Abstract
Background/Aim: This study aimed to analyze the correlation between microsatellite instability (MSI) and inflammatory markers during neoadjuvant CRT in rectal cancer and its influence on prognosis. Patients and Methods: A total of 549 patients with locally advanced rectal cancer underwent neoadjuvant CRT. Complete blood counts before CRT, and 4-8 weeks after CRT were used to measure neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR). Results: MSI was significantly associated with elevated NLR and PLR after CRT as well as with a change in NLR and PLR during CRT. Neither inflammatory markers nor MSI significantly related to survival. However, in patients with MSI, an increase in NLR and PLR before CRT was significantly correlated with poor overall survival and disease-free survival. Conclusion: There is correlation between inflammatory markers and MSI during CRT and it influences prognosis. Therefore, inflammatory markers might have a role in assessing the microenvironment related to MSI and the immunologic response in rectal cancer.
Keywords: Rectal cancer, inflammatory markers, chemoradiotherapy, microsatellite instability
Chemotherapy and radiotherapy have the potential to re-program the tumor microenvironment and induce immunostimulatory effects, possibly by encouraging tumor antigen-specific immune response (1). In rectal cancer, neoadjuvant chemoradiotherapy (CRT) has been widely used in patients with locally advanced rectal cancer. We have previously reported that inflammatory markers including a neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) can be used as a predictive marker of treatment response after neoajduvant CRT (2). In previous studies, inflammatory markers have been shown to have a direct correlation with the intra-tumoral levels of granulocyte myeloid-derived suppressor cells and regulatory T cells (3,4), which can suppress anti-tumor immune reaction (1). Furthermore, the inflammatory markers have been recently found to predict the response of immunotherapy (5,6).
Microsatellite instability (MSI) is one of the well-known immunologic markers in colorectal cancer, which tends to induce hyper-mutation and exhibit peri-tumoral immune cell infiltration/activation closely related to the change in tumor microenvironment (7). Furthermore, MSI in colorectal cancer has been found to be predictive of poor response to 5-fluorouracil based chemotherapy and better response to immunotherapy such as checkpoint inhibitors (8,9). As an immunogenic tumor, the anti-tumor immunity and pro-tumoral inflammation microenvironment plays an essential role in immunotherapy in colorectal cancer with MSI (7-9).
Based on previous findings, we hypothesized that MSI status could affect the immunologic status in rectal cancer during CRT and might lead to changes in the inflammatory markers and its prognosis. Although neoadjuvant CRT is part of the standard care in rectal cancer, it has not been studied whether MSI as a representative of microenvironments related to the dynamics and prognostic value of inflammatory markers. Thus, in the present study, we aimed to analyze the correlation between the MSI and inflammatory markers during neoadjuvant CRT in rectal cancer and its influence on prognosis.
Patients and Methods
Patients. The present study was approved by the Institutional Ethical Review Board of the Seoul National University Bundang Hospital (IRB no. B-1807-478-106) and Seoul National University Hospital (IRB no. J-2001-054-1093). We retrospectively reviewed the medical records of patients with rectal cancer who underwent neoadjuvant CRT followed by surgery in the two affiliated tertiary academic hospitals. The time of treatment for rectal cancer was from January 2004 to August 2015 in Seoul National University Bundang Hospital and from January 2004 to December 2011 in Seoul National University Hospital. Inclusion criteria were as follows: (i) Pathologically confirmed primary rectal cancer; (ii) neoadjuvant CRT followed by surgery; (iii) serial complete blood count (CBC) examination throughout CRT; (iv) immunologically competent before and during CRT, including absolute neutrophil count (ANC)>1,000/μl, white blood cell count (WBC) before and during CRT, including absolute neutrophil count (ANC)>1,000/μl, white blood cell count (WBC) between 4,000 and 10,000 μl; (v) negative for hepatitis B virus antigen, anti-hepatitis C virus, anti-human immunodeficiency virus, and venereal disease research laboratory results before CRT. We excluded patients showing complete regression in the surgical specimen, because the MSI could not be evaluated. Patients were staged according to the classification system of the American Joint Committee on Cancer (7th edition) (10). The number of total eligible patients meeting the inclusion criteria was 549.
Treatment. All patients underwent clinical examination, including digital rectal examination, routine laboratory tests with carcinoembryonic antigen (CEA) level, and colonoscopic examination, computed tomography of the chest and abdomen-pelvis, and rectal magnetic resonance imaging. The rectal cancer was histologically confirmed before neoadjuvant CRT.
For neoadjuvant CRT, the median radiation dose was 50.4 Gy (range=50.4-55.8 Gy) with conventional fractionations. Patients were mainly treated with the combination of chemotherapy with intravenous 5-fluorouracil or capecitabine throughout the radiation period. Following neoadjuvant CRT, all patients underwent curative radical surgery 4 to 8 weeks after neoadjuvant CRT.
MSI status determination. The DNA was extracted from each paraffin block after deparaffinization. To evaluate the MSI status, five quasi-monomorphic mononucleotide markers (BAT-25, BAT-26, NR-21, NR-24, and MONO-27) were coamplified in a single polymerase chain reaction (11,12). Samples with instability at two or more of the five markers were classified as high-degree microsatellite instability (MSI-H). In contrast, those with instability at one and no marker were classified as low-degree microsatellite instability (MSI-L) and microsatellite stability (MSS), respectively (11).
Blood test. CBC was performed throughout CRT. Laboratory index values, including neutrophil counts, lymphocyte counts, and CEA, were obtained for each patient before CRT (pre-CRT), and 4-6 weeks after CRT (post-CRT). NLR and PLR was calculated as the ratio of the neutrophil count and of the platelet count divided by the lymphocyte count. The ratio of change in NLR (cNLR) and PLR (cPLR) was calculated using the following formula: cNLR=(post-CRT NLR–pre-CRT NLR)/pre-CRT NLR, cPLR=(post-CRT NLR–pre-CRT NLR)/pre-CRT NLR.
Statistical analysis. The chi-square test was applied for categorical variables, and Student’s t-test was applied for continuous variables. Patients were divided into groups based on the median index value of inflammatory markers. Overall survival (OS) and progression-free survival (DFS) were calculated as the interval from the first date of treatment to the date of death and to the date that progression was detected, respectively. Survival curves were generated using the Kaplan-Meier method, and univariate survival comparison was performed using the log-rank test. Multivariate survival comparison was performed using the Cox regression analysis. A value of p<0.050 was considered statistically significant. Analyses were performed using SPSS version 18 (SPSS Inc., Chicago, IL, USA).
Results
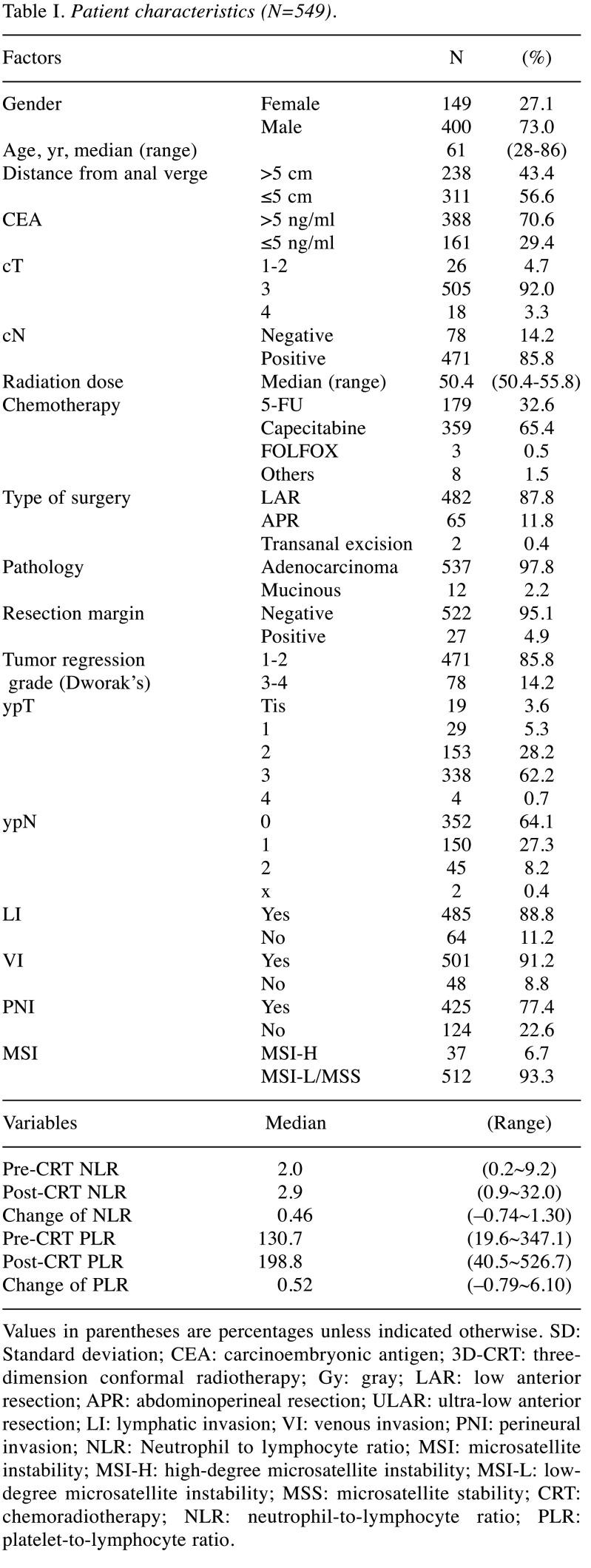
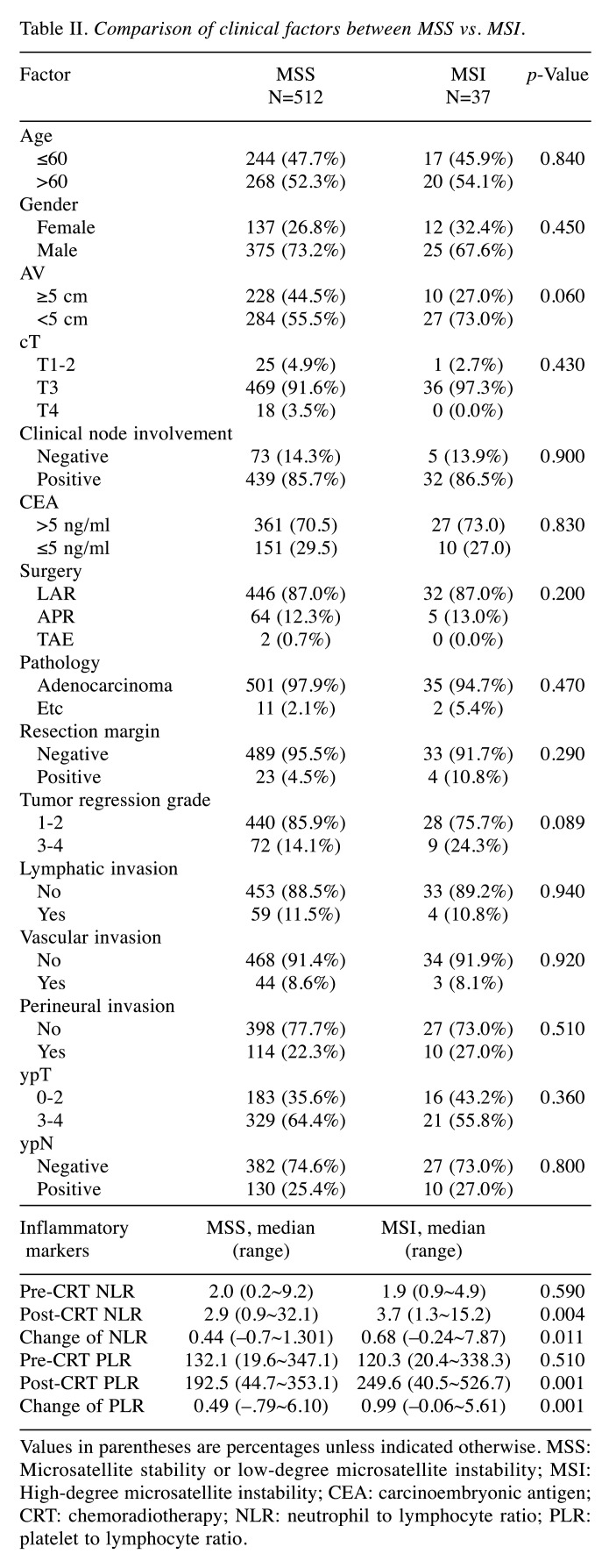
The relationship between MSI and inflammatory markers. Patient characteristics and NLR profiles are detailed in Table I. The proportions of MSI-H and MSI-L/MSS were 6.7% (n=37) and 93.3% (n=512), respectively. Compared with MSI-L/MSS (Table II), MSI-H patients had significantly higher post-CRT NLR, cNLR, post-CRT PLR, cPLR (p=0.004, 0.011, 0.00, and 0.001, respectively), and a non-significant trend for lower tumor location and better tumor regression (p=0.060 and 0.089, respectively). Otherwise, no statistical difference between the two groups was observed in clinical factors, including tumor regression grade (p=0.360 and 0.720, respectively).
Table I. Patient characteristics (N=549).
Values in parentheses are percentages unless indicated otherwise. SD: Standard deviation; CEA: carcinoembryonic antigen; 3D-CRT: threedimension conformal radiotherapy; Gy: gray; LAR: low anterior resection; APR: abdominoperineal resection; ULAR: ultra-low anterior resection; LI: lymphatic invasion; VI: venous invasion; PNI: perineural invasion; NLR: Neutrophil to lymphocyte ratio; MSI: microsatellite instability; MSI-H: high-degree microsatellite instability; MSI-L: lowdegree microsatellite instability; MSS: microsatellite stability; CRT: chemoradiotherapy; NLR: neutrophil-to-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio
Table II. Comparison of clinical factors between MSS vs. MSI.
Values in parentheses are percentages unless indicated otherwise. MSS: Microsatellite stability or low-degree microsatellite instability; MSI: High-degree microsatellite instability; CEA: carcinoembryonic antigen; CRT: chemoradiotherapy; NLR: neutrophil to lymphocyte ratio; PLR: platelet to lymphocyte ratio
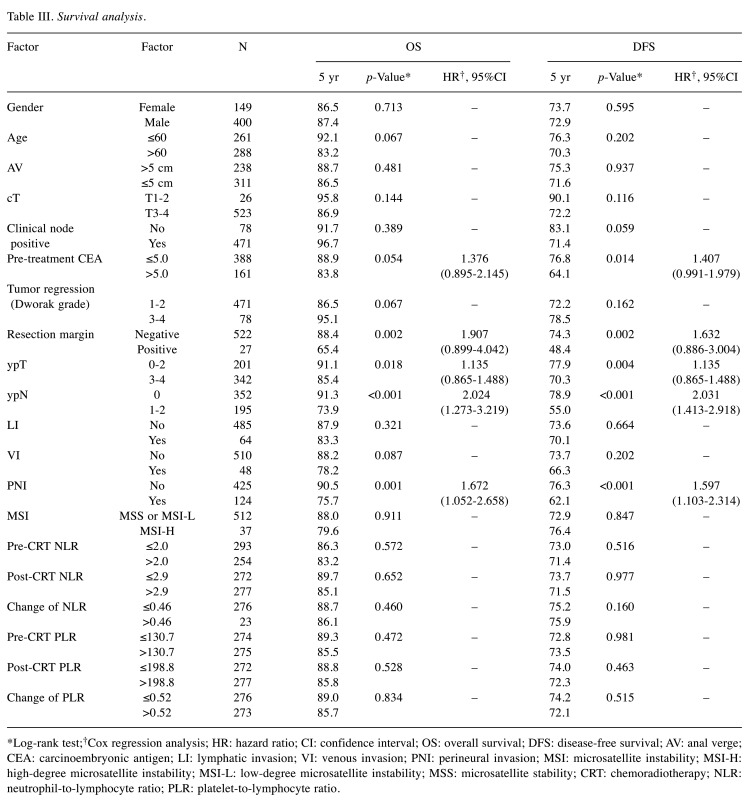
Analysis of prognostic factors. The analysis of survival outcomes is shown in Table III. Resection margin (RM), ypT, ypN, and perineural invasion (PNI) were significant prognostic factors that correlated with OS (p=0.002, 0.018, <0.001, and 0.001, respectively). Pre-treatment CEA, RM, ypT, ypN, and PNI were significant prognostic factors for DFS (p=0.014, 0.002, 0.004, <0.001, and <0.001, respectively). MSI status and inflammation markers were not significantly related to survival and disease control rate. In multivariate analysis, ypN and perineural invasion (PNI) are independently significant factors for OS and DFS.
Table III. Survival analysis.
*Log-rank test;†Cox regression analysis; HR: hazard ratio; CI: confidence interval; OS: overall survival; DFS: disease-free survival; AV: anal verge; CEA: carcinoembryonic antigen; LI: lymphatic invasion; VI: venous invasion; PNI: perineural invasion; MSI: microsatellite instability; MSI-H: high-degree microsatellite instability; MSI-L: low-degree microsatellite instability; MSS: microsatellite stability; CRT: chemoradiotherapy; NLR: neutrophil-to-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio
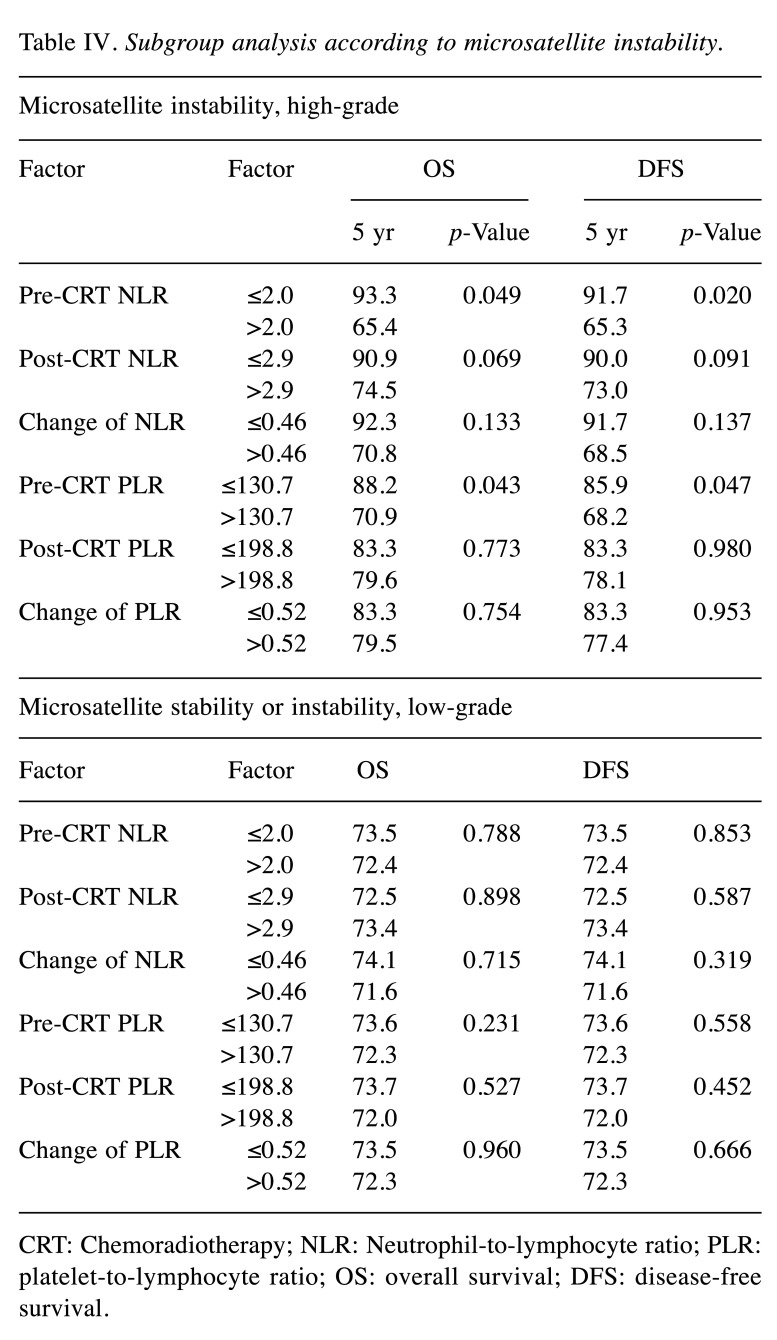
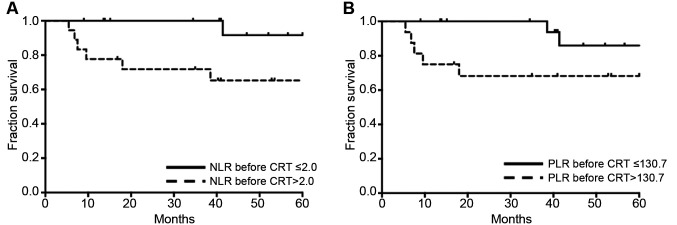
Different prognostic effects of NLR according to MSI status. In the subgroup of MSI-H, the increase of pre-CRT NLR and PLR was significantly correlated with poor OS and DFS (Table IV and Figure 1). However, in patients with MSI-L/MSS, NLR and PLR had no prognostic effect on all clinical endpoints. The distribution of significant factors in prognostic analysis was compared between patient groups dichotomized into pre-CRT NLR and PLR. Pre-treatment CEA, RM, ypT, ypN, and PNI, which were found to be significant factors in the prognostic analysis, were not statistically different between pre-CRT NLR and PLR.
Table IV. Subgroup analysis according to microsatellite instability.
CRT: Chemoradiotherapy; NLR: Neutrophil-to-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio; OS: overall survival; DFS: disease-free survival.
Figure 1. Disease-free survival according to inflammatory markers.
Discussion
In this study, MSI-H patients demonstrated a significant increase in NLR and PLR during CRT and high levels of NLR and PLR after CRT, whereas the baselines of inflammatory markers were not different according to MSI status. A previous study has also shown that baseline NLR in colorectal cancer was not significantly different according to MSI status (13). However, to the best of our knowledge, this is the first report to show the relationship between the change of inflammatory markers and MSI during neoadjuvant CRT for rectal cancer. Among the different molecular subtypes of colorectal cancer, the MIS-immune type tends to have hyper-mutation and be vulnerable to immune infiltration and inflammation (7). The response of rectal cancer to CRT can depend on the change of the immunologic and inflammatory microenvironment during CRT (14-16). The systemic inflammatory response can cause alterations in circulating leukocytes, especially neutrophilia and thrombophilia with a lymphopenia (17,18), which can be calculated as NLR and PLR. Therefore, our findings indicate that changes in NLR and PLR might reflect the dynamics of systemic inflammation and immune response induced by CRT, especially in patients with MSI.
If the change in inflammatory markers can reflect the degree of systemic inflammation and immune response during CRT, the clinical utility of inflammatory markers can be one of the predictive or prognostic markers of the response to immunologic agents for rectal cancer. Programmed cell death 1-blocking antibodies have improved survival in patients with metastatic colorectal cancer with MSI-H (9). However, there is weak background evidence regarding the primary treatment of rectal cancer with immunologic agents. Moreover, the big challenge of immunotherapy is the absence of predictive markers for a response, while only 10-20% of patients respond to immunotherapy even in immunogenic tumors (1,5,6). The close relationship between the change in inflammatory markers during CRT and MSI in our study indicates that inflammatory markers may reflect the immunologic change induced by CRT. Previous studies have also confirmed that inflammatory markers could be used as predictive markers of the response to immunogenic agents in immunogenic tumors such as renal cell carcinoma (5), and melanoma (6).
The prognostic effect of MSI and inflammatory markers after CRT in rectal cancer is controversial. Previous studies about the difference in clinical outcomes, according to MSI, have shown conflicting results (19-22). Also, only a limited number of studies have reported inconsistent data on the potential role of inflammatory markers as a predictive factor for survival (2,23-26). We could not find any significant prognostic effect of MSI or inflammatory markers in the rectal cancer patients. Increased NLR or PLR caused by neutrophilia, thrombophilia or lymphopenia denotes the enhanced accumulation of immune-suppressive myeloid cells and suppression of anti-tumor immunity. Our finding indicating that inflammatory markers had a robust prognostic effect only in MSI-H tumors support the conclusion that the clinical impact of inflammatory markers can be augmented in the immunogenic tumor such as MSI-H (27). Howard et al. have also confirmed that the prognostic value of NLR is heterogeneous among 18 cancer types and has the most significant effect on prognosis in the melanoma, one of the representative immunogenic tumors (28). Therefore, to refine the clinical utility of NLR from the previous inconsistent studies, research should focus on the role of inflammatory markers in the context of immunogenic tumors and immunotherapy.
The small sample size of MSI-H was the major limitation of this study. However, the incidence of MSI-H is infrequent, only about 2-8% in rectal cancer (19-22,29). Among the clinical studies dealing with this subject (19,21,22,29), our study included the largest number of MIS-H patients. Although Hassan et al. (20) have collected 636 MSI-positive patients through the National Cancer Database, they did not discriminate MSI-H against MSI-L, which is immunologically and clinically similar to MSS. Moreover, our cohort comprised homogeneous patients treated at two affiliated hospitals with consistent treatment guidelines regarding CRT, surgery and pathological review techniques. However, our study did not address whether MSI and inflammatory markers can affect the immunologic response to the combined strategies of standard CRT and immunotherapy. This should be addressed in future studies (30). Moreover, detailed analysis of the microenvironments and immune cells using specimens of rectal cancer can highlight the clinical value of inflammatory markers to predict the change between pro- and anti-tumoral immunity.
Conclusion
This study found a close correlation between inflammatory markers and MSI during CRT and its influence on prognosis. These findings suggest the potential role of inflammatory markers in assessing the immunologic microenvironments related to MSI and the immunologic response in rectal cancer.
Conflicts of Interest
The Authors declare that they have no competing interests regarding this study.
Authors’ Contributions
JHL, EKC, JSK conceived of and designed the study. JHL, BK, CS, SK, HSL, and KL provided data. JHL and BK performed the statistical analysis. JHL, EKC, JSK drafted the manuscript. All Authors read and approved the final manuscript.
References
- 1.Weichselbaum RR, Liang H, Deng L, Fu YX. Radiotherapy and immunotherapy: a beneficial liaison. Nat Rev Clin Oncol. 2017;14:365–379. doi: 10.1038/nrclinonc.2016.211. [DOI] [PubMed] [Google Scholar]
- 2.Lee JH, Song C, Kang SB, Lee HS, Lee KW, Kim JS. Predicting pathological complete regression with haematological markers during neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Anticancer Res. 2018;38:6905–6910. doi: 10.21873/anticanres.13067. [DOI] [PubMed] [Google Scholar]
- 3.Cheng H, Luo G, Lu Y, Jin K, Guo M, Xu J, Long J, Liu L, Yu X, Liu C. The combination of systemic inflammation-based marker NLR and circulating regulatory T cells predicts the prognosis of resectable pancreatic cancer patients. Pancreatology. 2016;16:1080–1084. doi: 10.1016/j.pan.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Ohki S, Shibata M, Gonda K, Machida T, Shimura T, Nakamura I, Ohtake T, Koyama Y, Suzuki S, Ohto H, Takenoshita S. Circulating myeloid-derived suppressor cells are increased and correlate to immune suppression, inflammation and hypoproteinemia in patients with cancer. Oncol Rep. 2012;28:453–458. doi: 10.3892/or.2012.1812. [DOI] [PubMed] [Google Scholar]
- 5.Lalani A-KA, Xie W, Martini DJ, Steinharter JA, Norton CK, Krajewski KM, Duquette A, Bossé D, Bellmunt J, Allen EMV. Change in neutrophil-to-lymphocyte ratio (NLR) in response to immune checkpoint blockade for metastatic renal cell carcinoma. JI Immunother Cancer. 2018;6:5. doi: 10.1186/s40425-018-0315-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Capone M, Giannarelli D, Mallardo D, Madonna G, Festino L, Grimaldi AM, Vanella V, Simeone E, Paone M, Palmieri G. Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J Immunother Cancer. 2018;6:74. doi: 10.1186/s40425-018-0383-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guinney J, Dienstmann R, Wang X, Reyniès A de, Schlicker A, Soneson C, Marisa L, Roepman P, Nyamundanda G, Angelino P, Bot BM, Morris JS, Simon IM, Gerster S, Fessler E, Melo FDSE, Missiaglia E, Ramay H, Barras D, Homicsko K, Maru D, Manyam GC, Broom B, Boige V, Perez-Villamil B, Laderas T, Salazar R, Gray JW, Hanahan D, Tabernero J, Bernards R, Friend SH, Laurent-Puig P, Medema JP, Sadanandam A, Wessels L, Delorenzi M, Kopetz S, Vermeulen L, Tejpar S. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21:nm.3967. doi: 10.1038/nm.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ribic CM, Sargent DJ, Moore MJ, Thibodeau SN, French AJ, Goldberg RM, Hamilton SR, Laurent-Puig P, Gryfe R, Shepherd LE, Tu D, Redston M, Gallinger S. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. New Engl J Med. 2003;349:247–257. doi: 10.1056/NEJMoa022289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, Chapelle A de la, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA. PD-1 blockade in tumors with mismatch-repair deficiency. New Engl J Medicine. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edge SB, Byrd DR, Carducci MA, Compton CC, Fritz A, Greene F. AJCC cancer staging manual. 2010 [Google Scholar]
- 11.Patil DT, Bronner MP, Portier BP, Fraser CR, Plesec TP, Liu X. A five-marker panel in a multiplex PCR accurately detects microsatellite instability-high colorectal tumors without control DNA. Diagnostic Mol Pathology Am J Surg Pathology Part B. 2012;21:127–133. doi: 10.1097/PDM.0b013e3182461cc3. [DOI] [PubMed] [Google Scholar]
- 12.Laiho P, Launonen V, Lahermo P, Esteller M, Guo M, Herman JG, Mecklin JP, Järvinen H, Sistonen P, Kim K-M, Shibata D, Houlston RS, Aaltonen LA. Low-level microsatellite instability in most colorectal carcinomas. Cancer Res. 2002;62:1166–1170. [PubMed] [Google Scholar]
- 13.Rashtak S, Ruan X, Druliner BR, Liu H, Therneau T, Mouchli M, Boardman LA. Peripheral neutrophil to lymphocyte ratio improves prognostication in colon cancer. Clin Colorectal Cancer. 2017;16:115–123. doi: 10.1016/j.clcc.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim YJ, Koh J, Kim S, Jeon SR, Chie EK, Kim K, Kang GH, Han SW, Kim T-Y, Jeong SY, Park KJ, Wu HG. Chemoradiation-induced alteration of programmed death-ligand 1 and CD8+tumor-infiltrating lymphocytes identified patients with poor prognosis in rectal cancer: Amatched comparison analysis. Int J Radiat Oncol Biology Phys. 2017;99:1216–1224. doi: 10.1016/j.ijrobp.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Lee JH, Chie EK, Jeong SY, Kim TY, Kim DY, Kim TH, Kim SY, Baek JY, Chang HJ, Kim MJ, Park SC, Oh JH, Kim SH, Lee JH, Choi DH, Park HC, Kang S-B, Kim JS. Redefining the positive circumferential resection margin by incorporating preoperative chemoradiotherapy treatment response in locally advanced rectal cancer: a multicenter validation study. Cancer Res Treat. 2018;50:506–517. doi: 10.4143/crt.2016.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JH, Chie EK, Kim K, Jeong SY, Park KJ, Park JG, Kang GH, Han SW, Oh DY, Im SA, Kim TY, Bang YJ, Ha SW. The influence of the treatment response on the impact of resection margin status after preoperative chemoradiotherapy in locally advanced rectal cancer. BMC Cancer. 2013;13:576. doi: 10.1186/1471-2407-13-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Epstein FH, Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. New Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 18.Imtiaz F, Shafique K, Mirza SS, Ayoob Z, Vart P, Rao S. Neutrophil lymphocyte ratio as a measure of systemic inflammation in prevalent chronic diseases in Asian population. Int Arch Med. 2012;5:2. doi: 10.1186/1755-7682-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Acar T, Acar N, Kamer E, Tekindal MA, Cengiz F, Kar H, Atahan K, Haciyanli M. Do microsatellite instability (MSI) and deficient mismatch repair (dMMR) affect the pathologic complete response (pCR) in patients with rectal cancer who received neoadjuvant treatment. Updates Surg. 2020;72(1):73–82. doi: 10.1007/s13304-019-00697-2. [DOI] [PubMed] [Google Scholar]
- 20.Hasan S, Renz P, Wegner RE, Finley G, Raj M, Monga D, McCormick J, Kirichenko A. Microsatellite instability (MSI) as an independent predictor of pathologic complete response (PCR) in locally advanced rectal cancer. Ann Surg. 2018 doi: 10.1097/SLA.0000000000003051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charara M, Edmonston TB, Burkholder S, Walters R, Anne P, Mitchell E, Fry R, Boman B, Rose D, Fishel R, Curran W, Palazzo J. Microsatellite status and cell cycle associated markers in rectal cancer patients undergoing a combined regimen of 5-FU and CPT-11 chemotherapy and radiotherapy. Anticancer Res. 2004;24:3161–3167. [PubMed] [Google Scholar]
- 22.Samowitz WS, Curtin K, Wolff RK, Tripp SR, Caan BJ, Slattery ML. Microsatellite instability and survival in rectal cancer. Cancer Causes Control. 2009;20:1763–1768. doi: 10.1007/s10552-009-9410-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carruthers R, Tho LM, Brown J, Kakumanu S, McCartney E, McDonald AC. Systemic inflammatory response is a predictor of outcome in patients undergoing preoperative chemoradiation for locally advanced rectal cancer. Colorectal Dis. 2012;14:e701–e707. doi: 10.1111/j.1463-1318.2012.03147.x. [DOI] [PubMed] [Google Scholar]
- 24.Yang J, Xu H, Guo X, Zhang J, Ye X, Yang Y, Ma X. Pretreatment inflammatory indexes as prognostic predictors for survival in colorectal cancer patients receiving neoadjuvant chemoradiotherapy. Sci Rep. 2018;8:3044. doi: 10.1038/s41598-018-21093-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee IH, Hwang S, Lee SJ, Kang BW, Baek D, Kim HJ, Park SY, Park JS, Choi GS, Kim JC, Cho SH, Kim JG. Systemic inflammatory response after preoperative chemoradiotherapy can affect oncologic outcomes in locally advanced rectal cancer. Anticancer Res. 2017;37:1459–1466. doi: 10.21873/anticanres.11470. [DOI] [PubMed] [Google Scholar]
- 26.Petrelli F, Ghidini M, Cabiddu M, Pezzica E, Corti D, Turati L, Costanzo A, Varricchio A, Ghidini A, Barni S, Tomasello Review: Microsatellite instability and survival in stage II colorectal cancer: A systematic review and meta-analysis. Anticancer Res. 2019;39:6431–6441. doi: 10.21873/anticanres.13857. [DOI] [PubMed] [Google Scholar]
- 27.Su P, Dong Y, Shi Y, He L. Prognostic significance of neutrophil-to-lymphocyte ratio in rectal cancer: a meta-analysis. Oncotargets Ther. 2016;9:3127–3134. doi: 10.2147/OTT.S103031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Howard R, Kanetsky PA, Egan KM. Heterogeneity in prognostic value of the neutrophil-to-lymphocyte ratio: a meta-analysis. MedRxiv. 2019;19011387 doi: 10.1101/19011387. [DOI] [Google Scholar]
- 29.Hong SP, Min BS, Kim TI, Cheon JH, Kim NK, Kim H, Kim WH. The differential impact of microsatellite instability as a marker of prognosis and tumour response between colon cancer and rectal cancer. Eur J Cancer. 2012;48:1235–1243. doi: 10.1016/j.ejca.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 30.Zhang J, Cai J, Deng Y, Wang H. Complete response in patients with locally advanced rectal cancer after neoadjuvant treatment with nivolumab. Oncoimmunology. 2019;8:1663108. doi: 10.1080/2162402X.2019.1663108. [DOI] [PMC free article] [PubMed] [Google Scholar]