Abstract
The first plant-derived, purified pharmaceutical-grade cannabidiol (CBD) medication, Epidiolex, was approved in the United States by the FDA on June 25, 2018. Its approval for patients ≥ 2 years of age with Dravet syndrome (DS) or Lennox-Gastaut syndrome (LGS) markedly altered the treatment of medically refractory seizures in these disorders. This state-of-the-art review will discuss the history of CBD, its current pharmacology and toxicology, evidence supporting its use in a variety of epileptic syndromes, common side effects and adverse effects, and pharmacokinetically based drug-drug interactions. Owing to the importance in considering side effects, adverse effects, and drug-drug interactions in patients with medically refractory epilepsy syndromes, this review will take a deeper look into the nuances of the above within a clinical context, as compared to the other antiepileptic medications. Furthermore, despite the limited data regarding clinically significant drug-drug interactions, potential pharmacokinetic drug-drug interactions with CBD and other antiepileptics are theorized on the basis of their metabolic pathways. The article will further elucidate future research in terms of long-term efficacy, safety, and drug interactions that is critical to addressing unanswered questions relevant to clinical practice.
Keywords: antiepileptics; cannabidiol; CBD; epilepsy; pediatrics; Lennox-Gastaut, Dravet syndrome
Introduction
On June 25, 2018, the first plant-derived, purified pharmaceutical-grade cannabidiol (CBD) medication, Epidiolex (Greenwich Biosciences, Inc, Carlsbad, CA), was approved by the FDA for patients ≥ 2 years of age with Dravet syndrome (DS) or Lennox-Gastaut syndrome (LGS).1–4
The road to therapeutic use of CBD, and its recent approval for seizure disorders, has been a long one.5–8 While the commercial availability of a regulated, pharmaceutical CBD product is a recent phenomenon, cannabinoid use has been documented within the United States Pharmacopeia as early as 18506,8 and internationally as early as 2000 BC.6–8 Furthermore, Δ9-tetrahydrocannabinol [Δ9-THC] has been identified in ashes, which directly links the medicinal use of cannabis to around 400 AD.6 The long-withstanding controversy about the therapeutic use of cannabis has resulted in federal regulations that have restricted use and hampered medical research of cannabis with subsequent ramifications.6–8
After the passage of the Marihuana Tax Act of 1937, the use of cannabis was federally restricted owing to the rise in hemp product use and the financial implications this could have for newspaper tycoon, William Randolph Hearst, and his paper products.7–9 This tax act penalized the prescribing physicians and dispensing pharmacists, despite its medicinal use prior to the act.7 The passage of the Cannabis Control Act in 1970 officially outlawed any use for marijuana, including medicinal, with its status as a schedule 1 controlled substance.10,11 Since 1986, support for the legalization of marijuana has steadily increased across all political parties according to the General Social Survey.9 Within the 33 US states that support the use of marijuana for either recreational or medicinal purposes, patients must have a legal qualifying condition to obtain the product for medical reasons.9,12 While there is variability amongst each state, these legal qualifying conditions, defined by the American College of Physicians in 1996, include HIV/AIDS, cancer, severe muscle spasm, amyotrophic lateral sclerosis, end of life (defined by <1 year life expectancy), Crohn disease, seizure disorders, glaucoma, and Tourette syndrome.10,13 Despite the recent FDA approval, controversy continues to surround the legality of purified CBD owing to the plethora of cannabis products available without complete understanding of the appropriate terminology.14
As the use of medical marijuana has increased, specifically in seizure disorders, the distinction between the various compounds, or phytocannabinoids, within marijuana plants has become more clearly defined.6,13,15 Phytocannabinoids are a class of compounds derived from the plants Cannabis sativa, Cannabis ruderalis, and Cannabis indica.11 These compounds can be psychoactive or non-psychoactive.6,11 The most well-known, psychoactive phytocannabinoid is Δ9-THC, which exerts various therapeutic and non-therapeutic effects through its interaction with CB1 receptors; however, CBD, the most well-known non-psychoactive phytocannabinoid, has a low affinity for both CB1 and CB2 receptors.6,11,13 While both compounds have therapeutic properties, purified CBD may have a more favorable role for some indications owing to absence of the psychoactive effects noted with the other cannabinoid compounds.3,4,16 While CBD is known to exhibit anti-epileptic properties, it may possess anti-inflammatory and antioxidant activity despite having a low affinity for the CB1 and CB2 receptors.6,17 While the mechanism for the anticonvulsant activity is still largely unknown, it is theorized to reduce seizure severity and frequency in a multifactorial fashion through the modulation of serotonin, gamma-amino butyric acid, T-type calcium channels, N-methyl-D-aspartate, and other neurochemicals.6,17 In recent animal studies, CBD has been shown to act independently of sodium channels and reflect more of a multimodal cell-type–dependent mechanism that terminates excitatory neuron action potentials.19 Despite the lack of in vivo anticonvulsant properties of the blocking of voltage-gated sodium channel, recent studies have shown that CBD may preferentially target resurgent sodium currents from mutant channels, which can be seen in syndromic epilepsies such as DS.17
Epidiolex, a plant-derived CBD product with the Δ9-THC synthetically extracted, was originally scheduled as a schedule 5 controlled substance however was descheduled by the Drug Enforcement Adminstration (DEA) in April 2020.1,3,4,18 Despite having an approved and regulated CBD pharmaceutical product available, current restrictions unfortunately foster controversy around the use of other products that are marketed as “CBD oil.” With prior authorizations and insurance hurdles, Epidiolex may be restricted to the 2 refractory epilepsy disorders with FDA-approved indications.1 As of March 2020, Greenwich Biosciences is pursuing FDA approval for an additional indication for tuberous sclerosis after the release of promising results from GWPCARE6.20,21 Consequently, there is difficulty in obtaining Epidiolex off-label for patients with other medically refractory epilepsy disorders.14,22 In lieu of obtaining Epidiolex, the widespread prevalence of other CBD products on the market has become more and more controversial owing to the lack of regulation by the FDA. To address these controversies, the FDA states they are “aware that some companies are marketing products containing cannabis and cannabis-derived compounds in ways that violate the Federal Food, Drug, and Cosmetic Act and that may put the health and safety of consumers at risk.”14 Further concerns surrounding these unregulated products include lack of standardization of nomenclature, compounds, and concentration of various compounds. Although specific concentrations may be advertised on these alternate products, the innate variability results in an inability to validate the concentration of CBD in these products.
Standard of Care for Medically Refractory Epilepsy Management
Currently, Epidiolex has a seemingly narrow niche with FDA approvals for 2 rare childhood epilepsy syndromes, DS and LGS.1–3 Dravet syndrome affects approximately 1:15,700 children, while LGS is even more rare affecting approximately 1:200,000 children.23–27 Patients with these aforementioned syndromes experience seizures that vary in subtype, severity, and frequency, with patients having up to hundreds of seizures a day that may cause a patient to pass prior to reaching adulthood.27,28 Seizure subtypes in DS include tonic, clonic, tonic-clonic, and atonic seizures.28 Patients with LGS can present with a multitude of seizure types; however, drop, or atonic seizures, and tonic seizures are the most common.4,24,26 Patients with LGS generally have a poor prognosis for seizure control and cognitive development.4,29 Drop seizures, also known as “drop attacks,” in which a patient suddenly falls to the ground, can be very dangerous for children, often leading to injury.4,24,30 Sudden unexplained death in epilepsy patients is the primary cause of death in these populations.28 Patients with DS and LGS are often on complicated regimens consisting of both pharmacologic and non-pharmacologic treatment modalities with persistent breakthrough seizures.27,31,32 In both DS and LGS, clinicians use a combination of antiepileptic drugs (AEDs) as well as non-pharmacologic therapies to reduce seizure severity and frequency.24,27 Nonpharmacologic therapies for refractory epilepsy such as ketogenic diet (KGD) and vagal nerve stimulation are recommended to be considered by both the American Epilepsy Society and the American Academy of Neurology.33,34 However, these modalities are not without their complications.33,34
First-line AED therapy in DS includes valproate (VPA) derivatives and CLB with second-line agents consisting of stiripentol and topiramate. Despite not being approved for use in specific seizure disorders such as LGS or DS, VPA derivatives are broad-spectrum AEDs that are one of the first-line agents used in DS and LGS.29,35–37 Stiripentol, having been used in Europe, has recently gained FDA approval in the United States on August 20, 2018, but has limited commercial availability to date.31,32 Furthermore, the limited evidence supporting the use of stiripentol in pediatric patients further compounds the lack of viable options for treatment in these patients. Clonazepam, levetiracetam, zonisamide, and ethosuximide can be considered when previous therapies prove to be ineffective or intolerable.25,28 Second-line agents for LGS include but are not limited to lamotrigine, topiramate, CLB, and phenytoin.24,25 Unfortunately there is not a single drug that has shown to be highly efficacious in DS, and monotherapy is rarely used, making optimal treatment uncertain and complex.29 Most patients do not achieve seizure freedom despite the use of multiple pharmacologic and non-pharmacologic treatment modalities.17,27,31
Additionally, the current therapies do not go without consequences. Adverse drug reactions (ADRs) are quite common with AEDs, including first-line agents such as VPA derivatives and CLB.35,38 Valproate derivatives have potentially life-threatening black box warnings including hepatic failure and acute pancreatitis.35 Other notable reasons for discontinuation of VPA include tremors, blood disorders, and the possibility for extensive drug interactions.33 Further considerations in our female patients of childbearing age include both the teratogenic risks, most notably manifesting as neural tube defects, and the reproductive and cosmetic risks including polycystic ovarian syndrome, menstrual abnormalities, severe weight gain, and alopecia.35 Clobazam may cause drowsiness, lethargy, and aggressiveness.38 Additionally, when used concomitantly with opioids, alcohol, antidepressants, or other central nervous system depressants, CLB has the serious life-threatening risk of profound sedation, respiratory depression, coma, and death.23,38 Furthermore, serious dermatologic reactions have been reported with other AED use (i.e., lamotrigine) such as Stevens-Johnson syndrome and toxic epidermal necrolysis.35,38 Patients who experience a drug-related rash not only must seek medical attention but now also have limited the number of viable AEDs.39–41 The primary cause for therapeutic failure of a medication is lack of tolerability or serious ADR, consequently increasing the difficulty of treating refractory seizure disorders.3,4 Owing to the complexities of these regimens and the ADRs associated with the treatments, patients with rare refractory epileptic conditions, such as LGS and DS, could benefit greatly from additional therapeutic modalities.
