Abstract
Backgound: This study aimed to determine the usefulness of the Controlling Nutritional Status (CONUT) scorescore for predicting postoperative pancreatic fistula (POPF). Patients and Methods: Data from 108 consecutive pancreaticoduodenectomy cases performed at the Surgery Department of Iwakuni Clinical Center, from April 2008 to May 2018, were included. Preoperative patient data and postoperative complication data were collected. Results: Of the 108 patients (male=65; female=43; mean age=70 years), 41 (37.9%) had indication for pancreaticoduodenectomy due to pancreatic carcinoma. Grade B or higher POPF was diagnosed in 32 patients (29.6%). In the multivariate analysis, body mass index ≥22 kg/m2 [odds ratio (OR)=5.24; p=0.005], CONUT score ≥4 (OR=3.28; p=0.042), non-pancreatic carcinoma (OR=47.17; p=0.001), and a low computed tomographic contrast attenuation value (late/early ratio) (OR=4.39; p=0.029) were independent risk factors for POPF. Conclusion: Patients with high CONUT score are at high risk for POPF. Preoperative nutritional intervention such as immunonutrition might help reduce the POPF risk in these patients.
Keywords: CONUT, pancreatic fistula, pancreaticoduodenectomy
Currently, pancreaticoduodenectomy (PD) is the primary treatment for malignant tumours involving the pancreatic head, lower bile duct, and duodenal ampulla (1,2). This procedure is technically difficult, highly invasive and is associated with high morbidity and mortality rates (3-5). The perioperative mortality rate is still up to 5% (6-8). The most important factor affecting morbidity and mortality after PD is the development of postoperative pancreatic fistula (POPF). According to recent studies, the incidence of POPF remains high, accounting for 11.4-64.3% of all PD cases (9-15). POPF is associated with delayed gastric emptying, intra-abdominal abscesses, surgical site infections, sepsis, and bleeding after PD (16-18). Several approaches may reduce the incidence of POPF. However, a definitive approach that prevents POPF is still not available.
The Controlling Nutritional Status (CONUT) score is an automatic tool used to assess nutritional status. It takes into account laboratory examination data including serum albumin level (indicating protein reserve), total cholesterol level (indicating calorie depletion), and total lymphocyte count (indicating loss of immune defence caused by immune malnutrition) (19). The CONUT score has been used to evaluate nutritional status objectively in patients with inflammatory diseases, chronic heart failure, and chronic liver diseases (20-22). Recently, the CONUT score was demonstrated to be a predictive or prognostic marker for patients with malignancies, including colorectal, oesophageal cancer, and hepatocellular carcinoma (23-26). However, to the best of our knowledge, the usefulness of the CONUT score to assess the risk of POPF after PD has not yet been determined. Therefore, this retrospective study aimed to assess whether the preoperative CONUT score might be a useful predictor of POPF.
Patients and Methods
Patient and data collection. We reviewed the data from 108 consecutive patients who underwent PD at the Department of Surgery of the Iwakuni Clinical Center from April 2008 to September 2018. Preoperative patient data collected were sex, age, hypertension, diabetes, alcohol consumption, smoking, body mass index (BMI), CONUT score, surgery indications, main pancreatic duct (MPD) diameter determined using preoperative computed tomography (CT) or magnetic resonance cholangiopancreatography, CT attenuation values [late/early (L/E) ratio] in the pancreatic body, blood transfusion, blood loss, operative time, and use of pancreaticojejunostomy technique. Neoadjuvant chemotherapy for patients with borderline resectable pancreatic cancer was performed from March 2015 to September 2018.
The Ethics Committee at the Iwakuni Clinical Center approved the study protocol (approval number: 0191). This study was performed in accordance with the protocols of the 1975 Declaration of Helsinki.
Preoperative calculation of the CONUT score and cutoff value. The CONUT score was calculated using the serum albumin level, total lymphocyte count, and total cholesterol level for each patient (19). Albumin concentrations ≥3.5, 3.0-3.49, 2.5-2.99, and <2.5 g/dl were scored as 0, 2, 4, and 6 points, respectively. Total lymphocyte counts ≥1,600, 1,200-1,599, 800-1,199, and <800/mm3 were scored as 0, 1, 2, and 3 points, respectively. Total cholesterol concentrations ≥180, 140-179, 100-139, and <100 mg/dl were scored as 0, 1, 2, and 3 points, respectively. The CONUT score was defined as the sum of these three sub-scores. Receiver operating characteristics (ROC) curves for these scores were analyzed for prediction of POPF by comparing the areas under the curves (AUCs). The cutoff value was considered optimal when the highest Youden index (sensitivity+specificity-1) was noted (26). Analysis showed that the optimal cutoff value for the CONUT score in POPF was 3 (sensitivity=50.00%; specificity=67.11%; AUC of ROC curve=0.604). Based on the cutoff value, the patients were categorized into two groups: score ≤3, low CONUT score; and score ≥4, high CONUT score (23,24).
In addition, data regarding postoperative complications were collected for analysis considering the incidence of POPF. No patients were excluded.
Assessment of pancreatic firmness. Reportedly, soft pancreas is a risk factor for POPF (27-32). However, pancreatic firmness solely assessed by the surgeon during surgery may not be accurate. The distinction between the end of the soft area and the start of the firm area is obscure. Pancreatic fibrosis reduces the softness of the gland. Hashimoto et al. reported that the ratio of the mean pancreatic CT contrast attenuation value (hepatic to pancreatic phase; L/E ratio) upstream from the tumour can help in the assessment of the histologic degree of pancreatic fibrosis (33). Therefore, we used the L/E ratio at the pancreatic body to assess pancreatic firmness.
