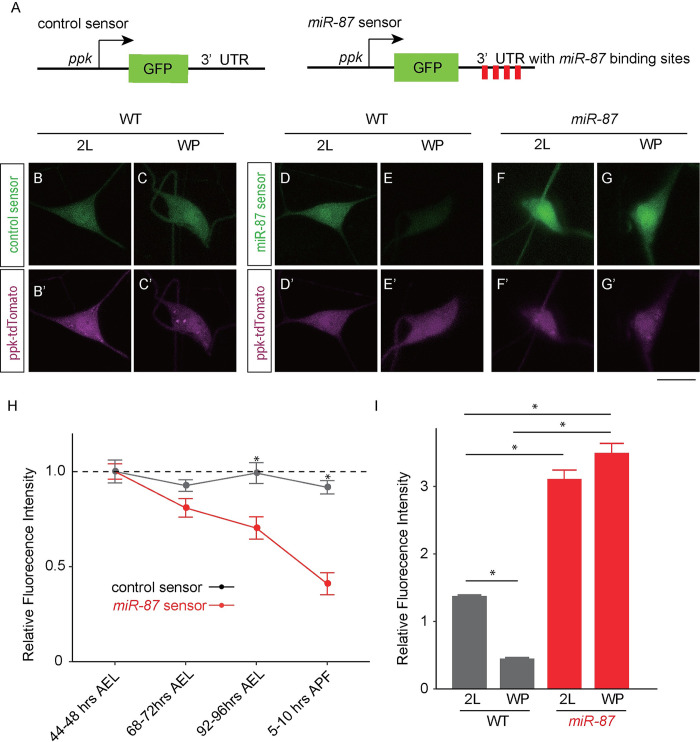
Given the temporal requirement for miR-87 for dendrite regrowth after ~27 hr APF, we next investigated whether miR-87 activity was induced to trigger regrowth. A previous northern blot analysis showed that miR-87 expression in whole animals is markedly increased during the larva-to-pupa transition [40], however the dynamics of miR-87 expression in C4da neurons have not been documented. To directly monitor miR-87 activity in C4da neurons, we generated a miR-87 activity sensor that contains four miR-87 binding sites in the 3′UTR of GFP, hence GFP expression is attenuated in cells expressing mature miR-87 (Fig 4A) [41]. As a control, we first monitored expression of a control GFP sensor that lacked miR-87 binding sites and therefore should be refractory to miR-87 expression levels. We found that this control sensor supported uniform levels of GFP expression in C4da neurons during larval and early pupal stages (Fig 4B and 4C and 4H). In contrast, whereas the miR-87 sensor produced high levels of GFP expression in C4da neurons during early larval stages, we observed significant attenuation of GFP expression by the miR-87 sensor beginning in 3rd instar larvae, resulting in a >50% reduction of GFP expression in early pupal stages (Fig 4D–4H). Notably, the temporal control of the miR-87 sensor was dependent on miR-87 activity, as GFP expression by the miR-87 sensor was not attenuated in miR-87 mutant C4da neurons (Fig 4I). Thus, miR-87 activity in C4da neurons is increased during the larva-to-pupa transition, in anticipation of miR-87 function in dendrite regrowth.
Fig 4. miR-87 expression is elevated in C4da neurons during the larvae-to-pupae transition.
(A) Schematic representations of control and miR-87 sensor constructs. (B-C) Control sensor is stably expressed in C4da neurons. Control sensor expression in animals additionally expressing ppk-CD4-tdTomato is shown in 2nd instar larval (2L) and white pupal (WP) C4da neurons. (D-G) Regulated expression of miR-87 sensor. Images show expression of the miR-87 sensor in 2nd instar larval (2L) and white pupal (WP) C4da neurons additionally expressing ppk-CD4-tdTomato. Note the marked downregulation of GFP expression over this interval. (F, G) miR-87 sensor expression is responsive to miR-87 activity. Image depicts miR-87 sensor expression in 2L and WP C4da neurons of miR-87 KO animals. Sensor expression remains high in miR-87 KO neurons, indicating that pupal downregulation of the sensor depends on miR-87 function. Scale bar = 20 μm. (H) Quantification of control and miR-87 sensor GFP expression in C4da neurons at the indicated developmental stages. AEL, after egg laying. The miR-87 sensor fluorescence intensity was normalized to the control sensor fluorescence intensity and represented as relative intensity to 2nd instar larval levels (44–48 hr AFL). n = 25, Error bars indicate mean ± S.D, *p<0.001 (Student’s t-test). (I) Quantification of relative fluorescence intensity of miR-87 sensor GFP in wild-type (WT) and miR-87 KO (miR-87) C4da neurons. The miR-87 sensor fluorescence intensity was normalized to the fluorescence intensity of the control sensor lacking miR-87 binding sites in the same genetic background and at the same developmental stage. n = 25, Error bars indicate mean ± S.D, *p<0.01 (Student’s t-test). Genotypes: control sensor in WT neurons: ppk::tdTomato; +/+; ppk-miR-87-control sensor; miR-87 sensor in WT neurons, ppk::tdTomato; +/+; ppk-miR-87 sensor; miR-87 sensor in miR-87 neurons, ppk::tdTomato; miR-87KO/miR-87KO; ppk-miR-87 sensor.
