Abstract
The adult mammalian heart lacks regenerative capacity and heals through activation of an inflammatory cascade that leads to the formation of a collagen-based scar. Although scar formation is important to preserve the structural integrity of the ventricle, unrestrained inflammation and excessive fibrosis have been implicated in the pathogenesis of adverse post-infarction remodeling and heart failure. Interstitial cells play a crucial role in the regulation of cardiac repair. Although recent studies have explored the role of fibroblasts and immune cells, the cardiac pericytes have been largely ignored by investigators interested in myocardial biology. This review manuscript discusses the role of pericytes in the regulation of inflammation, fibrosis and angiogenesis following myocardial infarction. During the inflammatory phase of infarct healing, pericytes may regulate microvascular permeability and may play an important role in leukocyte trafficking. Moreover, pericyte activation through Toll-like receptor-mediated pathways may stimulate cytokine and chemokine synthesis. During the proliferative phase, pericytes may be involved in angiogenesis and fibrosis. To what extent pericyte to fibroblast conversion and pericyte-mediated growth factor synthesis contribute to the myocardial fibrotic response remains unknown. During the maturation phase of infarct healing, coating of infarct neovessels with pericytes plays an important role in scar stabilization. Implementation of therapeutic approaches targeting pericytes in the infarcted and remodeling heart remains challenging, due to the lack of systematic characterization of myocardial pericytes, their phenotypic heterogeneity and the limited knowledge on their functional role.
Keywords: pericyte, myocardial infarction, inflammation, fibrosis, angiogenesis
Introduction
Pericytes are vascular mural cells that are embedded within the microvascular basement membrane, enwrapping capillaries, venules and terminal arterioles (1, 2, 3, 4). In contrast, larger vessels, such as arteries and veins, are coated by vascular smooth muscle cells. Pericytes are ubiquitously found in all organs and contribute to vascular maturation and stability, by regulating capillary permeability and by restraining endothelial cell proliferation. In addition to their role in microvascular homeostasis, a growing body of evidence implicates pericytes in a wide range of cellular responses, including inflammation (5, 6), fibrosis (7, 8), tissue repair and regeneration (9, 10). Understanding of the role of pericytes in the pathophysiology of disease is hampered by their functional and phenotypic heterogeneity (11), the lack of specific and reliable pericyte markers and the possible overlap between pericytes and other interstitial cell populations (12).
The myocardium is perfused through a rich microvascular network; in adult mouse hearts, endothelial cells are the most abundant non-cardiomyocytes (13) Thus, it is not surprising that pericytes represent a significant proportion of non-endothelial, non-hematopoietic interstitial myocardial cells (14, 13). Following myocardial infarction, sudden loss of up to a billion cardiomyocytes releases danger signals, activating an intense inflammatory response that serves to clear the wound from dead cells and extracellular matrix debris (15). Because the adult mammalian heart has negligible regenerative capacity, interstitial cell populations play a critical role in repair of the infarcted heart. However, excessive, prolonged or unrestrained activation of matrix-producing interstitial cells may contribute to adverse remodeling and to the pathogenesis of post-infarction heart failure (16). Considering their abundance, phenotypic plasticity and functional diversity, pericytes may be critically involved in regulating inflammatory, fibrotic, angiogenic and reparative responses following myocardial infarction. The current review manuscript discusses our current knowledge on the role of pericytes in injury, repair and remodeling of the infarcted heart.
Pericytes in the normal myocardium
The adult mammalian heart contains abundant interstitial cells, including fibroblasts, pericytes and macrophages (13, 14). Early ultrastructural studies have identified a large population of pericytes in normal adult mammalian hearts and suggested that these cells form an incomplete layer around the microvascular endothelium (17, 18). Due to the lack of specific and reliable markers to identify pericytes, and to differentiate them from other interstitial cells, studies systematically characterizing myocardial pericyte populations are lacking. Several molecular markers have been used to identify cardiac pericytes, including the proteoglycan neural/glial2 (NG2), platelet-derived growth factor β (PDGFRβ), CD146 and CD73, α-smooth muscle actin (SMA), desmin, nestin, alkaline phosphatase and the zinc finger protein Glioma-associated oncogene protein 1 (Gli1) (8, 19). Information on the specificity and sensitivity of these markers for detection of myocardial pericytes is limited and sometimes conflicting. Methodological differences between studies, species-specific properties of pericyte populations and differences in the definition of pericytes may account for conflicting findings.
Although NG2 is expressed by cardiomyocytes in embryonic hearts (20), in the adult mammalian myocardium NG2 expression specifically labels mural cells, both pericytes and vascular smooth muscle cells (Fig. 1). In normal hearts, PDGFRβ expression also seems to be restricted to vascular mural cells (21). In contrast, α-SMA expression marks vascular smooth muscle cells and not microvascular pericytes, whereas desmin is not expressed by cardiac pericytes (19). A growing body of evidence suggests that cardiac pericytes exhibit significant heterogeneity. In rat hearts, the majority of pericytes were found to co-express PDGFRβ and NG2, while a much smaller subset (around 6%) of PDGFRβ+ cells were NG2 negative (22). Studies in mice classified pericytes on the basis of expression of the intermediate filament protein, nestin. Nestin-positive and nestin-negative NG2-expressing pericytes were noted in normal hearts; however, only nestin-expressing cells exhibited significant expansion following infarction (23).
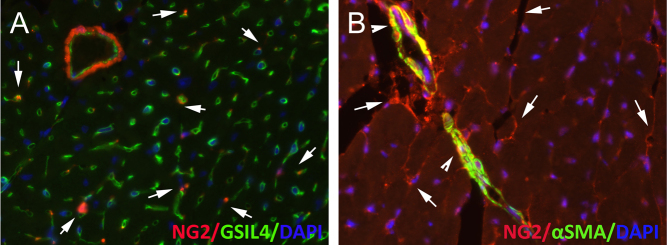
Figure 1.