Safety and Efficacy of CBD
Dravet Syndrome. In the 2 pivotal randomized double-blind, multicenter, clinical trials investigating the effect of CBD on patients with DS, Devinsky et al3,42 evaluated the dose-ranging safety, tolerability, pharmacokinetics, and efficacy as compared to placebo. The results of these trials ultimately led to the approval of CBD for the treatment of DS.3,4 In the GWPCARE1 trial, 120 patients with DS were enrolled (median age, 9.2 years [2.3–18.4]) who were taking an average of 2.9 ± 1 concomitant AEDs, having previously experienced failure with an average of 4.6 ± 3.8 AEDs.3 Of the 120 patients who underwent randomization to be included in the intention-to-treat and safety analysis sets, 59 patients were randomly assigned to the placebo arm and 61 patients were randomly assigned to the treatment arm of 20 mg/kg/day.3 Of note, placebo was an identical strawberry sesame oil solution without the active ingredient provided by the manufacturer, taken alongside standard of care.3 A statistically significant absolute reduction in the median number of convulsive seizures per month was observed with a difference of −22.8 (−41.1 to −5.4) percentage points (N = 120; p = 0.01) in the convulsive-seizure frequency for patients initiated on CBD as compared to placebo alone.3 Furthermore, 42.5% (n = 51, p = 0.08) of patients initiated on CBD achieved a 50% reduction in convulsive-seizure frequency.3 There were no statistically significant changes observed with other specific seizure subtypes such as tonic-clonic seizures (p = 0.07), tonic seizures (p = 0.20), clonic seizures (p = 0.84), atonic seizures (p = 0.24), myoclonic seizures (p = 0.20), countable partial seizures (p = 0.08), other partial seizures (p = 1.0), and absence seizures (p = 0.5).3 Investigators attribute this to a lack of power amongst these subtypes.3 While the overall study had enough participants to detect a difference amongst the primary outcome, these subtypes may have been underpowered and thus future research is warranted to determine efficacy in these seizure subtypes.3 In the case of absence seizures and total non-convulsive seizures, the lack of significant reduction may be due to the difficulty in the reliability of recording these episodes. In another safety analysis that evaluated CBD in DS,16 reductions in seizure frequency were not only similar to those observed within previous trials, but also demonstrated the persistent reduction in seizure frequency over 96 weeks. Given the common, and yet discouraging lack of efficacy of current treatments for these patients, a significant decrease in convulsive seizures with CBD is very promising.3,25,42
When assessing for benefits of seizure treatment it is vital to evaluate the benefits in both the reduction in convulsive-seizure frequency and subjective caregiver perception of quality of life (QoL). A statistically significant change in the Caregiver Global Impression of Change score was seen with 62% (N = 120; p = 0.02) of caregivers judging their child's overall condition as improved within the treatment group.3 The score uses a 7-point scale to rate the patients' illness from substantial worsening to substantial improvement and was compared against baseline.3 This indicates that the patients who received CBD had an improved QoL as compared to those who remained on the standard of care with placebo.3
In addition to demonstrating efficacy in terms of improved seizure control and QoL, CBD has shown to be tolerable, with the most common (≥10%) ADRs including somnolence (20%–38%), fatigue (11%–20%), maculopapular rash or erythema (11%–13%), decreased appetite (13%–44%), diarrhea (10%–31%), insomnia (5%– 11%), infection (11%–40%), and elevated transaminases (5%–22%), with less than 1% of patients experiencing aminotransferase (AST) or alanine transaminase (ALT) values greater than 20 times the upper limit of normal (ULN).1,3,16,42 Patients who experienced liver transaminase elevations 3 times the ULN of either AST or ALT were withdrawn from the studies.3 Of the 22 patients in GWPCARE1 who experienced somnolence, 81% (n = 18) were taking CLB concomitantly.3 Similarly, of the 12 patients in the CBD group who experienced elevated liver transaminases, all 12 patients were taking VPA concomitantly.3 In the GWPCARE1 trial, 10 patients in the treatment group (n = 61) withdrew either owing to ADRs (n = 9) or investigator discretion (n = 1), and 3 patients in the placebo group (n = 59) withdrew owing to ADRs (n = 1), parent/guardian discretion (n = 1), or loss of follow-up (n = 1).3 Most of these ADRs resolved spontaneously while others, such as elevated liver transaminases or increased somnolence, resolved with the cessation or dose reduction of CBD.3 Many of the ADRs are dose-dependent (Table 1).
Table 1.
| ADR | Study | 5 mg/kg/day, % | 10 mg/kg/day, % | 20 mg/kg/day, % |
|---|---|---|---|---|
| Rash | Safety Analysis (DS) | 0 | 13 | 11 |
| GWPCARE1 (DS) | — | — | — | |
| GWPCARE3 (LGS) | — | — | — | |
| GWPCARE4 (LGS) | — | — | 7 | |
| Pyrexia | Safety Analysis(DS) | 30 | 38 | 0 |
| GWPCARE1 (DS) | — | — | 15 | |
| GWPCARE3 (LGS) | — | 9 | 12 | |
| GWPCARE4 (LGS) | — | — | 13 | |
| Diarrhea | Safety Analysis (DS) | — | — | — |
| GWPCARE1 (DS) | — | — | 31 | |
| GWPCARE3 (LGS) | — | 10 | 15 | |
| GWPCARE4 (LGS) | — | — | 19 | |
| Vomiting | Safety Analysis (DS) | 10 | 13 | 11 |
| GWPCARE1 (DS) | — | — | 15 | |
| GWPCARE3 (LGS) | — | 6 | 12 | |
| GWPCARE4 (LGS) | — | — | 10 | |
| Upper respiratory tract infection | Safety Analysis (DS) | — | — | — |
| GWPCARE1 (DS) | — | — | 11 | |
| GWPCARE3 (LGS) | — | 16 | 13 | |
| GWPCARE4 (LGS) | — | — | 2 | |
| Decreased appetite | Safety Analysis (DS) | 0 | 13 | 44 |
| GWPCARE1 (DS) | — | — | 28 | |
| GWPCARE3 (LGS) | — | 16 | 26 | |
| GWPCARE4 (LGS) | — | — | 13 | |
| Somnolence | Safety Analysis (DS) | 20 | 38 | 0 |
| GWPCARE1 (DS) | — | — | 36 | |
| GWPCARE3 (LGS) | — | 21 | 30 | |
| GWPCARE4 (LGS) | — | — | 15 | |
| Increased AST/ALT | Safety Analysis (DS) | — | — | 44 |
| GWPCARE1 (DS) | — | — | 20 | |
| GWPCARE3 (LGS) | — | <10 | <10 | |
| GWPCARE4 (LGS) | — | — | 9 |
ADR, adverse drug reaction; ALT/AST, alanine transaminase; AST, aminotransferase; DS, Dravet syndrome; LGS, Lennox-Gastaut syndrome
