Abstract
Most patients with coronavirus disease 2019 (COVID-19) have just only mild symptoms, but about 5% are very severe. Although extracorporeal membranous oxygenation (ECMO) is sometimes used in critically patients with COVID-19, ECMO is only an adjunct, not the main treatment. If the patient's condition deteriorates and it is determined to be irreversible, it is necessary to decide to stop ECMO.
A 54-year-old man was admitted on day 6 of onset with a chief complaint of high fever and cough. Computed tomography (CT) showed a ground glass opacity in both lungs, and reverse transcription-polymerase chain reaction (RT-PCR) diagnosed COVID-19. He was admitted to the hospital and started to receive oxygen and favipiravir. After that, his respiratory condition deteriorated, and he was intubated and ventilated on day 9 of onset, and ECMO was introduced on day 12. Two days after the introduction of ECMO, C-reactive protein (CRP) increased, chest X-p showed no improvement in pneumonia, and PaO2/FiO2 decreased again. As D-dimer rose and found a blood clot in the ECMO circuit, we had to decide whether to replace the circuit and continue with ECMO or stop ECMO. At this time, the viral load by RT-PCR was drastically reduced to about 1/1750. We decided to continue ECMO therapy and replaced the circuit. The patient's respiratory status subsequently improved and ECMO was stopped on day 21 of onset.
In conclusion, viral load measurement by RT-PCR may be one of the indicators for promoting the treatment of severe COVID-19 patients.
Keywords: Coronavirus disease 2019 (COVID-19), Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), Reverse transcription-polymerase chain reaction (RT-PCR), Extracorporeal membranous oxygenation (ECMO)
1. Introduction
From the end of 2019, coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spread to the world. Most patients with COVID-19 have just only mild symptoms, but about 5% are critical [1,2]. There are several reports of using extracorporeal membranous oxygenation (ECMO) in severe cases. In an early report from China, 4 out of 138 (2.9%) inpatients needed ECMO [3]. Another group reported that 3% of patients received ECMO [4]. Other teams in China reported that 11.5% of COVID-19 cases in the intensive care unit (ICU) received ECMO as rescue therapy [5].
It is not easy to judge the ECMO indication. In addition to making decisions about each patient's indications, we must consider the appropriate allocation of socially limited medical resources.
Although there are some guidelines, it is difficult to select the patients and the timing of ECMO initiation and to manage monitoring and weaning of ECMO. The most difficult thing is the decision to discontinue ECMO if we could not save the patient. Guideline of Surviving Sepsis Campaign said that ECMO should only be considered in carefully selected patients with COVID-19 and severe acute respiratory distress syndrome (ARDS) [6]. Guideline of the World Health Organization (WHO) reported that ECMO should be offered only in expert centers with enough cases to maintain expertise [7].
The quantitative data like bacterial load in the culture have contributed to manage patients with active infectious diseases. For diagnosis of COVID-19, reverse transcription-polymerase chain reaction (RT-PCR) is widely used as a diagnostic method; however, only a small part of physicians can use the quantitative data because the results are usually reported as qualitative data. The viral loads can be an index for understanding the condition of the patient with COVID-19.
Here we present a COVID-19 case that we decided to continue ECMO using the viral load by RT-PCR as an index.
2. Case report
The case was a 54-years-old man who had a cough and high fever of 39° or higher on day 0. On day 1, he met a primary care doctor and started to take 500 mg/day azithromycin. But his symptom did not improve.
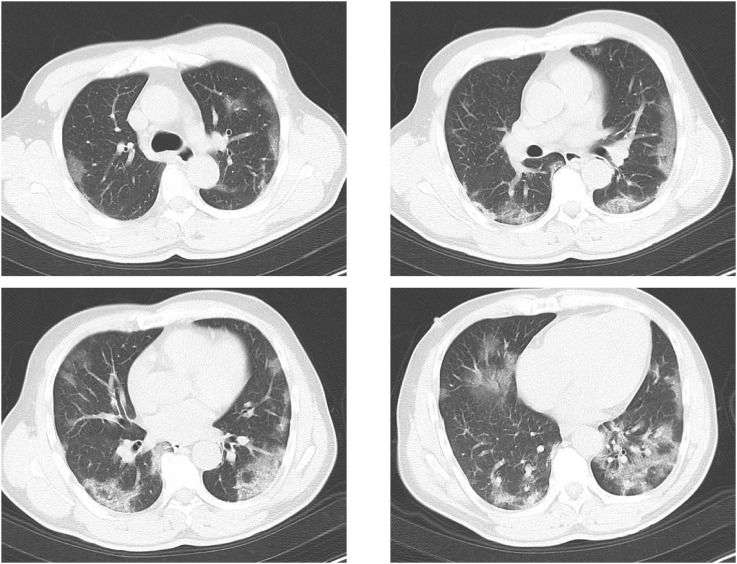
He came to our hospital on day 6. On physical examination, his vital signs were as follows: blood pressure, 102/78 mmHg; pulse, 96/min; respiratory rate, 32/min; oxygen saturation, 92% on room air; body temperature, 39.2 °C; and Glasgow Coma Scale (GCS), 15. White blood count (WBC) was 2650/μL (Neutro 72.4%, Lymph 23.4%), C-reactive protein (CRP) was 3.12 mg/mL. D-dimer was 1.3 μg/mL (normal range < 1 μg/mL). His computed tomography (CT) images showed ground glass opacity on both lungs (Fig. 1 ). RT-PCR test of the nasal specimen showed the positive result of SARS-CoV-2.