The adult mouse heart contains abundant pericytes. The immunofluorescence panels show myocardial sections from normal adult NG2Dsred reporter mice, stained for Griffonia Simplicifolia lectin (GSIL4) to identify endothelial cells (A), and for α-SMA to label vascular smooth muscle cells (B). A significant population of NG2+/α-SMA- peri-endothelial cells is noted (arrows). Arteriolar vascular smooth muscle cells express both NG2 and α-SMA (arrowheads).
The functional role of pericytes in cardiac homeostasis remains poorly understood. Pericytes play an important role in vascular patterning and maturation (24), restrain endothelial cell proliferation and control microvascular permeability (25). Although experiments depleting myocardial pericytes in adult animals have not been performed, several lines of evidence suggest that cardiac pericytes may support similar functions in the myocardium. First, mice lacking PDGF-B or PDGFRβ exhibit pericyte loss, associated with perturbed vascular development and formation of microaneurysms in many organs, including the heart (26). Second, administration of tyrosine kinase inhibitors (such as the anticancer agent, sunitinib) that target PDGF receptors induces cardiotoxicity, associated with pericyte loss and microvascular dysfunction (27). Third, mice with endothelial cell-specific overexpression of angiopoietin-2 have marked loss of capillary-associated pericytes, associated with cardiomyopathy (28). It should be emphasized that whether pericyte loss plays a causative role in the myocardial pathology associated with PDGF-B/PDGFRβ disruption or angiopoietin overexpression was not documented. Thus, these experimental studies provide only supportive evidence on the role of cardiac pericytes in homeostasis. Fourth, conditions characterized by perturbed pericyte function, such as diabetes are associated with myocardial capillary rarefaction that may limit the functional reserve of the heart under conditions of stress (29, 30).
Pericytes in infarcted hearts
The phases of cardiac repair: from inflammation to fibrosis
In adult mammals, myocardial infarction results in sudden loss of up to a billion cardiomyocytes, overwhelming the negligible regenerative capacity of the heart. Thus, the infarcted myocardium heals through a well-orchestrated inflammatory/reparative response that ultimately leads to formation of a collagen-based scar (15, 31). The cell biological response following myocardial infarction can be divided into three distinct, but overlapping phases: the inflammatory, the proliferative and the maturation phase.
During the early inflammatory phase, release of danger signals from dying cardiomyocytes and protease-induced generation of matrikines, matrix fragments with pro-inflammatory properties, activates Toll-like receptor signaling pathways in cardiomyocytes, interstitial cells and immune cells and inducing cytokine and chemokine expression (32). Recruitment of abundant and diverse leukocyte populations in the infarcted heart clears the wound from dead cells and matrix fragments, while setting the stage for expansion of reparative cells through macrophage and lymphocyte-mediated secretion of growth factors (33). Induction of anti-inflammatory signals, such as TGF-βs and IL-10, not only suppresses inflammation, but also activates fibroblasts and vascular cells (34, 35, 36) leading to the proliferative phase of infarct healing. Conversion of fibroblasts to myofibroblasts is the dominant cellular event during the proliferative phase of infarct healing (37, 38). Activated myofibroblasts secrete both structural and matricellular extracellular matrix proteins, preserving the structural integrity of the ventricle and protecting from cardiac rupture (36, 39). The maturation phase follows, as myofibroblasts become quiescent (40) and a dense collagenous network comprising cross-linked collagen is formed. As the scar matures, the non-infarcted segments remodel, exhibiting hypertrophy and interstitial fibrosis and may develop worsening dysfunction. Chronic remodeling of non-infarcted segments may involve sustained neurohumoral activation and the effects of pressure and volume loads, due to the loss of contractile muscle in the infarcted area, the elevated filling pressures and the progressive chamber dilation following infarction (31).
As the heart transitions through the phases of repair and remodeling following infarction, cardiac pericytes exhibit dynamic functional and phenotypic alterations in response to the dramatic microenvironmental changes (Fig. 2). Unfortunately, myocardial biologists have ignored the pericytes; for this reason, information on their functional role in cardiac injury and repair remains extremely limited.
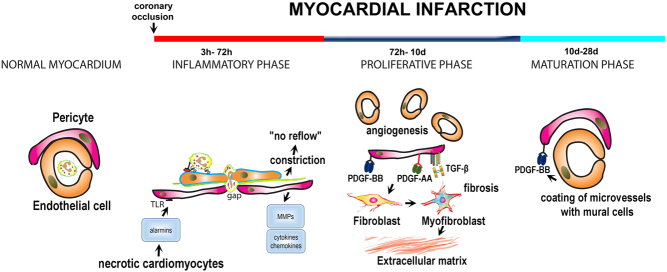
Figure 2.
The role of endogenous pericytes in myocardial infarction. The adult mammalian heart lacks regenerative capacity and heals through formation of a collagen-based scar. The reparative response can be divided into three distinct, but overlapping phases: the inflammatory phase, the proliferative phase and the maturation phase. The normal myocardium contains a large population of pericytes with peri-endothelial location. During the inflammatory phase, release of alarmins from dying cells may activate TLR-mediated pathways in pericytes, stimulating cytokine and chemokine secretion. Moreover, pericytes may produce matrix metalloproteinases, thus degrading the microvascular basement membrane and stimulating an angiogenic response. Formation of gaps between pericytes in the pro-inflammatory environment of the infarct may be required for leukocyte extravasation. Pericytes also regulate microvascular function. Constriction of pericytes in the ischemic and reperfused myocardium has been implicated in the pathogenesis of ‘no-reflow’. During the proliferative phase, pericytes activated by growth factors may regulate angiogenesis and fibrosis. To what extent pericyte to fibroblast conversion contributes to the expansion of myofibroblasts in healing infarcts remains unclear. Pericytes may also produce angiogenic and fibrogenic growth factors. During the maturation phase infarct neovessels recruit mural cells (both pericytes and vascular smooth muscle cells) through PDGFRβ-dependent pathways. Acquisition of a mural cell coat may be important to stabilize the infarct vasculature and to protect the infarcted heart from sustained inflammation.