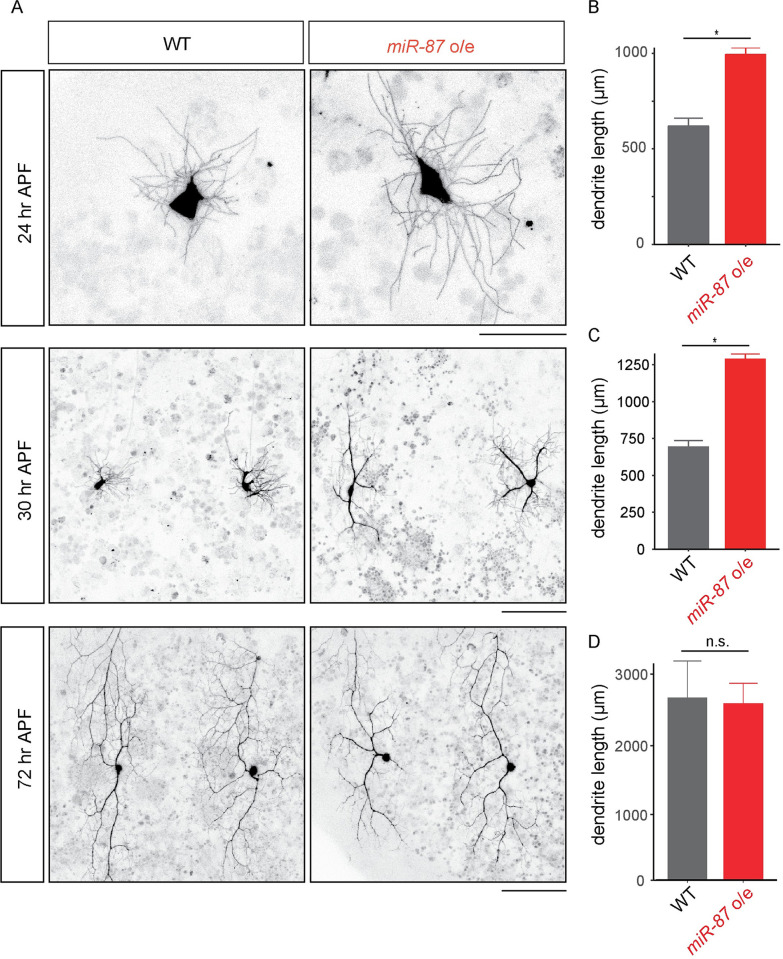
Given that miR-87 activity is upregulated and required for dendrite regeneration, we reasoned that elevation of miR-87 activity might promote C4da dendrite regeneration. To examine this possibility, we constitutively overexpressed miR-87 in C4da neurons from early developmental stages using the C4da-specific ppk-Gal4 driver. As described above, dendrite pruning is complete at 24 hr APF in wild-type and miR-87 KO C4da neurons (Fig 1B and 1C). We found that dendrite pruning was completed on a similar timescale in miR-87-overexpressing neurons (S2 Fig), however miR-87 overexpression led to precocious dendrite outgrowth following pruning. First, whereas control C4da neurons exhibited short, fine protrusions at 24 hr APF, the length of each protrusion was significantly increased in miR-87-overexpressing neurons (Fig 5A and 5B; wild-type, 12.4 ± 0.5 μm; miR-87 o/e, 35.2 ± 1.2 μm, p< 0.01, n = 30 protrusions from 10 different neurons). Second, whereas control neurons remained in the “pausing” phase of regrowth at 30 hr APF, with control dendrites exhibiting no significant net dendrite growth, miR-87-overexpressing neurons had extended 2–3 thick branches by 30 hr APF (Fig 5A and 5C; wild-type, 0.3 ± 0.1 branches; miR-87 o/e, 2.7 ± 0.6 branches, p< 0.01, n = 30). Finally, when we monitored the status of dendrite regrowth at 72 hr APF, we found that both wild type and miR-87-overexpressing neurons had regrown to a comparable degree (Fig 5A and 5D), demonstrating that miR-87 overexpression specifically regulates the timing of early stages of regrowth, not the overall extent of regrowth. These results together support the idea that elevation of the miR-87 expression during the larvae-to-pupae transition triggers dendrite regeneration in C4da neurons.
Fig 5. miR-87 overexpression in C4da neurons causes precocious dendrite regeneration.
(A) Live-imaging of wild-type (WT) and miR-87-overexpressing (miR-87 o/e) C4da dendrites. Scale bars = 20 μm for 24 hr APF, and 100 μm for 30hr and 72 hr APF. (B-D) Quantification of the total dendrite length in wild-type (WT) and miR-87-overexpressing (miR-87 o/e) C4da dendrites at 24 hr (B), 30 hr (C), and 72 hr (D) APF. n = 30, Error bars indicate mean ± S.D., *p<0.01 (Student’s t-test), n.s., not significant. Genotypes: WT, ppk-Gal4, UAS-mCD8GFP; miR-87o/e, ppk-GAL4, UAS-mCD8GFP; UAS-miR-87.
miR-87 targets the transcriptional repressor Tramtrack69 to promote dendrite regeneration
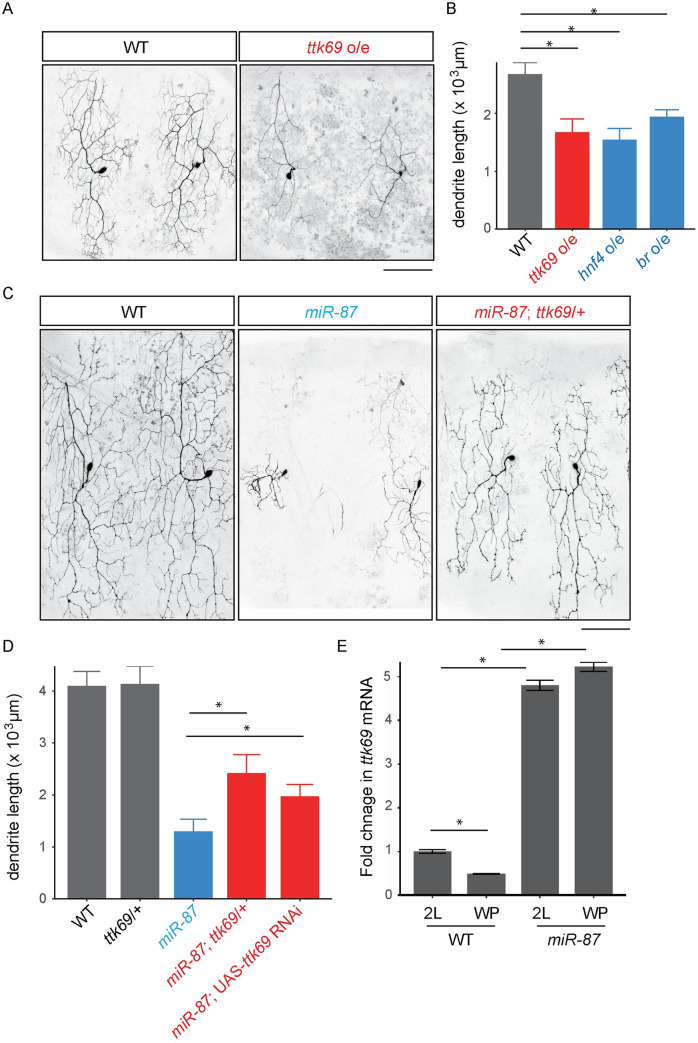
miRNAs typically exert their control via base-pairing interactions with 3’UTR sequences in their targets, leading to translational repression [28, 29]. These base-pairing interactions allow for computational prediction of miRNA targets; such a prediction has identified more than 100 putative miR-87 targets [42]. As a first step to determine the functionally relevant targets of miR-87 in C4da dendrite regeneration, we used gain-of-function approaches, reasoning that overexpression of bona fide miR-87 targets in a wild-type background should phenocopy loss of miR-87 function. Indeed, among 43 potential targets we tested (S2 Table), overexpression of 3 genes, hepatocyte nuclear factor 4 (hnf4), broad (br), and tramtrack69 (ttk69), caused significant defects in C4da dendrite regeneration (Fig 6A and 6B, S3 Fig, S5A and S5B Fig).
Fig 6. miR-87 targets the transcriptional repressor Tramtrack69 to promote dendrite regeneration.
