Abstract
Observational data suggest an acquired prothrombotic state may contribute to the pathophysiology of COVID-19. These data include elevated D-dimers observed among many COVID-19 patients. We present a retrospective analysis of admission D-dimer, and D-dimer trends, among 1065 adult hospitalized COVID-19 patients, across 6 New York Hospitals. The primary outcome was all-cause mortality. Secondary outcomes were intubation and venous thromboembolism (VTE). Three-hundred-thirteen patients (29.4%) died, 319 (30.0%) required intubation, and 30 (2.8%) had diagnosed VTE. Using Cox proportional-hazard modeling, each 1 μg/ml increase in admission D-dimer level was associated with a hazard ratio (HR) of 1.06 (95%CI 1.04–1.08, p < 0.0001) for death, 1.08 (95%CI 1.06–1.10, p < 0.0001) for intubation, and 1.08 (95%CI 1.03–1.13, p = 0.0087) for VTE. Time-dependent receiver-operator-curves for admission D-dimer as a predictor of death, intubation, and VTE yielded areas-under-the-curve of 0.694, 0.621, and 0.565 respectively. Joint-latent-class-modeling identified distinct groups of patients with respect to D-dimer trend. Patients with stable D-dimer trajectories had HRs of 0.29 (95%CI 0.17–0.49, p < 0.0001) and 0.22 (95%CI 0.10–0.45, p = 0.0001) relative to those with increasing D-dimer trajectories, for the outcomes death and intubation respectively. Patients with low-increasing D-dimer trajectories had a multivariable HR for VTE of 0.18 (95%CI 0.05–0.68, p = 0.0117) relative to those with high-decreasing D-dimer trajectories. Time-dependent receiver-operator-curves for D-dimer trend as a predictor of death, intubation, and VTE yielded areas-under-the-curve of 0.678, 0.699, and 0.722 respectively. Although admission D-dimer levels, and D-dimer trends, are associated with outcomes in COVID-19, they have limited performance characteristics as prognostic tests.
Keywords: COVID-19, D-dimer, Admission, Trend, Outcomes, Thrombosis
Highlights
-
•
We present a retrospective analysis of admission D-dimer, and D-dimer trends, among adults hospitalized for COVID-19.
-
•
1065 inpatients from 6 hospitals were included; outcomes included mortality, intubation, and VTE.
-
•
Admission D-dimers and D-dimer trends were associated with outcomes in COVID-19.
-
•
However, D-dimer levels and trends were limited prognostic tests in COVID-19.
-
•
The role of D-dimer levels in COVID-19 clinical decision making remains unclear.
1. Introduction
Amidst the current pandemic of severe acute respiratory coronavirus 2 (SARS-CoV-2), an impression has arisen among clinicians that some component of the associated syndrome (COVID-19), may be driven by an acquired prothrombotic state and venous thromboembolic disease [[1], [2], [3], [4], [5]]. This impression has been based on several clinical observations. These include high rates of venous thromboembolism described in some COVID-19 cohorts, reports of large vessel stoke among young patients with COVID-19, experiences of recurrent clotting of hemodialysis catheters among COVID-19 patients, reports of occult pulmonary emboli and pulmonary microangiopathy in some COVID-19 autopsy series, and observations of pulmonary physiology reminiscent of pulmonary vascular disease among some mechanically ventilated COVID-19 patients [[6], [7], [8], [9]]. Additionally, many patients with COVID-19 have been observed to present with prominently elevated D-dimers, findings which have been postulated to reflect underlying thromboembolic burden, and which have been associated with increased mortality among such patients [[10], [11], [12], [13], [14], [15]]. As a result, some centers have begun using D-dimer values to guide decisions regarding use of empiric therapeutic anticoagulation (AC) to treat COVID-19. However, the majority of studies associating D-dimer level with outcomes in COVID-19 have been limited by sample size and/or questionable methods, and their results require further validation in larger cohorts. Studies to date have only examined D-dimers as static variables (most often using only admission levels) and have not analyzed D-dimer trends over time. We present a retrospective analysis of the predictive characteristics of both admission D-dimer levels, and D-dimer trends, among a large cohort of patients hospitalized with COVID-19.