Pericytes in acute myocardial ischemia and during the inflammatory phase of cardiac repair
Induction of pro-inflammatory cytokines following infarction increases microvascular permeability accentuating cardiac injury. Evidence derived from experimental models of reperfused myocardial infarction suggests an important role for pericytes in regulation of capillary barriers. Although the molecular signals involved in pericyte activation remain poorly characterized, published evidence suggests an important role for nerve growth factor (NGF) and angiopoietin-2 in accentuation of pericyte-driven vascular hyperpermeability. Pro-NGF, an injury-induced cytokine, is rapidly upregulated in cardiomyocytes following myocardial ischemia and reperfusion and activates the p75 receptor in pericytes promoting microvascular dysfunction that extends the area of infarction (41). On the other hand, angiopoietin-2 is released by ischemic endothelial cells and promotes pericyte detachment, increasing microvascular permeability (42).
In addition to their effects in regulation of microvascular function, pericytes may actively participate in the post-infarction inflammatory response by producing pro-inflammatory cytokines and chemokines. Danger signals released following infarction may activate innate immune signaling cascades in pericytes promoting a pro-inflammatory phenotype. Brain and lung pericytes have been demonstrated to express TLRs and activate inflammatory cascades in response to stimulation with TLR ligands (43, 44). Stark and coworkers (45) found that cytokine- or lipopolysaccharide-stimulated human pericytes express TLR2 and TLR4, the G-protein-coupled receptor N-formyl peptide receptor (FPR) 2, tumor necrosis factor receptor 1 (TNFR1) and the cytoplasmic receptor NLR family pyrin domain-containing 3 (NLRP3). The significance of pro-inflammatory pericyte activation in myocardial infarction remains unknown. Whether pericytes in the infarcted myocardium can respond to the release of alarmins by dying cardiomyocytes has not been investigated. Considering the well-established contributions of other cell types as sources of inflammatory cytokines and chemokines (including endothelial cells (46), fibroblasts (47), macrophages (48) and cardiomyocytes (49, 50)), the relative role of pericytes in secretion of pro-inflammatory mediators remains unclear.
Despite the absence of direct evidence in models of myocardial injury, pericytes may serve as critical regulators of leukocyte transendothelial trafficking cascades. Recruitment of neutrophils, monocytes and lymphocytes in sites of injury is driven by chemokine-dependent adhesive interactions between endothelial cells and circulating leukocytes (51). Migrating neutrophils and monocytes are found to be polarized when in close vicinity of pericytes, suggesting a role for pericyte-derived factors to drive chemotaxis of leukocytes. Pericytes may contribute to leukocyte chemotaxis by releasing chemokines and by ‘instructing’ leukocytes exiting through venules with pattern-recognition and motility programs (45). Intravital microscopy experiments in cremaster muscle demonstrated that cytokine-mediated pericyte activation triggers transition to an ‘inflammatory phenotype’, characterized by the expression of adhesion molecules and chemoattractant mediators that provide molecular cues to transmigrating leukocytes. Moreover, inflammatory activation causes shape changes in pericytes, promoting enlargement of gaps between adjacent pericytes. Thus, leukocytes use the enlarged gaps as exit points during transmigration into the injured tissue (52). Whether alterations in the morphology and adhesive properties of pericytes are involved in leukocyte trafficking in infarcted hearts remains unknown.
In addition to their role in inflammation and in leukocyte trafficking, pericytes may also play an important role in degradation of the pericellular extracellular matrix. Pericyte-mediated breakdown of the basement membrane and detachment from endothelial cells is an important early step in angiogenesis (53). Although other cardiac interstitial cells, such as fibroblasts, acquire a matrix-degrading phenotype during the early inflammatory phase of healing, producing matrix metalloproteinases in response to stimulation with IL-1β (54), whether cardiac pericytes also participate in matrix degradation remains unknown.
Pericytes have also been implicated in the pathogenesis of the ‘no-reflow phenomenon’. Coronary no-reflow occurs when the myocardium fails to perfuse despite restoration of coronary flow (55). In experimental models of myocardial ischemia/reperfusion, endothelial injury and leukocyte plugging may cause microvessel occlusion, accentuating ischemic injury. In human patients, thrombi and atherosclerotic debris may embolize microvessels further reducing microvascular flow (56). A recently published study in a rat model of myocardial ischemia and reperfusion suggested that pericyte constriction contributes to the ‘no-reflow phenomenon’ by causing microvascular obstruction (22, 57). NG2-fluorescent labeling revealed that most of the blocked sites were spatially associated with pericytes. Coronary capillary lumen diameter was reduced by 37% at sites of pericyte juxtaposition upon ischemia, when compared to sham-operated hearts, suggesting a possible role for pericytes in coronary no-reflow phenomenon. Pericyte relaxation through administration of adenosine markedly increased perfusion in the ischemic and reperfused myocardium (22). Despite the limitations of the study, related to the broad effects of adenosine on many cell types, these intriguing observations support a potential role for pericyte constriction in mediating persistent reduction of microvascular flow following ischemia, despite reperfusion of the culprit vessel.
Pericytes during the proliferative phase of infarct healing: a role in fibrosis?
Transition to the proliferative phase of cardiac repair is preceded by suppression of pro-inflammatory signaling cascades (58). As pro-inflammatory signaling is attenuated, fibroblasts become activated and acquire a myofibroblast phenotype, incorporating α-SMA into cytoskeletal stress fibers and producing large amounts of extracellular matrix proteins (37, 59, 60). Myofibroblast activation is accompanied by an intense angiogenic response, presumably to support the metabolically active hypercellular wound with oxygen and nutrients (61). The potential role of pericytes in the proliferative phase of repair is poorly supported by experimental evidence. However, considering their abundance, phenotypic plasticity and responsiveness to growth factors and cytokines, pericytes may contribute to several of the cellular events associated with the proliferative phase of cardiac repair.