Lennox-Gastaut Syndrome. Two pivotal randomized double-blind, multicenter, clinical trials evaluated the use of CBD in patients with LGS with regard to the efficacy and safety in drop seizures (GWPCARE 3 and 4),4,30 A total of 225 patients with LGS were enrolled who were on average 15.5 ± 9.8 years old (2.6–48 years old), with 30% of the patients being older than 18 years, taking a median of 3 (0–5) concomitant AEDs, and having experienced treatment failure with a median of 6 (0–22) previous AEDs.4 Of the 225 patients who underwent randomization in the intention-to-treat analysis, 76 patients were assigned to the placebo, 73 received 10 mg/kg/day, and 76 received 20 mg/kg/day.4 Similar to the previous trials conducted in patients with DS, placebo was taken alongside standard of care. There was a median relative reduction of 21.6% (n = 152; 95% CI, 6.7–34.8) in drop seizures between the 20 mg/kg/day group and placebo and a median relative reduction of 19.2% (n = 149; 95% CI, 7.7–31.2) in drop seizures between the 10 mg/kg/day group and placebo.4 Furthermore, a median relative reduction of 18.8% (n = 152; 95% CI, 4.4–31.8) was seen in total seizure frequency between the 20 mg/kg/day group and placebo, and a median relative reduction of 19.5% (n = 149; 95% CI, 7.5–30.4) was seen in total seizure frequency between the 10 mg/kg/day group and placebo.30 GWPCARE3 demonstrated decreased frequency in seizures in patients initiated on 20 mg/kg/day, with 39% of all study participants in the treatment group (n = 72) experiencing 50% reduction in drop seizure frequency as compared to only 14% (n = 76) in the placebo group (p < 0.001).4 Furthermore, there was a statistically significant difference of reductions in drop seizures with both CBD doses, with a 19.2% reduction in the participants receiving 10 mg/kg/day compared to placebo (n = 73; p = 0.002) and a 21.6% reduction in the participants receiving 20 mg/kg/day (n = 76; p < 0.005).4 Adverse drug reactions were reported in both treatment groups in GWPCARE3 and demonstrated a potentially dose-dependent increased risk of developing ADRs as demonstrated by increase in the frequency of ADRs as the total daily dose increased.4,30 The dose-dependent ADRs of GWPCARE3 included somnolence (21%–30%), diarrhea (10%–15%), pyrexia (9%–12%), decreased appetite (16%–26%), upper respiratory tract infection (13%–16%), nasopharyngitis (4%–11%), and vomiting (6%–12%).3,4,30 Among the 149 patients who received CBD in the GWPCARE3 study, 14 (9%) patients (11 patients in the 20 mg/kg/day group and 3 in the 10 mg/kg/day group) experienced elevated liver transaminases. Eleven of the 14 were taking VPA concomitantly both in the 20 mg/kg/day group (n = 9) and 10 mg/kg/day group (n = 2).4 None of these patients met the criteria for severe drug-induced liver injury (DILI), and elevated liver transaminase levels returned to normal either spontaneously (n = 3) after entry into the open-label extension trial (n = 2), or after the dose of CBD was tapered or discontinued, or the dose of another AED was reduced (n = 9).4 In the GWPCARE3 trial, 9 participants (n = 76) in the 20 mg/kg/day treatment group withdrew owing to ADRs (n = 4), caregiver discretion (n = 1), meeting withdrawal criteria (n = 1), protocol deviation (n = 1), or investigator discretion (n = 1). In the 10 mg/kg/day treatment group (n = 73), 2 participants withdrew either owing to an ADR (n = 1) or investigator discretion (n = 1). Two participants (n = 76) in the placebo group withdrew for similar reasons: ADR (n = 1) and caregiver discretion (n = 1).4
In the GWPCARE4 trial, 171 patients with LGS were enrolled who were on average 15.4 ± 9.3 years old (2.7–45.1 years old), taking a median of 3 (1–5) concomitant AEDs, and having experienced treatment failure with a median of 6 (0–28) previous AEDs.4 Of the 171 patients who underwent randomization in the intention-to-treat analysis, 85 patients were assigned to the placebo and 86 received 20 mg/kg/day.43 Of the 156 patients who completed the treatment period, 84 were assigned to the placebo (1 discontinued study treatment) and 72 received 20 mg/kg/day (14 discontinued study treatment).30 Fourteen patients in the treatment group (n = 86) withdrew owing to ADR (n = 8), meeting withdrawal criteria (n = 4), or another unknown reason (n = 2), and 1 patient in the placebo group (n = 85) withdrew owing to an adverse event.43 Among the 72 study participants in the treatment group (20 mg/kg/day), a 17.21% decrease (p = 0.0135) in drop seizures and a 21.13% decrease (p = 0.0005) in total seizures was observed throughout the study period.30 Both studies had similar rates of ADRs as the trials that evaluated patients with DS.3,4,30,42 All patients on CBD in GWPCARE4 received 20 mg/kg/day, with the most common treatment-related ADRs including diarrhea (13%), somnolence (14%), decreased appetite (9%), and vomiting (7%).43 Increased risk of somnolence is likely attributed to a drug interaction between CBD and CLB, as 48% (n = 86) of patients initiated on CBD in GWPCARE4 were on concomitant CLB, with 22% (n = 41) of participants receiving both AEDs reporting somnolence as compared to 16% (n = 43) of participants in the placebo group on CLB.4 Twenty-three percent of patients (n = 20) in the group who received CBD experienced elevated liver transaminases (either AST or ALT).43 Of the 20 patients who experienced an elevation in either AST or ALT, 16 were taking VPA concomitantly.43 Liver transaminase levels resolved spontaneously or following a dose reduction or discontinuation of either VPA or CBD; neither caused DILI.43 Both somnolence and elevations in aminotransferases were the only 2 clinically significant ADRs potentially resulting from drug interactions that were reported in all the pivotal clinical trials, with concomitant CLB and valproic acid, respectively.3,4,42,43
Drug Interactions. Cannabidiol metabolism largely occurs through cytochrome P450 (CYP450) enzymes and is known to be a substrate of CYP3A4 and CYP2C19 (see Figure) with minor pathways via uridine 5′-diphospho-glucuronosyltransferase (UGT)1A7, UGT1A9, and UGT2B7.1 Furthermore, CBD affects the CYP2C family, which may contribute to the potential for drug-drug interactions with numerous medications, especially other concomitantly prescribed AEDs (Tables 2 and 3).4,6,23 Cannabidiol is metabolized to its active metabolite 7-hydroxy-CBD (7-OH-CBD) via CYP2C19 and subsequently converted to the inactive metabolite 7-carboxy-CBD (7-COOH-CBD) via CYP3A4 (Figure).1,42 CBD has the potential to inhibit CYP1A2, CYP2B6, CYP2C8, CYP2C9 (i.e., phenytoin), CYP2C19 (i.e., CLB), and UGT1A9 and UGT2B7 (i.e., lamotrigine, lorazepam), and induce CYP1A2 and CYP2B6, theoretically resulting in drug-drug interactions involving these pathways (Table 2).23,44 The only clinically significant pharmacokinetic drug-drug interaction with CBD involving CYP450 pathways that was reported in the pivotal clinical trials was with CLB, resulting in a 3-fold increase in plasma concentrations of N-desmethylclobazam (N-CLB), the active metabolite of CLB, presumably due to the inhibition of CYP2C19, which inactivates N-CLB.3,42 Other AEDs that also undergo CYP2C19 metabolism, including phenobarbital, phenytoin, and stiripentol, could theoretically interact with CBD; however, evidence has yet to be reported to indicate clinical significance.23,45–47 AEDs that induce or inhibit CYP2C19 metabolism, such as oxcarbazepine, felbamate, topiramate, or stiripentol, may also have interactions with CBD, but these interactions were not reported in these clinical trials.16,42,47 In the pivotal clinical trials, there were reports that CBD significantly affected concentrations of rufinamide, topiramate, zonisamide, and eslicarbazepine, but none were deemed as exceeding their therapeutic plasma concentrations.3,16,23,42,44,47 Unfortunately, there was no further information reported regarding specifics of the regimens, including doses and concomitant AED regimens, which would provide greater insight into these interactions.1,16,44 In the expanded access program (EAP) there was no difference in seizure severity and frequency with patients on any of these potentially interacting medications as compared to those who were not on any of these medications.44
Figure.
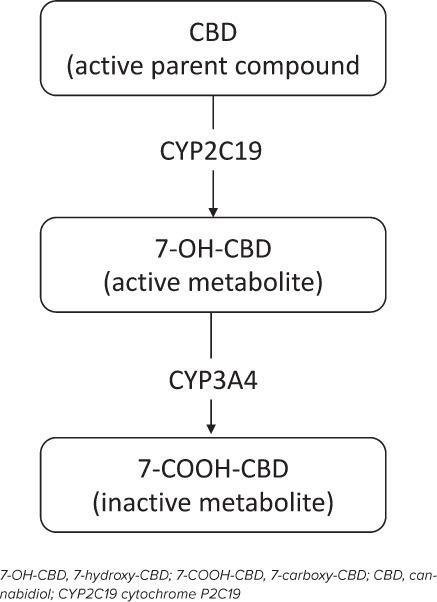
Substrates in the metabolism of CBD.