Operative procedure. All patients underwent subtotal, stomach-preserving PD via an open approach, and the degree of locoregional lymphadenectomy was determined according to the preoperative diagnosis. Surgical reconstruction was performed using a modification of Child’s method. The proximal jejunal stump was passed through the retrocolic pathway, and pancreaticojejunostomy, biliojejunostomy, and gastrojejunostomy were subsequently performed. Pancreaticojejunostomy was performed using the modified Kakita anastomosis (n=47; April 2008-May 2013) or the modified Blumgart anastomosis method (n=50; June 2015-present) (34). During the procedure, plastic stents were inserted into the MPD for internal drainage at the surgeon’s discretion. Two or three abdominal drains were placed either anteriorly or posteriorly to the pancreaticojejunostomy and hepaticojejunostomy anastomoses.
Classification and detailed definition of POPF. POPF was diagnosed and graded based on the International Study Group on Pancreatic Fistula classification (35). POPF was diagnosed when the amylase concentration in the drainage fluid on postoperative day 3 was more than three times the upper limit of its normal serum concentration. Grade A POPF is called a biochemical fistula and is defined as the measurable fluid output on or after postoperative day 3, with amylase content higher than three times the upper normal serum level. Grade A POPF has no clinical impact on the normal postoperative pathway. Clinically significant POPFs are classified as grades B and C. POPF with an elevated inflammatory response observed in blood examination and following the intravenous antibiotic administration was defined as grade B POPF caused by infection. POPF that required drain placement for >22 days and showed no elevated inflammatory response or did not require antibiotic administration was defined as grade B POPF caused by long-term drain placement. Whenever a major change in clinical management or deviation from the normal clinical pathway was required or organ failure occurs, the fistula was classified as grade C POPF. Latent POPF (36) was defined as POPF that initially lacked amylase-rich effluent but ultimately progressed to clinically relevant POPF.
Definition of operative mortality. Operative mortality was defined as any death, regardless of cause, occurring within 30 days after surgery in or out of hospital, and after 30 days during the same hospitalisation subsequent to the operation.
Statistical analysis. Statistical analyses were performed using the unpaired Student’s t-test and the chi-squared test with Fisher’s exact test. All variables were assessed using univariate analyses, and only those showing statistical significance (p<0.05) were evaluated using multivariate logistic analyses to determine the primary independent risk factors of POPF. Values of p<0.05 were considered statistically significant. Statistical analysis was undertaken using JMP version 9 software (SAS Institute, Cary, NC, USA).
Results
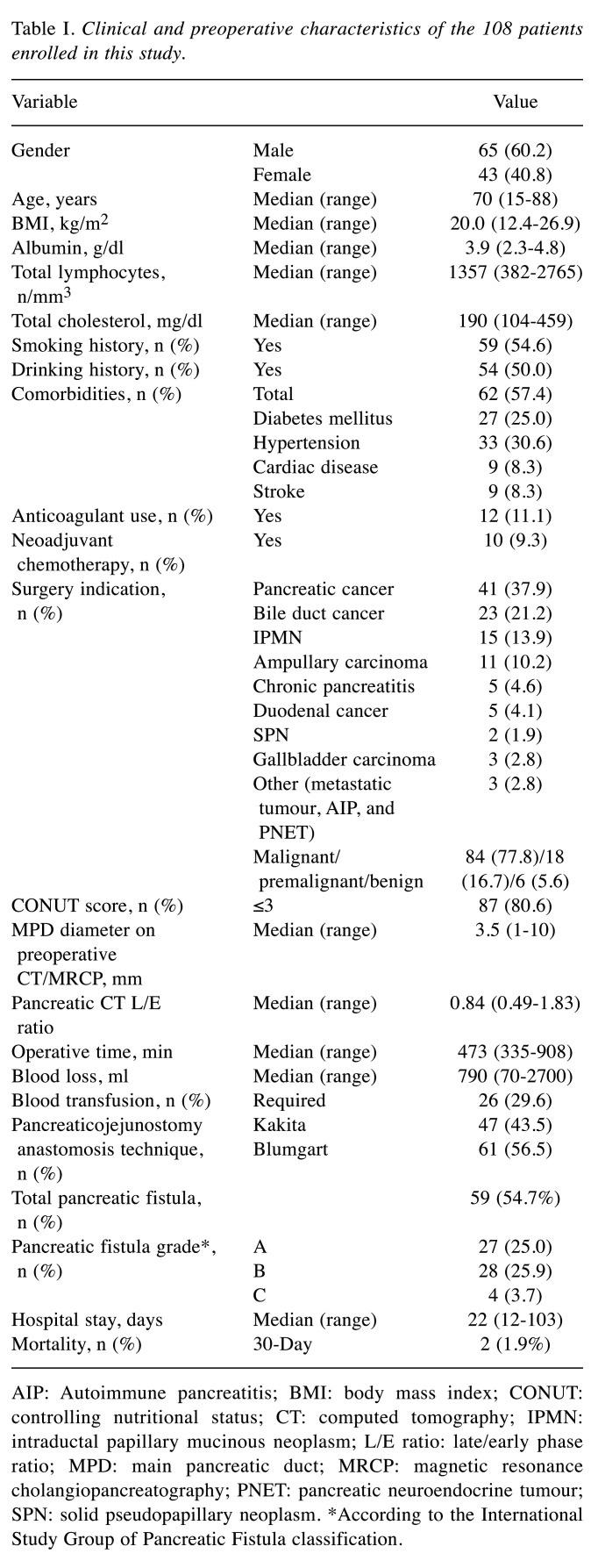
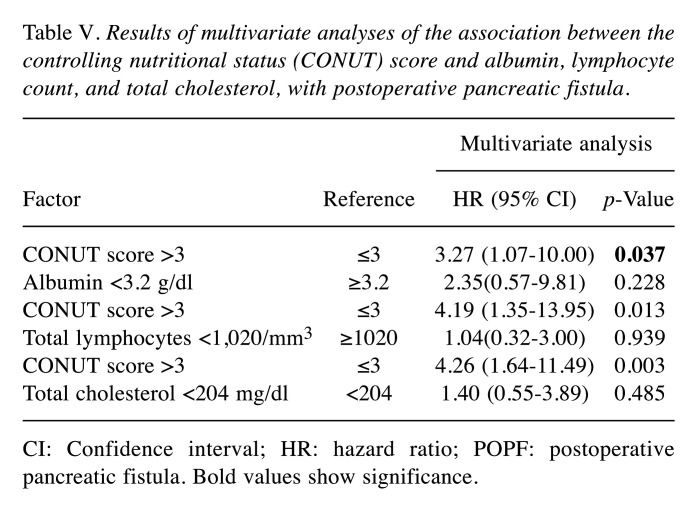
Clinical and preoperative characteristics of the 108 patients [65 males, 45 females; median age=70 years; interquartile range (IQR)=15-88 years] are summarized in Table I. The Median CONUT score was 2 (IQR=1-3; Figure 1). The low- and high-CONUT score groups included 87 (80.6%) and 21 (19.4%) patients, respectively. The indication for PD included pancreatic carcinoma in 41 (37.9%) patients. Malignant diseases, including pancreatic carcinoma, bile duct carcinoma, ampullary carcinoma, duodenal carcinoma, gallbladder carcinoma, and metastatic tumour occurred in 84 (77.8%) cases. For 10 patients with borderline resectable pancreatic cancer, neoadjuvant chemotherapy was performed. Mean postoperative hospital stay was 22.5 (12-103) days. A total of (54.7%) patients were diagnosed with POPF, with 27 (25.0%) classified as grade A, 28 (25.9%) classified as grade B, and 4 (3.7%) classified as grade C.