In fibrotic conditions involving the kidney (62), lung (63) and skin (64), pericytes have been directly implicated in myofibroblast activation and in deposition of collagenous matrix. Moreover, stimulation of pericytes with growth factors (such as PDGF-BB) has been suggested to induce pericyte-fibroblast transition in tumors (65) and in experimental models of retinopathy (66). However, evidence implicating pericytes in activation of fibrogenic pathways in the heart is lacking. Recent studies using robust lineage-tracing approaches suggest that resident fibroblast populations account for the expansion of myofibroblasts in the infarcted heart (67). Whether pericytes also contribute to cardiac fibrosis by undergoing conversion to myofibroblast-like cells remains controversial. Descriptive studies using pericyte-reporter mice suggested that, although myocardial infarction triggers expansion of a nestin-negative, NG2+ pericyte population, these cells did not exhibit collagen immunoreactivity (23). The finding was interpreted as suggestive of the absence of a role for pericytes in cardiac fibrosis. However, considering the challenges associated with the detection of collagen expression in tissue sections, the possibility that following injury pericyte-derived cells may no longer express canonical pericyte markers, and the possibility of fibrogenic effects of the pericytes that may be independent of collagen synthesis, these observations do not exclude a crucial role of pericytes in myocardial fibrosis. On the other hand, Kramann et al. found that a large number of myofibroblasts in remodeling and infarcted hearts are derived from Gli1+ ‘pericytes’ (8). The notion that pericytes may convert to myofibroblasts was also challenged by a study using lineage tracing of Tbx18+ ‘pericytes’, which demonstrated that these cells maintain their identity in pressure-overloaded hearts and do not significantly contribute to other lineages (68). Clearly, the lack of uniform criteria and reliable markers for defining pericytes contributes to the confusion regarding their role in myocardial fibrosis.
Pericytes during the maturation phase of cardiac repair
During the maturation phase of infarct healing, suppression of fibrogenic responses, microvessel maturation and extracellular matrix crosslinking result in the formation of a stable scar and prevent the expansion of fibrosis (39, 69). Mural cells (both pericytes and vascular smooth muscle cells) play a central role in vascular maturation, by coating infarct neovessels (70, 71), thus stabilizing the microvasculature of the scar and attenuating inflammation. Antibody inhibition experiments in a mouse model of reperfused myocardial infarction demonstrated that PDGF-BB/PDGFRβ signaling plays a crucial role in scar maturation (70). Disruption of PDGFRβ signaling perturbed coating of neovessels with mural cells and prolonged hemorrhagic and inflammatory changes in the healing infarct (70).
Pericytes as therapeutic targets in myocardial infarction
The absence of robust data on the role of endogenous pericytes greatly limits design of therapeutic approaches targeting these cells in infarcted and remodeling hearts. Most of the evidence suggesting important roles of pericytes in inflammation and fibrosis following myocardial infarction is based on associative data. Documentation of pericyte-specific effects in vivo is particularly challenging; thus, therapeutic targeting of endogenous pericyte actions is heavily based on speculation. Considering their potential role in regulating microvascular function, inhibition of pericyte constriction has been suggested as a promising strategy for protection of the ischemic and reperfused myocardium from ‘no-reflow’ (22, 57). Whether approaches targeting endogenous pericyte-mediated actions may protect from adverse post-infarction remodeling restrain inflammation or attenuate fibrosis remains unknown.
Considering their role in angiogenesis, microvascular function, regulation of inflammation and growth factor expression profile, cell therapy with pericytes has been suggested as an attractive strategy to improve repair of the infarcted heart. Experimental studies have suggested that intramyocardial delivery of pericytes may protect the infarcted heart from adverse remodeling by exerting angiogenic and anti-apoptotic actions (72, 73, 74) through paracrine effects on cardiomyocytes, fibroblasts and vascular cells. In a mouse model of myocardial infarction, transplantation of saphenous vein-derived pericytes into the peri-infarct zone attenuated adverse remodeling and improved systolic dysfunction, by secreting angiogenic mediators (such as VEGF and angiopoietin-1) and by releasing protective microRNAs (75). Pericyte-derived miR-132 was identified a key cardioprotective factor that improved cardiac function following infarction, increasing angiogenesis and attenuating fibrosis (75). Studies in large animal models have provided additional support to the beneficial effects of pericyte cell therapy following infarction. In a blind, randomized placebo-controlled study in a swine model of reperfused myocardial infarction, transplantation of human adventitial pericytes improved capillary dysfunction and reduced fibrosis (76). However, these presumably beneficial cell biological actions of pericytes were not associated with any effects on myocardial function. It has also been suggested that human myocardial pericytes, may serve as multipotent precursors and may have limited cardiomyogenic potential (19). However, robust documentation of pericyte-driven remuscularization in vivo is lacking.
Conclusions
Despite their abundance and their potential significance in regulating a wide range of functions, including microvascular permeability, angiogenesis, inflammation, repair, and fibrosis, cardiac pericytes have been largely ignored by the cardiovascular community. There is an urgent need for studies systematically characterizing cardiac pericyte populations, identifying subsets with distinct functional properties and exploring the role of pericyte-mediated actions in cardiac injury, repair and remodeling.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
Funding
Dr Frangogiannis’ laboratory is supported by NIH grants R01 HL76246 and R01 HL85440 and by Department of Defense grants PR151134 and PR151029.