Table 2.
| AED | CYP3A4 | CYP 2C19 | CYP 2C8/9 | CYP 2B6 | CYP 1A2 | UGT |
|---|---|---|---|---|---|---|
| Brivaracetam | Substrate | |||||
| Carbamazepine | Substrate* | Inducer§ | Substrate# | Inducer | Substrate# | — |
| Inducer§ | Inducer | Inducer | ||||
| Clobazam | Substrate* | Substrate*,#,†,¶ | — | Substrate*,# | — | — |
| Clonazepam | Substrate | — | — | — | — | |
| Diazepam | Substrate* | Substrate* | — | — | — | Substrate† |
| Eslicarbazepine | Inducer | Inhibitor | — | — | — | — |
| Ethosuximide | Substrate† | — | — | — | — | — |
| Everolimus | Substrate | — | — | — | — | — |
| Felbamate | Substrate‡ | Inhibitor‡ | — | — | — | — |
| Inducer | ||||||
| Lacosamide | Substrate† | Substrate† | Substrate† | — | — | — |
| Lamotrigine | — | — | — | — | — | Substrate |
| Inducer‡ | ||||||
| Levetiracetam | — | — | — | — | — | — |
| Midazolam | Substrate* | — | — | — | — | — |
| Oxcarbazepine | Substrate | Inhibitor‡ | — | — | — | Substrate |
| Inducer§ | ||||||
| Perampanel | Substrate¶ | — | Inhibitor ‡ | Substrate | Substrate | Inhibitor‡ |
| Inhibitor‡ | Inducer‡ | Inducer‡ | ||||
| Inducer‡ | ||||||
| Phenobarbital | Inducer§ | Substrate | Substrate | — | — | Inducer |
| Inducer | Inducer | |||||
| Phenytoin | Substrate# | Substrate | Substrate | — | — | Inducer |
| Inducer§ | Inducer§ | Inhibitor | ||||
| Inducer | ||||||
| Primidone | Substrate | Substrate | Substrate | — | — | Inducer |
| (active to PB) | Inducer§ | Inhibitor | ||||
| Inducer | Inducer | |||||
| Rufinamide | Inducer§ | — | — | — | — | — |
| Stiripentol | Substrate | Substrate | Inhibitor | Inhibitor Inducer | Substrate | |
| Inhibitor | Inhibitor | Inhibitor | ||||
| Inducer | Inducer | |||||
| Topiramate | Inducer‡ | Inhibitor‡ | — | — | — | — |
| Valproate | — | Substrate | Substrate¶ | Inhibitor | Inhibitor | |
| Inhibitor‡ | Inhibitor | |||||
| Vigabatrin | — | Inducer‡ | Inducer‡ | — | — | — |
| Zonisamide | Substrate | Substrate# | — | — | — | — |
AED, antiepileptic drug
* Pharmacologically active metabolite
† Pharmacologically inactive metabolite
‡ Weak effect of enzyme
§ Strong effect of enzyme
¶ Major metabolic pathway
# Minor metabolic pathway
Table 3.
| Antiepileptic | Affect of CBD | Affect on CBD |
|---|---|---|
| Brivaracetam | Decrease in clearance and increase in concentrations of brivaracetam | None |
| Carbamazepine | Decrease in clearance and increase in concentrations of carbamazepine (minor) | Increase in conversion of parent compound to active metabolite Decrease in concentration of active metabolite and increase in formation of inactive metabolite |
| Clobazam | Increase in concentration of the parent compound. Decrease in clearance of the active metabolite (NCLB). | None |
| Clonazepam | None | None |
| Diazepam | Increase in conversion of parent compound to active metabolite Decrease in clearance of active metabolites |
None |
| Eslicarbazepine | None | Increase in clearance of active metabolite Decrease in conversion of parent compound to active metabolite |
| Ethosuximide | None | None |
| Everolimus | None | None |
| Felbamate | None | Increase in clearance of active metabolite Decrease in conversion of parent compound to active metabolite |
| Lacosamide | Decrease in clearance and increase in concentrations of lacosamide | None |
| Lamotrigine | Decrease in clearance and increase in concentrations of lamotrigine | None |
| Levetiracetam | None | None |
| Midazolam | None | None |
| Oxcarbazepine | Decrease in clearance and increase in concentrations of oxcarbazepine | Increase in clearance of active metabolite Decrease in conversion of parent compound to active metabolite |
| Perampanel | Decrease in clearance and increase in concentrations of perampanel | Decrease or increase in conversion of active to inactive metabolite |
| Phenobarbital | Decrease in clearance and increase in concentrations of phenobarbital | Increase in conversion of parent compound to active metabolite Decrease in concentration of active metabolite and increase in formation of inactive metabolite |
| Phenytoin | Decrease in clearance and increase concentrations of phenytoin | Increase in formation of active metabolite. Decrease in concentration of active metabolite and increase in formation of inactive metabolite. |
| Primidone | Decrease in clearance and increase in concentrations of active metabolite (phenobarbital) | Increase in conversion of parent compound to active metabolite Decrease in concentration of active metabolite and increase in formation of inactive metabolite. |
| Rufinamide | None | None |
| Stiripentol | Decrease in clearance and increase in concentration of stiripentol | Increase in concentration of parent compound and decrease in formation of active metabolite Increase/decrease in formation of inactive metabolite |
| Topiramate | None | Decrease in conversion of parent compound to active metabolite Decrease in concentration of active metabolite and increase in formation of inactive metabolite |
| Valproate | Decrease in clearance and increase in concentrations of valproic acid | Decrease in conversion of parent compound to active metabolite |
| Vigabatrin | None | Increase in conversion of parent compound to active metabolite |
| Zonisamide | Decrease in clearance and increase in concentration of zonisamide (minor) | None |
CBD, cannabidiol; N-CLB, N-desmethylclobazam
Laux et al16 conducted an EAP that allowed for a safety analysis set that analyzed the concomitant use of AEDs with CBD in LGS and DS. In this safety analysis set, the 152 patients with LGS or DS were on average 12.8 (1.7–51) years old and were taking an average of 3 (0–10) concomitant AEDs.16 Among the 152 patients in the LGS/DS cohort, CLB (65%), VPA (42%), and levetiracetam (32%) were the most common concomitant AEDs.16 This study demonstrated that 46% (n = 99) of patients taking CLB and 52% (n = 64) of patients taking VPA concomitantly reduced their dose during CBD initiation. However, of those patients taking levetiracetam concomitantly, only 16% (n = 50) underwent a dose reduction.16 In total, 38% of all patients (n = 57) in this cohort reduced their dose of CBD at some point during the EAP. Despite those reductions, the average dose of CBD actually increased from an average of 21 mg/kg/day at 12 weeks to an average of 25 mg/kg/day at 96 weeks.16 Although there were no reports of the rationale behind the dose adjustments in this study, 38% (n = 101) of those taking CLB concomitantly experienced somnolence, compared to 18% (n = 51) for those not taking CLB.16 Despite the decrease in concomitant AEDs, study participants still observed a reduction in seizures, with nearly half of patients with LGS showing ≥50% reduction in seizures. Despite dose reductions of CLB with CBD initiation, the interaction between CLB and CBD can potentially elucidate the continued seizure control in these patients.16
To investigate this theory, the EAP conducted by Gaston et al44 evaluated whether the treatment response, measured as seizure frequency and severity, was affected by the presence of concomitant CLB. In the EAP, 132 patients with an average age of 19.5 ± 12.9 years were enrolled who were then divided into 4 groups—CBD + interacting AED (iAED), CBD + iAED + CLB, CBD (no iAED), and CBD + CLB (no iAED)—on the basis of their previously stable regimens.44 With the initiation of CBD, each group demonstrated a statistically significant absolute reduction in seizure severity and frequency (p < 0.05) without a significant difference between the groups at 12 weeks (p = 0.4395 and p = 0.5505, respectively).44 Thus, the results suggest that the efficacy of CBD may be independent of the presence of other concomitant AEDs; however, there was no further discussion regarding ADRs and the effect of concomitant administration of AEDs, particularly CLB, on said ADRs.44
In the analysis of plasma pharmacokinetics of CLB and N-CLB, Devinsky et al42 confirmed an increase of the active metabolite N-CLB in the presence of CBD. Patients with DS who participated were on average 7.6 ± 1.8 years of age, ranging from 4 to 10.8 years, and taking an average of 2.6 ± 0.9 concomitant AEDs. The 2 most common AEDs that were reported within the baseline characteristics and taken concomitantly with the study drug were CLB, which 68% of patients (n = 23) were taking, and VPA, which 65% (n = 22) of patients were taking, across all groups.42 Of the 32 patients who completed the study period, 7 patients were assigned to the placebo group, 10 patients received 5 mg/kg/day, 7 received 10 mg/kg/day, and 8 received 20 mg/kg/day.42 Among the 32 patients, 17 were taking CLB concomitantly.42 Amongst these patients there was a notable mean increase (>166%) in N-CLB across all dose groups as compared to placebo (n =17). When quantifying the change in serum concentration of N-CLB baseline (no CBD) to the first dose of CBD, there was a statistically significant increase in the mean concentration from 2207.5 ng/mL (no CBD) to 3727.7 ng/mL (first dose of CBD) when the dose was left unchanged (p < 0.001; n = 26).44 There was not a dose-dependent trend of inhibition observed, and all treatment groups had a notable increase in N-CLB concentrations as compared to placebo.44 The increased serum concentration of active metabolite could contribute to the increased reports of sedation in these patients.3,42,44