2. Methods
2.1. Patients and outcomes
We retrospectively reviewed all hospitalized patients, at least 18 years of age, with laboratory-confirmed COVID-19, and a D-dimer level assessed either within 3 days of admission (for admission D-dimer analysis), or at least 3 D-dimer levels assessed prior to outcome of interest (for D-dimer trend analysis), admitted to 6 New York hospitals between March 1, 2020 and April 1, 2020. Hospitalized patients carrying a confirmed diagnosis of COVID-19 were identified via the health system's electronic medical record. Confirmed COVID-19 was defined by a positive result on a reverse-transcriptase–polymerase-chain-reaction (RT-PCR) SARS-CoV-2 assay of a nasopharyngeal swab specimen. The availability and results of D-dimer levels were determined and extracted via our health system clinical laboratory database.
D-dimer testing was performed across 6 clinical laboratories. All 6 clinical laboratories performed D-dimer testing using the same methods, reagents, and instruments. Specifically, all D-dimer measurements were performed via immune-turbidometric assay using STA®-Liatest D-Di ® kits (Cat. No. 00515) on STA®-analyzers (Diagnostica Stago S.A.S, 3 allee Theresa, 92600 Asnieres sur Seine, France).
Only those patients with a D-dimer level available within 3 days of admission were included in the admission D-dimer analysis. If more than one D-dimer level was available within 3 days of admission for a given patient, the initial level (earliest) was used for admission D-dimer analysis. Only those patients with at least 3 D-dimer levels within 21 days of diagnosis or prior to the outcome of interest were included in the D-dimer trend analysis.
The primary outcome of interest was all-cause mortality. Relevant secondary outcomes included need for invasive mechanical ventilation (i.e. intubation), and venous thrombotic event (VTE, defined via the availability of definitive imaging evidence). Clinical information regarding these outcomes was extracted from physician documentation via review of the electronic medical record by study investigators. This study was approved by the Program for Protection of Human Subjects by the Program for the Protection of Human Subjects (PPHS) of the Icahn School of Medicine at Mount Sinai.
Covariates of interest included age, sex, race (Black, White, Other, or Unknown), smoking status, obesity, prior VTE history, use of AC prior to admission, and comorbidity status (assessed using the Charlson Comorbidity Index (CCI)).
2.2. Statistical methods
This project had two primary objectives which required distinct statistical methods: (1) to assess the association between admission D-dimer levels with each outcome of interest and (2) to assess the association between longitudinally measured D-dimer levels (D-dimer trends), with outcome of interest.
To address (1) above, univariable and multivariable Cox-proportional hazards models were used to estimate the hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) for each outcome, for every 1 μg/mlincrease in the admission D-dimer level. Time-dependent receiver operator characteristic (ROC) curves for right censored survival data were estimated using the inverse probability of censoring weighting method previously described by Uno et al. [16]. Areas under the 21-day ROC curves (AUCs) as well as integrated time-dependent AUCs (IAUC − average of all time-dependent AUC) were provided as metrics of model discrimination.
To address (2) univariable and multivariable joint latent class models (JLCMs) were used to estimate the association between longitudinally measured D-dimer trajectories and the hazard of each outcome. The joint model consisted of longitudinal and survival sub-model components. For the longitudinal sub-model, a latent class analysis was used to identify two subgroups of patients with distinct quadratic trajectories of D-dimer values measured over time. Each class had its own survival sub-model with the class specific baseline hazard approximated by a 7-equi piecewise constant function. With this approach, the latent class membership is the link between the longitudinal and survival sub-models.
Under the JLCM modeling framework, the cohort size for each outcome was different as each patient needed to have at least 3 D-dimer values either before the observation of the outcome of interest or within the first 21 days to allow for estimation of D-dimer values as a quadratic polynomial function of time. As intubation can occur earlier in the hospitalization period, the cohort for intubation had the fewest patients. Once the latent classes were estimated, patient characteristics were compared among classes using the chi-square test for categorical variables and the Wilcoxon rank sum test for continuous variables. Any covariate significant at the 0.05 level was included in the multivariable JLCM. Time-dependent ROC curves for right censored survival data were estimated using the inverse probability of censoring weighting method previously described by Uno et al. [16]. Areas under the 21-day ROC curves (AUCs) as well as integrated time-dependent AUCs (IAUC − average of all time-dependent AUC) were provided as metrics of model discrimination.
Hypothesis testing was two-sided and conducted at the 5% level of significance. All statistical analyses were performed with SAS v9.4 (SAS Institute, Cary, NC) and R software packages (Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/).