References
- 1.Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Developmental Cell 2011. 21 193–215. ( 10.1016/j.devcel.2011.07.001) [DOI] [PubMed] [Google Scholar]
- 2.Dulmovits BM, Herman IM. Microvascular remodeling and wound healing: a role for pericytes. International Journal of Biochemistry and Cell Biology 2012. 44 1800–1812. ( 10.1016/j.biocel.2012.06.031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamazaki T, Mukouyama YS. Tissue specific origin, development, and pathological perspectives of pericytes. Frontiers in Cardiovascular Medicine 2018. 5 78 ( 10.3389/fcvm.2018.00078) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sims DE. The pericyte – a review. Tissue and Cell 1986. 18 153–174. ( 10.1016/0040-8166(86)90026-1) [DOI] [PubMed] [Google Scholar]
- 5.Nehme A, Edelman J. Dexamethasone inhibits high glucose-, TNF-alpha-, and IL-1beta-induced secretion of inflammatory and angiogenic mediators from retinal microvascular pericytes. Investigative Ophthalmology and Visual Science 2008. 49 2030–2038. ( 10.1167/iovs.07-0273) [DOI] [PubMed] [Google Scholar]
- 6.Wang S, Cao C, Chen Z, Bankaitis V, Tzima E, Sheibani N, Burridge K. Pericytes regulate vascular basement membrane remodeling and govern neutrophil extravasation during inflammation. PLoS ONE 2012. 7 e45499 ( 10.1371/journal.pone.0045499) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ren S, Johnson BG, Kida Y, Ip C, Davidson KC, Lin SL, Kobayashi A, Lang RA, Hadjantonakis AK, Moon RT, et al. LRP-6 is a coreceptor for multiple fibrogenic signaling pathways in pericytes and myofibroblasts that are inhibited by DKK-1. PNAS 2013. 110 1440–1445. ( 10.1073/pnas.1211179110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kramann R, Schneider RK, DiRocco DP, Machado F, Fleig S, Bondzie PA, Henderson JM, Ebert BL, Humphreys BD. Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell 2015. 16 51–66. ( 10.1016/j.stem.2014.11.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campagnolo P, Katare R, Madeddu P. Realities and misconceptions on the pericytes role in tissue repair. Regenerative Medicine 2018. 13 119–122. ( 10.2217/rme-2017-0091) [DOI] [PubMed] [Google Scholar]
- 10.Paquet-Fifield S, Schluter H, Li A, Aitken T, Gangatirkar P, Blashki D, Koelmeyer R, Pouliot N, Palatsides M, Ellis S, et al. A role for pericytes as microenvironmental regulators of human skin tissue regeneration. Journal of Clinical Investigation 2009. 119 2795–2806. ( 10.1172/JCI38535) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O. Skeletal muscle pericyte subtypes differ in their differentiation potential. Stem Cell Research 2013. 10 67–84. ( 10.1016/j.scr.2012.09.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caporali A, Martello A, Miscianinov V, Maselli D, Vono R, Spinetti G. Contribution of pericyte paracrine regulation of the endothelium to angiogenesis. Pharmacology and Therapeutics 2017. 171 56–64. ( 10.1016/j.pharmthera.2016.10.001) [DOI] [PubMed] [Google Scholar]
- 13.Pinto AR, Ilinykh A, Ivey MJ, Kuwabara JT, D'Antoni ML, Debuque R, Chandran A, Wang L, Arora K, Rosenthal NA, et al. Revisiting cardiac cellular composition. Circulation Research 2016. 118 400–409. ( 10.1161/CIRCRESAHA.115.307778) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nees S, Weiss DR, Senftl A, Knott M, Forch S, Schnurr M, Weyrich P, Juchem G. Isolation, bulk cultivation, and characterization of coronary microvascular pericytes: the second most frequent myocardial cell type in vitro. American Journal of Physiology: Heart and Circulatory Physiology 2012. 302 H69–H84. ( 10.1152/ajpheart.00359.2011) [DOI] [PubMed] [Google Scholar]
- 15.Frangogiannis NG. The immune system and the remodeling infarcted heart: cell biological insights and therapeutic opportunities. Journal of Cardiovascular Pharmacology 2014. 63 185–195. ( 10.1097/FJC.0000000000000003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nature Reviews: Cardiology 2014. 11 255–265. ( 10.1038/nrcardio.2014.28) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forbes MS, Rennels ML, Nelson E. Ultrastructure of pericytes in mouse heart. American Journal of Anatomy 1977. 149 47–70. ( 10.1002/aja.1001490105) [DOI] [PubMed] [Google Scholar]
- 18.Nag AC. Study of non-muscle cells of the adult mammalian heart: a fine structural analysis and distribution. Cytobios 1980. 28 41–61. [PubMed] [Google Scholar]
- 19.Chen WC, Baily JE, Corselli M, Diaz ME, Sun B, Xiang G, Gray GA, Huard J, Peault B. Human myocardial pericytes: multipotent mesodermal precursors exhibiting cardiac specificity. Stem Cells 2015. 33 557–573. ( 10.1002/stem.1868) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ozerdem U, Grako KA, Dahlin-Huppe K, Monosov E, Stallcup WB. NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Developmental Dynamics 2001. 222 218–227. ( 10.1002/dvdy.1200) [DOI] [PubMed] [Google Scholar]