As stated in the previous studies, in patients on concomitant VPA, the initiation of CBD was associated with an increase in liver transaminases (either AST or ALT) >3 times the ULN, up to 12 times the ULN.3,30,42,44 In the EAP, 6 patients of 27 in the CBD groups, all receiving concomitant VPA, had elevations in liver transaminases >3 times ULN, with 4 of the 6 patients belonging to the 20 mg/kg/day group.42 However, there was not an association with DILI in these patients despite the large increase in transaminases.3,42 Furthermore, the reduction in either VPA or CBD led to normalization of liver transaminase levels in some patients, whereas some levels resolved spontaneously without a change in medications.3,42,44 Furthermore, there was no reported increase of bilirubin that was >2 times the ULN or increase in international normalized ratio (INR) indicating preserved liver function.42 Elevation of liver transaminases were largely observed with CBD use in the presence of VPA, suggesting the interaction is due to the potentiation of a VPA-induced elevation in transaminase levels.3,42
Drug-drug interactions with CBD have been well described with concomitant CLB and VPA, but data are emerging with potential interaction with other medications. To date, there have been 2 case reports/series demonstrating potential interactions with brivaracetam (BRV) and tacrolimus.3,42,48,49 Brivaracetam is a new AED approved as adjunctive therapy in the treatment of partial-onset seizures in patients with epilepsy who are 16 years of age and older.50,51 Brivaracetam has a 15- to 30-fold higher affinity for synaptic vesicle 2A, which has rapid brain penetration owing to its high lipid solubility.51 Klein et al51 describe the weak inhibition that BRV has on CYP2C19 in context with its interaction with phenytoin. However, this inhibition did not affect the steady-state plasma concentrations of other CYP2C19 substrates (i.e., phenobarbital and topiramate), thus demonstrating that BRV is neither an inducer nor an inhibitor of CYP2C19.51 In a case series of 5 patients, Klotz et al48 observed an increase in BRV concentrations when administered concomitantly with CBD, which may be due to its inhibition of CYP2C19. These 5 patients all had preexisting BRV therapy and were then initiated on CBD at 5 mg/kg/day with titration to 20 mg/kg/day according to tolerance and efficacy.48 With the addition of CBD to the stable regimen, 2 of the 5 patients reported potentially related adverse events with 1 patient reporting diarrhea and the other reporting somnolence.48 However, all 5 patients showed an increase of BRV plasma concentration of between 95% to 280%.48 This interaction is theorized to be due to the decreased CYP2C19-meditated oxidation of BRV as a result of the potent inhibition of CYP2C19 by CBD.48
Drug interactions with CBD are not exclusive to AEDs. A recent case report49 described a 3-fold increase in a previously therapeutic tacrolimus concentration in 1 patient after CBD initiation. A 32-year-old woman with refractory epilepsy was receiving 2000 to 2900 mg/day (20–25 mg/kg/day) of CBD while enrolled in a clinical trial for epilepsy while taking tacrolimus concomitantly for interstitial nephritis.49 After the initiation of CBD at 20 mg/kg/day, this patient experienced a 3-fold increase in serum tacrolimus concentration from 4.1 ng/mL to 13.3 ng/mL.49 After the increase in serum concentration, tacrolimus was held and restarted at 3 mg twice daily (previously 5 mg twice daily).49 When the CBD dose was titrated to 25 mg/kg/day (2900 mg/day), the tacrolimus dose had to be subsequently reduced further to 1 mg twice daily.49 In addition to the supratherapeutic serum concentration of tacrolimus, the patient was potentially exhibiting signs of tacrolimus toxicity with a serum creatinine SCr of 1.92 mg/dL (baseline of 1.2 mg/dL).49 The interaction was theorized to be due to the inhibition of CYP3A4 and/or P-glycoprotein (P-gp) inhibition.49 Although there is currently no confirmatory evidence that CBD elicits CYP3A4 inhibition, it is a substrate of CYP3A4. Furthermore, the development of diarrhea could alter P-gp efflux, which could theoretically explain the elevations in tacrolimus concentrations. Diarrhea was not reported in this case report.49 However, as this is a known side effect of CBD and could potentially alter tacrolimus concentrations, monitoring may be warranted.49 Owing to the use of multiple medications, including but not limited to AEDs, in patients with medically refractory epilepsy, further research on the clinical implication of other theoretical drug-drug interactions is warranted and clinical monitoring in these patients is essential.28,29,32,44
Niche in Therapy
With any new medication approval, the question becomes, where does it fit in therapy? Currently there is no evidence to support the use of CBD as monotherapy for epilepsy, given that the studies were conducted in patients with either LGS or DS who had tried several AEDs and other non-pharmacologic treatment modalities (i.e., vagal nerve stimulation and/or KGD) with either suboptimal therapeutic responses or intolerability of adverse effects. However, the literature is promising as regards its use as an adjunct therapy with standard of care to reduce seizure frequency in medically refractory generalized convulsive and drop seizures in pediatric and/or adult patients in DS and LGS, respectively.
While FDA approval is currently for patients ≥ 2 years of age with either LGS or DS, there is growing use of CBD for medically refractory epilepsy that does not satisfy the diagnostic criteria of LGS or DS. The current hurdle to chronic off-label use is its logistic attainment. Off-label use of CBD is emerging with compassionate use, open-label prospective studies and case series describing its use for genetic epilepsy syndromes (i.e., CDKL5 deficiency disorder and Aicardi, Dup15q, and Doose syndromes)52 and in febrile infection-related epilepsy syndrome (FIRES) for both the acute and chronic management of seizures.53 In an open-label study with 55 patients, similar efficacy and safety of CBD for these 4 syndromes (i.e., CDKL5 deficiency disorder and Aicardi, Dup15q, and Doose syndromes) were reported with doses up to 50 mg/kg/day (mean maximum dose of 29.6 mg/kg/day).52 Statistically significant percentage seizure changes in median seizure frequency were reported in all patients: 51.4% (IQR, 9%–85%) at 12 weeks and 59.1% (IQR, 14%–86%) at 48 weeks (p = 0.00001).52 Similar percentage reductions were reported for each syndrome at both 12 and 48 weeks with the most significant reduction at 12 weeks, but negligible change from 12 to 48 weeks.52 Similar rates of adverse events were noted as reported for the pivotal studies in patients with LGS and DS.4 In an open-label case series of 7 patients with FIRES, treatment with CBD at doses up to 25 mg/kg/day in both the acute and chronic phase of therapy, after failure of conventional therapies and continuous status epilepticus for a minimum of 2 weeks, resulted in the weaning of several AEDs (decreased from a mean of 7.1 AEDs to 2.8 AEDs after the initiation of CBD; p = 0.002), with only 3 patients remaining on the KGD, which is currently the only treatment known to have any efficacy in FIRES.53
Given promising results of pivotal studies and open-label studies for off-label indications, there is emerging literature in the pipeline surrounding the potential use of CBD for other indications, including other medically refractory epilepsy syndromes.
Studies in the Pipeline
While the GWPCARE study series has led to the long-awaited approval of the first FDA-approved, purified, plant-derived, CBD medication in the United States, there is a long road ahead with many studies in the pipeline. Perusing the National Library of Medicine Clinical Trials, there are over 100 studies investigating the use of CBD nationally and internationally. Most of these pending studies are investigating potential future indications for CBD.
Studies that are active and no longer recruiting include a phase III trial, an extension of the GWPCARE series investigating the use of CBD for infantile spasms with an open-label extension phase (GWPCARE7)54,55 that has been long awaited, as the original estimated completion date was the end of 2018. Other active, but no longer recruiting clinical trials include those investigating its use in medically refractory Sturge-Weber syndrome,56 safety and tolerability in pediatric patients (1–19 years old) with intractable epilepsy,57 seizures in tuberous sclerosis complex with an open-label extension phase (GWPCARE6),17,19 and a study assessing the safety and tolerability of fenfluramine hydrochloride in children and young adults with DS and LGS on concomitant CBD.58 Most of these studies have approximate completion dates in 2019–2020.
