Abstract
Upon implantation, engineered tissues rely on the supply with oxygen and nutrients as well as the drainage of interstitial fluid. This prerequisite still represents one of the current challenges in the engineering and regeneration of tissues. Recently, different vascularization strategies have been developed. Besides technical approaches like 3D printing or laser processing and de-/recelluarization of natural scaffolds, mainly co-cultures of endothelial cells (ECs) with supporting cell types are being used. This mini-review provides a brief overview of different co-culture systems for the engineering of blood and lymphatic microvascular networks.
Keywords: co-culture, vascularization, endothelial cells, lymphangiogenesis, scaffolds, microvasculature, mesenchymal stem/stromal cells, fibroblast, fibrin
Necessity for prevascularization
Tissue engineering and regenerative medicine are emerging disciplines focusing on the repair and regeneration of injured or diseased tissues. Except very few tissues like cartilage, epidermis, the cornea and the lens in the eye, most of the organs in the human body rely on a functional supply with vascular structures to provide the cells with oxygen and nutrients on the one side (blood vessels) and to drain interstitial fluid back into the venous circulation (lymphatic vessels) on the other side. Similar to solid tumors, tissues which grow beyond the diffusion limit of oxygen (100–200 µm) are in need of blood vessels for oxygen and nutrient supply. In the last two decades a plethora of approaches have been developed in order to engineer vascular structures, both of blood vascular and lymphatic nature.
Technical approaches for vascularization
In order to achieve a ‘pre-patterned’ extracellular matrix (ECM), technological solutions comprise the use of 3D printing in order to establish ‘vascular trees’ in biocompatible hydrogels (1) or the decellularization of larger vascular structures for instance from the small intestine of the pig (2) or from the human placenta (3) (Fig. 1). The generated grafts or scaffolds can be reseeded with autologous ECs on the inside and supporting cells (fibroblasts, smooth muscle cells, etc.) on the outside of the tubes. Moreover, microfluidic systems have been established integrating a vascular network in pre-fabricated channels, which turns out to be suitable for basic biological studies of cell-cell communication, and further might serve as a model system for drug testing. In addition, more sophisticated models utilize different cell types to create organoids/mini-organs, resulting in organ-on-a-chip systems, which rely on functional vasculature as well (4). As a rather new process 3D bioprinting has also been considered as an approach for successful vascularization with a wide range of applicability (5).
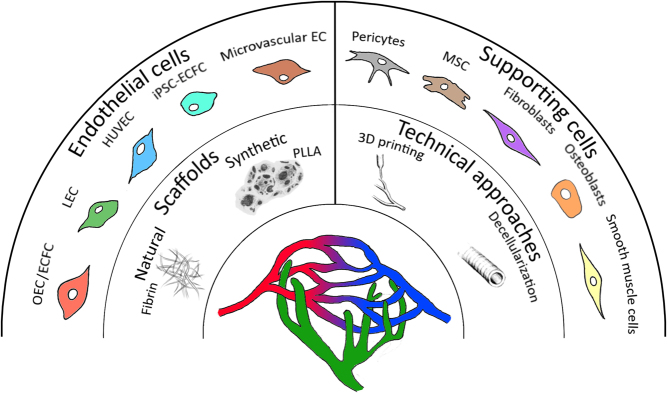
Figure 1.
Overview of different vascularization strategies. Functional blood and lymphatic microvasculature can be achieved by co-culturing of endothelial and supporting cells from different origins. Natural or synthetic scaffolds are generated from different materials to provide a 3D structure. Furthermore, technical approaches such as 3D printing or decellularization aid in the fabrication of these structures.