- 21.Chen Q, Zhang H, Liu Y, Adams S, Eilken H, Stehling M, Corada M, Dejana E, Zhou B, Adams RH. Endothelial cells are progenitors of cardiac pericytes and vascular smooth muscle cells. Nature Communications 2016. 7 12422 ( 10.1038/ncomms12422) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Farrell FM, Mastitskaya S, Hammond-Haley M, Freitas F, Wah WR, Attwell D. Capillary pericytes mediate coronary no-reflow after myocardial ischaemia. eLife 2017. 6 e29280 ( 10.7554/eLife.29280) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Birbrair A, Zhang T, Files DC, Mannava S, Smith T, Wang ZM, Messi ML, Mintz A, Delbono O. Type-1 pericytes accumulate after tissue injury and produce collagen in an organ-dependent manner. Stem Cell Research and Therapy 2014. 5 122 ( 10.1186/scrt512) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simonavicius N, Ashenden M, van Weverwijk A, Lax S, Huso DL, Buckley CD, Huijbers IJ, Yarwood H, Isacke CM. Pericytes promote selective vessel regression to regulate vascular patterning. Blood 2012. 120 1516–1527. ( 10.1182/blood-2011-01-332338) [DOI] [PubMed] [Google Scholar]
- 25.Edelman DA, Jiang Y, Tyburski J, Wilson RF, Steffes C. Pericytes and their role in microvasculature homeostasis. Journal of Surgical Research 2006. 135 305–311. ( 10.1016/j.jss.2006.06.010) [DOI] [PubMed] [Google Scholar]
- 26.Hellstrom M, Kalen M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 1999. 126 3047–3055. [DOI] [PubMed] [Google Scholar]
- 27.Chintalgattu V, Rees ML, Culver JC, Goel A, Jiffar T, Zhang J, Dunner K, Jr, Pati S, Bankson JA, Pasqualini R, et al. Coronary microvascular pericytes are the cellular target of sunitinib malate-induced cardiotoxicity. Science Translational Medicine 2013. 5 187ra69 ( 10.1126/scitranslmed.3005066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ziegler T, Horstkotte J, Schwab C, Pfetsch V, Weinmann K, Dietzel S, Rohwedder I, Hinkel R, Gross L, Lee S, et al. Angiopoietin 2 mediates microvascular and hemodynamic alterations in sepsis. Journal of Clinical Investigation 2013. 123 3436–3445. ( 10.1172/JCI66549) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hinkel R, Howe A, Renner S, Ng J, Lee S, Klett K, Kaczmarek V, Moretti A, Laugwitz KL, Skroblin P, et al. Diabetes mellitus-induced microvascular destabilization in the myocardium. Journal of the American College of Cardiology 2017. 69 131–143. ( 10.1016/j.jacc.2016.10.058) [DOI] [PubMed] [Google Scholar]
- 30.Gonzalez-Quesada C, Cavalera M, Biernacka A, Kong P, Lee DW, Saxena A, Frunza O, Dobaczewski M, Shinde A, Frangogiannis NG. Thrombospondin-1 induction in the diabetic myocardium stabilizes the cardiac matrix in addition to promoting vascular rarefaction through angiopoietin-2 upregulation. Circulation Research 2013. 113 1331–1344. ( 10.1161/CIRCRESAHA.113.302593) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frangogiannis NG. Pathophysiology of myocardial infarction. Comprehensive Physiology 2015. 5 1841–1875. ( 10.1002/cphy.c150006) [DOI] [PubMed] [Google Scholar]
- 32.Arslan F, Smeets MB, O'Neill LA, Keogh B, McGuirk P, Timmers L, Tersteeg C, Hoefer IE, Doevendans PA, Pasterkamp G, et al. Myocardial ischemia/reperfusion injury is mediated by leukocytic toll-like receptor-2 and reduced by systemic administration of a novel anti-toll-like receptor-2 antibody. Circulation 2010. 121 80–90. ( 10.1161/CIRCULATIONAHA.109.880187) [DOI] [PubMed] [Google Scholar]
- 33.Dutta P, Nahrendorf M. Monocytes in myocardial infarction. Arteriosclerosis, Thrombosis, and Vascular Biology 2015. 35 1066–1070. ( 10.1161/ATVBAHA.114.304652) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frangogiannis NG, Mendoza LH, Lindsey ML, Ballantyne CM, Michael LH, Smith CW, Entman ML. IL-10 is induced in the reperfused myocardium and may modulate the reaction to injury. Journal of Immunology 2000. 165 2798–2808. ( 10.4049/jimmunol.165.5.2798) [DOI] [PubMed] [Google Scholar]
- 35.Frangogiannis NG. The role of transforming growth factor (TGF)-beta in the infarcted myocardium. Journal of Thoracic Disease 2017. 9 S52–S63. ( 10.21037/jtd.2016.11.19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kong P, Shinde AV, Su Y, Russo I, Chen B, Saxena A, Conway SJ, Graff JM, Frangogiannis NG. Opposing actions of fibroblast and cardiomyocyte Smad3 signaling in the infarcted myocardium. Circulation 2018. 137 707–724. ( 10.1161/CIRCULATIONAHA.117.029622) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frangogiannis NG, Michael LH, Entman ML. Myofibroblasts in reperfused myocardial infarcts express the embryonic form of smooth muscle myosin heavy chain (SMemb). Cardiovascular Research 2000. 48 89–100. ( 10.1016/S0008-6363(00)00158-9) [DOI] [PubMed] [Google Scholar]
- 38.Shinde AV, Humeres C, Frangogiannis NG. The role of alpha-smooth muscle actin in fibroblast-mediated matrix contraction and remodeling. Biochimica et Biophysica Acta: Molecular Basis of Disease 2017. 1863 298–309. ( 10.1016/j.bbadis.2016.11.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frangogiannis NG. The extracellular matrix in myocardial injury, repair, and remodeling. Journal of Clinical Investigation 2017. 127 1600–1612. ( 10.1172/JCI87491) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fu X, Khalil H, Kanisicak O, Boyer JG, Vagnozzi RJ, Maliken BD, Sargent MA, Prasad V, Valiente-Alandi I, Blaxall BC, et al. Specialized fibroblast differentiated states underlie scar formation in the infarcted mouse heart. Journal of Clinical Investigation 2018. 