Actively recruiting clinical trials, with anticipated results in 2020, include those investigating its efficacy and long-term safety in pediatric patients with treatment-resistant childhood absence seizures59; its efficacy, safety, and tolerability with vigabatrin for the initial treatment in patients with infantile spasms60; and its use in children and adolescents with fragile X syndrome (CONNECT-FX).61 The above studies are summarized in Table 4.
Table 4.
Pipeline Studies for Epidiolex (Cannabidiol)72
| Status | Estimated Completion Date | Study Title |
|---|---|---|
| Not yet recruiting | June 2022 | An Open-label Extension Study of Cannabidiol Oral Solution (GWP42003-P, CBD-OS) in Patients With Rett Syndrome (ARCH) |
| Recruiting | February 2022 | Open Label Extension to Assess the Long-Term Safety and Tolerability of ZYN002 in Children and Adolescents With FXS |
| Enrolling by invitation | February 2022 | An Open-label Extension Trial of Cannabidiol (GWP42003-P, CBD) for Seizures in Tuberous Sclerosis Complex (GWPCARE6) |
| Recruiting | September 2021 | Efficacy and Safety of Cannabidiol Oral Solution (GWP42003-P, CBD-OS) in Patients With Rett Syndrome |
| Active, not recruiting | February 2021 | Safety, and Tolerability of CBD in Patients (Ages 1–19 Years) With Intractable Epilepsy |
| Active, not recruiting | February 2020 | A Study to Assess the Safety and Tolerability of ZX008 (Fenfluramine Hydrochloride) in Children and Young Adults With DS or LGS Currently Taking CBD |
| Completed | January 2020 | An Open Label, Multi-Center Study to Investigate the Safety of Cannabinoid (GWP42003-P) in Children With Medication Resistant Epilepsy |
| Recruiting | November 2019 | Long-Term Safety of Pharmaceutical Grade Synthetic Cannabidiol Oral Solution in Pediatric Participants With Treatment-Resistant Childhood Absence Seizures |
| Active, not recruiting | December 2019 | Study to Assess the Efficacy, Safety, and Tolerability of Cannabidiol Oral Solution With Vigabatrin as Initial Therapy in Participants With Infantile Spasms |
| Active, not recruiting | October 2019 | Clinical Study of Cannabidiol in Children and Adolescents With Fragile X (CONNECT-FX) |
| Recruiting | June 2019 | Cannabidiol Oral Solution in Pediatric Participants With Treatment-Resistant Childhood Absence Seizures |
| Active, not recruiting | June 2019 | Cannabidiol Expanded Access Study in Medically Refractory Sturge-Weber Syndrome |
| Completed | February 2019 | A Randomized Controlled Trial of Cannabidiol (GWP42003-P, CBD) for Seizures in Tuberous Sclerosis Complex (GWPCARE6) |
| Active, not recruiting | December 2018 | Phase 3 Trial of Cannabidiol (CBD; GWP42003-P) for Infantile Spasms: Open-label Extension Phase (GWPCARE7) |
| Active, not recruiting | October 2018 | Trial of Cannabidiol (CBD; GWP42003-P) for Infantile Spasms (GWPCARE7) |
| Terminated | September 2016 | Cannabidiol Oral Solution for Treatment of Refractory Infantile Spasms |
Future Questions
While there are several studies in the pipeline that will answer many questions regarding the use of CBD in several other indications, there are still many other unanswered questions that warrant further research. Several of the unanswered questions regarding the use of CBD in our patients include drug and food interactions, and long-term efficacy and safety outcomes.
Concomitant Drug Therapy. Currently there is no evidence to support the use of CBD as monotherapy in patients with epilepsy disorders. Thus, it is fundamental that further research investigate the use of CBD with other medications. As depicted in the Figure, CBD's primary metabolic pathway is through hepatic metabolism by CYP2C19 to its active metabolite 7-OH-CBD and further metabolization to its inactive metabolite 7-COOH-CBD by CYP3A4. In vitro studies further demonstrate that CBD is a potent inhibitor of both CYP2C19 and 3A4, and to a weaker extent CYP2D6.62–64 These pharmacokinetic parameters yield the potential for numerous drug interactions overlapping with many other medications, including several AEDs, as summarized in Table 2. Therefore, further studies investigating the clinical implications of the effect of coadministration of CBD and other compounds that are metabolized by, inhibit, and/or induce these hepatic enzymes are essential. There are several pending trials, and their corresponding open-label studies that emerged from the GWPCARE series investigating the potential for drug-drug interactions with CBD, including with stiripentol, valproic acid, and CLB.65–68 While most clinical significant drug interactions that emerged from the GWPCARE study series were with valproic acid and CLB, there are several important limitations with these studies: 1) those were the most common concomitant medications in the study, which is appropriate as they are standard of care; 2) while interactions were implicated, data are insufficient as to the details regarding the therapeutic regimens, pharmacokinetic and pharmacodynamic parameters, which would be needed to modify therapies in clinical practice; and 3) no information was provided on other AEDs that have theoretical potential for drug-drug interactions with CBD. Owing to the discovery of these drug interactions, future studies have sought to investigate the interactions with concomitant CLB both as an open-label extension study68 and another randomized controlled trial67 as well as concomitant VPA and/or stiripentol.6,66 While the results of the above studies are very much anticipated, there are still several other antiepileptics that have theoretical interactions based on known metabolic pathways (Table 2), and investigation into their clinical significance is warranted. Furthermore, there are other medications, outside of AEDs, that are potent inhibitors, inducers, and substrates of these hepatic metabolic pathways that need to be investigated, as evidenced by the recent case report demonstrating a 3-fold increase in tacrolimus concentrations with concomitant CBD therapy.49 While that interaction was hypothesized to be due to CBD's effect on P-gp or CYP3A4, there are currently no recommendations for dose adjustments with medications that are substrates of CYP3A4 in the package insert,1,44 thus further demonstrating the need for research regarding the potential for drug interactions with CBD.
Long-Term Efficacy. Safety is at the forefront when considering drug-drug interactions; however, efficacy should not be neglected. Drug-drug interactions can indeed result in decreased efficacy, and loss of seizure control in the case of clinically significant reductions in plasma concentrations and/or induction of metabolism or elimination. Multimodal mechanisms of action are warranted to minimize seizure burden for patients with medially refractory epilepsy. However, which drug combinations are most efficacious in treating patients with refractory epilepsy? While we may not have a standard regimen for treating medically refractory epilepsy, there are drug combinations that are considered the backbone or “first-line” for various medically refractory epilepsy syndromes, including but not limited to DS and LGS. While very few would dispute the potential role that CBD has in the treatment of medically refractory epilepsy, it is important to investigate which AEDs in combination with CBD have the most promising outcomes. For those patients who attain seizure control, there is the plea to taper or discontinue other AEDs whether due to their lack of efficacy or intolerability and safety profile. Post-marketing data and future research are warranted to be able to assess which drug combinations have the most promising outcomes to maximize seizure control and minimize adverse effects. Likewise, while many of the pivotal clinical trials assessed the reduction of seizure frequency in different seizure subtypes as secondary outcomes, the data are still inconclusive as to whether CBD has potential benefit in other seizure subtypes outside of atonic or drop attacks in LGS and/or convulsive seizures in DS.3,4,30,42,69 With emerging use of CBD in intractable epilepsy outside of these syndromes, data regarding efficacy in other seizure subtypes would be beneficial. Furthermore, a small subset of patients experienced worsened seizure frequency in the clinical trials, eliciting the question of whether or not CBD worsens particular seizure subtypes.3,4,30,42,69
Long-Term Safety. The most common ADRs reported in clinical studies included gastrointestinal effects (i.e., diarrhea, decreased appetite, and vomiting), somnolence, and increased aminotransferases. As alluded to previously, adverse events of somnolence and elevations in aminotransferases were precipitated by concomitant AED therapy, notably CLB and VPA derivatives, respectively. In addition to unanswered questions regarding implications of other drug-drug interactions and ADRs, there are still limited data regarding effects on reproductive health, including pregnancy and lactation. In animal studies, ADRs including embryo-fetal mortality, developmental toxicity, reduction of spermatogenesis and male reproduction impairment, and other neurotoxicities were demonstrated with higher doses than used in humans.70
Conclusions
The approval of the first FDA-approved pharmaceutical-grade cannabidiol product has revolutionized the management of medically refractory epilepsy in both adult and pediatric patients. While the results of the pivotal, multicenter, clinical trials that led to its approval are promising, there is much left to be investigated before definitive recommendations can be made in clinical practice. Further research is warranted to investigate potential impact of these unanswered questions in clinical practice.
ABBREVIATIONS
- 7-OH-CBD
7-hydroxy-CBD
- 7-COOH-CBD
7-carboxy-CBD
- ADR
adverse drug reaction
- AED
antiepileptic drug
- ALT
alanine transaminase
- AST
aminotransferase
- BRV
brivaracetam
- CBD
cannabidiol
- CLB
clobazam
- CY
cytochrome
- DILI
drug-induced liver injury
- Δ9-THC
Δ9-tetrahydrocannabinol
- DS
Dravet syndrome
- EAP
expanded access program
- FIRES
febrile infection-related epilepsy syndrome
- HIV/AIDS
human immunodeficiency virus/acquired immunodeficiency syndrome
- iAED
interacting antiepileptic drug
- INR
international normalized ratio
- KGD
ketogenic diet
- LGS
Lennox-Gastaut syndrome
- N-CLB
N-desmethylclobazam
- P-gp
P-glycoprotein
- QoL
quality of life
- UGT
uridine 5'-diphospho-glucuronosyltransferase
- ULN
upper limit of normal
- VPA
valproate
Footnotes
Disclosure The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
Ethical Approval and Informed Consent Given the nature of this study, the project was exempt from institution review board/ethics committee review.
REFERENCES
- 1.Epidiolex [package insert] Carlsbad, CA: Greenwich Biosciences; 2018. [Google Scholar]
- 2.Corroon J, Kight R. Regulatory status of cannabidiol in the United States: a perspective. Cannabis Cannabinoid Res. 2018;3(1):190–194. doi: 10.1089/can.2018.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devinsky O. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med. 2017;376(21):2011–2020. doi: 10.1056/NEJMoa1611618. [DOI] [PubMed] [Google Scholar]
- 4.Devinsky O, Patel AD, Cross JH et al. Effect of cannabidiol on drop seizures in the Lennox–Gastaut syndrome. N Engl J Med. 2018;378(20):1888–1897. doi: 10.1056/NEJMoa1714631. [DOI] [PubMed] [Google Scholar]
- 5.Brooke K, O’Connell DG, Devinsky O. Cannabinoids in treatment-resistant epilepsy: a review. Epilepsy Behav. 2016;70(pt B):341–348. doi: 10.1016/j.yebeh.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 6.Bridgeman MB, Abazia DT. Medicinal cannabis: history, pharmacology, and implications for the acute care setting. PT. 2017;42(3):180–188. [PMC free article] [PubMed] [Google Scholar]
- 7.French LMM. NAFTA & Neocolonialism: Comparative Criminal, Human & Social Justice. University Press of America; 2004. [Google Scholar]
- 8.Earlywine M. Understanding Marijuana: A New Look at the Scientific Evidence. Oxford University Press; 2005. [Google Scholar]
- 9.Denham BE. Attitudes toward legalization of marijuana in the United States, 1986–2016: changes in determinants of public opinion. Int J Drug Policy. 2019;71:78–90. doi: 10.1016/j.drugpo.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 10.National Conference of State Legislatures State Medical Marijuana Laws. 2019. Accessed April 1, 2020. http://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx.
- 11.National Institute on Drug Abuse Drug Facts: Marijuana. Bethesda, MD: NIDA; 2018. [Google Scholar]
- 12. Governing. State marijuana laws in 2019 map. 2019. Accessed April 1, 2020. https://www.governing.com/gov-data/state-marijuana-laws-map-medical-recreational.html.
- 13.Reznikoff C. Medical cannabis update. American College of Physicians; Minnesota Chapter Annual Scientific Meeting; 2015; In. https://www.acponline.org/about-acp/chapters-regions/united-states/minnesota-chapter/news-meetings/american-college-of-physicians2015-minnesota-chapter-annual-scientific-meeting. [Google Scholar]
- 14.FDA FDA regulation of cannabis and cannabis-derived products: questions and answers. 2019. www.fda.gov Accessed April 1, 2020.
- 15.Gupta DS, editor. WEED. WEED: Why I Changed My Mind on Weed. Atlanta, GA: CNN; 2014. [Google Scholar]
- 16.Laux LC, Bebinb EM, Checkettsc D, et al. on behalf of CBD EAP Study Group Long-term safety and efficacy of cannabidiol in children and adults withtreatment resistant Lennox-Gastaut syndrome or Dravet syndrome: expanded access program results. Epilepsy Res. 2019;154:7. doi: 10.1016/j.eplepsyres.2019.03.015. doi. [DOI] [PubMed] [Google Scholar]
- 17.Tyler E, Gaston DF. Pharmacology of cannabinoids in the treatment of epilepsy. Epilepsy Behav. 2017;70(pt B):313–318. doi: 10.1016/j.yebeh.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 18. Pharmaceuticals plc and Its U.S. Subsidiary Greenwich Biosciences, Inc. Announce That EPIDIOLEX® (cannabidiol) Oral Solution Has Been Descheduled And Is No Longer A Controlled Substance. GlobalNewswire. 06 April 2020. Accessed 17 July 2020.
- 19.Sun Y, Dolmetsch RE. Investigating the therapeutic mechanism of cannabidiol in a human induced pluripotent stem call (iPSC)-based cellular model of Dravet Syndrome. Cold Spring Harb Symp Quant Biol. 2018;83:185–191. doi: 10.1101/sqb.2018.83.038174. [DOI] [PubMed] [Google Scholar]
- 20. A randomized controlled trial of cannabidiol (GWP42003-P, CBD) for seizures in tuberous sclerosis complex (GWPCARE6). ClinicalTrials.gov. identifier: NCT02544763. https://ClinicalTrials.gov/show/NCT02544763 Accessed April 1, 2020.
- 21. An open-label extension trial of cannabidiol (GWP42003-P, CBD) for seizures in tuberous sclerosis complex (GWPCARE6). ClinicalTrials.gov. identifier: NCT02544750. https://ClinicalTrials.gov/show/NCT02544750 Accessed April 1, 2020.
- 22.LaVito A. The cannabis industry is begging the FDA for some CBD regulaiton as Gottlieb heads to the Hill. CNBC. 2019 Feb 27; Accessed April 1, 2020 https://www.cnbc.com/2019/02/27/the-cannabis-industry-is-begging-the-fda-for-some-cbd-regulations.html.
- 23.Morrison G, Crockett J, Blakey G, Sommerville K. A phase 1, open-label, pharmacokinetic trial to investigate possible drug-drug interactions between clobazam, stiripentol, or valproate and cannabidiol in healthy subjects. Clin Pharmacol Drug Dev. 2019;8(8):1009–1031. doi: 10.1002/cpdd.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Appleton R, Macleod S. Lennox-Gastaut syndrome. Epilepsy Action. 2016 https://www.epilepsy.org.uk/info/syndromes/lennox-gastaut-syndromeAccessed April 1, 2020.
- 25.Sullivan J, Knupp K, Wirrell E. Dravet syndrome. 2018. https://rarediseases.org/rare-diseases/dravet-syndrome-spectrum/ Accessed April 1, 2020.
- 26.National Organization for Rare Disorders Lennox-Gastaut syndrome. 2017. https://rarediseases.org/rare-diseases/lennox-gastaut-syndrome/ Accessed April 1, 2020.
- 27.LGS Foundation Epidemiology of Lennox-Gastaut syndrome. 2019. https://www.lgsfoundation.org/ Accessed April 1, 2020.
- 28.News D. Dravet syndrome prognosis. 2019. https://dravetsyndromenews.com/dravet-syndrome-prognosis/ Accessed April 1, 2020.