Fig. 1.
CT images on admission (day 6 from onset). Computed tomography (CT) images showed typical ground glass opacity on both lungs.
He admitted to the ICU and received non-invasive ventilation. Also, he started to take an anti-viral agent, Favipiravir. But, because his respiratory condition was worse sharply on day 9, he needed intubation and invasive mechanical ventilation. The respiratory status worsened further and bilateral pneumonia on the chest X-ray also worsened (Fig. 2 ). ECMO was introduced on day 12. Furthermore, his renal function worsened, continuous hemodiafiltration (CHDF) was also introduced on day 13. His condition did not improve immediately after the ECMO introduction. PaO2/FiO2 had increased temporally but decreased again (Fig. 3 ). CRP increased to 32.42 mg/dL. There was a coagulation abnormality. Clots were found in the circuit of ECMO, and D-dimer increased to over 30 μg/mL. Thus, we had to decide whether to make a circuit switch and continue ECMO, or stop ECMO. At that time, the RT-PCR test of nasopharyngeal swab samples still showed positive. However, the viral load decreased from 8.33 × 104 copies/μL (day 6) to 4.80 × 10 copies/μL (day 14). Viral road decreased to about 1/1750 compared to that on admission. We decided to continue the ECMO therapy and replace the circuit of the ECMO (day 15). After that, the patient's condition improved and pneumonia improved on chest X-ray (Fig. 2). ECMO and CHDF were terminated on day 21. RT-PCR of nasal specimen showed a negative result (Fig. 3).
Fig. 2.
Serial chest-X-ray of the patient. (A) Day 6; chest-X-ray of the patient on admission. (B) Day 12; after introduction of ECMO. (C) Day 15; After replacement of ECMO. (D) Day 21; after termination of ECMO.
Fig. 3.
Clinical course of the patient. Viral loads of nasopharyngeal swab samples were measured by RT-PCR.
Favipiravir was administered 3600 mg/day on day 6 and 1600 mg/day from day 7 to day 19 for a total of 14 days. A respirator was not needed on day 24 and the patients moved to the general ward from ICU on day 29.
3. Discussion
If ECMO was introduced for the patient with severe COVID-19 pneumonia and the patient's general condition did not improve, it is very difficult to judge whether to stop ECMO use or not. Extracorporeal Life Support Organization (ELSO) guidance document said that clinicians should be continuously evaluating when ECMO no longer provides a benefit and should at that point return to conventional management [8].
One of the complications of COVID-19 was the venous thrombotic event [9]. It was reported that 27 out of 54 (50%) non-survivor showed coagulopathy meanwhile 10 out of 137 (7%) survivor showed coagulopathy [10]. Another group in New York reported five cases of large-vessel stroke in patients younger than 50 years [11]. Coagulopathy may associate with the severity of COVID-19 patients.
In our case, clots were found in the circuit and the patient's D-dimer increased to over 30 mg/mL immediately after the introduction of ECMO. PaO2/FiO2 was decreased to less than 100 mmHg and CRP increased to over 30 mg/dL. Coagulopathy might be due to the placement of the cannulas for ECMO. We judged that the patient's condition was very severe. We needed to decide to stop ECMO or to replace the circuit and continue ECMO. Viral load by RT-PCR of nasal specimen decreased to about 1/1750 at that time, we could decide to continue ECMO and replaced the circuit.
RT-PCR is now generally used to detect SARS-CoV-2 or confirm negative for SARS-CoV-2. The result of the RT-PCR test shows usually just positive or negative, viral load is not shown. One of the papers about viral load showed that the viral load early after onset was high, but a sputum sample collected on day 8 post-onset from a patient who died had a very high viral load [12]. Another report revealed that viral loads in mild cases decreased, while viral loads in severe cases did not change or increased [13]. Patients in our hospital showed that viral loads of symptomatic patients were higher than those of asymptomatic patients [14]. If the persistently high viral load in patients with COVID-19 could lead to severe disease; preparation for invasive ventilation and ECMO therapy may be necessary.
For determining the severity of COVID-19, it is better to use a blood sample to accurately measure the viral load. However, it has been reported that RNAemia was only found in patients with critical COVID-19 [15]. Nasopharyngeal swab samples are usually used for initial RT-PCR testing, and we use nasal specimens to see changes in viral load over time. The viral load of SARS-CoV-2 in nasopharyngeal swab samples may be a useful marker for assessing disease severity and prognosis.