128 2127–2143. ( 10.1172/JCI98215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Siao CJ, Lorentz CU, Kermani P, Marinic T, Carter J, McGrath K, Padow VA, Mark W, Falcone DJ, Cohen-Gould L, et al. ProNGF, a cytokine induced after myocardial infarction in humans, targets pericytes to promote microvascular damage and activation. Journal of Experimental Medicine 2012. 209 2291–2305. ( 10.1084/jem.20111749) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee SJ, Lee CK, Kang S, Park I, Kim YH, Kim SK, Hong SP, Bae H, He Y, Kubota Y, et al. Angiopoietin-2 exacerbates cardiac hypoxia and inflammation after myocardial infarction. Journal of Clinical Investigation 2018. 128 5018–5033. ( 10.1172/JCI99659) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nyul-Toth Á, Kozma M, Nagyoszi P, Nagy K, Fazakas C, Hasko J, Molnar K, Farkas AE, Vegh AG, Varo G, et al. Expression of pattern recognition receptors and activation of the non-canonical inflammasome pathway in brain pericytes. Brain, Behavior, and Immunity 2017. 64 220–231. ( 10.1016/j.bbi.2017.04.010) [DOI] [PubMed] [Google Scholar]
- 44.Edelman DA, Jiang Y, Tyburski JG, Wilson RF, Steffes CP. Lipopolysaccharide activation of pericyte’s Toll-like receptor-4 regulates co-culture permeability. American Journal of Surgery 2007. 193 730–735. ( 10.1016/j.amjsurg.2006.08.086) [DOI] [PubMed] [Google Scholar]
- 45.Stark K, Eckart A, Haidari S, Tirniceriu A, Lorenz M, von Bruhl ML, Gartner F, Khandoga AG, Legate KR, Pless R, et al. Capillary and arteriolar pericytes attract innate leukocytes exiting through venules and ‘instruct’ them with pattern-recognition and motility programs. Nature Immunology 2013. 14 41–51. ( 10.1038/ni.2477) [DOI] [PubMed] [Google Scholar]
- 46.Frangogiannis NG, Mendoza LH, Lewallen M, Michael LH, Smith CW, Entman ML. Induction and suppression of interferon-inducible protein 10 in reperfused myocardial infarcts may regulate angiogenesis. FASEB Journal 2001. 15 1428–1430. ( 10.1096/fj.00-0745fje) [DOI] [PubMed] [Google Scholar]
- 47.Anzai A, Choi JL, He S, Fenn AM, Nairz M, Rattik S, McAlpine CS, Mindur JE, Chan CT, Iwamoto Y, et al. The infarcted myocardium solicits GM-CSF for the detrimental oversupply of inflammatory leukocytes. Journal of Experimental Medicine 2017. 214 3293–3310. ( 10.1084/jem.20170689) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bajpai G, Bredemeyer A, Li W, Zaitsev K, Koenig AL, Lokshina I, Mohan J, Ivey B, Hsiao HM, Weinheimer C, et al. Tissue resident CCR2− and CCR2+ cardiac macrophages differentially orchestrate monocyte recruitment and fate specification following myocardial injury. Circulation Research 2019. 124 263–278. ( 10.1161/CIRCRESAHA.118.314028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gwechenberger M, Mendoza LH, Youker KA, Frangogiannis NG, Smith CW, Michael LH, Entman ML. Cardiac myocytes produce interleukin-6 in culture and in viable border zone of reperfused infarctions. Circulation 1999. 99 546–551. ( 10.1161/01.CIR.99.4.546) [DOI] [PubMed] [Google Scholar]
- 50.Mezzaroma E, Toldo S, Farkas D, Seropian IM, Van Tassell BW, Salloum FN, Kannan HR, Menna AC, Voelkel NF, Abbate A. The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. PNAS 2011. 108 19725–19730. ( 10.1073/pnas.1108586108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vestweber D. How leukocytes cross the vascular endothelium. Nature Reviews: Immunology 2015. 15 692–704. ( 10.1038/nri3908) [DOI] [PubMed] [Google Scholar]
- 52.Proebstl D, Voisin MB, Woodfin A, Whiteford J, D’Acquisto F, Jones GE, Rowe D, Nourshargh S. Pericytes support neutrophil subendothelial cell crawling and breaching of venular walls in vivo. Journal of Experimental Medicine 2012. 209 1219–1234. ( 10.1084/jem.20111622) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Hinsbergh VW, Koolwijk P. Endothelial sprouting and angiogenesis: matrix metalloproteinases in the lead. Cardiovascular Research 2008. 78 203–212. ( 10.1093/cvr/cvm102) [DOI] [PubMed] [Google Scholar]
- 54.Saxena A, Chen W, Su Y, Rai V, Uche OU, Li N, Frangogiannis NG. IL-1 induces proinflammatory leukocyte infiltration and regulates fibroblast phenotype in the infarcted myocardium. Journal of Immunology 2013. 191 4838–4848. ( 10.4049/jimmunol.1300725) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kloner RA, Ganote CE, Jennings RB. The ‘no-reflow’ phenomenon after temporary coronary occlusion in the dog. Journal of Clinical Investigation 1974. 54 1496–1508. ( 10.1172/JCI107898) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rezkalla SH, Stankowski RV, Hanna J, Kloner RA. Management of no-reflow phenomenon in the catheterization laboratory. JACC: Cardiovascular Interventions 2017. 10 215–223. ( 10.1016/j.jcin.2016.11.059) [DOI] [PubMed] [Google Scholar]
- 57.O'Farrell FM, Attwell D. A role for pericytes in coronary no-reflow. Nature Reviews: Cardiology 2014. 11 427–432. ( 10.1038/nrcardio.2014.58) [DOI] [PubMed] [Google Scholar]
- 58.Chen W, Saxena A, Li N, Sun J, Gupta A, Lee DW, Tian Q, Dobaczewski M, Frangogiannis NG. Endogenous IRAK-M attenuates postinfarction remodeling through effects on macrophages and fibroblasts. Arteriosclerosis, Thrombosis, and Vascular Biology 2012. 32 2598–2608. ( 10.1161/ATVBAHA.112.300310) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Willems IE, Havenith MG, De Mey JG, Daemen MJ. The alpha-smooth muscle actin-positive cells in healing human myocardial scars. American Journal of Pathology 1994. 145 868–875. [PMC free article] [PubMed] [Google Scholar]