(A, B) Overexpression of ttk69 causes dendrite regeneration defects. Morphology of wild-type control (WT) and ttk69 overexpressing (ttk69 o/e) dendrites at 72 hr APF (A). Scale bar = 100 μm. Quantification of total dendrite length in wild-type (WT), ttk69 overexpressing (ttk69 o/e), hnf4 overexpressing (fnf4 o/e), and br overexpressing (br o/e) neurons (B). n = 15, Error bars indicate mean ± S.D., *p<0.01 (ANOVA with a post hoc Bonferroni correction). (C, D) Reduction of ttk69 dosage significantly rescues dendrite regeneration defects in miR-87 KO C4da neurons. Morphology of control wild-type (WT), miR-87 KO (miR-87), and miR-87 KO with reduction of one copy of ttk69 (miR-87; ttk69+/-) dendrites at 96 hr APF (C). Scale bar = 100 μm. Quantification of total dendrite length in control wild-type (WT), miR-87 KO (miR-87), miR-87 KO with reduction of one copy of ttk69 (miR-87; ttk69+/-), and miR-87 KO with ttk69 RNAi (miR-87; UAS-ttk69 RNAi) dendrites at 96 hr APF (D). n = 15, Error bars indicate mean ± S.D., *p<0.01 (ANOVA with a post hoc Bonferroni correction). (E) Quantitative PCR showing ttk69 mRNA levels from brain extracts of the indicated genotypes at second instar (2L) or white pupal (WP) stages. Data were normalized to rp49 and represent the average of 5 independent experiments. Error bars indicate mean ± S.D., *p<0.01, (Student’s t-test). Genotypes: WT, ppk-Gal4, UAS-mCD8GFP; ttk69 o/e, ppk-GAL4, UAS-mCD8GFP; UAS-ttk69/+; miR-87, ppk-Gal4, UAS-mCD8GFP; miR-87KO/ miR-87KO; miR-87; ttk69/+, ppk-Gal4, UAS-mCD8GFP; miR-87KO/ miR-87KO; ttk1e11/+.
To further define the functional relevance of these putative miR-87 targets, we next tested for dosage-sensitive interactions between the candidate genes and miR-87, assaying whether heterozygous loss-of-function mutations in the target genes could suppress the dendrite regeneration defect in miR-87 KO neurons. Strikingly, removal of one copy of ttk69 significantly restored dendrite growth during regeneration in miR-87 KO animals whereas removal of one copy of hnf4 or br caused no significant rescue in the dendrite regeneration defect in miR-87 KO neurons (Fig 6C and 6D, S3B and S3C Fig). Similarly, targeted expression of UAS-ttk69-RNAi in C4da neurons partially rescued dendrite regrowth in miR-87 KO neurons (Fig 6D), demonstrating that ttk69 cell-autonomously regulates C4da dendrite regeneration. These results strongly suggest that Ttk69 is a key target of miR-87 in C4da dendrite regeneration, and we further tested this possibility by assaying effects of miR-87 on nervous system expression of ttk69 using qPCR. In wild-type control larvae, we found that the level of ttk69 mRNA was reduced during the larva-to-pupa transition, but this reduction was not observed in miR-87 KO animals (Fig 6E), consistent with a role for miR-87 in control of ttk69 expression.
Alterations in ttk69 levels could reflect transcriptional or post-transcriptional regulation of gene expression. To discriminate between these possibilities, we assayed whether miR-87 affected expression of a ubiquitously expressed GFP sensor containing the ttk69 3’UTR (S4A Fig), which was previously shown to mediate miRNA-dependent post-transcriptional control of ttk69 expression [43]. We found that ttk69 GFP sensor expression was under developmental control in C4da neurons, with sensor expression significantly reduced during the larva-to-pupa transition in wild-type controls (S4B and S4C Fig). In contrast, this developmental control of ttk69 sensor expression was lost in mir-87 KO mutants, which exhibited sustained ttk69 sensor expression at significantly higher levels than in wild type controls. Altogether, these results suggest that mir-87 repression of ttk69 expression is mediated through the ttk69 3’UTR. Taken together with genetic interaction studies, these findings suggest that miR-87 function in dendrite regeneration depends in part on control of ttk69 expression in C4da neurons.
miR-87 is required for injury-induced dendrite regeneration
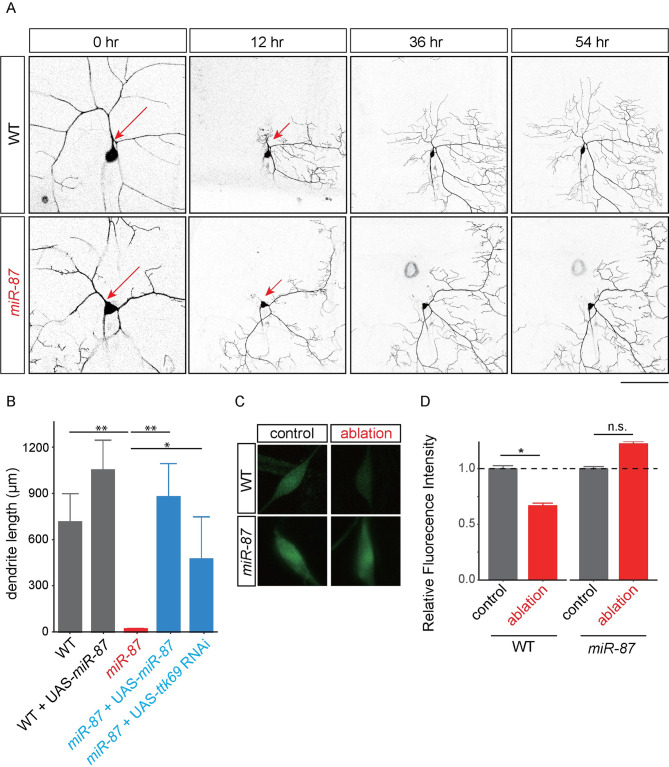
Previous studies indicate that larval C4da neurons are capable of regenerating dendrites after injury [20–22]. To determine whether miR-87 might also play a role in injury-induced dendrite regeneration, we assayed injury-induced dendrite regeneration in miR-87 KO larvae. To this end, we severed dendrites of individual C4da neurons using a two-photon laser at ~48 hr AEL, with the lesion site restricted to the primary dendritic branch point, and monitored dendrite regeneration using time-lapse confocal microscopy (Fig 7A). Consistent with previous studies, we observed robust dendrite regrowth in wild-type larvae, with significant new growth evident at 12 hr after lesioning and dendrite regrowth continuing through the end of our imaging paradigm, 54 hr after lesioning (Fig 7A and 7B). In contrast, we observed no sign of dendrite regeneration in miR-87 KO C4da neurons at either early or late time points after laser severing, suggesting that miR-87 plays an essential role in injury-induced C4da dendrite regeneration (Fig 7A and 7B). We found that these degeneration defects in miR-87 KO neurons were rescued to wild-type levels of regeneration by C4da neuron-specific expression of miR-87. Further, overexpression of miR-87 in C4da neurons tended to enhance dendrite regeneration after injury (Fig 7B; WT, 746.71 ± 190.13 μm; WT + UAS-miR87, 1101.13 ± 201.33 μm). These data suggest that miR-87 promotes larval dendrite regeneration following injury in a cell-autonomous manner. Similar to developmental dendrite regeneration, ttk69 knockdown significantly enhanced injury-induced dendrite regrowth in miR-87 mutant C4da neurons (Fig 7B), whereas overexpression of ttk69 in C4da neurons caused dendrite regrowth defects in otherwise wild-type C4da neurons (S5C and S5D Fig). These data suggest that, similar to developmentally programmed dendrite regrowth, dendrite regrowth after injury critically depends on miR-87 control of a gene expression program that involves ttk69.