3. Results
3.1. Patient characteristics and outcomes
We identified 2032 unique COVID-19 patients, aged >18 years, admitted during the study period. A total of 1065 (52%) of these patients had at least one D-dimer level measured within 3 days of admission, and were therefor included in the admission D-dimer analysis. The baseline characteristics of these patients are shown in Table 1 . The median admission D-dimer among all included patients was 1.39 μg/ml (IQR 0.75–2.75 μg/ml). Three-hundred-seventy-four patients (35.1%) had an admission D-dimer >2 μg/ml, 138 (13.0%) had an admission D-dimer >5 μg/ml, and 73 (6.9%) had an admission D-dimer >10 μg/ml. The primary outcome (all-cause mortality) occurred in 313 patients (29.4%) during the study period. Regarding secondary outcomes, 319 patients (30.0%) required intubation, and 30 (2.8%) demonstrated imaging evidence of VTE during the study period.
Table 1.
The baseline characteristics of patients in the admission D-dimer analysis are shown.
| Total (n = 1065) | |
|---|---|
| Age, median (IQR) | 66.3 (54.7–76.3) |
| Sex | |
| Female | 423 (39.7%) |
| Male | 642 (60.3%) |
| Race | |
| Black | 258 (24.2%) |
| Non-Hispanic White | 255 (23.9%) |
| Other | 501 (47.0%) |
| Unknown | 51 (4.8%) |
| CCI, median (IQR) | 3 (2–5) |
| Smoking | |
| Yes | 47 (4.4%) |
| No | 950 (89.2%) |
| Unknown | 68 (6.4%) |
| Obesity (BMI > 30 kg/m2) | |
| Yes | 394 (37.0%) |
| No | 648 (60.9%) |
| Unknown | 23 (2.2%) |
| Prior VTE | 49 (4.6%) |
| Baseline anticoagulant use | 97 (9.1%) |
| Admission D-dimer (μg/ml), median (IQR) | 1.39 (0.75–2.75) |
Abbreviations: BMI – body mass index; CCI – Charleson Comorbidity Index; IQR – interquartile range.
Of note, 97 patients (9.1%) were receiving AC prior to admission (all for non-COVID-19 indications such as prior history of VTE or atrial fibrillation). There was no significant correlation between pre-admission AC and admission D-dimer level (r = −0.121, p = 0.739). All of these patients were continued on therapeutic-dose AC following admission. A further 42 patients (3.9%) received at least some therapeutic AC (for varying courses) during admission (almost exclusively for confirmed or suspected venous thromboembolism). The vast majority of the remaining patients (n = 901, 97%) received prophylactic AC during admission. There was no significant association between therapeutic AC during admission and D-dimer trend (chi-square statistic = 0.859, p = 0.354).
3.2. Admission D-dimer analysis
The results of univariable and multivariable Cox proportional hazards models relating admission D-dimer to the primary outcome of all-cause mortality are shown in Table 2 . All variables found to be significantly associated with the outcome on univariable analysis (age, smoking status, CCI, admission AC use, and admission D-dimer) were included in the multivariable model. On multivariable analysis, admission D-dimer (treated as a continuous variable) was associated with a HR of 1.06 (95% CI 1.04–1.08, p < 0.0001) for all-cause mortality. That is to say, every 1 μg/ml increase in the admission D-dimer level was associated with a 6% increase in the risk of all-cause mortality.
Table 2.
The results of univariable and multivariable Cox proportional hazards models for the primary outcome of all-cause mortality. All variables found to be significantly associated with the outcome on univariable analysis were used in the multivariable model. Some patients were excluded from univariable analyses due missing data (as noted in the table). Abbreviations; AC – anticoagulation; CCI – Charleson Comorbidity Index; CI – confidence interval; VTE – venous thromboembolism.
| Univariable analysis (n = 1065) |
Multivariable analysis (n = 997) |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Age | 1.04 | 1.03–1.05 | <0.0001 | 1.03 | 1.02–1.05 | <0.0001 |
| Sex | ||||||
| Female | 0.82 | 0.65–1.04 | 0.1010 | |||
| Male | Reference | |||||
| Race | 51 excluded | |||||
| Non-Hispanic White | Reference | |||||
| Black | 0.94 | 0.70–1.28 | 0.7063 | |||
| Other | 0.74 | 0.56–0.97 | 0.0307 | |||
| CCI | 1.17 | 1.13–1.21 | <0.0001 | 1.08 | 1.03–1.14 | 0.0037 |
| Smoking | 68 excluded | |||||
| Yes | 1.68 | 1.06–2.69 | 0.0285 | 1.86 | 1.16–2.98 | 0.0096 |
| No | Reference | Reference | ||||
| Obesity | 23 excluded | |||||
| Yes | 0.87 | 0.69–1.10 | 0.2474 | |||
| No | Reference | |||||
| Prior VTE | 0.95 | 0.55–1.66 | 0.8660 | |||
| AC use at admission | 1.58 | 1.12–2.21 | 0.0086 | 1.00 | 0.69–1.45 | 0.9933 |
| Admission D-dimer | 1.05 | 1.04–1.07 | <0.0001 | 1.06 | 1.04–1.08 | <0.0001 |
The results of similar univariable and multivariable Cox proportional hazards models relating admission D-dimer to the secondary outcome of intubation are shown in Supplemental Table 1. All variables found to be significantly associated with the outcome on univariable analysis (obesity and admission D-dimer) were included in the multivariable model. On multivariable analysis, admission D-dimer (treated as a continuous variable) was associated with a HR of 1.08 (95% CI 1.06–1.10, p < 0.0001) for intubation. That is to say, every 1 μg/ml increase in the admission D-dimer level was associated with an 8% increase in the risk of intubation.