- 60.Cleutjens JP, Verluyten MJ, Smiths JF, Daemen MJ. Collagen remodeling after myocardial infarction in the rat heart. American Journal of Pathology 1995. 147 325–338. [PMC free article] [PubMed] [Google Scholar]
- 61.Ren G, Michael LH, Entman ML, Frangogiannis NG. Morphological characteristics of the microvasculature in healing myocardial infarcts. Journal of Histochemistry and Cytochemistry 2002. 50 71–79. ( 10.1177/002215540205000108) [DOI] [PubMed] [Google Scholar]
- 62.Leaf IA, Nakagawa S, Johnson BG, Cha JJ, Mittelsteadt K, Guckian KM, Gomez IG, Altemeier WA, Duffield JS. Pericyte MyD88 and IRAK4 control inflammatory and fibrotic responses to tissue injury. Journal of Clinical Investigation 2017. 127 321–334. ( 10.1172/JCI87532) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sava P, Ramanathan A, Dobronyi A, Peng X, Sun H, Ledesma-Mendoza A, Herzog EL, Gonzalez AL. Human pericytes adopt myofibroblast properties in the microenvironment of the IPF lung. JCI Insight 2017. 2 96352 ( 10.1172/jci.insight.96352) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sundberg C, Ivarsson M, Gerdin B, Rubin K. Pericytes as collagen-producing cells in excessive dermal scarring. Laboratory Investigation 1996. 74 452–466. [PubMed] [Google Scholar]
- 65.Hosaka K, Yang Y, Seki T, Fischer C, Dubey O, Fredlund E, Hartman J, Religa P, Morikawa H, Ishii Y, et al. Pericyte-fibroblast transition promotes tumor growth and metastasis. PNAS 2016. 113 E5618–E5627. ( 10.1073/pnas.1608384113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kitahara H, Kajikawa S, Ishii Y, Yamamoto S, Hamashima T, Azuma E, Sato H, Matsushima T, Shibuya M, Shimada Y, et al. The novel pathogenesis of retinopathy mediated by multiple RTK signals is uncovered in newly developed mouse model. EBioMedicine 2018. 31 190–201. ( 10.1016/j.ebiom.2018.04.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kanisicak O, Khalil H, Ivey MJ, Karch J, Maliken BD, Correll RN, Brody MJ, J Lin SC, Aronow BJ, Tallquist MD, et al. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nature Communications 2016. 7 12260 ( 10.1038/ncomms12260) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guimaraes-Camboa N, Cattaneo P, Sun Y, Moore-Morris T, Gu Y, Dalton ND, Rockenstein E, Masliah E, Peterson KL, Stallcup WB, et al. Pericytes of multiple organs do not behave as mesenchymal stem cells in vivo. Cell Stem Cell 2017. 20 345.e5–359.e5. ( 10.1016/j.stem.2016.12.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Frangogiannis NG. Regulation of the inflammatory response in cardiac repair. Circulation Research 2012. 110 159–173. ( 10.1161/CIRCRESAHA.111.243162) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zymek P, Bujak M, Chatila K, Cieslak A, Thakker G, Entman ML, Frangogiannis NG. The role of platelet-derived growth factor signaling in healing myocardial infarcts. Journal of the American College of Cardiology 2006. 48 2315–2323. ( 10.1016/j.jacc.2006.07.060) [DOI] [PubMed] [Google Scholar]
- 71.Dobaczewski M, Akrivakis S, Nasser K, Michael LH, Entman ML, Frangogiannis NG. Vascular mural cells in healing canine myocardial infarcts. Journal of Histochemistry and Cytochemistry 2004. 52 1019–1029. ( 10.1369/jhc.3A6210.2004) [DOI] [PubMed] [Google Scholar]
- 72.Avolio E, Madeddu P. Discovering cardiac pericyte biology: from physiopathological mechanisms to potential therapeutic applications in ischemic heart disease. Vascular Pharmacology 2016. 86 53–63. ( 10.1016/j.vph.2016.05.009) [DOI] [PubMed] [Google Scholar]
- 73.Avolio E, Meloni M, Spencer HL, Riu F, Katare R, Mangialardi G, Oikawa A, Rodriguez-Arabaolaza I, Dang Z, Mitchell K, et al. Combined intramyocardial delivery of human pericytes and cardiac stem cells additively improves the healing of mouse infarcted hearts through stimulation of vascular and muscular repair. Circulation Research 2015. 116 e81–e94. ( 10.1161/CIRCRESAHA.115.306146) [DOI] [PubMed] [Google Scholar]
- 74.Cathery W, Faulkner A, Maselli D, Madeddu P. Concise review: the regenerative journey of pericytes toward clinical translation. Stem Cells 2018. 36 1295–1310. ( 10.1002/stem.2846) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Katare R, Riu F, Mitchell K, Gubernator M, Campagnolo P, Cui Y, Fortunato O, Avolio E, Cesselli D, Beltrami AP, et al. Transplantation of human pericyte progenitor cells improves the repair of infarcted heart through activation of an angiogenic program involving micro-RNA-132. Circulation Research 2011. 109 894–906. ( 10.1161/CIRCRESAHA.111.251546) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alvino VV, Fernandez-Jimenez R, Rodriguez-Arabaolaza I, Slater S, Mangialardi G, Avolio E, Spencer H, Culliford L, Hassan S, Sueiro Ballesteros L, et al. Transplantation of allogeneic pericytes improves myocardial vascularization and reduces interstitial fibrosis in a swine model of reperfused acute myocardial infarction. Journal of the American Heart Association 2018. 7 e006727 ( 10.1161/JAHA.117.006727) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a