- 29.Michoulas A, Farrell K. Medical management of Lennox-Gastaut syndrome. CNS Drugs. 2010;24(5):363–374. doi: 10.2165/11530220-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 30.Thiele EA, Marsh ED, French JA et al. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391(10125):1085–1096. doi: 10.1016/S0140-6736(18)30136-3. [DOI] [PubMed] [Google Scholar]
- 31.Varlet V, Concha-Lozano N, Berthet A et al. Drug vaping applied to cannabis: is “Cannavaping” a therapeutic alternative to marijuana? Sci Rep. 2016;6:25599. doi: 10.1038/srep25599. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dravet Syndrome Foundation Cherry Hill, NJ: 2019. What is Dravet sydnrome? https://www.dravetfoundation.org/what-is-dravet-syndrome/Accessed April 1, 2020. [Google Scholar]
- 33.Cervenka MC, Hocker S, Koenig M et al. Phase I/II multicenter ketogenic diet study for adult superrefractory status epilepticus. Neurology. 2017;88(10):938–943. doi: 10.1212/WNL.0000000000003690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morris GL, III, Gloss D, Buchhalter J et al. Evidence-based guideline update: vagus nerve stimulation for the treatment of epilepsy: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;81(16):1453–1459. doi: 10.1212/WNL.0b013e3182a393d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Depakote (valproate sodium) [package insert] North Chicago, IL: Hospira for AbbVie Inc; 2013. [Google Scholar]
- 36.Verrotti A, Striano P, Iapadre G et al. The pharmacological management of Lennox-Gastaut syndrome and critical literature review. Seizure. 2018;63:17–25. doi: 10.1016/j.seizure.2018.10.016. [DOI] [PubMed] [Google Scholar]
- 37.Cross JH, Auvin S, Falip M et al. Expert opinion on the management of Lennox-Gastaut syndrome: treatment algorithms and practical considerations. Front Neurol. 2017;8:505. doi: 10.3389/fneur.2017.00505. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Onfi (clobazam) [package insert] Deerfield, IL Lundbeck: 2019. [Google Scholar]
- 39.Lamictal [package insert] Research Triangle Park, NC: GlaxoSmithKline; 2009. [Google Scholar]
- 40.Borrelli EP, Lee EY, Descoteaux AM et al. Stevens-Johnson syndrome and toxic epidermal necrolysis with antiepileptic drugs: an analysis of the US Food and Drug Administration adverse event reporting system. Epilepsia. 2018;59(12):2318–2324. doi: 10.1111/epi.14591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trivedi BS, Darji NH, Malhotra SD, Patel PR. Antiepileptic drugs-induced Stevens-Johnson syndrome: a case series. J Basic Clin Pharm. 2016;8(1):42–44. doi: 10.4103/0976-0105.195130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Devinsky OM, Patel AD, Thiele EDM et al. Randomized, dose-ranging safety trial of cannabidiol in Dravet syndrome. Neurology. 2018;90(14):1204–1211. doi: 10.1212/WNL.0000000000005254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thiele EA, French JA, Mazurkiewicz-Beldzinska M et al. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391(10125):1085–1096. doi: 10.1016/S0140-6736(18)30136-3. [DOI] [PubMed] [Google Scholar]
- 44.Gaston TE, Bebin EM, Cutter GR et al. Interactions between cannabidiol and commonly used antiepileptic drugs. Epilepsia. 2017;58(9):1586–1592. doi: 10.1111/epi.13852. [DOI] [PubMed] [Google Scholar]
- 45.New York, NY: Pfizer: 2009. Phenytoin USP. [Google Scholar]
- 46.Boucherville, Canada: Sandoz Canada Inc; 2015. Phenobarbital sodium USP. [Google Scholar]
- 47.Titusville, NJ: Janssen Pharmaceuticals, Inc; 2017. Topirimate USP. [Google Scholar]
- 48.Klotz KA, Hirsch M, Heers M et al. Effects of cannabidiol on brivaracetam plasma levels. Epilepsia. 2019;60(7):e74–e77. doi: 10.1111/epi.16071. [DOI] [PubMed] [Google Scholar]
- 49.Leino AD, Emoto C, Fukuda T et al. Evidence of a clinically significant drug-drug interaction between cannabidiol and tacrolimus. Am J Transplant. 2019;19(10):2944–2948. doi: 10.1111/ajt.15398. [DOI] [PubMed] [Google Scholar]
- 50.Smyrna, GA: UCB, Inc; 2016. Brivaracetam USP. [Google Scholar]
- 51.Klein P, Diaz A, Gasalla T, Whitesides J. A review of the pharmacology and clinical efficacy of brivaracetam. Clin Pharmacol. 2018;10:1–22. doi: 10.2147/CPAA.S114072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Devinsky O, Verducci C, Thiele EA et al. Open-label use of highly purified CBD (Epidiolex®) in patients with CDKL5 deficiency disorder and Aicardi, Dup15q, and Doose syndromes. Epilepsy Behav. 2018;86:131–137. doi: 10.1016/j.yebeh.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 53.Gofshteyn JS, Wilfong A, Devinsky O et al. Cannabidiol as a potential treatment for febrile infection-related epilepsy syndrome (FIRES) in the acute and chronic phases. J Child Neurol. 2017;32(1):35–40. doi: 10.1177/0883073816669450. [DOI] [PubMed] [Google Scholar]
- 54.Phase 3 trial of cannabidiol (CBD; GWP42003-P) for infantile spasms: open-label extension phase (GWPCARE7). ClinicalTrials.gov.identifier: NCT02954887. https://ClinicalTrials.gov/show/NCT02954887Accessed April 1, 2020.
- 55.Trial of cannabidiol (CBD; GWP42003-P) for infantile spasms (GWPCARE7). ClinicalTrials.gov.identifier: NCT02953548. https://ClinicalTrials.gov/show/NCT02953548 Accessed April 1, 2020.
- 56.Cannabidiol expanded access study in medically refractory Sturge-Weber syndrome. ClinicalTrials.gov.identifier: NCT02332655. https://ClinicalTrials.gov/show/NCT02332655 Accessed April 1, 2020.
- 57.Safety, and tolerability of Epidiolex in patients (ages 1–19 years) with intractable epilepsy. ClinicalTrials.gov. identifier: NCT02695537. https://ClinicalTrials.gov/show/NCT02695537 Accessed April 1, 2020.
- 58.A study to assess the safety and tolerability of ZX008 in children and young adults with DS or LGS currently taking CBD. ClinicalTrials.gov.identifier: NCT03467113. https://ClinicalTrials.gov/show/NCT03467113 Accessed April 1, 2020.
- 59.Cannabidiol oral solution in pediatric participants with treatment-resistant childhood absence seizures. Clinical-Trials.gov.identifier: NCT03336242. https://ClinicalTrials.gov/show/NCT03336242 Accessed April 1, 2020.
- 60.A study to assess the efficacy, safety, and tolerability of cannabidiol oral solution with vigabatrin as initial therapy in participants with infantile spasms. ClinicalTrials.gov. identifier: NCT03421496. https://ClinicalTrials.gov/show/NCT03421496 Accessed April 1, 2020.
- 61.Clinical study of cannabidiol in children and adolescents with fragile X (CONNECT-FX). ClinicalTrials.gov. identifier: NCT03614663. https://ClinicalTrials.gov/show/NCT03614663 Accessed April 1, 2020.
- 62.Jiang R, Yamaori S, Okamoto Y et al. Cannabidiol is a potent inhibitor of the catalytic activity of cytochrome P450 2C19. Drug Metab Pharmacokinet. 2013;28(4):332–338. doi: 10.2133/dmpk.dmpk-12-rg-129. [DOI] [PubMed] [Google Scholar]
- 63.Yamaori S, Okamoto Y, Yamamoto I, Watanabe K. Cannabidiol, a major phytocannabinoid, as a potent atypical inhibitor for CYP2D6. Drug Metab Dispos. 2011;39(11):2049–2056. doi: 10.1124/dmd.111.041384. [DOI] [PubMed] [Google Scholar]
- 64.Yamaori S, Ebisawa J, Okushima Y et al. Potent inhibition of human cytochrome P450 3A isoforms by cannabidiol: role of phenolic hydroxyl groups in the resorcinol moiety. Life Sci. 2011;88(15–16):730–736. doi: 10.1016/j.lfs.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 65.An open-label extension trial to investigate possible drug-drug interactions between stiripentol or valproate and cannabidiol in patients with epilepsy. ClinicalTrials.gov. identifier: NCT02607904. https://ClinicalTrials.gov/show/NCT02607904 Accessed April 1, 2020.
- 66.A study to investigate possible drug-drug interactions between stiripentol or valproate and cannabidiol in patients with epilepsy.ClinicalTrials.gov. identifier: NCT02607891. https://ClinicalTrials.gov/show/NCT02607891 Accessed April 1, 2020.
- 67.A randomized controlled trial to investigate possible drug-drug interactions between clobazam and cannabidiol. ClinicalTrials.gov. identifier: NCT02565108. https://ClinicalTrials.gov/show/NCT02565108 Accessed April 1, 2020.
- 68.An open-label extension study to investigate possible drug-drug interactions between clobazam and cannabidiol. ClinicalTrials.gov. identifier: NCT02564952. https://ClinicalTrials.gov/show/NCT02564952 Accessed April 1, 2020.
- 69.A dose-ranging pharmacokinetics and safety study of GWP42003-P in children with Dravet syndrome (GWPCARE1). ClinicalTrials.gov. identifier: NCT02091206. https://ClinicalTrials.gov/show/NCT02091206 Accessed April 1, 2020.
- 70.Huestis MA, Solimini R, Pichini S et al. Cannabidiol adverse effects and toxicity. Curr Neuropharmacol. 2019;17(10):974–989. doi: 10.2174/1570159X17666190603171901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Anderson GD. A mechanistic approach to antiepileptic drug interactions. Ann Pharmacother. 1998;32(5):554–563. doi: 10.1345/aph.17332. [DOI] [PubMed] [Google Scholar]
- 72.US National Library of Medicine NIH clinical trials. 2019. Accessed April 1, 2020. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/results?term=epidiolex&Search=Search Accessed April 1, 2020.