The results of univariable Cox proportional hazards models relating admission D-dimer to the secondary outcome of VTE are shown in Supplemental Table 2. On univariable analysis, admission D-dimer (treated as a continuous variable) was associated with a HR of 1.08 (95% CI 1.03–1.13, p = 0.0087) for VTE. That is to say, every 1 μg/ml increase in the admission D-dimer level was associated with an 8% increase in the risk of VTE. Multivariable analysis was not performed for this outcome as admission D-dimer was the only variable significantly associated with VTE on univariable analysis.
In order to assess the performance characteristics of admission D-dimer as a prognostic test among patients with COVID-19, time dependent ROC analyses were then used to estimate IAUCs. Time-dependent ROCs for admission D-dimer as a predictor of death, intubation, and VTE yielded areas-under-the-curve of 0.694, 0.621, and 0.565 respectively, consistent with relatively poor discriminating ability as a predictor of these outcomes. The optimal cutoff admission D-dimer levels for prediction of all-cause mortality, intubation, and VTE were 2.38 μg/ml, 2.32 μg/ml, and 2.98 μg/ml respectively. Performance characteristics of admission D-dimer as a predictor of mortality included; sensitivity 51%, specificity 78%, positive predictive value (PPV) 48%, negative predictive value (NPV) 80%, positive likelihood ration (PLR) 2.33, and negative likelihood ration (NLR) 0.64. Performance characteristics of admission D-dimer as a predictor of intubation included; sensitivity 43%, specificity 75%, PPV 43%, NPV 75%, PLR 1.76, NLR 0.75. Performance characteristics of admission D-dimer as a predictor of VTE included; sensitivity 67%, specificity 58%, PPV 4%, NPV 98%, PLR 1.61, NLR 0.57.
3.3. D-dimer trend analysis
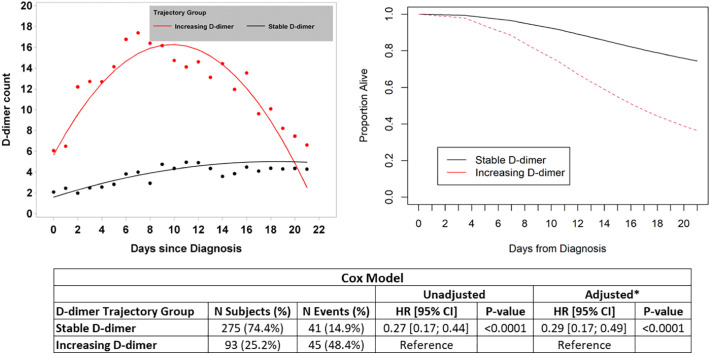
Longitudinal rends in D-dimer over time, and their association with each outcome of interest, were then assessed via joint latent class modeling (JLCM). Three-hundred-sixty-eight patients were included in the model of all-cause mortality. JLCM identified two distinct groups of patients with respect to D-dimer trend; those with relatively stable D-dimer (n = 275, 74.7%), and those with increasing D-dimer (n = 93, 25.3%) (depicted in the upper-left panel of Fig. 1 ). The baseline characteristics of patients in these 2 distinct trajectory groups are shown in Table 3 . A total of 41/275 (14.9%) patients in the stable trajectory group died during follow-up compared with 45/93 (48.4%) patients in the increasing trajectory group. The adjusted (multivariable) HR for death among patients in the stable D-dimer group relative to those in the increasing D-dimer group was 0.29 (95% CI 0.17–0.49, p < 0.0001) (Fig. 1).