Scaffolds for 3D engineering
Based on the necessity for 3D co-culture to engineer vasculature, different scaffolds have been used to provide the required stability in 3D and allow for angiogenic/vasculogenic remodeling and thus the formation of functional vessels (Fig. 1). For that purpose, both synthetic and natural scaffolds have been described. Synthetic scaffolds include materials such as poly-l-lactic acid (PLLA), poly-lactic-co-glycolic acid (PLGA) or polycaprolactone fumarate (PCLF) (6, 7, 8) as well as self-assembling nanopeptides (9). Their main advantages are accessibility, high reproducibility and an eminently controllable degradation rate; however, low cell adhesion represents the main disadvantage (10). Nevertheless, this difficulty can be mastered by binding of cell recognition motifs in form of small immobilized peptides such as the RGD sequence, which stimulates cell adhesion via integrins (11). The most employed natural scaffolds for engineering vascular networks are collagens (12, 13) or fibrin matrices (14, 15, 16). These types of scaffolds have a high degree of biocompatibility and provide superior adhesion sites leading to improved growth and differentiation capability of the cells (17). Since both types of materials – natural and synthetic – can be fine-tuned with high precision, they are also utilized to deliver different proangiogenic factors such as vascular endothelial growth factor (VEGF) for the recruitment of EC (18, 19). Nevertheless, natural scaffolds represent the predominant type used due to their physiological characteristics resulting in improved cellular functions (20).
EC for vascular tissue engineering
Due to their ease in isolation and availability, ECs isolated from the human umbilical cord (HUVEC) have become the ‘gold standard’ in several areas of vascular biology including vascular tissue engineering (21). In addition to HUVEC, ECs from microvascular origin (brain, dermis) have been successfully employed in 3D co-culture models (Table 1). However, these cells cannot be translated into clinical settings, making autologous tissue sources like fat or peripheral blood more interesting for the use of ECs in prevascularization strategies. Thus, cells like endothelial colony-forming cells (ECFCs, also described as outgrowth endothelial cells (OECs)), induced pluripotent stem cell (iPSC)-derived ECs will be able to account for organotypic vascular beds (21). Another possibility is the direct reprogramming of differentiated human cells, such as fibroblast (22, 23) or mature amniotic cells (24) making these cells attractive for tissue-specific vascular bioengineering.
Table 1.
Cell types used in co-culture models for microvascular network formation.
| Endothelial cell type | Supporting cell type | Reference |
|---|---|---|
| ECFC | MSC (from different sources) | (12, 15, 35) |
| Fibroblast | (36) | |
| HUVEC | Fibroblast | (8, 16, 37, 38) |
| MSC (from different sources) | (4, 8, 14, 26, 27, 37) | |
| Human embryonic stem cells/iPSC-fibroblast | (25) | |
| Osteoblast | (13, 39) | |
| Smooth muscle cells | (40, 41) | |
| Human brain vascular pericytes | (41) | |
| Human embryonic stem cell-derived pericytes | (42) | |
| iPSC-EC | Fibroblast | (16, 43) |
| iPSC-EC, cardiac tissue EC, pulmonary artery EC | MSC (adipose derived) | (27) |
| LEC | Fibroblast | (32, 44) |
| LEC, BEC | ASC | (33) |
| Microvascular EC | Fibroblast | (32, 45, 46) |
| Dental pulp stem cells | (45) | |
| Outgrowth EC | Osteoblast | (39, 47) |
| MSC | (17, 48, 49) |
Endothelial cells and supporting cell types from different tissue sources mediate the formation of vascular structures.
Different sources of supporting cell types
Initially, fibroblasts were utilized as supporting cell types for capillary formation in co-culture with ECs (16, 25). Later, also mesenchymal stromal/stem cells (MSCs) mainly from bone marrow (14, 26) and adipose tissue (14, 17, 27) were used to provide ECs with the cues for vascular network formation (Table 1). These microcapillaries show characteristics of mature vessels, such as pericyte coverage or cell-cell junctions and are capable of blood perfusion when implanted subcutaneously in animal models (12, 28), therefore suggesting functionality of these tissue-engineered constructs. Moreover, a number of studies investigated the complex interplay with the ECM. Different proteases like plasmin or matrix metalloproteinases (16) have been shown to be key players in the morphogenesis and the remodeling of their 3D environment. In addition, these microcapillary structures can produce their own ECM consisting of perlecan, collagen IV and laminin (14). Interestingly, the analysis of biomechanical properties of the ECM revealed local stiffness to be quite heterogeneous (29).
The advent of lymphatic networks
Despite its presence and importance in nearly all organs with blood vasculature, the lymphatic system only recently became the research focus in vascular tissue engineering. Due to this neglect, lymphatic vascular markers like VEGFR3, PODOPLANIN, LYVE-1 and PROX-1 have only been cloned and functionally characterized years after respective markers on blood vascular cells (30). Consequently, the engineering of these structures lags behind. The group of Melody Swartz was among the first who took up this topic and integrated lymphatic ECs in 3D matrices to build lymphatic capillaries (31). Later on the Reichmann group (32) used ECs from the human dermis (comprising both, blood and lymphatic ECs – BEC/LEC) and integrated them in a fibrin matrix together with supporting cells (fibroblasts). Interestingly, the results show separate vascular network formation. Moreover, these vascular structures turned out to be biologically functional, as evidenced in a mouse skin model (32). In addition, our group has shown lymphatic and blood capillary morphogenesis in fibrin, when MSCs from fat tissue were co-integrated in the 3D matrix (33). Recently, the group of Anja Boos described vascular tube formation of LECs when cultured in conditions, where the MSC secretome, but not the MSC themselves were in contact with the LECs (34). Taken together, the importance of engineering of lymphatic microcapillaries is increasingly recognized, but still at the beginning.
Future directions of co-cultures and outlook
Based on the current knowledge on co-cultures for ex vivo vascular tissue engineering, many other aspects are currently discussed. For example, the spatio-temporal distribution of gradients which are necessary for vascular network formation can be monitored by microfluidic approaches (50). Furthermore, vascularization of multi-organ-chips is studied among others by the groups of Donald Ingber (51), Ali Khademhosseini (52) and Uwe Marx (4, 53). Moreover, the emerging role of extracellular vesicles (comprising ecto- and exosomes) in the cell-cell communication of ECs and supporting cell types will become of interest in the future. Our understanding of microcapillary morphogenesis in engineered vascular networks has constantly increased over the last two decades. Ex vivo engineered blood and lymphatic microcapillary structures will be of utmost importance in nearly every tissue engineering approach to provide larger constructs with the necessary oxygen and nutrient supply on the one hand, but also the lymphatic drainage system on the other hand. Integrating organotypic vessels into tissue-specific organoids will further pave the way to transplantable tissues suitable for tissue repair and regeneration.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Author contribution statement
J S, M P and W H designed the outline and wrote the manuscript.
Acknowledgments
The authors thank Severin Mühleder and Johannes Oesterreicher for critical comments on the manuscript.
References
- 1.Muehleder S, Ovsianikov A, Zipperle J, Redl H, Holnthoner W. Connections matter: channeled hydrogels to improve vascularization. Frontiers in Bioengineering and Biotechnology 2014. 2 52 ( 10.3389/fbioe.2014.00052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schanz J, Pusch J, Hansmann J, Walles H. Vascularised human tissue models: a new approach for the refinement of biomedical research. Journal of Biotechnology 2010. 148 56–63. ( 10.1016/j.jbiotec.2010.03.015) [DOI] [PubMed] [Google Scholar]
- 3.Schneider KH, Aigner P, Holnthoner W, Monforte X, Nürnberger S, Rünzler D, Redl H, Teuschl AH. Decellularized human placenta chorion matrix as a favorable source of small-diameter vascular grafts. Acta Biomaterialia 2016. 29 125–134. ( 10.1016/j.actbio.2015.09.038) [DOI] [PubMed] [Google Scholar]
- 4.Hasenberg T, Mühleder S, Dotzler A, Bauer S, Labuda K, Holnthoner W, Redl H, Lauster R, Marx U. Emulating human microcapillaries in a multi-organ-chip platform. Journal of Biotechnology 2015. 216 1–10. ( 10.1016/j.jbiotec.2015.09.038) [DOI] [PubMed] [Google Scholar]
- 5.Duan B. State-of-the-art review of 3D bioprinting for cardiovascular tissue engineering. Annals of Biomedical Engineering 2017. 45 195–209. ( 10.1007/s10439-016-1607-5) [DOI] [PubMed] [Google Scholar]
- 6.Nör JE, Peters MC, Christensen JB, Sutorik MM, Linn S, Khan MK, Addison CL, Mooney DJ, Polverini PJ. Engineering and characterization of functional human microvessels in immunodeficient mice. Laboratory Investigation 2001. 81 453–463. ( 10.1038/labinvest.3780253) [DOI] [PubMed] [Google Scholar]
- 7.Wagner ER, Parry J, Dadsetan M, Bravo D, Riester SM, Van Wijnen AJ, Yaszemski MJ, Kakar S. VEGF-mediated angiogenesis and vascularization of a fumarate-crosslinked polycaprolactone (PCLF) scaffold. Connective Tissue Research 2018. 59 542–549. ( 10.1080/03008207.2018.1424145) [DOI] [PubMed] [Google Scholar]
- 8.Freiman A, Shandalov Y, Rozenfeld D, Shor E, Segal S, Ben-David D, Meretzki S, Egozi D, Levenberg S. Adipose-derived endothelial and mesenchymal stem cells enhance vascular network formation on three-dimensional constructs in vitro. Stem Cell Research and Therapy 2016. 7 5 ( 10.1186/s13287-015-0251-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang TW, Chang K, Chen L, Liao S, Yeh C, Chuang Y. Effects of an injectable functionalized self-assembling nanopeptide hydrogel on angiogenesis and neurogenesis for regeneration of the central nervous system. Nanoscale 2017. 9 16281–16292. ( 10.1039/C7NR06528K) [DOI] [PubMed] [Google Scholar]
- 10.Mima Y, Fukumoto S, Koyama H, Okada M, Tanaka S, Shoji T, Emoto M, Furuzono T, Nishizawa Y, Inaba M. Enhancement of cell-based therapeutic angiogenesis using a novel type of injectable scaffolds of hydroxyapatite-polymer nanocomposite microspheres. PLoS ONE 2012. 7 e35199 ( 10.1371/journal.pone.0035199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hersel U, Dahmen C, Kessler H. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials 2003. 24 4385–4415. ( 10.1016/S0142-9612(03)00343-0) [DOI] [PubMed] [Google Scholar]
- 12.Allen P, Kang K, Bischoff J. Rapid onset of perfused blood vessels after implantation of ECFCs and MPCs in collagen, PuraMatrix and fibrin provisional matrices. Journal of Tissue Engineering and Regenerative Medicine 2015. 9 632–636. ( 10.1002/term.1803) [DOI] [PubMed] [Google Scholar]
- 13.Wenger A, Stahl A, Weber H, Finkenzeller G, Augustin HG, Stark GB, Kneser U. Modulation of in vitro angiogenesis in a three-dimensional spheroidal coculture model for bone tissue engineering. Tissue Engineering 2004. 10 1536–1547. ( 10.1089/ten.2004.10.1536) [DOI] [PubMed] [Google Scholar]
- 14.Pill K, Melke J, Mühleder S, Pultar M, Rohringer S, Priglinger E, Redl HR, Hofmann S, Holnthoner W. Microvascular networks from endothelial cells and mesenchymal stromal cells from adipose tissue and bone marrow: a comparison. Frontiers in Bioengineering and Biotechnology 2018. 6 156 ( 10.3389/fbioe.2018.00156) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mühleder S, Pill K, Schaupper M, Labuda K, Priglinger E, Hofbauer P, Charwat V, Marx U, Redl H, Holnthoner W. The role of fibrinolysis inhibition in engineered vascular networks derived from endothelial cells and adipose-derived stem cells. Stem Cell Research and Therapy 2018. 9 35 ( 10.1186/s13287-017-0764-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bezenah JR, Kong YP, Putnam AJ. Evaluating the potential of endothelial cells derived from human induced pluripotent stem cells to form microvascular networks in 3D cultures. Scientific Reports 2018. 8 2671 ( 10.1038/s41598-018-20966-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holnthoner W, Hohenegger K, Husa AM, Muehleder S, Meinl A, Peterbauer-Scherb A, Redl H. Adipose-derived stem cells induce vascular tube formation of outgrowth endothelial cells in a fibrin matrix. Journal of Tissue Engineering and Regenerative Medicine 2015. 9 127–136. ( 10.1002/term.1620) [DOI] [PubMed] [Google Scholar]
- 18.Rosa AR, Steffens D, Santi B, Quintiliano K, Steffen N, Pilger DA, Pranke P. Development of VEGF-loaded PLGA matrices in association with mesenchymal stem cells for tissue engineering. Brazilian Journal of Medical and Biological Research 2017. 50 e5648 ( 10.1590/1414-431X20175648) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nör JE, Christensen J, Mooney DJ, Polverini PJ. Vascular endothelial growth factor (VEGF)-mediated angiogenesis is associated with enhanced endothelial cell survival and induction of Bcl-2 expression. American Journal of Pathology 1999. 154 375–384. ( 10.1016/S0002-9440(10)65284-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Serbo JV, Gerecht S. Vascular tissue engineering: biodegradable scaffold platforms to promote angiogenesis. Stem Cell Research and Therapy 2013. 4 8 ( 10.1186/scrt156) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang K, Lin RZ, Melero-Martin JM. Bioengineering human vascular networks: trends and directions in endothelial and perivascular cell sources. Cellular and Molecular Life Sciences 2019. 76 421–439. ( 10.1007/s00018-018-2939-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee S, Park C, Han JW, Kim JY, Cho K, Kim EJ, Kim S, Lee SJ, Oh SY, Tanaka Y, et al. Direct reprogramming of human dermal fibroblasts into endothelial cells using ER71/ETV2. Circulation Research 2017. 120 848–861. ( 10.1161/CIRCRESAHA.116.309833) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han JK, Chang S, Cho H, Choi S, Ahn H, Lee J, Jeong H, Youn S, Lee H, Kwon Y, et al. Direct conversion of adult skin fibroblasts to endothelial cells by defined factors. Circulation 2014. 130 1168–1178. ( 10.1161/CIRCULATIONAHA.113.007727) [DOI] [PubMed] [Google Scholar]
- 24.Ginsberg M, James D, Ding B, Nolan D, Geng F, Butler J, Schachterle W, Pulijaal V, Mathew S, Chasen S, et al. Efficient direct reprogramming of mature amniotic cells into endothelial cells by ETS factors and TGFβ suppression. Cell 2012. 151 559–575. ( 10.1016/j.cell.2012.09.032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shamis Y, Silva EA, Hewitt KJ, Brudno Y, Levenberg S, Mooney DJ, Garlick JA. Fibroblasts derived from human pluripotent stem cells activate angiogenic responses in vitro and in vivo. PLoS ONE 2013. 8 e83755 ( 10.1371/journal.pone.0083755) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carrion B, Kong YP, Kaigler D, Putnam AJ. Bone marrow-derived mesenchymal stem cells enhance angiogenesis via their α6β1 integrin receptor. Experimental Cell Research 2013. 319 2964–2976. ( 10.1016/j.yexcr.2013.09.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manikowski D, Andrée B, Samper E, Saint-Marc C, Olmer R, Vogt P, Strauß S, Haverich A, Hilfiker A. Human adipose tissue-derived stromal cells in combination with exogenous stimuli facilitate three-dimensional network formation of human endothelial cells derived from various sources. Vascular Pharmacology 2018. 106 28–36. ( 10.1016/j.vph.2018.02.003) [DOI] [PubMed] [Google Scholar]
- 28.Pill K, Hofmann S, Redl H, Holnthoner W. Vascularization mediated by mesenchymal stem cells from bone marrow and adipose tissue: a comparison. Cell Regeneration 2015. 4 4–8. ( 10.1186/s13619-015-0025-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Juliar BA, Keating MT, Kong YP, Botvinick EL, Putnam AJ. Sprouting angiogenesis induces significant mechanical heterogeneities and ECM stiffening across length scales in fibrin hydrogels. Biomaterials 2018. 162 99–108. ( 10.1016/j.biomaterials.2018.02.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature 2005. 438 946–953. ( 10.1038/nature04480) [DOI] [PubMed] [Google Scholar]
- 31.Swartz MA, Skobe M. Lymphatic function, lymphangiogenesis, and cancer metastasis. Microscopy Research and Technique 2001. 55 92–99. ( 10.1002/jemt.1160) [DOI] [PubMed] [Google Scholar]
- 32.Marino D, Luginbuhl J, Scola S, Meuli M, Reichmann E. Bioengineering dermo-epidermal skin grafts with blood and lymphatic capillaries. Science Translational Medicine 2014. 6 221ra14 ( 10.1126/scitranslmed.3006894) [DOI] [PubMed] [Google Scholar]
- 33.Knezevic L, Schaupper M, Mühleder S, Schimek K, Hasenberg T, Marx U, Priglinger E, Redl H, Holnthoner W. Engineering blood and lymphatic microvascular networks in fibrin matrices. Frontiers in Bioengineering and Biotechnology 2017. 5 25 ( 10.3389/fbioe.2017.00025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robering JW, Weigand A, Pfuhlmann R, Horch RE, Beier JP, Boos AM. Mesenchymal stem cells promote lymphangiogenic properties of lymphatic endothelial cells. Journal of Cellular and Molecular Medicine 2018. 22 3740–3750. ( 10.1111/jcmm.13590) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shafiee A, Patel J, Wong HY, Donovan P, Hutmacher DW, Fisk NM, Khosrotehrani K. Priming of endothelial colony-forming cells in a mesenchymal niche improves engraftment and vasculogenic potential by initiating mesenchymal transition orchestrated by NOTCH signaling. FASEB Journal 2017. 31 610–624. ( 10.1096/fj.201600937) [DOI] [PubMed] [Google Scholar]
- 36.Moya ML, Hsu Y, Lee AP, Hughes CCW, George SC. In vitro perfused human capillary networks. Tissue Engineering Part C 2013. 19 730–737. ( 10.1089/ten.tec.2012.0430) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marshall J, Barnes A, Genever P. Analysis of the intrinsic self-organising properties of mesenchymal stromal cells in three-dimensional co-culture models with endothelial cells. Bioengineering 2018. 5 E92 ( 10.3390/bioengineering5040092) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kreimendahl F, Köpf M, Thiebes AL, Duarte Campos DF, Blaeser A, Schmitz-Rode T, Apel C, Jockenhoevel S, Fischer H. Three-dimensional printing and angiogenesis: tailored agarose-type I collagen blends comprise three-dimensional printability and angiogenesis potential for tissue-engineered substitutes. Tissue Engineering Part C 2017. 23 604–615. ( 10.1089/ten.tec.2017.0234) [DOI] [PubMed] [Google Scholar]
- 39.Fuchs S, Hofmann A, Kirkpatrick CJ. Microvessel-like structures from outgrowth endothelial cells from human peripheral blood in 2-dimensional and 3-dimensional co-cultures with osteoblastic lineage cells. Tissue Engineering 2007. 13 2577–2588. ( 10.1089/ten.2007.0022) [DOI] [PubMed] [Google Scholar]
- 40.Bischel LL, Young EWK, Mader BR, Beebe DJ. Tubeless microfluidic angiogenesis assay with three-dimensional endothelial-lined microvessels. Biomaterials 2013. 34 1471–1477. ( 10.1016/j.biomaterials.2012.11.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zheng Y, Chen J, Craven M, Choi NW, Totorica S, Diaz-Santana A, Kermani P, Hempstead B, Fischbach-Teschl C, Lopez JA, et al. In vitro microvessels for the study of angiogenesis and thrombosis. PNAS 2012. 109 9342–9347. ( 10.1073/pnas.1201240109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van der Meer AD, Orlova VV, ten Dijke P, van den Berg A, Mummery CL. Three-dimensional co-cultures of human endothelial cells and embryonic stem cell-derived pericytes inside a microfluidic device. Lab on A Chip 2013. 13 3562–3568. ( 10.1039/c3lc50435b) [DOI] [PubMed] [Google Scholar]
- 43.Kurokawa YK, Yin RT, Shang MR, Shirure VS, Moya ML, George SC. Human induced pluripotent stem cell-derived endothelial cells for three-dimensional microphysiological systems. Tissue Engineering Part C 2017. 23 474–484. ( 10.1089/ten.tec.2017.0133) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gibot L, Galbraith T, Kloos B, Das S, Lacroix DA, Auger FA, Skobe M. Cell-based approach for 3D reconstruction of lymphatic capillaries in vitro reveals distinct functions of HGF and VEGF-C in lymphangiogenesis. Biomaterials 2016. 78 129–139. ( 10.1016/j.biomaterials.2015.11.027) [DOI] [PubMed] [Google Scholar]
- 45.Landau S, Guo S, Levenberg S. Localization of engineered vasculature within 3D tissue constructs. Frontiers in Bioengineering and Biotechnology 2018. 6 2 ( 10.3389/fbioe.2018.00002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ponec M, Ghalbzouri AE, Dijkman R, Kempenaar J, Pluijm Gvd, Koolwijk P. Endothelial network formed with human dermal microvascular endothelial cells in autologous multicellular skin substitutes. Angiogenesis 2004. 7 295–305. ( 10.1007/s10456-004-6315-3) [DOI] [PubMed] [Google Scholar]
- 47.Li M, Fuchs S, Böse T, Schmidt H, Hofmann A, Tonak M, Unger R, Kirkpatrick CJ. Mild heat stress enhances angiogenesis in a co-culture system consisting of primary human osteoblasts and outgrowth endothelial cells. Tissue Engineering Part C 2014. 20 328–339. ( 10.1089/ten.tec.2013.0087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rohringer S, Hofbauer P, Schneider KH, Husa A, Feichtinger G, Peterbauer-Scherb A, Redl H, Holnthoner W. Mechanisms of vasculogenesis in 3D fibrin matrices mediated by the interaction of adipose-derived stem cells and endothelial cells. Angiogenesis 2014. 17 921–933. ( 10.1007/s10456-014-9439-0) [DOI] [PubMed] [Google Scholar]
- 49.Sun W, Motta A, Shi Y, Seekamp A, Schmidt H, Gorb SN, Migliaresi C, Fuchs S. Co-culture of outgrowth endothelial cells with human mesenchymal stem cells in silk fibroin hydrogels promotes angiogenesis. Biomedical Materials 2016. 11 035009 ( 10.1088/1748-6041/11/3/035009) [DOI] [PubMed] [Google Scholar]
- 50.Bachmann B, Spitz S, Rothbauer M, Jordan C, Purtscher M, Zirath H, Schuller P, Eilenberger C, Ali SF, Mühleder S, et al. Engineering of three-dimensional pre-vascular networks within fibrin hydrogel constructs by microfluidic control over reciprocal cell signaling. Biomicrofluidics 2018. 12 042216 ( 10.1063/1.5027054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ingber DE. Developmentally inspired human ‘organs on chips’. Development 2018. 145 18 ( 10.1242/dev.156125) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miri AK, Khalilpour A, Cecen B, Maharjan S, Shin SR, Khademhosseini A. Multiscale bioprinting of vascularized models. Biomaterials 2019. 198 204–216. ( 10.1016/j.biomaterials.2018.08.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Materne EM, Ramme AP, Terrasso AP, Serra M, Alves PM, Brito C, Sakharov DA, Tonevitsky AG, Lauster R, Marx U. A multi-organ chip co-culture of neurospheres and liver equivalents for long-term substance testing. Journal of Biotechnology 2015. 205 36–46. ( 10.1016/j.jbiotec.2015.02.002) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a