Abstract
Ghrelin is a small peptide with important roles in the regulation of appetite, gut motility, glucose homeostasis as well as cardiovascular protection. This review highlights the role that acyl ghrelin plays in maintaining normal endothelial function by maintaining the balance of vasodilator-vasoconstrictor factors, inhibiting inflammatory cytokine production and immune cell recruitment to sites of vascular injury and by promoting angiogenesis.
Keywords: ghrelin, GHS-R1a, vasodilation, angiogenesis, inflammation
Ghrelin is a small peptide that was first noted for its actions in central regulation of appetite, adiposity (orexigenic pathways), gut motility and growth hormone release (1, 2), but is now recognized as having important protective roles in both the central nervous system and the cardiovascular system and is particularly important for the protection of endothelial cell function (3). The gut is the largest producer of this peptide, but the heart and vasculature also express ghrelin at much lower levels. Dependent on metabolic status, most of the circulating ghrelin is of the unacylated or desacyl ghrelin (DAG) form, which was initially considered to be an inactive form. Following cleavage of pre-proghrelin, ghrelin O-acyltransferase (GOAT) catalyzes the attachment of an acyl side branch at Serine 3 residue of proghrelin to produce the acyl ghrelin (AG) form (Fig. 1), enabling it to bind the growth hormone secretagogue receptor 1a (GHS-R1a) (1). Neural and cardioprotective roles for endogenous and exogenous AG have been described by numerous research groups. Most of these actions have been attributed or directly shown experimentally to be mediated by activation of GHS-R1a or to a lesser extent, mediated indirectly by release of growth hormone. However, it is important to recognize that other endogenous peptides are also considered to be released when pre-proghrelin is cleaved. Obestatin is a peptide derived from this cleavage that might have protective actions, particularly regarding pancreatic function and glucose homeostasis, but is yet to be clearly resolved (4). This brief review focuses on the roles that AG and its known receptor pathway play in modulating endothelial cell function and perfusion during conditions when vascular function becomes compromised and the known mechanisms involving immune cells and the endothelium.
Figure 1.
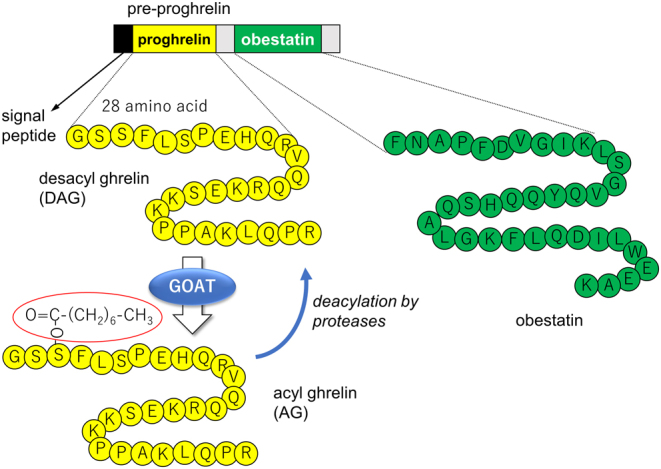
Pre-proghrelin produced within ghrelin cells is cleaved to produce ghrelin and obestatin. Ghrelin peptide is then present in these cells as unacylated or desacyl ghrelin (DAG) or becomes acylated with an 8-carbon fatty acid side chain (shown in red ring) at Serine 3 residue by ghrelin O-acyltransferase (GOAT) to become acyl ghrelin (AG). Proteases are responsible for the degradation of AG to DAG.
Ghrelin acts to preserve energy balance and long-term regulation of body weight (5). Indeed, AG has been consistently shown to negatively regulate proinflammatory pathways and reduce oxidative and nitrosative stress, as described below. Since both acute and chronic inflammation and an excess of reactive oxygen and nitrogen species impair metabolic, neural, cardiac and vascular function, these important inhibitory actions of AG can be considered to act to maintain organ homeostasis and survival. Herein, we will focus on the actions of ghrelin peptides that modulate vascular function and describe briefly some of the studies that have demonstrated the therapeutic potential of ghrelin treatment, or pretreatment in some cases, for cardiovascular protection (summarized in Fig. 2 and Table 1). However, it is important to state that the vascular protection potential of exogenous ghrelin, and particularly AG, needs to be weighed against the potentially maladaptive actions of ghrelin signaling in promoting adiposity and reducing glucose-stimulated insulin secretion and promoting glucose intolerance (6, 7).