Fig. 1.
The results of joint latent class modeling (JLCM) of D-dimer trend and its association with the primary outcome of all-cause mortality are depicted. D-dimer level is expressed in units of μg/ml. The two distinct D-dimer trajectories (increasing and stable) are shown in the upper left corner. Survival curves for these two groups are shown in the upper-right corner. The results of Cox proportional hazards models are shown in the lower panel (* the multivariable model adjusted for race and anticoagulant use prior to diagnosis, as these were the only baseline characteristics different between groups). Abbreviations: CI – confidence interval; HR – hazard ratio.
Table 3.
The baseline characteristics of all patients who could be assessed for the association between D-dimer trend and all-cause mortality via group-based trajectory modeling. Abbreviations: IQR – interquartile range; VTE – venous thromboembolism.
| Total (n = 368) | Stable D-dimer (n = 275) | Increasing D-dimer (n = 93) | p-Value | |
|---|---|---|---|---|
| Age at diagnosis, median (IQR) | 65 (53–74) | 65 (52–74) | 66 (58–73) | 0.2861 |
| Female, n (%) | 128 (0.0%) | 95 (34.5%) | 33 (35.5%) | 0.8695 |
| Race, n (%) | 0.0014 | |||
| Non-Hispanic White | 111 (30.2%) | 89 (32.4%) | 22 (23.7%) | |
| Black | 79 (21.5%) | 46 (16.7%) | 33 (35.5%) | |
| Other | 162 (44.0%) | 129 (46.9%) | 33 (35.5%) | |
| Unknown | 16 (4.3%) | 11 (4.0%) | 5 (5.4%) | |
| Charlson comorbidity index, median (IQR) | 3 (1–4) | 3 (1–5) | 3 (2–4) | 0.9012 |
| Current smoker | 13 (3.5%) | 11 (4.0%) | 2 (2.2%) | 0.3915 |
| Obese | 144 (39.1%) | 100 (36.4%) | 44 (47.3%) | 0.1566 |
| Prior VTE | 13 (3.5%) | 11 (4.0%) | 2 (2.2%) | 0.4036 |
| On anticoagulant prior to diagnosis | 29 (7.9%) | 27 (9.8%) | 2 (2.2%) | 0.0177 |
| First D-dimer value within 1 week of diagnosis, median (IQR) | 1.4 (0.8–3.1) | 1.1 (0.7–2.1) | 3.0 (1.4–8.0) | <0.0001 |
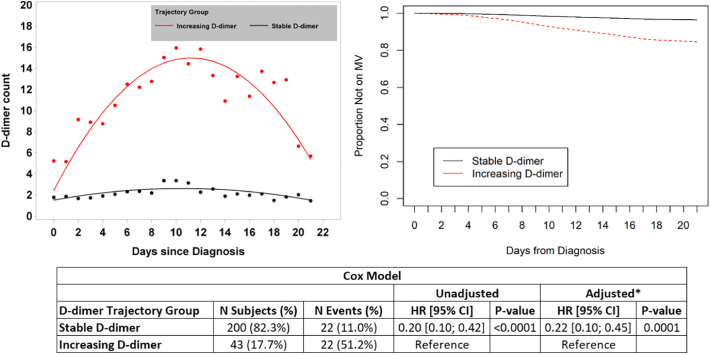
Two-hundred-forty-three patients were included in the model of mechanical ventilation (intubation). JLCM identified two distinct groups of patients with respect to D-dimer trend; those with relatively stable D-dimer (n = 200, 82.3%), and those with increasing D-dimer (n = 43, 17.7%) (depicted in the upper-left panel of Fig. 2 ). The baseline characteristics of patients in these 2 distinct trajectory groups are shown in Supplemental Table 3. A total of 22/200 (11.0%) patients in the stable trajectory group required mechanical ventilation during follow-up compared with 22/43 (51.2%) of patients in the increasing trajectory group. The adjusted (multivariable) HR for mechanical ventilation among patients in the stable D-dimer group relative to those in the increasing D-dimer group was 0.22 (95% CI 0.10–0.45, p = 0.0001) (Fig. 2).
Fig. 2.
The results of joint latent class modeling (JLCM) of D-dimer trend and its association with the secondary outcome of need for mechanical ventilation are depicted. D-dimer level is expressed in units of μg/ml. The two distinct D-dimer trajectories (increasing and stable) are shown in the upper left corner. Curves depicting proportion of patients not on mechanical ventilation over time are shown in the upper-right corner. The results of Cox proportional hazards models are shown in the lower panel (* the multivariable model adjusted for age, and Charlson Comorbidity Index, as these were the only baseline characteristics different between groups). Abbreviations: CI – confidence interval; HR – hazard ratio; MV – mechanical ventilation.