Some researchers showed the number of cycles of PCR (Ct value) as viral load [[16], [17], [18], [19]]. Our results of RT-PCR show the number of RNA copies/μL, it may be possible to monitor more detail of viral load. Our methods were as follows. Nasal swab specimens were pretreated with 500 μL of Sputazyme. After centrifugation, the supernatant was used for RNA extraction. RNA solution was obtained from the supernatant by using QIAamp ViralRNA Mini Kit or Nippongene Isospin RNA Virus according to the manufactural instructions. The viral loads of SARS-CoV-2 were quantified based on N2-gene-specific primer-probe set by reverse transcriptase-quantitative polymerase chain reaction using THUNDERBIRD Probe One-step qRT-PCR Kit according to the Japan National Institute of Infectious Diseases protocol [20]. The quality of quantification was controlled by AcroMetrix COVID-19 RNA Control.
We should evaluate the clinical advantages of viral loads-guided monitoring because it can be useful for planning the management of asymptomatic carriers as well as patients.
In conclusion, viral load measurement by RT-PCR of nasopharyngeal swab samples can be one of the indicators for management of COVID-19 patients.
Ethics approval and Consent for publication
The administration of Favipiravir and RT-PCR tests as clinical studies were approved by the Ethics Committee of the Toyama University Hospital. Written informed consent was obtained from the patient for publication of this case report.
Funding
No funding for this study.
Contributors
IS, YM, HO and YY contributed to study design, data collection and writing the report. IS, YF, HK, AU, YM, MW, TK, KH, TH and TS contributed to taking care of the patient. YM and HT performed RT-PCR. All authors reviewed and approved the final version of the report.
Conflict of interest declaration
None.
Footnotes
All authors meet the ICMJE authorship criteria.
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 Feb 15;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease Control and prevention. J Am Med Assoc. 2020 Feb 24 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. J Am Med Assoc. 2020 Feb 7;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020 Feb 24;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alhazzani W., Møller M.H., Arabi Y.M., Loeb M., Gong M.N., Fan E., et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19) Intensive Care Med. 2020;46:854–887. doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization . 2020. Clinical management of severe acute respiratory infection when COVID-19 is suspected. Interim guidance.https://www.who.int/publications-detail/clinical-management-of-severe-acuterespiratory-infection-when-novel-coronavirus-(ncov)- infection-is-suspected accessed March 20, 2020. [Google Scholar]
- 8.Bartlett R.H., Ogino M.T., Brodie D., McMullan D.M., Lorusso R., MacLaren G., et al. Initial ELSO guidance document: ECMO for COVID-19 patients with severe cardiopulmonary Failure. Am Soc Artif Intern Organs J. 2020 May;66(5):472–474. doi: 10.1097/MAT.0000000000001173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X., et al. High risk of thrombosis in patients in severe SARS-CoV-2 infection: a multicentre prospective cohort study. Intensive Care Med. 2020 Jun;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 Mar 28;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oxley T.J., Mocco J., Majidi S., Kellner C.P., Shoirah H., Singh I.P., et al. Large-vessel stroke as a presenting Feature of covid-19 in the young. N Engl J Med. 2020 Apr 28;382:e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan Y., Zhang D., Yang P., Poon L.L.M., Wang Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect Dis. 2020 Apr;20(4):411–412. doi: 10.1016/S1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y., Yan L.M., Wan L., Xiang T., Le A., Liu J., et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020 Jun;20(6):656–657. doi: 10.1016/S1473-3099(20)30232-2. Epub 2020 Mar 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawasuji Hitoshi, Takegoshi Yusuke, Kaneda Makito, Ueno Akitoshi, Miyajima Yuki, Kawago Koyomi, et al. High nasopharyngeal viral load around the onset may contributes to secondary transmission of COVID-19. medRxiv. 2020;4 doi: 10.1101/2020.06.02.20120014. preprint posted June. [DOI] [Google Scholar]
- 15.Chen X., Zhao B., Qu Y., Chen Y., Xiong J., Feng Y., et al. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. Clin Infect Dis. 2020 Apr 17 doi: 10.1093/cid/ciaa449. Published online 2020 Apr 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z., et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020 Mar 19;382(12):1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He X., Lau E.H.Y., Wu P., Deng X., Wang J., Hao X., et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020 Apr 15 doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 18.Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J., et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. J Am Med Assoc. 2020 Mar 27;323(16):1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan Y., Long L., Zhang D., Yan T., Cui S., Yang P., et al. Potential false-negative nucleic acid testing results for Severe Acute Respiratory Syndrome Coronavirus 2 from thermal inactivation of samples with low viral loads. Clin Chem. 2020 Jun 1;66(6):794–801. doi: 10.1093/clinchem/hvaa091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shirato K., Nao N., Katano H., Takayama I., Saito S., Kato F., et al. Development of genetic diagnostic methods for novel coronavirus 2019 (nCoV-2019) in Japan. Jpn J Infect Dis. 2020 Jul 22;73(4):304–307. doi: 10.7883/yoken.JJID.2020.061. Epub 2020 Feb 18. [DOI] [PubMed] [Google Scholar]