Figure 2.
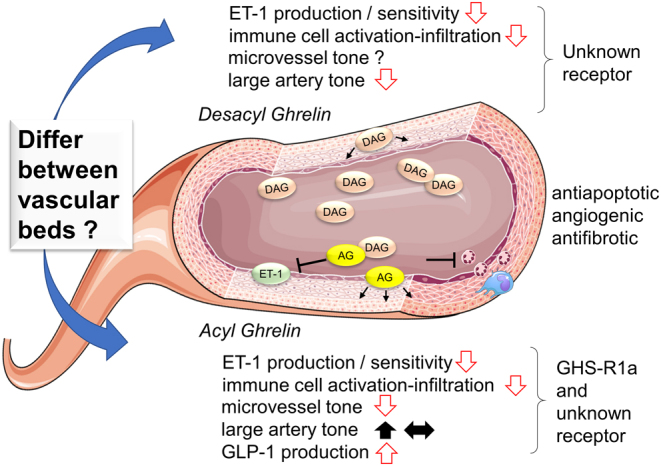
Summary of the reported effects of endogenous and exogenous acyl ghrelin (AG) and desacyl ghrelin (DAG) in the vasculature and their receptor pathways. ET-1, endothelin-1; GHS-R1a, growth hormone secretagogue receptor 1a; GLP-1, glucagon-like peptide-1.
Table 1.
Summary of the main studies and the reported vascular actions of acyl and desacyl ghrelin.
| Acyl ghrelin (AG) | Desacyl ghrelin (DAG) | |||
|---|---|---|---|---|
| Actions | Reference | Actions | Reference | |
| Cell types expressing ghrelin protein and receptor pathway | Endothelium | (10, 11) | ||
| VSMC | ||||
| T lymphocytes and macrophages | (5) | |||
| Low level in plasma | Plasma levels 3–4 fold greater than AG | |||
| Evokes GH release | (11) | Does not evoke GH release | (11) | |
| GHS-R1a on VSMC | Does not bind GHS-R1a | |||
| Unknown receptor also likely | (3) | Receptor pathway unknown | ||
| Suppression of ET-1 signaling and modulation of vascular tonea | Acute and chronic inhibition of ET-1 mediated vasoconstriction | (10, 11, 19, 33) | Acute inhibition of ET-1-mediated vasoconstriction | (10, 11) |
| Resistance vessel dilation in rat and human | (10, 11, 16) | |||
| Coronary arteriole constriction in dog | ||||
| Conduit artery constriction or no change in rat and human | (15, 16, 17) | |||
| Coronary flow ↓ in pig | Nitric oxide dependent coronary flow ↑ in pig | (13) | ||
| Coronary perfusion pressure ↑ in rat | (12) | |||
| Systemic hypotension via inhibition of adrenergic tone | (8, 9, 12, 14, 15) | |||
| Restored endothelium-mediated dilation and inhibited superoxide in disease states | (24, 32, 33) | |||
| Suppression of endothelial activation and inflammation | Cytokine-mediated vascular inflammation and oxidative stress ↓ | (21, 22, 23, 24, 25, 26) | Restored endothelial antioxidant balance and oxidative stress ↓ | (38) |
| Suppression of monocyte and T-lymphocyte activation and infiltration | (5, 26) | |||
| Suppression of monocyte and T-lymphocyte production of cytokines | (23, 27) | |||
| Sympathetic potentiation of cytokine release ↓ | (28) | |||
| Amelioration of vascular injury and rarefaction | Antiapoptotic, antifibrotic, proangiogenic | (3, 24, 25) | Antiapoptotic, antifibrotic, proangiogenic | (3, 38) |
| Upregulation of GHS-R1a density | (31) | |||
| Reduced rarefaction in pulmonary disease | (19) | |||
aSomewhat contrasting findings are indicated in bold, which suggest that the responses probably differ between vascular beds and vessel sizes.
ET-1, endothelin-1; GH, growth hormone; GHS-R1a, growth hormone secretagogue receptor 1a; VSMC, vascular smooth muscle cells.
AG inhibits endothelin-1 and directly reduces vasomotor tone
It was established in early studies following the discovery of ghrelin that acute administration of AG lowers arterial blood pressure (8, 9); however, there has been considerable debate whether AG directly modulates vascular tone or alternatively, evokes hypotension through direct inhibition of either sympathetic outflow or sympathetic afferent activity. Utilizing endothelium-denuded internal mammary artery segments, it was shown that AG and DAG both potently inhibit endothelin-1 (ET-1)-mediated vasoconstriction and that AG protein was expressed in endothelial cells of coronary microvessels (10, 11). In contrast, in anesthetized pigs, net coronary flow decreased during intracoronary AG infusion, which was inhibited by phentolamine (12). The same authors later showed that intracoronary DAG infusion evoked a nitric oxide (NO)-dependent increase in coronary flow (13). Furthermore, AG increased coronary perfusion pressure in the isolated rat heart and evoked constriction in isolated coronary arterioles (14). Callaghan and associates (15) have reasoned that AG does not directly alter vasomotor tone, since mesenteric and aortic segments did not dilate to AG, but rather AG might evoke hypotension by inhibiting sympathetic afferent activity to release tonic constrictor tone. Nonetheless, we have shown with in vivo microangiography that vessel responses evoked by AG appear to be dependent on vessel size as AG constricted large conduit arteries while dilating arterioles in the same vascular bed in rats following either induction of myocardial infarction or sham procedure (16). Beta-blockade with propranolol did not significantly alter vessel responses to AG. However, selective GHS-R1a inhibition blocked both dilator and constrictor responses to AG in sham-treated rats (16). In the same study, AG evoked dose-dependent dilation of human internal mammary arteries. Interestingly, AG-mediated coronary constrictor responses in canine arterioles were found to be independent of GHS-R1a (17). Hence, AG appears to evoke systemic hypotension but not all vascular beds exhibit vasodilator responses to acute AG administration. The reductions in coronary blood flow reported in some studies might be the result of GHS-R1a mediated vasoconstriction of the large arteries. In addition, there appears to be significant differences between species in GHS-R1a distribution and the acute vascular effects of AG and DAG, with some responses likely to be mediated by a yet unidentified receptor pathway. However, it needs to be emphasized that the receptor pathway for DAG actions remains to be identified.
In patients with chronic heart failure repeated intravenous AG infusion for 3 weeks improved left ventricular function, muscle wasting and exercise capacity (18). However, it is not known whether the beneficial actions of AG treatment involved changes in coronary function. Chronic AG treatment does appear to ameliorate endothelial dysfunction and vascular smooth muscle sensitivity to constriction by ET-1. In the chronic hypoxia model of pulmonary hypertension, we found that concurrent treatment with AG reduced the exaggerated constrictor responses across the pulmonary arterial bed, reduced ET-1 protein expression and vessel rarefaction (19). Furthermore, chronic AG treatment restored the impairment of acetylcholine-mediated vasodilation in thoracic aortas in growth hormone-deficient rats (20). Thus, chronic AG treatment improves endothelium-independent and -dependent vasodilation and the regulation of perfusion.
AG reduces vascular injury and enhances angiogenesis
Various lines of evidence indicate that AG and DAG both exert cytoprotective effects on endothelial cells, although DAG is not well understood. Both ghrelin forms inhibit cardiomyocyte and endothelial cell apoptosis through activation of extracellular signal-related kinase 1/2 (ERK1/2) and an unknown ghrelin receptor pathway (3). As noted earlier, we reported that AG prevented vessel pruning in the lungs conceivably by reducing apoptosis (19). Ischemia, septic shock and cardiopulmonary bypass-induced injury all involve pronounced cytokine mediated vascular inflammation and oxidative stress, which have been shown to be ameliorated by AG treatment (21, 22, 23, 24, 25, 26). AG suppresses interleukin-6 and tumor necrosis factor-α generation by lymphocytes and monocytes in part through vagal efferent release of acetylcholine, which turns off production (23, 27), and in part by central inhibition of sympathetic potentiation of cytokine release (28) and has been shown to suppress immune cell recruitment to vascular injury sites following endothelial activation (26). Indeed, T lymphocytes and monocytes express both AG and GHS-R1a, which function as negative regulators of these key cytokines (5). In the gastric submucosa it has been shown by microdialysis that local catecholamines evoke ghrelin release from the abundant ghrelin cells (29). It is not clear if these neurotransmitters regulate ghrelin secretion to a similar extent in the cardiovascular system, but the autonomic nervous system clearly plays important and complex roles in ghrelin’s actions.
Bearing in mind that circulating levels of ghrelin are low (10–20 fmol/mL) and that AG are lower than DAG, the levels of production of these peptides varies considerably among disease states, but various evidence suggests exogenous AG administration is able to improve endothelial dysfunction and promote angiogenesis. Ghrelin levels are low in obesity but increase during physiological and psychological conditions that evoke sympathetic overactivation (30). Interestingly, plasma levels of ET-1 and thromboxane have been reported to be elevated in patients with coronary artery disease and atherosclerotic coronary arteries show not only elevated thromboxane receptor density but also four-fold upregulation of GHS-R1a density in vascular smooth muscle layer relative to non-diseased arteries (31). As suggested by the authors of the latter study, increased expression of the receptor for AG might reflect a compensatory action of ghrelin signaling to ameliorate ET-1-mediated vasoconstriction and vascular remodeling. Furthermore, AG treatment was found to restore NO bioavailability and endothelial function in small arteries isolated from biopsies taken from essential hypertension patients, in part by inhibition of superoxide generation (32). In obese human subjects with metabolic syndrome even an acute infusion of AG was shown to normalize vasoconstrictor actions of ET-1 through increased NO-mediated dilation, potentially restoring vasodilator–constrictor balance (33). Indeed, chronic AG treatment rescued endothelial function and promoted angiogenesis in a hindlimb ischemia mouse model through potentiation of proangiogenic, antifibrotic and antiapoptotic pathways including activation of PI3K/Akt, vascular endothelial growth factor and modulation of miRNA (potentiation of miR-126, -132, -30a and inhibition of miR-21, -92a and -206) (24). While the proangiogenic properties of ghrelin are beneficial in pathological states where microvascular density is reduced (25), it is also clear that ghrelin signaling is central to the progression of some carcinomas (34) even though AG treatment has shown benefits in increasing body weight and reducing muscle wasting in cancer cachexia.
Effects of AG on glucose homeostasis and associated vascular complications
The roles of ghrelin peptides in glucose homeostasis have been a hot area of research in recent years and is beyond the scope of this review on vascular protection. However, it is noteworthy that ghrelin peptides have opposing effects, as DAG promotes insulin secretion and insulin sensitivity while AG inhibits glucose-stimulated insulin release and increases lipogenesis, contributing to glucose intolerance (6, 35); see recent review (7). For these reasons, based on various experimental and clinical evidence, some consider ghrelin antagonism to be a therapeutic target for type 2 diabetes (7). Nevertheless, it is important to understand that impaired incretin signaling contributes to glucose intolerance and poor glycemic control in type 2 diabetes. It has been suggested that ghrelin might not be the cause of metabolic dysfunction, but rather impaired endogenous AG/GHS-R1a regulation of glucagon-like peptide-1 (GLP-1) release following meal ingestion (36). Indeed, these authors showed with multiple lines of evidence that AG administration enhanced GLP-1 release and glucose tolerance. Here, it is important to acknowledge that it is now well established that GLP-1 itself has been shown to have important cardiovascular protective actions. Moreover, one study has shown that AG infusion in patients with metabolic syndrome acutely enhanced endothelium-dependent dilation (brachial artery) (37). Whether sustained improvements in vascular function can be obtained with ghrelin treatment remains unknown. On a final note, while studies of ghrelin effects on diabetic vascular complications are few, it has been shown that DAG treatment in a mouse model of glucose intolerance and hindlimb ischemia restored antioxidant superoxide dismutase-2 expression, sirtuin 1 and proangiogenic miR-126 levels and hindlimb vessel density while reducing inflammation (38).
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
Funding
The authors are supported by intramural funds of the National Cerebral and Cardiovascular Center (30-1-13) and a Grant-in-Aid for Scientific Research (KAKEN 19H03405) from the Ministry of Education, Culture, Sports, Sciences and Technology of Japan.
References
- 1.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999. 656–660. ( 10.1038/45230) [DOI] [PubMed] [Google Scholar]
- 2.Tschöp M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature 2000. 908–913. ( 10.1038/35038090) [DOI] [PubMed] [Google Scholar]
- 3.Baldanzi G, Filigheddu N, Cutrupi S, Catapano F, Bonissoni S, Fubini A, Malan D, Baj G, Granata R, Broglio F. et al. Ghrelin and des-acyl ghrelin inhibit cell death in cardiomyocytes and endothelial cells through ERK1/2 and PI 3-kinase/AKT. Journal of Cell Biology 2002. 1029–1037. ( 10.1083/jcb.200207165) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cowan E, Burch KJ, Green BD, Grieve DJ. Obestatin as a key regulator of metabolism and cardiovascular function with emerging therapeutic potential for diabetes. British Journal of Pharmacology 2016. 2165–2181. ( 10.1111/bph.13502) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dixit VD, Schaffer EM, Pyle RS, Collins GD, Sakthivel SK, Palaniappan R, Lillard JW, Jr, Taub DD. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. Journal of Clinical Investigation 2004. 57–66. ( 10.1172/JCI21134) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dezaki K, Hosoda H, Kakei M, Hashiguchi S, Watanabe M, Kangawa K, Yada T. Endogenous ghrelin in pancreatic islets restricts insulin release by attenuating Ca2+ signaling in β-cells. Diabetes 2004. 3142–3151. ( 10.2337/diabetes.53.12.3142) [DOI] [PubMed] [Google Scholar]
- 7.Poher AL, Tschöp MH, Müller TD. Ghrelin regulation of glucose metabolism. Peptides 2018. 236–242. ( 10.1016/j.peptides.2017.12.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagaya N, Kojima M, Uematsu M, Yamagishi M, Hosoda H, Oya H, Hayashi Y, Kangawa K. Hemodynamic and hormonal effects of human ghrelin in healthy volunteers. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 2001. R1483–R1487. ( 10.1152/ajpregu.2001.280.5.R1483) [DOI] [PubMed] [Google Scholar]
- 9.Okumura H, Nagaya N, Enomoto M, Nakagawa E, Oya H, Kangawa K. Vasodilatory effect of ghrelin, an endogenous peptide from the stomach. Journal of Cardiovascular Pharmacology 2002. 779–783. ( 10.1097/00005344-200206000-00001) [DOI] [PubMed] [Google Scholar]
- 10.Wiley KE, Davenport AP. Comparison of vasodilators in human internal mammary artery: ghrelin is a potent physiological antagonist of endothelin-1. British Journal of Pharmacology 2002. 1146–1152. ( 10.1038/sj.bjp.0704815) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kleinz MJ, Maguire JJ, Skepper JN, Davenport AP. Functional and immunocytochemical evidence for a role of ghrelin and des-octanoyl ghrelin in the regulation of vascular tone in man. Cardiovascular Research 2006. 227–235. ( 10.1016/j.cardiores.2005.09.001) [DOI] [PubMed] [Google Scholar]
- 12.Grossini E, Molinari C, Mary DA, Ghigo E, Bona G, Vacca G. Intracoronary ghrelin infusion decreases coronary blood flow in anesthetized pigs. Endocrinology 2007. 806–812. ( 10.1210/en.2006-1230) [DOI] [PubMed] [Google Scholar]
- 13.Grossini E, Raina G, Farruggio S, Camillo L, Molinari C, Mary D, Walker GE, Bona G, Vacca G, Moia S. et al. Intracoronary des-acyl ghrelin acutely increases cardiac perfusion Through a nitric oxide-related mechanism in female anesthetized pigs. Endocrinology 2016. 2403–2415. ( 10.1210/en.2015-1922) [DOI] [PubMed] [Google Scholar]
- 14.Pemberton CJ, Tokola H, Bagi Z, Koller A, Pöntinen J, Ola A, Vuolteenaho O, Szokodi I, Ruskoaho H. Ghrelin induces vasoconstriction in the rat coronary vasculature without altering cardiac peptide secretion. American Journal of Physiology-Heart and Circulatory Physiology 2004. H1522–H1529. ( 10.1152/ajpheart.00193.2004) [DOI] [PubMed] [Google Scholar]
- 15.Callaghan B, Hunne B, Hirayama H, Sartor DM, Nguyen TV, Abogadie FC, Ferens D, McIntyre P, Ban K, Baell J. et al. Sites of action of ghrelin receptor ligands in cardiovascular control. American Journal of Physiology-Heart and Circulatory Physiology 2012. H1011–H1021. ( 10.1152/ajpheart.00418.2012) [DOI] [PubMed] [Google Scholar]
- 16.Pearson JT, Collie N, Lamberts RR, Inagaki T, Yoshimoto M, Umetani K, Davis P, Wilkins G, Jones PP, Shirai M. et al. Ghrelin preserves ischemia-induced vasodilation of male rat coronary vessels following beta-adrenergic receptor blockade. Endocrinology 2018. 1763–1773. ( 10.1210/en.2017-03070) [DOI] [PubMed] [Google Scholar]
- 17.Sax B, Nadasy GL, Turi K, Hirschberg K, Furjesz D, Nagy A, Merkely B, Szabo G, Monos E, Kekesi V. Coronary vasoconstrictor effect of ghrelin is not mediated by growth hormone secretagogue receptor 1a type in dogs. Peptides 2011. 362–367. ( 10.1016/j.peptides.2010.11.020) [DOI] [PubMed] [Google Scholar]
- 18.Nagaya N, Moriya J, Yasumura Y, Uematsu M, Ono F, Shimizu W, Ueno K, Kitakaze M, Miyatake K, Kangawa K. Effects of ghrelin administration on left ventricular function, exercise capacity, and muscle wasting in patients with chronic heart failure. Circulation 2004. 3674–3679. ( 10.1161/01.CIR.0000149746.62908.BB) [DOI] [PubMed] [Google Scholar]
- 19.Schwenke DO, Gray EA, Pearson JT, Sonobe T, Ishibashi-Ueda H, Campillo I, Kangawa K, Umetani K, Shirai M. Exogenous ghrelin improves blood flow distribution in pulmonary hypertension-assessed using synchrotron radiation microangiography. Pflugers Archiv 2011. 397–406. ( 10.1007/s00424-011-0992-8) [DOI] [PubMed] [Google Scholar]
- 20.Shimizu Y, Nagaya N, Teranishi Y, Imazu M, Yamamoto H, Shokawa T, Kangawa K, Kohno N, Yoshizumi M. Ghrelin improves endothelial dysfunction through growth hormone-independent mechanisms in rats. Biochemical and Biophysical Research Communications 2003. 830–835. ( 10.1016/j.bbrc.2003.09.085) [DOI] [PubMed] [Google Scholar]
- 21.Wu R, Dong W, Zhou M, Zhang F, Marini CP, Ravikumar TS, Wang P. Ghrelin attenuates sepsis-induced acute lung injury and mortality in rats. American Journal of Respiratory and Critical Care Medicine 2007. 805–813. ( 10.1164/rccm.200604-511OC) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao Y, Tang J, Yang T, Ma H, Yi D, Gu C, Yu S. Cardioprotective effect of ghrelin in cardiopulmonary bypass involves a reduction in inflammatory response. PLoS ONE 2013. e55021 ( 10.1371/journal.pone.0055021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheyuo C, Wu R, Zhou M, Jacob A, Coppa G, Wang P. Ghrelin suppresses inflammation and neuronal nitric oxide synthase in focal cerebral ischemia via the vagus nerve. Shock 2011. 258–265. ( 10.1097/SHK.0b013e3181f48a37) [DOI] [PubMed] [Google Scholar]
- 24.Katare R, Rawal S, Munasinghe PE, Tsuchimochi H, Inagaki T, Fujii Y, Dixit P, Umetani K, Kangawa K, Shirai M. et al. Ghrelin promotes functional angiogenesis in a mouse model of critical limb ischemia through activation of proangiogenic microRNAs. Endocrinology 2016. 432–445. ( 10.1210/en.2015-1799) [DOI] [PubMed] [Google Scholar]
- 25.Neale JPH, Pearson JT, Katare R, Schwenke DO. Ghrelin, microRNAs, and critical limb ischemia: hungering for a novel treatment option. Frontiers in Endocrinology 2017. 350 ( 10.3389/fendo.2017.00350) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sukumaran V, Tsuchimochi H, Fujii Y, Hosoda H, Kangawa K, Akiyama T, Shirai M, Tatsumi E, Pearson JT. Ghrelin pre-treatment attenuates local oxidative stress and end organ damage During cardiopulmonary bypass in anesthetized rats. Frontiers in Physiology 2018. 196 ( 10.3389/fphys.2018.00196) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu R, Dong W, Cui X, Zhou M, Simms HH, Ravikumar TS, Wang P. Ghrelin down-regulates proinflammatory cytokines in sepsis through activation of the vagus nerve. Annals of Surgery 2007. 480–486. ( 10.1097/01.sla.0000251614.42290.ed) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu R, Zhou M, Das P, Dong W, Ji Y, Yang D, Miksa M, Zhang F, Ravikumar TS, Wang P. Ghrelin inhibits sympathetic nervous activity in sepsis. American Journal of Physiology-Endocrinology and Metabolism 2007. E1697–E1702. ( 10.1152/ajpendo.00098.2007) [DOI] [PubMed] [Google Scholar]
- 29.de la Cour CD, Norlén P, Håkanson R. Secretion of ghrelin from rat stomach ghrelin cells in response to local microinfusion of candidate messenger compounds: a microdialysis study. Regulatory Peptides 2007. 118–126. ( 10.1016/j.regpep.2007.05.001) [DOI] [PubMed] [Google Scholar]
- 30.Muller TD, Nogueiras R, Andermann ML, Andrews ZB, Anker SD, Argente J, Batterham RL, Benoit SC, Bowers CY, Broglio F. et al. Ghrelin. Molecular Metabolism 2015. 437–460. ( 10.1016/j.molmet.2015.03.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katugampola SD, Kuc RE, Maguire JJ, Davenport AP. G-protein-coupled receptors in human atherosclerosis: comparison of vasoconstrictors (endothelin and thromboxane) with recently de-orphanized (urotensin-II, apelin and ghrelin) receptors. Clinical Science 2002. 171S–175S. ( 10.1042/CS103S171S) [DOI] [PubMed] [Google Scholar]
- 32.Virdis A, Duranti E, Colucci R, Ippolito C, Tirotta E, Lorenzini G, Bernardini N, Blandizzi C, Taddei S. Ghrelin restores nitric oxide availability in resistance circulation of essential hypertensive patients: role of NAD(P)H oxidase. European Heart Journal 2015. 3023–3030. ( 10.1093/eurheartj/ehv365) [DOI] [PubMed] [Google Scholar]
- 33.Tesauro M, Schinzari F, Rovella V, Di Daniele N, Lauro D, Mores N, Veneziani A, Cardillo C. Ghrelin restores the endothelin 1/nitric oxide balance in patients with obesity-related metabolic syndrome. Hypertension 2009. 995–1000. ( 10.1161/HYPERTENSIONAHA.109.137729) [DOI] [PubMed] [Google Scholar]
- 34.Okada Y, Sugita Y, Ohshima K, Morioka M, Komaki S, Miyoshi J, Abe H. Signaling of ghrelin and its functional receptor, the growth hormone secretagogue receptor, promote tumor growth in glioblastomas. Neuropathology 2016. 535–543. ( 10.1111/neup.12315) [DOI] [PubMed] [Google Scholar]
- 35.Dezaki K, Sone H, Koizumi M, Nakata M, Kakei M, Nagai H, Hosoda H, Kangawa K, Yada T. Blockade of pancreatic islet–derived ghrelin enhances insulin secretion to prevent high-fat diet–induced glucose intolerance. Diabetes 2006. 3486–3493. ( 10.2337/db06-0878) [DOI] [PubMed] [Google Scholar]
- 36.Gagnon J, Baggio LL, Drucker DJ, Brubaker PL. Ghrelin is a novel regulator of GLP-1 secretion. Diabetes 2015. 1513–1521. ( 10.2337/db14-1176) [DOI] [PubMed] [Google Scholar]
- 37.Tesauro M, Schinzari F, Iantorno M, Rizza S, Melina D, Lauro D, Cardillo C. Ghrelin improves endothelial function in patients with metabolic syndrome. Circulation 2005. 2986–2992. ( 10.1161/CIRCULATIONAHA.105.553883) [DOI] [PubMed] [Google Scholar]
- 38.Togliatto G, Trombetta A, Dentelli P, Gallo S, Rosso A, Cotogni P, Granata R, Falcioni R, Delale T, Ghigo E. et al. Unacylated ghrelin induces oxidative stress resistance in a glucose intolerance and peripheral artery disease mouse model by restoring endothelial cell miR-126 expression. Diabetes 2015. 1370–1382. ( 10.2337/db14-0991) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a