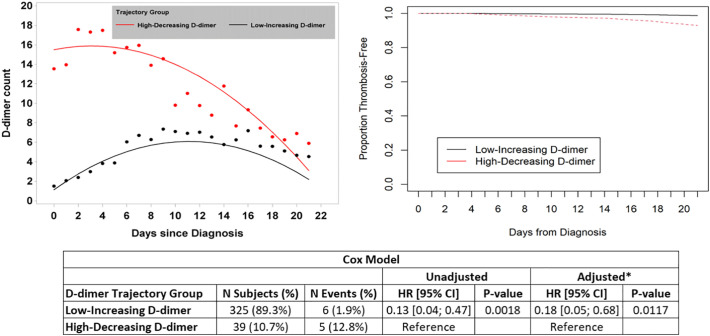
Three-hundred-sixty-four patients were included in the model of VTE. JLCM of D-dimer over time identified two distinct groups of patients with respect to D-dimer trend; those with low though increasing D-dimer (n = 325, 89.2%), and those with high though decreasing D-dimer (n = 39, 10.7%) (depicted in the upper-left panel of Fig. 3 ). The baseline characteristics of patients in these 2 distinct trajectory groups are shown in Supplemental Table 4. A total of 6/325 (1.9%) patients in the low-increasing trajectory group were diagnosed with VTE during follow-up compared with 5/39 (12.8%) of patients in the high-decreasing trajectory group. The adjusted (multivariable) HR for VTE among patients in the low-increasing D-dimer group relative to those in the high-decreasing D-dimer group was 0.18 (95% CI 0.05–0.68, p = 0.0117) (Fig. 3).
Fig. 3.
The results of joint latent class modeling (JLCM) of D-dimer trend and it association with the secondary outcome of diagnosed VTE are depicted. D-dimer level is expressed in units of μg/ml. The two distinct D-dimer trajectories (low-increasing and high-decreasing) are shown in the upper left corner. Curves depicting proportion of patients remaining venous-thrombosis-free over time are shown in the upper-right corner. The results of Cox proportional hazards models are shown in the lower panel (* the multivariable model adjusted for race as this was the only baseline characteristic different between groups). Abbreviations: CI – confidence interval; HR – hazard ratio.
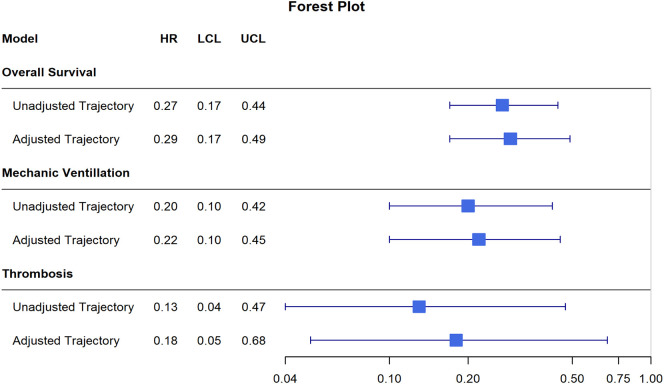
A forest plot depicting the unadjusted and adjusted HRs (with corresponding 95% CIs) comparing D-dimer trajectories for each outcome of interest is shown in Fig. 4 .
Fig. 4.
A forest plot depicting the unadjusted and adjusted HRs (with corresponding 95% CIs) comparing D-dimer trajectories for each outcome of interest. HRs represent the hazard of event in the lower and more stable D-dimer group relative to the hazard of event in higher increasing D-dimer group. All depicted HRs were significant. Abbreviations: HR – hazard ratio; LCL – lower confidence limit; UCL – upper confidence limit.
In order to assess the performance characteristics of D-dimer trend as a prognostic test among patients with COVID-19, time dependent ROC analyses were then used to estimate IAUCs. Time-dependent ROCs for admission D-dimer as a predictor of death, intubation, and VTE yielded areas-under-the-curve of 0.678, 0.699, and 0.722 respectively, consistent with relatively poor discriminating ability as a predictor of death and intubation, and a fair discriminating ability as a predictor of VTE.