Table I. Clinical and preoperative characteristics of the 108 patients enrolled in this study.
AIP: Autoimmune pancreatitis; BMI: body mass index; CONUT: controlling nutritional status; CT: computed tomography; IPMN: intraductal papillary mucinous neoplasm; L/E ratio: late/early phase ratio; MPD: main pancreatic duct; MRCP: magnetic resonance cholangiopancreatography; PNET: pancreatic neuroendocrine tumour; SPN: solid pseudopapillary neoplasm. *According to the International Study Group of Pancreatic Fistula classification
Figure 1. Distribution of controlling nutritional status (CONUT) scores.
The operative mortality rate in the study population was 1.9% (2/108). One patient died of aspiration pneumonia and sepsis, and another died of abdominal bleeding, both associated with POPF.
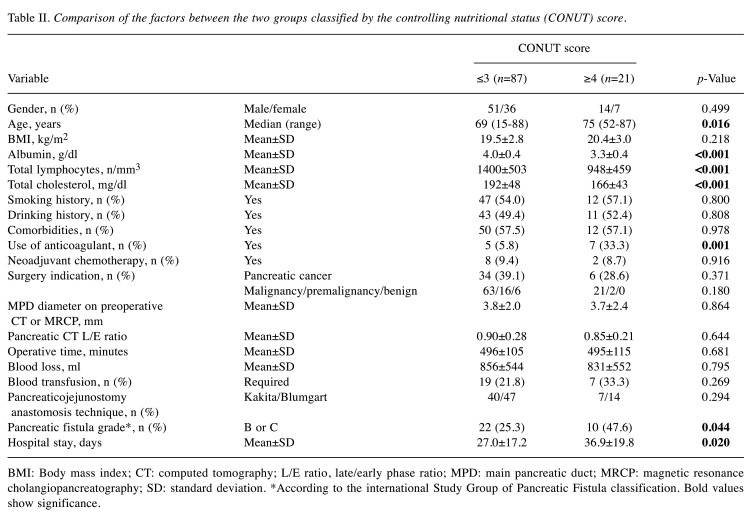
The demographic and clinical variables of the two groups classified based on the CONUT score are shown in Table II. The high-CONUT score group comprised patients who were significantly older, and had lower serum albumin level, lower lymphocyte count, lower serum cholesterol level, and a higher incidence of grade B or higher POPF than those in the low-CONUT score group. The proportion of anticoagulant use was also significantly higher in the high-CONUT score group than that in the low-CONUT score group. The duration of hospital stay of the high-CONUT score group was significantly longer than of the low-CONUT score group. There was no significant difference in other factors between these two groups.
Table II. Comparison of the factors between the two groups classified by the controlling nutritional status (CONUT) score.
BMI: Body mass index; CT: computed tomography; L/E ratio, late/early phase ratio; MPD: main pancreatic duct; MRCP: magnetic resonance cholangiopancreatography; SD: standard deviation. *According to the international Study Group of Pancreatic Fistula classification. Bold values show significance
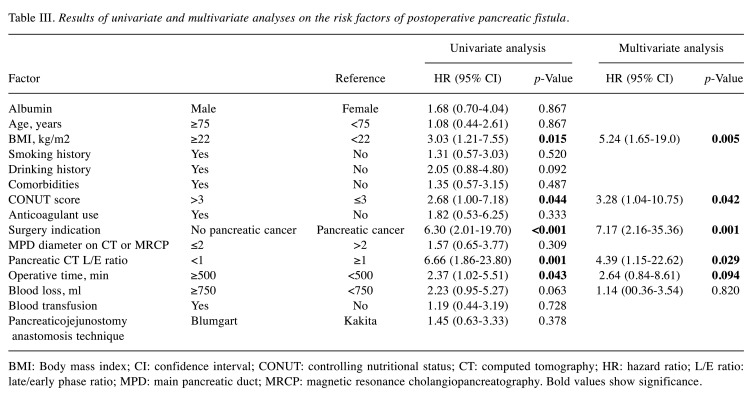
When the demographic and clinical variables were assessed using univariate analysis to determine their relationship with POPF, no statistical significance was observed for age, sex, hypertension, diabetes mellitus, smoking history, and anticoagulant use (Table III). POPF was significantly associated with BMI ≥22 kg/m2 (p=0.015), CONUT score ≥4 (p=0.044), non-pancreatic carcinoma (p<0.001), low L/E ratio in the pancreatic body (p=0.001), and operative time ≥500 min (p=0.043).
Table III. Results of univariate and multivariate analyses on the risk factors of postoperative pancreatic fistula.
BMI: Body mass index; CI: confidence interval; CONUT: controlling nutritional status; CT: computed tomography; HR: hazard ratio; L/E ratio: late/early phase ratio; MPD: main pancreatic duct; MRCP: magnetic resonance cholangiopancreatography. Bold values show significance
The significant preoperative risk factors of POPF identified using the univariate analysis were incorporated into logistic regression analysis. The results showed that BMI ≥22 kg/m2 [odds ratio (OR)=5.24; p=0.005], CONUT score ≥4 (OR=3.28; p=0.042), non-pancreatic carcinoma (OR=7.17; p=0.001), and low L/E ratio in the pancreatic body (OR=4.39; p=0.029) were independent risk factors for POPF (Table III).
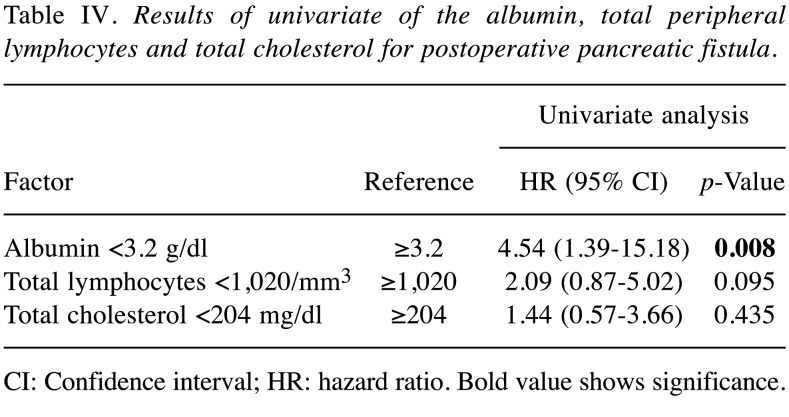
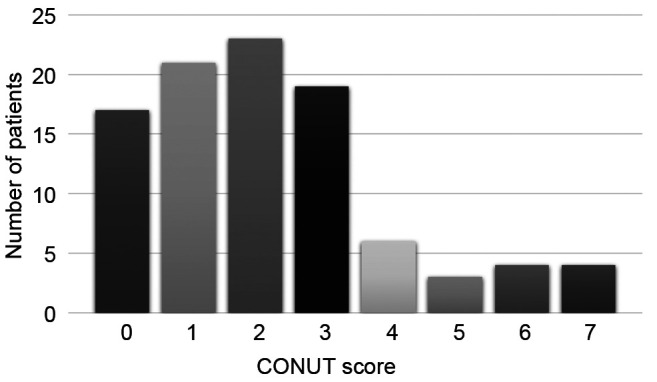
The cutoff values for each of the factors were determined by their respective ROC curves predicting POPF. The cutoff value for the albumin level was 3.2 g/dl, that of the total lymphocyte count was 1,020/mm3 and that of the total cholesterol level was 204 mg/dl. In the univariate analysis for POPF, the albumin level was found to be a predictive factor (Table IV). The multivariate analysis showed the CONUT score to be superior to serum albumin, total lymphocyte count and total cholesterol for predicting POPF. This study suggested that the CONUT score is more useful for predicting POPF than the individual factors that comprise the CONUT score (Table V).
Table IV. Results of univariate of the albumin, total peripheral lymphocytes and total cholesterol for postoperative pancreatic fistula.
CI: Confidence interval; HR: hazard ratio. Bold value shows significance
Table V. Results of multivariate analyses of the association between the controlling nutritional status (CONUT) score and albumin, lymphocyte count, and total cholesterol, with postoperative pancreatic fistula.
CI: Confidence interval; HR: hazard ratio; POPF: postoperative pancreatic fistula. Bold values show significance.
Discussion
The association between preoperative nutritional status and the outcomes of surgical interventions has been considerably researched (37,38). Based on the results of such studies, a poor preoperative nutritional status was considered to correlate with the incidence of postoperative complications. A meta-analyses and systematic reviews suggest that nutritional intervention reduces a number of postoperative complications (39,40).
In this study, the results of multivariate logistic regression analysis showed that CONUT score ≥4, BMI ≥22 kg/m2, non-pancreatic carcinoma, and low L/E ratio in the pancreatic body were the independent risk factors for POPF. Thus, we found a correlation between the CONUT score and POPF. Patients with a high CONUT score had a significantly higher incidence of POPF than those with a low CONUT score. To the best of our knowledge, this is the first report to investigate the relationship between the CONUT score and incidence of POPF. The CONUT score is easily determined using preoperative, blood examinations and is a useful tool to predict the incidence of POPF.
With regard to CONUT score parameters, the serum albumin level isa representative nutrition marker and used frequently to assess nutrition status for prediction of POPF (41). Hypoalbuminemia is often linked to poor tissue healing, reduced collagen synthesis at anastomoses, and impairment of cell-mediated immune response, such as macrophage activation and granuloma formation (42). Therefore, surgical site infection is commonly observed in hypoalbuminemic patients.
The total lymphocyte count is also an important marker of nutrition and immunity. Menges et al. revealed that lymphopenia caused by the systemic inflammatory response is characterized by significant depression of innate cellular immunity (43). A meta-analysis demonstrated that intervention with immune-enhancing nutrition increased the total lymphocyte count and reduced postoperative complications (44).
Studies suggested that a low serum cholesterol level correlated with morbidity and mortality after gastroenterological surgery (45,46) but the reason for this remains unclear. A decrease in cholesterol level implies not only a calorie deficiency but also that cells are being deprived of an essential nutrient required to maintain metabolic and hormonal equilibrium and membrane integrity (47). Tissue fragility may explain why the cholesterol level is associated with POPF.
POPF (grades B and C) is the most common and challenging complication of PD and has the potential to trigger life-threatening, delayed, massive intra-abdominal haemorrhage and septicaemia. The early prediction of this complication may thus improve the postoperative monitoring of patients who are at high risk for POPF. The predictors of POPF have been extensively studied. Factors that have been reported to be related to POPF are male, old age, preoperative jaundice, intraoperative blood loss, low albumin level, high American Society of Anesthesiologists score, long operative time, soft pancreas, high BMI, small MPD diameter, and pancreaticojejunal anastomosis (14,18,48-50).
It may be that modifying a patient’s CONUT score will reduce the incidence of POPF. To maintain or improve preoperative nutrition, several approaches are being investigated. However, studies with a large cohort aiming to establish the risk factors of postoperative morbidity did not include theserum cholesterol level as a variable (3,51). The possible usefulness of cholesterol level as a predictive marker should be confirmed by future study.
Soft pancreas and increased BMI have already been widely accepted as a patient-related risk factor that predisposes to POPF (27-32). We assessed pancreatic firmness using the L/E ratio, which has been proposed to be associated with the texture of the pancreatic parenchyma. The L/E ratio positively correlated with pancreatic firmness, which reflected the histological degree of pancreatic fibrosis. Our data showed that the L/E ratio was significantly lower in patients with POPF. As the result, a soft pancreas was associated with POPF. The high incidence of POPF in patients with high BMI or a soft pancreas may lead to increased difficulty in exposing the pancreas during surgery owing to a higher volume of abdominal and peripancreatic fat, to a higher risk of damage to the pancreatic capsule during separation due to a soft and brittle pancreas, and to a higher risk of pancreatic leakage caused by damage to pancreatic tissue and the fine pancreatic ducts because of suturing and knotting during pancreaticojejunal anastomosis (52).
Non-pancreatic cancer, such as ampullary carcinoma, bile duct carcinoma, or intraductal papillary mucinous neoplasms, was found to be a risk factor of POPF because these diseases clearly reflect the characteristics of the pancreatic remnant, such as the soft texture of the pancreas, a thin pancreatic body, and a nonfibrotic pancreatic parenchyma, which greatly increase the risk of POPF (53).
This study had some limitations. Firstly, this was a retrospective, single-centre study; therefore, there may be potential selection bias in the enrollment of patients for PD. Secondly, the sample size was small. Compared to patients with benign disease, those with malignant disease have a very different presentation and time course. Thus, the mixture of diagnoses may be a confounding factor and potentially add bias to the study. Thirdly, although the CONUT score conventionally describes the four classes of undernutrition, we used other cutoff values reported in a previous study (23,24). In this study, the cutoff value of the CONUT score associated with PF was determined using ROCs and was also 3 (AUC=0.61). Further studies are warranted to determine more adequate cutoff values of the CONUT score to predict the incidence of POPF. Finally, this study did not compare the efficiency of the CONUT score with that of other screening systems. Further studies are required to assess the efficacy of screening systems to evaluate patient status. CONUT score ≥4, BMI ≥22 kg/m2, non-pancreatic carcinoma, and low L/E ratio in the pancreatic body were the independent risk factors of PF after PD. The CONUT score is an effective tool for assessing the preoperative nutritional status and predicting the incidence of PF after PD.
Consent for Publication
Patients were not required to provide informed consent for the study because the analysis used anonymous data obtained after the patient agreed to treatment by written consent.
Conflicts of Interest
The Authors declare that they have no competing interests in regard to this study.
Authors’ Contributions
MU, HA, SN, SN, YU, HK, YK, FT, TA, KK and KT designed the study. HA, MU and YK treated and observed the patients. MU prepared the article and performed the literature search. HA corrected and revised the article. All Authors read and approved the final article.
Acknowledgements
The Authors would like to thank Enago (www.enago.jp) for English language review.
References
- 1.Brown EG, Yang A, Canter RJ, Bold RJ. Outcomes of pancreaticoduodenectomy: Where should we focus our efforts on improving outcomes. JAMA Surg. 2014;149(7):694–699. doi: 10.1001/jamasurg.2014.151. [DOI] [PubMed] [Google Scholar]
- 2.Yamashita Y, Shirabe K, Tsujita E, Takeishi K, Ikeda T, Yoshizumi T, Furukawa Y, Ishida T, Maehara Y. Surgical outcomes of pancreaticoduodenectomy for periampullary tumors in elderly patients. Langenbecks Arch Surg. 2013;398(4):539–545. doi: 10.1007/s00423-013-1061-x. [DOI] [PubMed] [Google Scholar]
- 3.Kimura W, Miyata H, Gotoh M, Hirai I, Kenjo A, Kitagawa Y, Shimada M, Baba H, Tomita N, Nakagoe T, Sugihara K, Mori M. A pancreaticoduodenectomy risk model derived from 8575 cases from a national single-race population (japanese) using a web-based data entry system: The 30-day and in-hospital mortality rates for pancreaticoduodenectomy. Ann Surg. 2014;259(4):773–780. doi: 10.1097/SLA.0000000000000263. [DOI] [PubMed] [Google Scholar]
- 4.Fong ZV, Ferrone CR, Thayer SP, Wargo JA, Sahora K, Seefeld KJ, Warshaw AL, Lillemoe KD, Hutter MM, Fernandez-Del Castillo C. Understanding hospital readmissions after pancreaticoduodenectomy: Can we prevent them?: A 10-year contemporary experience with 1,173 patients at the massachusetts general hospital. J Gastrointest Surg. 2014;18(1):137–144. doi: 10.1007/s11605-013-2336-9. discussion 144-135. [DOI] [PubMed] [Google Scholar]
- 5.Glazer ES, Amini A, Jie T, Gruessner RW, Krouse RS, Ong ES. Recognition of complications after pancreaticoduodenectomy for cancer determines inpatient mortality. JOP. 2013;14(6):626–631. doi: 10.6092/1590-8577/1883. [DOI] [PubMed] [Google Scholar]
- 6.Topal B, Aerts R, Hendrickx T, Fieuws S, Penninckx F. Determinants of complications in pancreaticoduodenectomy. Eur J Surg Oncol. 2007;33(4):488–492. doi: 10.1016/j.ejso.2006.10.041. [DOI] [PubMed] [Google Scholar]
- 7.Wang Q, Gurusamy KS, Lin H, Xie X, Wang C. Preoperative biliary drainage for obstructive jaundice. Cochrane Database Syst Rev. 2008;3:CD005444. doi: 10.1002/14651858.CD005444.pub2. [DOI] [PubMed] [Google Scholar]
- 8.Winter JM, Cameron JL, Yeo CJ, Alao B, Lillemoe KD, Campbell KA, Schulick RD. Biochemical markers predict morbidity and mortality after pancreaticoduodenectomy. J Am Coll Surg. 2007;204(5):1029–1036. doi: 10.1016/j.jamcollsurg.2007.01.026. discussion 1037-1028. [DOI] [PubMed] [Google Scholar]
- 9.Hiyoshi M, Chijiiwa K, Fujii Y, Imamura N, Nagano M, Ohuchida J. Usefulness of drain amylase, serum c-reactive protein levels and body temperature to predict postoperative pancreatic fistula after pancreaticoduodenectomy. World J Surg. 2013;37(10):2436–2442. doi: 10.1007/s00268-013-2149-8. [DOI] [PubMed] [Google Scholar]
- 10.Ansorge C, Nordin JZ, Lundell L, Strommer L, Rangelova E, Blomberg J, Del Chiaro M, Segersvard R. Diagnostic value of abdominal drainage in individual risk assessment of pancreatic fistula following pancreaticoduodenectomy. Br J Surg. 2014;101(2):100–108. doi: 10.1002/bjs.9362. [DOI] [PubMed] [Google Scholar]
- 11.Andrianello S, Pea A, Pulvirenti A, Allegrini V, Marchegiani G, Malleo G, Butturini G, Salvia R, Bassi C. Pancreaticojejunostomy after pancreaticoduodenectomy: Suture material and incidence of post-operative pancreatic fistula. Pancreatology. 2016;16(1):138–141. doi: 10.1016/j.pan.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Sugimoto M, Takahashi S, Gotohda N, Kato Y, Kinoshita T, Shibasaki H, Konishi M. Schematic pancreatic configuration: A risk assessment for postoperative pancreatic fistula after pancreaticoduodenectomy. J Gastrointest Surg. 2013;17(10):1744–1751. doi: 10.1007/s11605-013-2320-4. [DOI] [PubMed] [Google Scholar]
- 13.Addeo P, Delpero JR, Paye F, Oussoultzoglou E, Fuchshuber PR, Sauvanet A, Sa Cunha A, Le Treut YP, Adham M, Mabrut JY, Chiche L, Bachellier P, French Surgical A. Pancreatic fistula after a pancreaticoduodenectomy for ductal adenocarcinoma and its association with morbidity: A multicentre study of the french surgical association. HPB (Oxford) 2014;16(1):46–55. doi: 10.1111/hpb.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu QY, Zhang WZ, Xia HT, Leng JJ, Wan T, Liang B, Yang T, Dong JH. Analysis of risk factors for postoperative pancreatic fistula following pancreaticoduodenectomy. World J Gastroenterol. 2014;20(46):17491–17497. doi: 10.3748/wjg.v20.i46.17491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberts KJ, Sutcliffe RP, Marudanayagam R, Hodson J, Isaac J, Muiesan P, Navarro A, Patel K, Jah A, Napetti S, Adair A, Lazaridis S, Prachalias A, Shingler G, Al-Sarireh B, Storey R, Smith AM, Shah N, Fusai G, Ahmed J, Abu Hilal M, Mirza DF. Scoring system to predict pancreatic fistula after pancreaticoduodenectomy: A uk multicenter study. Ann Surg. 2015;261(6):1191–1197. doi: 10.1097/SLA.0000000000000997. [DOI] [PubMed] [Google Scholar]
- 16.Reid-Lombardo KM, Farnell MB, Crippa S, Barnett M, Maupin G, Bassi C, Traverso LW, Pancreatic Anastomotic Leak Study G. Pancreatic anastomotic leakage after pancreaticoduodenectomy in 1,507 patients: A report from the pancreatic anastomotic leak study group. J Gastrointest Surg. 2007;11(11):1451–1458. doi: 10.1007/s11605-007-0270-4. discussion 1459. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt CM, Choi J, Powell ES, Yiannoutsos CT, Zyromski NJ, Nakeeb A, Pitt HA, Wiebke EA, Madura JA, Lillemoe KD. Pancreatic fistula following pancreaticoduodenectomy: Clinical predictors and patient outcomes. HPB Surg. 2009;2009:404520. doi: 10.1155/2009/404520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lermite E, Pessaux P, Brehant O, Teyssedou C, Pelletier I, Etienne S, Arnaud JP. Risk factors of pancreatic fistula and delayed gastric emptying after pancreaticoduodenectomy with pancreaticogastrostomy. J Am Coll Surg. 2007;204(4):588–596. doi: 10.1016/j.jamcollsurg.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 19.Ignacio de Ulibarri J, Gonzalez-Madrono A, de Villar NG, Gonzalez P, Gonzalez B, Mancha A, Rodriguez F, Fernandez G. Conut: A tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp. 2005;20(1):38–45. [PubMed] [Google Scholar]
- 20.Ueno T, Hirayama S, Ito M, Nishioka E, Fukushima Y, Satoh T, Idei M, Horiuchi Y, Shoji H, Ohmura H, Shimizu T, Miida T. Albumin concentration determined by the modified bromocresol purple method is superior to that by the bromocresol green method for assessing nutritional status in malnourished patients with inflammation. Ann Clin Biochem. 2013;50(Pt 6):576–584. doi: 10.1177/0004563213480137. [DOI] [PubMed] [Google Scholar]
- 21.Nakagomi A, Kohashi K, Morisawa T, Kosugi M, Endoh I, Kusama Y, Atarashi H, Shimizu W. Nutritional status is associated with inflammation and predicts a poor outcome in patients with chronic heart failure. J Atheroscler Thromb. 2016;23(6):713–727. doi: 10.5551/jat.31526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taniguchi E, Kawaguchi T, Otsuka M, Uchida Y, Nagamatsu A, Itou M, Oriishi T, Ishii K, Imanaga M, Suetsugu T, Otsuyama J, Ibi R, Ono M, Tanaka S, Sata M. Nutritional assessments for ordinary medical care in patients with chronic liver disease. Hepatol Res. 2013;43(2):192–199. doi: 10.1111/j.1872-034X.2012.01055.x. [DOI] [PubMed] [Google Scholar]
- 23.Iseki Y, Shibutani M, Maeda K, Nagahara H, Ohtani H, Sugano K, Ikeya T, Muguruma K, Tanaka H, Toyokawa T, Sakurai K, Hirakawa K. Impact of the preoperative controlling nutritional status (conut) score on the survival after curative surgery for colorectal cancer. PLoS One. 2015;10(7):e0132488. doi: 10.1371/journal.pone.0132488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takagi K, Yagi T, Umeda Y, Shinoura S, Yoshida R, Nobuoka D, Kuise T, Araki H, Fujiwara T. Preoperative controlling nutritional status (conut) score for assessment of prognosis following hepatectomy for hepatocellular carcinoma. World J Surg. 2017;41(9):2353–2360. doi: 10.1007/s00268-017-3985-8. [DOI] [PubMed] [Google Scholar]
- 25.Yoshida N, Harada K, Baba Y, Kosumi K, Iwatsuki M, Kinoshita K, Nakamura K, Sakamoto Y, Miyamoto Y, Karashima R, Mima K, Sawayama H, Ohuchi M, Chikamoto A, Imamura Y, Watanabe M, Baba H. Preoperative controlling nutritional status (conut) is useful to estimate the prognosis after esophagectomy for esophageal cancer. Langenbecks Arch Surg. 2017;402(2):333–341. doi: 10.1007/s00423-017-1553-1. [DOI] [PubMed] [Google Scholar]
- 26.Harimoto N, Yoshizumi T, Sakata K, Nagatsu A, Motomura T, Itoh S, Harada N, Ikegami T, Uchiyama H, Soejima Y, Maehara Y. Prognostic significance of preoperative controlling nutritional status (conut) score in patients undergoing hepatic resection for hepatocellular carcinoma. World J Surg. 2017;41(11):2805–2812. doi: 10.1007/s00268-017-4097-1. [DOI] [PubMed] [Google Scholar]
- 27.Hashimoto Y, Traverso LW. Incidence of pancreatic anastomotic failure and delayed gastric emptying after pancreatoduodenectomy in 507 consecutive patients: Use of a web-based calculator to improve homogeneity of definition. Surgery. 2010;147(4):503–515. doi: 10.1016/j.surg.2009.10.034. [DOI] [PubMed] [Google Scholar]
- 28.Yang YM, Tian XD, Zhuang Y, Wang WM, Wan YL, Huang YT. Risk factors of pancreatic leakage after pancreaticoduodenectomy. World J Gastroenterol. 2005;11(16):2456–2461. doi: 10.3748/wjg.v11.i16.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kiyochi H. Pathologic assessment of pancreatic fibrosis for objective prediction of pancreatic fistula and management of prophylactic drain removal after pancreaticoduodenectomy: Reply. World J Surg. 2016;40(6):1522–1523. doi: 10.1007/s00268-015-3395-8. [DOI] [PubMed] [Google Scholar]
- 30.Lin JW, Cameron JL, Yeo CJ, Riall TS, Lillemoe KD. Risk factors and outcomes in postpancreaticoduodenectomy pancreaticocutaneous fistula. J Gastrointest Surg. 2004;8(8):951–959. doi: 10.1016/j.gassur.2004.09.044. [DOI] [PubMed] [Google Scholar]
- 31.Kawai M, Kondo S, Yamaue H, Wada K, Sano K, Motoi F, Unno M, Satoi S, Kwon AH, Hatori T, Yamamoto M, Matsumoto J, Murakami Y, Doi R, Ito M, Miyakawa S, Shinchi H, Natsugoe S, Nakagawara H, Ohta T, Takada T. Predictive risk factors for clinically relevant pancreatic fistula analyzed in 1,239 patients with pancreaticoduodenectomy: Multicenter data collection as a project study of pancreatic surgery by the Japanese Society of Hepato-biliary-pancreatic Surgery. J Hepatobiliary Pancreat Sci. 2011;18(4):601–608. doi: 10.1007/s00534-011-0373-x. [DOI] [PubMed] [Google Scholar]
- 32.El Nakeeb A, Salah T, Sultan A, El Hemaly M, Askr W, Ezzat H, Hamdy E, Atef E, El Hanafy E, El-Geidie A, Abdel Wahab M, Abdallah T. Pancreatic anastomotic leakage after pancreaticoduodenectomy. Risk factors, clinical predictors, and management (single-center experience) World J Surg. 2013;37(6):1405–1418. doi: 10.1007/s00268-013-1998-5. [DOI] [PubMed] [Google Scholar]
- 33.Hashimoto Y, Sclabas GM, Takahashi N, Kirihara Y, Smyrk TC, Huebner M, Farnell MB. Dual-phase computed tomography for assessment of pancreatic fibrosis and anastomotic failure risk following pancreatoduodenectomy. J Gastrointest Surg. 2011;15(12):2193–2204. doi: 10.1007/s11605-011-1687-3. [DOI] [PubMed] [Google Scholar]
- 34.Kawakatsu S, Inoue Y, Mise Y, Ishizawa T, Ito H, Takahashi Y, Saiura A. Comparison of pancreatojejunostomy techniques in patients with a soft pancreas: Kakita anastomosis and Blumgart anastomosis. BMC Surg. 2018;18(1):88. doi: 10.1186/s12893-018-0420-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M, International Study Group on Pancreatic Fistula D. Postoperative pancreatic fistula: An international study group (ISGPF) definition. Surgery. 2005;138(1):8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 36.Pratt WB, Callery MP, Vollmer CM Jr. The latent presentation of pancreatic fistulas. Br J Surg. 2009;96(6):641–649. doi: 10.1002/bjs.6614. [DOI] [PubMed] [Google Scholar]