Fig 7. miR-87 is required for injury-induced dendrite regeneration.
(A) Time-lapse images of dendrite regeneration after injury in wild-type (WT) and miR-87 KO (miR-87) neurons. Red arrows indicate the site of dendrite severing in 2nd instar larvae (44–48 hr AEL). Scale bar = 25 μm and 50 μm for 0 hr and 12–54 hrs after severing, respectively. (B) Quantification of regenerated dendrite length after severing in control wild-type (WT), wild-type overexpressing miR-87 (WT + UAS-miR-87) miR-87 KO (miR-87), miR-87 KO with C4da-specific rescue of miR-87 (miR-87 + UAS-miR-87), and miR-87 KO with ttk69 RNAi (miR-87; UAS-ttk69 RNAi) dendrites at 54 hr after severing. n = 25, Error bars indicate mean ± S.D., **p<0.01, *p<0.05 (ANOVA with a post hoc Bonferroni correction). (C) Expression of miR-87 sensor in control and injured neurons showed a marked down-regulation of GFP expression 12 hrs after dendrite severing. miR-87 sensor expression remains high in miR-87 KO neurons after dendrite severing, indicating that the downregulation depends on miR-87 function. Scale bar = 20 μm. (D) Quantification of miR-87 sensor GFP in control and injured C4da neurons. The miR-87 sensor fluorescence intensity was normalized to the control sensor fluorescence intensity in the same genetic background and at the same developmental stage. n = 15 neurons from independent animals for each genotype/condition, Error bars indicate mean ± S.D., *p<0.01 (unpaired t-test), n.s., not significant. Genotypes: WT, ppk-Gal4, UAS-mCD8GFP; WT + UAS-miR-87, ppk-GAL4, UAS-mCD8GFP; UAS-miR-87/+; miR-87, ppk-Gal4, UAS-mCD8GFP; miR-87KO/ miR-87KO; miR-87 + UAS-miR-87, ppk-Gal4, UAS-mCD8GFP; miR-87KO/ miR-87KO; UAS-miR-87/+; miR-87 + UAS-ttk69 RNAi, ppk-Gal4, UAS-mCD8GFP; miR-87KO/ miR-87KO; UAS-ttk69 RNAi /+.
Finally, to test whether dendrite injury induces expression of this regeneration program, we monitored effects of dendrite severing on miR-87 sensor expression in C4da neurons. Indeed, miR-87 sensor fluorescent intensity was significantly reduced 12 hr after dendrite severing in control but not miR-87 mutant larvae, suggesting that dendrite damage induced miR-87 activity in C4da neurons (Fig 7C and 7D). Altogether, these data suggest that miR-87 plays a central role in dendrite regrowth, potentiating dendrite regrowth following both injury-induced dendrite regeneration and developmental dendrite regeneration by suppressing expression of the transcription factor Ttk69.
Discussion
In this study, through a non-biased miRNA screen in Drosophila sensory neurons, we have for the first time identified the miRNA miR-87 as a critical regulator of dendrite regeneration. Despite the many links between miRNAs and neuronal development, including axon regeneration [30–35], these studies provide one of the first connections between miRNAs and dendrite regeneration. In Drosophila, miR-87 KO C4da neurons fail to regenerate dendritic arbors, resulting in incomplete coverage of the adult epidermis (Fig 1). These defects are ameliorated substantially by expression of miR-87 in KO C4da neurons (Fig 2), indicating that miR-87 functions in a cell-autonomous manner to regulate dendrite regeneration. Furthermore, our time-lapse analysis showed that miR-87 mutant C4da neurons are defective in the initial elongation of dendritic branches (Fig 3, S1 & S2 Movies), suggesting that miR-87 is required for pruned dendrites to reinitiate the developmental program for dendrite outgrowth. Given that miR-87 activity in C4da neurons is elevated during the larvae-to-pupae transition (Fig 4), we propose a model in which upregulation of miR-87 is a critical determinant for dendrite regrowth. In support of this model, forced expression of miR-87 in C4da neurons in the early larval stages causes precocious initiation of dendrite regeneration (Fig 5).
How could miR-87 promote dendrite regeneration in C4da neurons? Given that miRNAs typically bind to 3’UTR of particular mRNAs and suppress their expression [28, 29], the most likely scenario is that miR-87 promotes dendrite regeneration by suppressing target gene expression in C4da neurons. Our genetic and biochemical evidence indicates that the transcriptional repressor Ttk69 is a key functional target for miR-87 in dendrite regeneration. Overexpression of ttk69 in C4da neurons leads to dendrite regeneration defects (Fig 6). Further, both heterozygosity for ttk69 and RNAi knockdown of ttk69 significantly rescue the dendrite regeneration defects in miR-87 KO C4da neurons (Fig 6). Our qPCR data demonstrate that ttk69 mRNA levels are developmentally regulated in the nervous system and subject to regulation by miR-87. Intriguingly, ttk69 mRNA levels are reduced in the larvae-to-pupae transition and significantly increased in miR-87 mutants (Fig 6), suggesting that the increased activity of miR-87 during the larvae-to-pupae transition could suppress pupal ttk69 expression. Whether this reciprocal expression relationship reflects direct control of ttk69 expression by miR-87 remains to be determined. And while our results support a key role for Ttk69 in dendrite regeneration, we cannot rule out the possibility that additional miR-87 targets contribute to regeneration. Further studies comparing gene expression between wild-type and miR-87 KO C4da neurons might help to identify additional miR-87 targets in dendrite regeneration.
Although functions for Ttk69 in C4da dendrite morphogenesis or regeneration have not been previously described, prior studies provide some insights into likely control of dendrite regeneration by Ttk69. In the developing eye and the embryonic PNS, Ttk69 prevents progenitor cell differentiation by suppressing expression of genes required for neural fate specification [44–47]. In the context of regeneration, Ttk69 could be controlling expression of an adult C4da neuron “differentiation” program required for dendrite regrowth. In this model, miR-87 repression of ttk69 in pupal C4da neurons would facilitate differentiation. One key feature of this model is the precise temporal control; dendrite regrowth occurs during a stereotyped developmental window, with miR-87 required for neurons to transition from the “pausing” to elongation phases of dendrite regrowth. Indeed, the few studies of Ttk69 function in post-mitotic neurons demonstrate roles for Ttk69 in precisely timed developmental events. For example, during embryonic development of C1da dendrite arbors, ttk69 depletion prevents the timely transition from dorsal outgrowth to lateral branching [48]. Ttk69 likewise regulates a late event of R7 photoreceptor axon targeting, namely bouton formation after axons reach the medulla [49, 50].
How is this timing achieved? As in dendrite regeneration, many of the other precisely timed Ttk69 functions are gated by miRNA control including miR-7 in oogenesis, miR-184 in blastoderm embryos, and miR-310 cluster miRNAs under conditions of nutrient stress [43, 51–52], suggesting that Ttk69 may be particularly susceptible to miRNA control. The question therefore becomes: how is the temporal activity of miRNAs that regulate ttk69 gated? Developmental timing in insects is controlled by several hormonal cues, most notably including ecdysone, with spikes in ecdysone titer preceding and required for major developmental transitions [53]. Intriguingly, ecdysone titers drop at the onset of pupal development, and then peak again at ~24 h APF [54], concomitant with the timing of dendrite regrowth. It therefore seems likely that miR-87 and hence ttk69 expression dynamics are controlled in part by the larval ecdysone pulse that triggers pupation and/or the early pupal pulse that precedes dendrite regrowth. Studies in S2 cells suggest that a direct connection between ecdysone and miR-87 is unlikely [40]. Instead, ecdysone-responsive genes that are absent in S2 cells likely induce miR-87 expression to trigger dendrite regrowth. Indeed, temporal specificity of ecdysone signaling is imparted by stage-specific induction of ecdysone-responsive transcription factors including Eip75B, HR3, and HR4, each of which are induced by the late larval ecdysone pulse [55] and absent or lowly expressed in S2 cells [56].
Our data demonstrate that miR-87 is required for dendrite regeneration after acute injury as well as during developmental remodeling (Fig 7). We thus propose that the miR-87-mediated Ttk69 downregulation might be a core mechanism to reactivate dendrite regrowth in both developmental and injury induced regeneration in C4da neurons. Our observation that miR-87 activity is induced by dendrite damage raises several significant questions with far-reaching implications for dendrite regeneration. What controls miR-87 activity in sensory neurons? How are dendrite injuries sensed and transduced to induce miR-87 activation? The latter questions are particularly significant, and the single cell resolution provided by our miR-87 sensor should allow for systematic characterization of the mechanisms involved in damage sensing and signal transduction.
Our findings suggest that modulating miRNA activity in neurons might be a potential therapeutic strategy for promoting dendrite regeneration and functional repair after nervous system damage. Recent studies indicate that expression of a variety of miRNAs are induced following traumatic brain injury, spinal cord injury, and peripheral nerve injury [30, 31]; the physiological roles of these miRNAs in injury responses remain to be determined.
Materials and methods
Fly stocks
Ppk-GFP reporter and Ppk-Gal4 were used in previous studies [5, 16]. miR-87KO, ttk691e11, hnf4Δ33, brnpr-3, UAS-ttk69, UAS-br, UAS-hnf4, UAS-ttk69 RNAi were obtained from Bloomington Stock Center. UAS-miR-87 was obtained from FlyORF. The ttk69 3’UTR sensor stock was obtained from Dr. Deng (Florida State University).
miR-87 sensor
We designed our miR-87 sensor following a previous report [41]. In brief, we generated P-element-based sensors containing an unmodified 3’UTR or a 3’UTR with high-affinity miR-87 binding sites, and then shuttled the sensors into an attB-containing plasmid containing the C4da-specific ppk-promoter. First, four copies of the complementary sequence of the putative miR-87 targeting sequence (TCACACACCTGAAATTTTGCTCAA) were cloned downstream of EGFP in the previously described miRNA sensor construct, a modified pCaSpeR4 vector with tub-EGFP inserted in 3’ end of the P element [41]. The resulting pCaSpeR4-tub-EGFP with 4 x miR-87 target sequences was used as a template to amplify EGFP (for control sensor) and EGFP with 4 x miR-87 targets (for miR-87 sensor) fragments. The following primers were used: for control sensor, 5’-GGTACCAACTTAAAAAAAAAAATCAAAATGGTGAGCAAGGGCGAGGA and 5’- CTCGAGTTACTTGTACAGCTCGTCCA; for miR-87 sensor, 5’-GGTACCAACTTAAAAAAAAAAATCAAAATGGTGAGCAAGGGCGAGGA and 5’- CTCGAGTCCGGTTGAGCAAA. PCR products were cloned into pENTR (Thermo Fisher Scientific) as a KpnI/XhoI fragment. Using Gateway LR reaction (Thermo Fisher Scientific), EGFP and EGFP with 4 x miR-87 targets were recombined into the pDEST-APPHIH vector (a gift from Chun Han) to generate the control (ppk-EGFP-UTR) and mir-87 (ppk-EGFP-miR-87 binding sites-UTR) sensors used in these studies. Transgenic flies with the construct integrated at the attP2 landing site were generated by BestGene Inc.
MARCM analysis
For MARCM analyses of miR-87 KO C4da neurons, FRT40A (control) and miR-87KO, FRT40A flies were crossed to ppk-Gal4, UAS-mCD8GFP, hs-flp; tub-Gal80, FRT40A flies. Embryos were collected for 2 hr at 25°C and allowed to develop for 3 hr in yeasted agar plates, then heat shocked for 1 hr at 37°C. Heat-shocked embryos were kept at 25°C and C4da MARCM clones were analyzed at third instar larvae. The animals with clones were further aged until the desired developmental stage.
In vivo imaging
Dendrite regeneration in C4da neurons during the pupa-to-adult stages was live-imaged by confocal microscopy (Leica SP8) using a prep previously described [15, 37].
Quantitative RT-PCR
mRNA preparation from larval and pupal brains and qRT-PCR were performed as previously described [38]. In brief, 2nd instar larvae (40–48 AEL) and 24 hr APF pupa were dissected and brains were extracted in cold PBS. Total RNA was extracted using a RNAqueous-Micro Total RNA Isolation Kit (Invitrogen) from 50–70 larval brains and 20–30 pupal brains, respectively. First-strand cDNA was synthesized using ReverTra Ace qPCR RT Master Mix (Toyobo). qRT-PCR was performed using THUNDERBIRD SYBR qPCR Mix (Toyobo). Primers used to assay ttk69 RNA levels were F5′-ATCAAAGAACTCCAAGGATCACCG-3′ and R 5′-ATGATGTGTCCAGACCTTCGC-3′. Measurements were normalized to ribosomal protein 49 (rp49: F 5′-GCTAAGCTGTCGCACAAA-3′ and R 5′-TCCGGTGGGCAGCATGTG-3′). Data were analyzed by the Pfaffl method.
Dendrite lesion
A 2nd instar larva (48–52 hr AEL) was mounted on a coverslip, and single primary branches of C4da dendrites (~50 μm away from the soma) were targeted using a focused 900 nm two photon laser (Leica MP8). Following the lesion, animals were recovered on yeasted apple juice agar plates and live-imaged at appropriate stages.
Quantification
For quantification, C4da neurons in segments A3-5 were used. Dendrites of C4da neurons were traced using the ImageJ plug-in NeuroJ (NIH, Bethesda, MD). Total dendritic length was calculated from the traces. Dendritic fields were calculated by measuring the area bounded by a polygon formed by connecting all dendritic terminals of the fields by using Fiji/ImageJ (NIH, Bethesda, MD).
Statistical analysis
The data are presented as the means ± standard deviation, and statistical significance was determined via unpaired Student’s t-test, one sample t-test, non-repeated measures ANOVA with a post hoc Bonferroni correction using Microsoft Excel (Microsoft). For quantification, at least three cells per larvae and five different animals per condition were analyzed. The statistical significance was set at p<0.05.
Supporting information
Dendrite regeneration in wild-type control (WT) and miR-87 knockout (miR-87) C4da neurons at the indicated time points. Scale bar = 100 μm. Genotypes: WT, ppk-GFP; miR-87, ppk-GFP; miR-87KO/miR-87KO.
(PDF)
Total dendrite length of wild-type control (WT) and miR-87 overexpressing (miR-87 o/e) C4da neurons at white pupa (WP), 12 hours after pupal formation (12 hr APF), and 24 hours after pupal formation (24 hr APF). Note that no obvious difference was observed in dendrite pruning processes between WT and miR-87o/e neurons. Error bars indicate mean ± S.D; n = 15; n.s., not significant (unpaired t-test). Genotypes: WT, ppk-Gal4, UAS-mCD8GFP; miR-87o/e, ppk-GAL4, UAS-mCD8GFP;+/+; UAS-miR-87/+.
(PDF)
(A) Overexpression of hnf4 or br causes dendrite regeneration defects. Morphology of hnf4 overexpressing (hnf4 o/e) and br overexpressing (br o/e) dendrites at 72 hr APF. Scale bar = 100 μm. (B) Reduction of hnf4 or br dosage causes no obvious rescue in dendrite regeneration defects in miR-87 KO C4da neurons. Morphology of miR-87 KO with reduction of one copy of hnf4 (miR-87, hnf4/ miR-87) or br (br/+; miR-87/ miR-87) dendrites at 96 hrs APF. Scale bar = 100 μm. (C) Quantification of total dendrite length in control wild-type (WT), miR-87 KO (miR-87), miR-87 KO with reduction of one copy of ttk69 (miR-87; ttk69/+), miR-87 KO with reduction of one copy of hnf4 (miR-87, hnf4/miR-87), miR-87 KO with reduction of one copy of br (br/+; miR-87) dendrites at 96 hrs APF. n = 15, Error bars indicate mean ± S.D., *p<0.01 (ANOVA with a post hoc Bonferroni correction). Genotypes: WT, ppk-Gal4, UAS-mCD8GFP/+; miR-87, ppk-Gal4, UAS-mCD8GFP/+; miR-87KO/ miR-87KO; miR-87; ttk69/+, ppk-Gal4, UAS-mCD8GFP/+; miR-87KO/ miR-87KO; ttk1e11/+; miR-87, hnf4/miR-87, ppk-Gal4, UAS-mCD8GFP/+; miR-87KO, hnf4Δ33/ miR-87KO; br/+; miR-87/miR-87, ppk-Gal4, UAS-mCD8GFP/brnpr-3; miR-87KO/ miR-87KO.
(PDF)
(A) A schematic illustration of the ttk69 3’UTR sensor construct. (B) Regulated expression of ttk69 3’UTR sensor. Images show expression of the ttk69 3’UTR sensor in wild-type (WT) and miR-87 KO mutant (miR-87) C4da neurons at 2nd instar larval (2L) and white pupal (WP) additionally expressing ppk-CD4-tdTomato. Scale bar = 20 μm. (C) Quantification of the ttk69 3’UTR sensor GFP expression in C4da neurons at the indicated developmental stages. The ttk69 3’UTR sensor fluorescence intensity was normalized to the control sensor fluorescence intensity. n = 25, Error bars indicate mean ± S.D, *p<0.001 (Student’s t-test). Genotypes: ppk-CD4-tdTomato; miR-87KO/miR-87KO; tub-GFP-ttk69 3’UTR /+
(PDF)
(A, B) Overexpression of ttk69 causes dendrite regeneration defects in adult. Morphology of wild-type control (WT) and ttk69 overexpressing (ttk69 o/e) dendrites at adult 1 day (A). Scale bar = 100 μm. Quantification of total dendrite length in wild-type (WT), ttk69 overexpressing (ttk69 o/e) neurons (B). n = 15, Error bars indicate mean ± S.D, *p<0.01 (Student’s t-test). (C, D) Time-lapse images of dendrite regeneration after injury in wild-type (WT) and ttk69-overexpressed (ttk69 o/e) neurons. Red arrows indicate the site of dendrite severing in 2nd instar larvae (44–48 hr AEL) (C). Scale bar = 50 μm. Quantification of regenerated dendrite length after severing in control wild-type (WT) and ttk69-overexpressed (ttk69 o/e) neurons at 54 hr after severing. n = 6, Error bars indicate mean ± S.D., *p<0.05 (unpaired t-test). Genotypes: WT, ppk-Gal4, UAS-mCD8GFP; ttk69 o/e, ppk-GAL4, UAS-mCD8GFP; +/+; UAS-ttk69/+.
(PDF)
To visualize C4da dendrites, we introduced ppk-GFP reporter into miRNAs KO stocks. C4da dendrites of miRNAs KO homozygote animals were observed at 24, 48, 72 hr APF. Five C4da neurons from at least 3 independent animals were observed.
(TIFF)
Potential miR-87 targets were listed using the target prediction program (Stark et al. 2003). Among the top 100 potential candidates, UAS stocks were available for 43 candidates. The potential target genes were driven by the C4da neuron-specific ppk-GAL4. Dendrite phenotypes were observed at 72 hr APF.
(TIFF)
(MOV)
(MOV)
Acknowledgments
We would like to thank D. Anderson, T. Kitamoto, G. Rubin, W.M. Deng, Bloomington Stock Center, and Kyoto Stock Center for fly stocks; the member of the Emoto and Parrish labs for critical comments and discussion; M. Miyahara and H. Itoh for technical assistance.
Data Availability
All relevant data are within the manuscript and its supporting information files.
Funding Statement
This work was supported by MEXT Grants-in-Aid for Scientific Research on Innovative Areas ‘‘Dynamic regulation of brain function by Scrap & Build system’’ (KAKENHI 16H06456), JSPS (KAKENHI 16H02504), WPI-IRCN, AMED-CREST (JP18gm0610014), JST-CREST, the Strategic Research Program for Brain Sciences, Toray Foundation, Naito Foundation, Takeda Science Foundation, and Uehara Memorial Foundation to KE; and by grants from the National Institutes of Health (NINDS R01 NS076614) and a JSPS invitational fellowship to JZP. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Takeo YH, Kakegawa W, Miura E, Yuzaki M (2015) RORα regulates multiple aspects of dendrite development in cerebellar Purkinje cells in vivo. J Neurosci 35: 12518–12534. 10.1523/JNEUROSCI.0075-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mizuno H, et al. (2014) NMDA-regulated dynamics of layer 4 neuronal dendrites during thalamocortical reorganization in neonates. Neuron 82: 365–379. 10.1016/j.neuron.2014.02.026 [DOI] [PubMed] [Google Scholar]
- 3.Nakazawa S, Mizuno H, Iwasato T (2018) Different dynamics of cortical neuron dendrite trees revealed by long-term in vivo imaging in neonates. Nat Commun 9: 3106 10.1038/s41467-018-05563-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Emoto K, Parrish JZ, Jan LY, Jan YN (2006) The tumour suppressor Hippo acts with the NDR kinases in dendrite tiling and maintenance. Nature 443: 210–213. 10.1038/nature05090 [DOI] [PubMed] [Google Scholar]
- 5.Parrish JZ, Emoto K, Jan LY, Jan YN (2007) Polycomb genes interact with the tumor suppressor genes hippo and warts in the maintenance of Drosophila sensory neuron dendrites. Genes Dev 21: 956–972. 10.1101/gad.1514507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koleske AJ. (2013) Molecular mechanisms of dendrite stability. Nat Rev Neurosci 14: 536–550. 10.1038/nrn3486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy TH, Corbett D (2009) Plasticity during stroke recovery: from synapse to behavior. Nat Rev Neurosci 10: 861–872. 10.1038/nrn2735 [DOI] [PubMed] [Google Scholar]
- 8.Ruan YWR, et al. (2006) Dendritic plasticity of CA1 pyramidal neurons after transient grobal ischemia. Neuroscience 140: 191–201. 10.1016/j.neuroscience.2006.01.039 [DOI] [PubMed] [Google Scholar]
- 9.Spigelman I, Yan XX, Obenaus A, Lee EY, Wasterlain CG, Ribak CE (1998) Dentate granule cells form novel basal dendrites in a rat model of temporal lobe epilepsy. Neuroscience 86: 109–120. 10.1016/s0306-4522(98)00028-1 [DOI] [PubMed] [Google Scholar]
- 10.Jan YN, Jan LY (2010) Branching out: mechanisms of dendrite arborization. Nat Rev Neurosci 11: 316–328. 10.1038/nrn2836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu F, Schuldiner O (2014) Axon and dendrite pruning in Drosophila. Curr Opin Neurobiol 27: 192–198. 10.1016/j.conb.2014.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanamori T, Togashi K, Koizumi H, Emoto K (2015) Dendrite remodeling: lessons from invertebrate models. Int Rev Cell Mol Biol 318: 1–15. 10.1016/bs.ircmb.2015.05.001 [DOI] [PubMed] [Google Scholar]
- 13.Kuo CT, Jan LY, Jan YN (2005) Dendrite-specific remodeling of Drosophila sensory neurons requires matrix metalloproteases, ubiquitin-proteasome, and ecdysone signaling. Proc Natl Acad Sci USA 102: 15230–15235. 10.1073/pnas.0507393102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams DW, Truman JW (2005) Remodeling dendrites during insect metamorphosis. J Neurobiol 64: 24–33. 10.1002/neu.20151 [DOI] [PubMed] [Google Scholar]
- 15.Kanamori T, Kanai MI, Dairyo Y, Yasunaga KI, Morikawa RK, Emoto K (2013) Compartmentalized calcium transients trigger dendrite pruning in Drosophila sensory neurons. Science 340: 1475–1478. 10.1126/science.1234879 [DOI] [PubMed] [Google Scholar]
- 16.Shimono K, et al. (2009) Multidendritic sensory neurons in the adult Drosophila abdomen: origin, dendritic morphology, and segment- and age-dependent programmed cell death. Neural Dev 4: 37 10.1186/1749-8104-4-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yasunaga KI, Kanamori T, Morikawa R, Emoto K (2010) Dendrite reshaping of adult Drosophila sensory neurons requires matrix metalloproteinase- mediated modification of the basement membranes. Dev Cell 18: 621–632. 10.1016/j.devcel.2010.02.010 [DOI] [PubMed] [Google Scholar]
- 18.Yasunaga KI, et al. (2015) Adult Drosophila sensory neurons specify dendritic territories independently of dendritic contacts through the Wnt5-Drl signaling pathway. Genes Dev 29: 1763–1775. 10.1101/gad.262592.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lyons GR, Anderson RO, Abdi K, Song WS, Kuo CT (2014) Cysteine proteinase-1 and cut protein isoform control dendritic innervation of two distinct sensory fields by a single neuron. Cell Rep 6: 783–791. 10.1016/j.celrep.2014.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song Y, Ori-McKenney KM, Zheng Y, Han C, Jan LY, Jan YN (2012) Regeneration of Drosophila sensory neuron axons and dendrites is regulated by the AKT pathway involving Pten and microRNA bantam. Genes Dev 26: 1612–1625. 10.1101/gad.193243.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stone MC, Albertson RM, Chen L, Rolls MM (2014) Dendrite injury triggers DLK-independent regeneration. Cell Rep 6: 247–253. 10.1016/j.celrep.2013.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thompson-Peer K, DeVault L, Li T, Jan LY, Jan YN (2016) In vivo dendrite regeneration after injury is different from dendrite development. Genes Dev 30: 1776–1789. 10.1101/gad.282848.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Devaut L, Li T, Izabel S, Thompson-Peer K, Jan LY, Jan YN (2018) Dendrite regeneration of adult Drosophila sensory neurons diminishes with aging and is inhibited by epidermal-derived matrix metalloproteinase 2. Genes Dev 32: 402–414. 10.1101/gad.308270.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang S, Johnstone RJ, Frokjer-Jensen C, Lockery S, Hobert O (2004) MicroRNAs act sequentially and asymmetrically to control chemosensory laterality in the nematode. Nature 430: 785–789. 10.1038/nature02752 [DOI] [PubMed] [Google Scholar]
- 25.Zou Y, Chiu H, Domenger D, Chuang CF, Chang C (2012) The lin-4 microRNA targets the LIN-14 transcription factor to inhibit Netrin-mediated axon attraction. Sci Signal 5: ra43 10.1126/scisignal.2002437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun AX, Crabtree GR, Yoo AS (2013) MicroRNAs: regulations of neuronal fate. Curr Opin Cell Biol 25: 215–221. 10.1016/j.ceb.2012.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shenoy A, Blelloch RH (2014) Regulation of microRNA function in somatic stem cell proliferation and differentiation. Nat Rev Mol Cell Biol 15: 565–576. 10.1038/nrm3854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo H, Ingolia NT, Weissman JS, Bartel DP (2010) Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 466: 835–840. 10.1038/nature09267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Djuranovic S, Nahvi A, Green R (2012) miRNA-mediated gene silencing by translational repression followed by mRNA deadenylation and decay. Science 336: 237–240. 10.1126/science.1215691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghibaudi M, Boido M, Vercelli A (2017) Functional integration of complex miRNA networks in central and peripheral lesion and axonal regeneration. Prog Neurobiol 158: 69–93. 10.1016/j.pneurobio.2017.07.005 [DOI] [PubMed] [Google Scholar]
- 31.Mahar M, Cavalli V (2018) Intrinsic mechanisms of neuronal axon regeneration. Nat Rev Neurosci 19: 323–337. 10.1038/s41583-018-0001-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaudet AD, et al. (2016) miR-155 deletion in mice overcomes neuron-intrinsic and neuron-extrinsic barriers to spinal cord repair. J Neurosci 36: 8516–8532. 10.1523/JNEUROSCI.0735-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu YW, Jiang JJ, Gao Y, Wang RY, Tu GJ (2016) MicroRNA-210 promotes sensory axon regeneration of adult mice in vivo and in vitro. Neurosci Lett 622: 61–66. 10.1016/j.neulet.2016.04.034 [DOI] [PubMed] [Google Scholar]
- 34.Yu YM, et al. (2011) MicroRNA miR-133b is essential for functional recovery after spinal cord injury in adult zebrafish. Eur J Neurosci 33: 1587–1597 (2011). 10.1111/j.1460-9568.2011.07643.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu CM, Wang RY, Saijilafu, Jiao ZX, Zhang BY, Zhou FQ(2013) MicroRNA-138 and SIRT1 form a mutual negative feedback loop to regulate mammalian axon regeneration. Genes Dev 27: 1473–1483. 10.1101/gad.209619.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen YW, et al. (2014) Systematic study of Drosophila microRNA functions using a collection of targeted knockout mutations. Dev Cell 31: 784–800. 10.1016/j.devcel.2014.11.029 [DOI] [PubMed] [Google Scholar]
- 37.Kanamori T, Yoshino J, Yasunaga KI, Dairyo Y, Emoto K (2015) Local endocytosis triggers dendritic thinning and pruning in Drosophila sensory neurons. Nat Commun 6: 6515 10.1038/ncomms7515 [DOI] [PubMed] [Google Scholar]
- 38.Parrish JZ, Xu P, Kim CC, Jan LY, Jan YN (2009) The microRNA bantam functions in epithelial cells to regulate scaling growth of dendrite arbors in Drosophila sensory neurons. Neuron 63: 788–802. 10.1016/j.neuron.2009.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee T, Luo L (1999) Mosaic analysis with repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron 22: 451–461. 10.1016/s0896-6273(00)80701-1 [DOI] [PubMed] [Google Scholar]
- 40.Sempere LF, Sokol NS, Dubrosky EB, Berger EM, Ambros V (2003) Temporal regulation of microRNA expression in Drosophila melanogaster mediated by hormonal signals and broad-Complex gene activity. Dev Biol 259: 9–18. 10.1016/s0012-1606(03)00208-2 [DOI] [PubMed] [Google Scholar]
- 41.Brennecke J, Hipfner DR, Stark A, Russell RB, Cohen SM (2003) bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell 113: 25–36. 10.1016/s0092-8674(03)00231-9 [DOI] [PubMed] [Google Scholar]
- 42.Stark A, Brennecke J, Russell RB, Cohen SM (2003) Identification of Drosophila MicroRNA targets. PLoS Biol 1: E60 10.1371/journal.pbio.0000060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang YC, Smith L, Poulton J, Deng WM (2013) The microRNA miR-7 regulates Tramtrack69 in a developmental switch in Drosophila follicle cells. Development 140: 897–905. 10.1242/dev.080192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiong WC, Montell C (1993) tramtrack is a transcriptional repressor required for cell fate determination in the Drosophila eye. Genes Dev 7: 1085–1096. 10.1101/gad.7.6.1085 [DOI] [PubMed] [Google Scholar]
- 45.Guo M, Bier E, Jan LY, Jan YN (1995) tramtrack acts downstream of numb to specify distinct daughter cell fates during asymmetric cell divisions in the Drosophila PNS. Neuron 14: 913–925. 10.1016/0896-6273(95)90330-5 [DOI] [PubMed] [Google Scholar]
- 46.Li S, Li Y, Carthew RW, Lai ZC (1997) Photoreceptor cell differentiation requires regulated proteolysis of the transcriptional repressor Tramtrack. Cell 90: 469–478. 10.1016/s0092-8674(00)80507-3 [DOI] [PubMed] [Google Scholar]
- 47.Lai ZC, Li Y (1999) Tramtrack69 is positively and autonomously required for Drosophila photoreceptor development. Genetics 152: 299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parrish JZ, Kim MD, Jan LY, Jan YN (2006) Genome-wide analyses identify transcription factors required for proper morphogenesis of Drosophila sensory neuron dendrites. Genes Dev 20: 820–835. 10.1101/gad.1391006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kniss JS, Holbrook S, Mergman TG (2013) R7 photoreceptor axon growth is temporally controlled by the transcription factor Ttk69, which inhibits growth in part by promoting transforming growth factor-β/activin signaling. J Neurosci 33: 1509–1520. 10.1523/JNEUROSCI.2023-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Feoktistov AI, Herman TG (2016) Wallenda/DLK protein levels are temporally downregulated by Tramtrack69 to allow R7 growth cones to become stationary boutons. Development 143: 2983–2993. 10.1242/dev.134403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iovino N, Pane A, Gaul U (2009) miR-184 has multiple roles in Drosophila female germline development. Dev Cell 17: 123–133. 10.1016/j.devcel.2009.06.008 [DOI] [PubMed] [Google Scholar]
- 52.Cicek IO, Karaca S, Brankatschk M, Eaton S, Urlaub H, Shcherbata HR (2016) Hedgehog signaling strength is orchestrated by the mir-310 cluster of microRNAs in response to diet. Genetics 202: 1167–1183. 10.1534/genetics.115.185371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamanaka N, Rewitz KF, O’Connor MB (2013) Ecdysone control of developmental transition: lessons from Drosophila research. Annu Rev Entomol 58: 497–516. 10.1146/annurev-ento-120811-153608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Handler AM (1982) Ecdysteroid titers during pupal and adult development in Drosophila melanogaster. Dev Biol 93: 73–82. 10.1016/0012-1606(82)90240-8 [DOI] [PubMed] [Google Scholar]
- 55.Thummel CS (2001) Molecular mechanisms of developmental timing in C. elegans and Drosophila. Dev Cell 4: 453–465. [DOI] [PubMed] [Google Scholar]
- 56.Cherbas L, Willingham A, Zhag D, Yang L, Zou Y, Eads BD, Carlson JW, Landolin JM, Kapranov P, Dumais J, Samsonova A, Choi JH, Roberts J, Davis C, Tang H, van Baren MJ, Ghosh S, Dobin A, Bell K, Lin W, Langton L, Duff MO, Tenney AE, Zaleski C, Brent MR, Hoskins RA, Kaufman TC, Andrews J, Gravely BR, Perrimon N, Celniker SE, Ginveras TR, and Cherbas P (2011) The transcriptional diversity of 25 Drosophila cell lines. Genome Res 2: 301–314. [DOI] [PMC free article] [PubMed] [Google Scholar]