4. Discussion
Great interest has arisen regarding the role of VTE in the pathophysiology of COVID-19, and of the potential role of D-dimer levels as COVID-19 biomarkers and outcome-predictors [17,18]. In this retrospective study of 1065 hospitalized COVID-19 patients, higher admission D-dimer was associated with greater risk of all-cause mortality, need for mechanical ventilation (intubation), and VTE. However, admission D-dimer, as an isolated measure, did not appear to be a reliable prognostic test for outcomes among COVID-19 patients. Particular D-dimer trends following admission were strongly associated with these same outcomes. However D-dimer trend/trajectory, as an isolated measure, did not appear to be a reliable prognostic test for these outcomes.
Some recent studies have indicated that D-dimer may be a relevant predictor of outcomes among patients with COVID-19, however most of these studies have been limited by sample size and/or questionable methods, and all have assessed only static D-dimer values (with none examining D-dimer trends) [[10], [11], [12], [13], [14]]. A study of 343 inpatients with COVID-19 from Wuhan Asia General Hospital reported that an AUC of 0.89 for a ROC of admission D-dimer as a predictor of in-hospital mortality. Further, they reported that their optimal cutoff D-dimer of 2 μg/ml yield a sensitivity and specificity for prediction of death 92% and 93% respectively. However these findings were based on only 13 death events in the cohort, hardly sufficient to construct a rigorous and reliable ROC [10]. In a retrospective study of 191 patients from 2 other centers in Wuhan, China, a D-dimer level greater than 1 μg/ml was suggested as a predictor of poor prognosis [11]. Here again, this conclusion was drawn based on relatively few death events (n = 54). More importantly, this conclusion was drawn based on comparing small groups of patients who fell within arbitrarily selected D-dimer ranges. A prospective study of 41 COVID-19 patients admitted to a single center in Wuhan, China included mention that patients requiring intensive care unit (ICU) admission had higher d-D-dimer values than those who were not, however this comparison included only 13 ICU patients, and was based on a limited univariate analysis [12]. A retrospective series of 138 consecutive COVID-19 patients from Zhongnan Hospital in Wuhan China indicated that “D-dimer was higher in non-survivors than in survivors” however this was based on a subgroup analysis which included only 33 patients, only 5 of whom were non-survivors [13]. Most recently, a study of 400 hospitalized COVID-19 patients from Massachusetts, USA reported that admission D-dimer levels falling in the ranges of 1–2.5 μg/ml, and >2.5 μg/ml were associated with greater risk of “critical illness” and mortality relative to a reference admission d-dimer of 1 μg/ml [14]. Although it was unclear why these specific cutoffs were chosen, and there was no information regarding the performance characteristics of these cutoffs as diagnostic tools, nor any analysis of D-dimer trends following admission.
The observation that patients with COVID-19 frequently have markedly elevated D-dimer levels has been used to further the argument that a significant component of the pathophysiology of this syndrome results from thromboembolic phenomena. In the minds of many, these D-dimer elevations go hand-in-hand with several other empiric observations among COVID-19 patients including high rates of VTE described in some cohorts, reports of stoke in the young, experiences of recurrently clotting hemodialysis catheters, reports of VTE on autopsy studies, and suggestions of pulmonary physiology akin to that in pulmonary vascular disease [[6], [7], [8], [9]]. However, such observations remain largely anecdotal, and reliable data implicating an acquired prothrombotic state and/or VTE as a primary factor in COVID-19 pathogenesis remains lacking with further studies needed in this regard. Additionally, it should be noted that elevated D-dimer is a highly non-specific marker of VTE and may be a manifestation of inflammation rather than thrombosis [19,20]. In a study of 449 COVID-19 patients, there was no difference in admission D-dimer as compared with 104 patients with non-COVID pneumonia [21]. In the above mentioned retrospective cohort from Massachusetts, USA D-dimer levels were shown to correlate with levels of inflammatory markers (such as C-reactive protein and procalcitonin) [14]. Nevertheless, D-dimers levels are currently being used to help make management decisions, particularly decisions regarding therapeutic AC, for COVID-19 patients at some centers. Although our findings show that both admission D-dimer and D-dimer trend are significantly associated with COVID-19 outcomes, it remains unclear if, and in what way, these measures should influence treatment decisions.
Our results were notable for a relatively low rate of overt venous thrombosis than has been described in some prior COVID-19 cohorts [14,22]. This may be attributable to resource utilization issues, infection control precautions, and the high frequency of clinically unstable patients during the current pandemic, all of which may have limited the employment of diagnostic imaging studies such as CT pulmonary angiography.