- 37.Meguid MM, Mughal MM, Debonis D, Meguid V, Terz JJ. Influence of nutritional status on the resumption of adequate food intake in patients recovering from colorectal cancer operations. Surg Clin North Am. 1986;66(6):1167–1176. doi: 10.1016/s0039-6109(16)44080-6. [DOI] [PubMed] [Google Scholar]
- 38.Detsky AS, Baker JP, O'Rourke K, Johnston N, Whitwell J, Mendelson RA, Jeejeebhoy KN. Predicting nutrition-associated complications for patients undergoing gastrointestinal surgery. J Parenter Enteral Nutr. 1987;11(5):440–446. doi: 10.1177/0148607187011005440. [DOI] [PubMed] [Google Scholar]
- 39.Mazaki T, Ishii Y, Murai I. Immunoenhancing enteral and parenteral nutrition for gastrointestinal surgery: A multiple-treatments meta-analysis. Ann Surg. 2015;261(4):662–669. doi: 10.1097/SLA.0000000000000935. [DOI] [PubMed] [Google Scholar]
- 40.Lawrence VA, Cornell JE, Smetana GW, American College of Physicians Strategies to reduce postoperative pulmonary complications after noncardiothoracic surgery: Systematic review for the American College of Physicians. Ann Intern Med. 2006;144(8):596–608. doi: 10.7326/0003-4819-144-8-200604180-00011. [DOI] [PubMed] [Google Scholar]
- 41.Fujiwara Y, Shiba H, Shirai Y, Iwase R, Haruki K, Furukawa K, Futagawa Y, Misawa T, Yanaga K. Perioperative serum albumin correlates with postoperative pancreatic fistula after pancreaticoduodenectomy. Anticancer Res. 2015;35(1):499–503. doi: 10.7326/0003-4819-144-8-200604180-00011. [DOI] [PubMed] [Google Scholar]
- 42.Bhatti I, Peacock O, Lloyd G, Larvin M, Hall RI. Preoperative hematologic markers as independent predictors of prognosis in resected pancreatic ductal adenocarcinoma: Neutrophil-lymphocyte versus platelet-lymphocyte ratio. Am J Surg. 2010;200(2):197–203. doi: 10.1016/j.amjsurg.2009.08.041. [DOI] [PubMed] [Google Scholar]
- 43.Menges T, Engel J, Welters I, Wagner RM, Little S, Ruwoldt R, Wollbrueck M, Hempelmann G. Changes in blood lymphocyte populations after multiple trauma: Association with posttraumatic complications. Crit Care Med. 1999;27(4):733–740. doi: 10.1097/00003246-199904000-00026. [DOI] [PubMed] [Google Scholar]
- 44.Nochioka K, Sakata Y, Takahashi J, Miyata S, Miura M, Takada T, Fukumoto Y, Shiba N, Shimokawa H, for the CHART-2 Investigators Prognostic impact of nutritional status in asymptomatic patients with cardiac diseases: A report from the chart-2 study. Circ J. 2013;77(9):2318–2326. doi: 10.1253/circj.cj-13-0127. [DOI] [PubMed] [Google Scholar]
- 45.Sanz L, Ovejero VJ, Gonzalez JJ, Laso CA, Azcano E, Navarrete F, Martinez E. Mortality risk scales in esophagectomy for cancer: Their usefulness in preoperative patient selection. Hepatogastroenterology. 2006;53(72):869–873. [PubMed] [Google Scholar]
- 46.Wang Q, Lau WY, Zhang B, Zhang Z, Huang Z, Luo H, Chen X. Preoperative total cholesterol predicts postoperative outcomes after partial hepatectomy in patients with chronic hepatitis B- or C-related hepatocellular carcinoma. Surgery. 2014;155(2):263–270. doi: 10.1016/j.surg.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 47.de Ulibarri Perez JI, Fernandez G, Rodriguez Salvanes F, Diaz Lopez AM. Nutritional screening; control of clinical undernutrition with analytical parameters. Nutr Hosp. 2014;29(4):797–811. doi: 10.3305/nh.2014.29.4.7275. [DOI] [PubMed] [Google Scholar]
- 48.Gaujoux S, Cortes A, Couvelard A, Noullet S, Clavel L, Rebours V, Levy P, Sauvanet A, Ruszniewski P, Belghiti J. Fatty pancreas and increased body mass index are risk factors of pancreatic fistula after pancreaticoduodenectomy. Surgery. 2010;148(1):15–23. doi: 10.1016/j.surg.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 49.Fu SJ, Shen SL, Li SQ, Hu WJ, Hua YP, Kuang M, Liang LJ, Peng BG. Risk factors and outcomes of postoperative pancreatic fistula after pancreatico-duodenectomy: An audit of 532 consecutive cases. BMC Surg. 2015;15:34. doi: 10.1186/s12893-015-0011-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Machado NO. Pancreatic fistula after pancreatectomy: Definitions, risk factors, preventive measures, and management-review. Int J Surg Oncol. 2012;2012:602478. doi: 10.1155/2012/602478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kenjo A, Miyata H, Gotoh M, Kitagawa Y, Shimada M, Baba H, Tomita N, Kimura W, Sugihara K, Mori M. Risk stratification of 7,732 hepatectomy cases in 2011 from the National Clinical Database for Japan. J Am Coll Surg. 2014;218(3):412–422. doi: 10.1016/j.jamcollsurg.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 52.Hu BY, Wan T, Zhang WZ, Dong JH. Risk factors for postoperative pancreatic fistula: Analysis of 539 successive cases of pancreaticoduodenectomy. World J Gastroenterol. 2016;22(34):7797–7805. doi: 10.3748/wjg.v22.i34.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aoki S, Miyata H, Konno H, Gotoh M, Motoi F, Kumamaru H, Wakabayashi G, Kakeji Y, Mori M, Seto Y, Unno M. Risk factors of serious postoperative complications after pancreaticoduodenectomy and risk calculators for predicting postoperative complications: A nationwide study of 17,564 patients in japan. J Hepatobiliary Pancreat Sci. 2017;24(5):243–251. doi: 10.1002/jhbp.438. [DOI] [PMC free article] [PubMed] [Google Scholar]