This study has several limitations resulting from its retrospective nature. It is possible that patients who were less ill at time of admission may have been less likely to have D-dimer levels assessed, thus such patients may be underrepresented in this cohort. It is possible that some treatment decisions may have been influenced by D-dimer levels and subsequently impacted outcomes, leading to the possibility of confounding. For instance, it is possible that D-dimer levels may have influenced patterns of AC use, and had an independent impact on outcomes (although this was not supported on multivariable analysis). Although this study sought to evaluate the most clinically relevant outcomes (mortality, intubation), it did not evaluate other often encountered outcomes such as intensive care unit (ICU) admission, nor any COVID-19 severity indices. Finally, it is possible that combining D-dimer levels with other laboratory parameters might yield better prognostic accuracy. Strengths of this study include a large sample size with high rates of relevant outcomes, and rigorous statistical analysis.
In conclusion higher admission D-dimer levels, and certain (particularly increasing) D-dimer trends, are associated with significantly greater risk of all-cause mortality, need for mechanical ventilation (intubation), and VTE, among patients with COVID-19. However, D-dimer values (either as static values or trends over time) have limited performance characteristics as prognostic tests in COVID-19 when taken in isolation. Further research is needed to clarify whether the high D-dimers so commonly encountered among COVID-19 patients are the result of thrombotic burden/tendency, inflammation, or a combination therein, as well as how D-dimers might be incorporated into multifactorial diagnostic paradigms, and if/how they should influence clinical decision making.
Declaration of competing interest
No funding was received for the preparation of this manuscript. The authors have no relevant conflicts of interest to report.
Acknowledgments
Acknowledgements
The authors acknowledge the support of the Biostatistics Shared Resource Facility, Icahn School of Medicine at Mount Sinai, and National Institutes of Health, National Cancer Institute Cancer Center Support Grant P30 CA196521-01.
Funding
No funding was received for the preparation of this manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.thromres.2020.08.032.
Appendix A. Supplementary data
Supplementary tables
References
- 1.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. (ahead of print) [DOI] [PubMed] [Google Scholar]
- 2.Tang N. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrett C.D. ISTH interim guidance on recognition and management of coagulopathy in COVID-19: a comment. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14860. (ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N. Engl. J. Med. 2020;382(17):e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dolhnikoff M. Pathological evidence of pulmonary thrombotic phenomena in severe COVID-19. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14844. (ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cui S. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14830. (ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oxley T.J. Large-vessel stroke as a presenting feature of Covid-19 in the young. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2009787. (ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barton L.M. COVID-19 Autopsies, Oklahoma, USA. Am. J. Clin. Pathol. 2020 doi: 10.1093/ajcp/aqaa062. (ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu X. Ventilatory ratio in hypercapnic mechanically ventilated patients with COVID-19 associated ARDS. Am. J. Respir. Crit. Care Med. 2020 doi: 10.1164/rccm.202002-0373LE. (ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang L. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14859. (ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou F. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang C. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang D. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. (ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Samkari H. COVID and coagulation: bleeding and thrombotic manifestations of SARS-CoV2 infection. Blood. 2020 doi: 10.1182/blood.2020006520. (ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thachil J. All those D-dimers in COVID-19. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14939. (ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uno H. Evaluating prediction rules for t-year survivors with censored regression models. J. Am. Stat. Assoc. 2007;102(478):527–537. [Google Scholar]
- 17.Khan I.H. The need to manage the risk of thromboembolism in COVID-19 patients. J. Vasc. Surg. 2020 doi: 10.1016/j.jvs.2020.05.015. (ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kermali M. The role of biomarkers in diagnosis of COVID-19 - a systematic review. Life Sci. 2020;254 doi: 10.1016/j.lfs.2020.117788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borowiec A. Elevated levels of d-dimer are associated with inflammation and disease activity rather than risk of venous thromboembolism in patients with granulomatosis with polyangiitis in long term observation. Adv. Med. Sci. 2020;65(1):97–101. doi: 10.1016/j.advms.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 20.Shorr A.F. D-dimer correlates with proinflammatory cytokine levels and outcomes in critically ill patients. Chest. 2002;121(4):1262–1268. doi: 10.1378/chest.121.4.1262. [DOI] [PubMed] [Google Scholar]
- 21.Yin S. Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2. J. Thromb. Thrombolysis. 2020 doi: 10.1007/s11239-020-02105-8. (ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nahum J. Venous thrombosis among critically ill patients with coronavirus disease 2019 (COVID-19) JAMA Netw. Open. 2020;3(5) doi: 10.1001/jamanetworkopen.2020.10478. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables