Abstract
The Bacille Calmette Guérin (BCG) vaccine was developed over a century ago and has become one of the most used vaccines without undergoing a modern vaccine development life cycle. Despite this, the vaccine has protected many millions from severe and disseminated forms of tuberculosis (TB). In addition, BCG has cross-mycobacterial effects against non-tuberculous mycobacteria and off-target (also called non-specific or heterologous) effects against other infections and diseases. More recently, BCG’s effects on innate immunity suggest it might improve the immune response against viral respiratory infections including SARS-CoV-2. New TB vaccines, developed over the last 30 years, show promise, particularly in prevention of progression to disease from TB infection in young adults. The role of BCG in the context of new TB vaccines remains uncertain as most participants included in trials have been previously BCG immunised. BCG replacement vaccines are in efficacy trials and these may also have off-target effects.
Keywords: Latent TB, Active TB, Trained immunity, COVID-19, SARS-CoV-2, Cross-protective
BCG development and early trials
The Bacille Callmette-Guérin (BCG) vaccine is one of the oldest and most commonly administered vaccines worldwide. The vaccine was developed by Albert Calmette and Camille Guérin in the early 1900s by attenuating a strain of Mycobacterium bovis – a mycobacterium closely related to Mycobacterium tuberculosis causing bovine tuberculosis (TB). The first use of BCG in humans in 1921 was long before standardised pre-clinical and clinical trials were required. Today, first-in-man phase I trials of new vaccines are generally small trials in healthy adults. The first time the BCG vaccine was used in humans was in an infant whose mother had died of TB only a few hours after birth [1]. The infant was fed a mixture of milk and oral BCG on day 3, 5 and 7 after birth and remained well over the following six-months. Encouraged by this, further infants with and without TB exposure were given BCG in France with an up to 4-year follow-up with no evidence of adverse effects [1]. This led to mass production of BCG at the Pasteur Institute in Lille (France) and Calmette and co-workers subsequently immunised over 52,000 children with BCG in France between 1924 and 1927. Of those over 6000 were born in families with TB cases and Calmette reported that BCG reduced TB mortality in infants from 25% to less than 1% [2]. Despite many researchers questioning the scientific approach by Calmette, Turpin and Weill-Hallé, the vaccine continued to be used in numerous studies in children and adults. For example, in Sweden the head of the children’s hospital in Gothenburg, Arvid Walgreen, studied the intradermal application of BCG as this route of administration resulted in a tuberculin skin test (TST) positivity, which at the time was considered a correlate of protection against TB [3]. Earlier work by Turpin and Weill-Hallé, using subcutaneous and intradermal routes of BCG administration, has been discontinued as they observed more frequent local adverse reactions. Despite continuing controversy about the protective efficacy and the optimal route of administration of the BCG vaccine, the vaccine was promoted after 1948 by the World Health Organization (WHO) and the United Nations International Children’s Emergency Fund (UNICEF) [4].
Specific effects: protective efficacy of BCG against tuberculosis
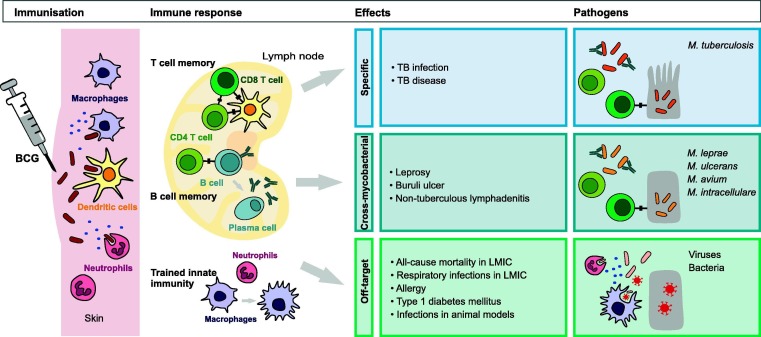
Large trials with more robust trial design evaluating the protective efficacy of BCG started in the 1930s in several countries and settings. Importantly, the protective efficacy varied considerably between studies, in particular for pulmonary forms of TB [5]. For example, the largest BCG vaccine trial including over 260,000 participants in Chingleput (India) starting in 1968 showed no evidence of protection against pulmonary TB compared with placebo in over 7 years follow-up [6]. Contrary to this, one of the earliest BCG vaccine trials, with a robust design done in North American Indians between 1935 and 1938, showed long-term protective efficacy for pulmonary TB of 82% after 20 years and 52% after 60 years follow-up [7]. Factors that might explain such heterogeneous results include study design (the Chingleput trial was criticised for methodological flaws), variation in vaccine strains used and exposure to environmental non-tuberculous mycobacteria, as well as host and other geographical factors. Despite the variably reported efficacy against pulmonary TB, BCG has consistently shown high (over 70%) protective efficacy against disseminated forms of TB, including TB meningitis and miliary TB [5], [8], [9], [10]. In addition, evidence from more recent studies suggest that BCG also protects against TB infection and progression from infection to disease [11].
Cross-mycobacterial effects: protective efficacy of BCG against non-tuberculous mycobacterial infections
In the late 1930s it was noted that BCG immunisation not only led to a positive TST but also to a positive skin reaction following intradermal injection of heat-killed Mycobacterium leprae. Subsequently studies investigating the protective efficacy of BCG against leprosy showed a 26% to 61% efficacy (summarised in two meta-analyses) [12], [13]. Further to this, BCG has been shown to protect against Buruli ulcer (caused by Mycobacterium ulcerans) in two randomised controlled trials in Uganda with a pooled protective efficacy of 47%, and variable protection in case-control studies in a recent meta-analysis [14].
Protection against other non-tuberculous mycobacteria, including Mycobacterium avium and Mycobacterium intracellulare (which can cause cervical lymphadenitis in pre-school children) has also been investigated. In Finland, the incidence of non-tuberculous mycobacterial infections increased from 0.2 to 3.9 per 100,000 person-years following a change from universal BCG to selective BCG immunisation [15]. Similar observations were made in Sweden [16] and the Czech Republic [17]. A meta-analysis based on three cohort studies in high-income countries showed that BCG-immunised children had a 96% lower risk of non-tuberculous mycobacterial lymphadenitis compared with BCG-unimmunised children [14].
Off-target effects of BCG on neonatal and infant mortality
In addition to protecting against mycobacterial infection, a growing body of evidence indicates that BCG vaccine has a protective effect against viruses, bacteria and parasites through heterologous lymphocyte activation, boosting of innate immunity and enhanced cytokine production (Fig. 1 ) [18], [19], [20], [21]. These ‘off-target’ (also called ‘non-specific’ or ‘heterologous’) effects were described soon after the first use of BCG in humans. Calmette reported an at least 4-fold reduction in non-TB related mortality (from 16–26% to 4%) in the first year of life in children immunised with BCG between 1926 and 1928 [22]. Similar observations were reported in other countries. For example, Carl Naeslund in Sweden was puzzled by the reduction in mortality associated with BCG vaccination in the first year of life considering TB being mainly responsible for deaths in children beyond the first year of life. He introduced the term ‘non-specific’ immunity to describe this BCG-induced protection against other diseases [23]. In the following decades a reduction in all-cause mortality was found in a number of controlled trials in the US, UK, Canada, India and Papua New Guinea, and more recently in observational and randomised controlled studies mainly in Guinea-Bissau [24]. A WHO commissioned systematic review, which analysed studies available up until 2013, concluded that BCG reduced all-cause mortality by 30% (95% confidence interval: −1% to 51%) in clinical trials [25]. Since then, two further randomised controlled trials have been published. The first one, in Guinea-Bissau, showed a reduction in mortality in the first 28 days of life by 30% (95% confidence interval: −6% to 53%), which decreased to 12% at 6 and 12 months of age [26]. The second trial, done in intensive care units in India, showed no significant change in reduction of mortality in the first 28 days of life of 5% (95% confidence interval: −7% to 20%) in the BCG-immunised infants [27]. It has been proposed that the difference in the results from Guinea-Bissau and India might be attributable to the use of different vaccine strains (BCG-Denmark in Guinea-Bissau and BCG-Russia in India) [28]. In addition, the infants in India were of lower birth weight (<2000 g in India compared with <2500 g in Guinea-Bissau) and potentially sicker as they were in an intensive care unit.
Fig. 1.
Summary of the specific, cross-mycobacterial and off-target effects of BCG relevant to children.
Off-target effects of BCG on acute respiratory tract infections
The studies on reduction in infant mortality by BCG triggered studies on its protective effect against respiratory tract infections, these being one of the most common causes of mortality in children under 5 years of age. A matched case-control study from Guinea-Bissau compared acute lower respiratory tract infections in 772 children under 5 years of age in BCG-immunised and non-immunised children in the late 1990’s, as BCG was temporarily unavailable in the country [29]. The study found that children with acute lower respiratory infections were more likely to be BCG unimmunised. Similarly, hospitalisation rates for respiratory infection from a Spanish registry were analysed by BCG status, as routine BCG immunisation at birth was part of the Basque country region immunisation schedule until 2012, whereas the rest of Spain stopped using BCG in 1982 [30]. In the 464,611 admissions between 1992 and 2011 the study found a 41.4% (95% confidence interval: 40.3% to 42.5%) reduction in hospital admissions for respiratory infections in BCG-immunised children. A study from Greenland with a similar design included population-based data from 1989 to 2004, as BCG immunisation was temporarily discontinued from 1991 to 1996 [31]. In the 19,363 children, admission for respiratory infections did not differ between BCG-immunised and BCG-unimmunised children [31]. However as highlighted in a commentary, there was a 28% (95% confidence interval: −6% to 51%) reduced risk of infectious disease admission the subgroup of infants aged 3 days to 3 months [32]. This important comment relates to the concept that administration of non-live vaccines, usually given at the age at 2 to 3 months of life, may mitigate or negate the off-target effects of BCG at birth. The largest population-based study investigating the effect of BCG on acute lower respiratory tract infections in children under 5 years of age included data from representative home-based interviews in 33 low- and middle-income countries [33]. Of the over 150,000 children included the majority (96–97%) were BCG immunised with a calculated 17% to 37% reduction in suspected acute lower respiratory tract infections (defined by guardians reporting a child as having a cough accompanied by rapid or difficult breathing during the two weeks before the interview). Importantly this study included data recorded up 2010, meaning none of the countries had introduced routine immunisations for Haemophilus influenza type B or Streptococcus pneumoniae in their national immunisation programs, which potentially influenced the results.
Contrary to the findings from most of these population-based studies, two studies originating from the randomised Danish Calmette Study showed no overall influence of BCG on hospital admission rates and parent-reported infections. In 4262 infants randomised to BCG immunisation or no intervention within 7 days after birth between 2012 and 2013, hospital admission rates for infections up to 15 months of age were not influenced by BCG immunisation [34]. Similarly, for guardian-reported infections at 3- and 13-month telephone interviews, there was no influence of BCG on the frequency of fever, pneumonia or cold episodes [35]. Interestingly, in a post hoc subset analysis of infants whose mothers had received BCG, there was a 32% (95% confidence interval: 1% to 54%) reduction in infections in the first three months of life in the unadjusted hazard ration analysis [36]. A randomised study in South African adolescents investigating a novel TB vaccine (H4:IC31) also included a BCG revaccination arm. [37] Lower respiratory tract infections were rare in all groups over the 24 months follow-up period and there was no difference between BCG-revaccinated and non-revaccinated individuals. However, there was a reduction in upper respiratory tract infections in BCG-immunised individuals compared with those who received the new TB vaccine or placebo (2.1%, 9.4%, and 7.9% respectively).
Off-target effects of BCG on viral infections
Animal studies suggest that BCG has protective effects against viruses including influenza, herpes simplex, hepatitis B and Japanese encephalitis [18]. Studies in humans that have investigated prevention of viral diseases associated with BCG immunisation are rare. An interesting randomised placebo-controlled study in 30 healthy male adults (not previously BCG immunised) compared the immune response to a yellow fever immunisation as a “viral challenge model” one month after BCG immunisation [38]. Viremia is usually detectable after yellow fever immunisation in the first few days. The study showed a difference on day 5 after yellow fever immunisation with higher viral loads in the non-BCG-immunised adults compared to the BCG-immunised adults but this was not the case when viral loads were compared on day 3 and 7. They also investigated in-vitro cytokine expression after BCG immunisation and showed that with some stimulants and cytokines this was increased in the BCG-immunised group [38]. Further, monocytes from BCG-immunised individuals responded (transcriptionally) differently to a secondary in-vitro stimulus and suggesting that BCG-immunisation induces trained immunity in innate immune cells through epigenetic changes. Innate immune responses have been shown to be important in both specific and off-target effects of BCG [39], [40]. In the light of the evidence for off-target effects on viral respiratory tract infections, it is therefore not surprising that BCG-immunisation has been proposed as a potential prophylaxis against the novel severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). It is hypothesised that induction of a trained immunity by BCG immunisation may reduce severity of coronavirus disease 2019 (COVID-19) and randomised controlled trials are underway in several countries worldwide to assess whether BCG immunisation reduces the incidence and severity of COVID-19 in healthcare workers [19], [41]. However, although BCG immunisation is generally safe in children and adults, it is also possible that up-regulation of innate immunity by BCG immunisation will exacerbate COVID-19 and therefore BCG immunisation for COVID-19 should only be given within clinical trials [41], [42]. Cautious interpretation is also needed for pre-publication released ecological studies suggesting that countries with longstanding and universal BCG immunisation policy have reduced mortality and numbers of COVID-19 cases [43], [44]. Such ecological studies are prone to significant bias from many confounders, including differences in national demographics and disease burden, different definitions used for COVID-19 (confirmed cases only versus inclusion of presumed cases), testing rates for SARS-CoV-2, and the stage of the pandemic in each country [43], [45]. In addition, the current expert opinion is that BCG vaccine given many years earlier is unlikely to protect against COVID-19 as trained immunity induced by BCG might not be long-lasting and is likely abrogated by other vaccines [46]. These and several other ecological studies should therefore be interpreted with caution as BCG is - including during the COVID-19 pandemic - essential for the prevention of TB in infants and young children. It therefore should not be used for the prevention of COVID-19 before solid evidence for its effectiveness for this indication is available [47].
Other off-target effects of BCG
Previous or concurrent BCG also influences immune responses to other immunisations. For example, when healthy volunteers received BCG two weeks prior to a trivalent influenza vaccine, antibody responses against the influenza A[H1N1]pdm09 were enhanced though not for the two other strains A[H3N2]2012 and B/2012 [48]. This effect was most pronounced in individuals with low baseline antibody titres. Previous or concurrent administration of BCG with other routine infant immunisation has shown variable effects. BCG immunisation increases concentrations of antibodies against pneumococcus [49], has variable effects on antibodies against hepatitis B and Haemophilus influenzae type b (Hib) [49], [50], [51] and was not associated with significant changes in antibody responses to diphtheria, tetanus, pertussis, typhoid, measles and mumps [51], [52]. Multiple other studies suggest that BCG immunisation has numerous other potential beneficial off-target effects. Of interest to paediatrics are the prevention of childhood leukaemia, and allergic asthma, and the treatment for type 1 diabetes (reviewed elsewhere [53], [54]).
BCG immunisation policies worldwide
The World Health organization (WHO) recommends administration of a single dose of BCG to infants shortly after birth in countries with high TB incidence. In countries with low TB incidence, mainly in Europe, North America and Australia, universal routine BCG immunisation has been replaced over the last few decades by a targeted approach with immunisation restricted to neonates and infants at increased risk [55], [56], [57]. According to the WHO Global Tuberculosis Report, 154 of 180 countries recommend universal BCG vaccination, while 20 have had this policy in the past and the remaining six use selective immunisation for at-risk individuals. Data on BCG coverage were available for 153 countries, of which 113 reported a BCG coverage of at least 90% of the population [58]. In Europe, recent data shows that indications for BCG immunisation varied considerably between countries, reflecting national TB incidence rates, immigration and other factors influencing TB control strategies. Apart from universal BCG immunisation at birth, other strategies included: immunisation at birth or older age for high-risk groups, universal immunisation at older age, immunisation for immigrants and as a travel vaccine [56], [59].
BCG vaccine strain
Several different BCG vaccine strains are in use today [57], [60]. This is the result of the worldwide distribution of BCG in the 1920s following its development with further sub-culturing under different conditions leading to genetic and metabolic variability and the consequent evolution of different BCG strains [61], [62]. To standardise BCG production in the 1950s, seed lot systems were established and freeze dried storage was recommended by WHO [63]. Different BCG vaccine strains are associated with different immune responses, expressed as proportion of polyfunctional CD4 T cells [64], [65]. However, in the absence of proven immunological correlates or biomarkers of protection, the significance of these findings for protective efficacy are unknown. One important observational study in Kazakhstan compared four birth cohorts that were subject to immunisation with different BCG vaccine strains (BCG-Russia, BCG-Serbia, BCG-Japan) or no BCG due to changes in national immunisation policies [66]. The cohorts included between 138,059 and 168,664 children with a 29-months follow-up. Based on TB case notifications, a substantial difference in protection was observed with 69%, 43%, and 22% efficacy against clinical TB and 92%, 82% and 51% efficacy against culture-positive TB for BCG-Japan, BCG-Serbia and BCG-Russia, respectively. However, in the absence of randomised controlled trials comparing currently available strains there is currently insufficient evidence to favour one BCG vaccine strain over another [67]. Naturally, in addition to protection against TB, the question of BCG vaccine strain also applies to BCG’s off-target effects [28]. A recently published randomised trial in Guinea-Bissau using BCG-Denmark, BCG-Japan and BCG-Russia found no statistically significant difference in the 6-week mortality of infants but unfortunately only had a power to show a ≥30% reduction as a result of having to change vaccine strains (BCG-Denmark was replaced by BCG-Japan) as the former became unavailable during the trial in 2015 [68].
BCG vaccine shortages
BCG is produced only by a limited number of manufactures worldwide. UNICEF procures BCG vaccine on behalf of 70 mainly resource-limited countries. In 2017, 160 million doses were distributed by UNICEF, accounting for 57% of the market [69]. Due to production difficulties between 2013 and 2015, there was a global BCG shortage in 2015 and 2016. Several actions to mitigate the shortage were coordinated by WHO and UNICEF but the dependence on limited suppliers underlines the importance of a well-coordinated distribution and avoidance of misuse of BCG. Resource-limited countries with higher TB incidences have, following WHO recommendations, higher coverage with BCG and are therefore most affected from supply shortages. Therefore, as shown in a mathematical model, even relatively small BCG shortages of 6.3–27.3% may result in 7400–24,900 excess TB deaths per birth cohort [70]. This is exemplified by the increased rates of TB meningitis reported in South Africa attributed with the 2015/16 shortage [71]. BCG shortages have also affected European countries and led to changes in immunisation policies and BCG vaccine strains used [57]. Various strategies to reduce BCG wastage have been proposed including optimising the use of multidose BCG vaccine vials by centralising and cohorting BCG immunisations.
BCG-revaccination
A single dose of BCG shortly after birth is recommended by the WHO in countries or settings with a high incidence of TB and revaccination is not recommended [72]. Despite this, BCG re-vaccination is done in some TB endemic countries. Evidence from a systematic review published in 2013 including four randomised controlled studies, three cohort and two case-control studies suggests that BCG revaccination overall confers no additional protection from TB and an updated systematic review is underway [73], [74]. Recent evidence from a phase 2 trial of a novel TB vaccine (H4:IC31), which included a BCG revaccination arm, however, suggests potential beneficial effects. BCG-revaccinated adolescents had a 45% reduced rate of TB infection (assessed by interferon-gamma release assay (IGRA) conversion) compared with non-revaccinated controls [37].
Intravenous and oral administration of BCG
Today the BCG vaccine is universally administered as an intradermal injection, but the original BCG was given orally. In Brazil, the BCG-Moreau strain was used as an oral vaccine until 1976 [75]. In addition, animals studies done almost 50 years ago, showed superior protection when intravenous and aerosol application were used compared with intradermal administration [76]. Recent data from a study in macaques compared TB prevention after intradermal, intravenous and aerosol BCG immunisation (given via a paediatric mask attached to a Pari eFlow nebuliser) [77]. The study had a complex design with several BCG administration routes: low-dose and high-dose intradermal, intravenous, aerosol and a combination of aerosol and intradermal. To compare the effects of intravenous and intradermal administration, only the high-dose intradermal and the intravenous groups should be compared as these received 5 × 107 colony forming units (CFU) of BCG-Denmark. Intravenous BCG administration resulted in a higher frequency of specific CD4 and CD8 T cells in blood and parenchymal lung tissue compared with the two other routes of immunisation. After the challenge with M. tuberculosis Erdman 6 to 10 months after BCG immunisation, macaques that had received intravenous BCG showed superior protection against TB measured by lung PET-CT, number of granulomas and M. tuberculosis CFU in necropsy. Similar results were seen in an earlier study using the macaque challenge model comparing intravenous (using a 10-fold higher dose compared to the standard intradermal dose), intradermal (standard dose) and intradermal-intratracheal-boost BCG immunisation [78]. Intravenous and the intradermal-intratracheal-boost administration showed improved protection and increased frequency of specific multifunctional CD4 T cells. For clinical applications, both intravenous and aerosol application of BCG are impractical for large scale immunisation. In addition, both studies used a 10–100-fold higher dose for intravenous administration than the current standard intradermal dose (2–8 × 105 CFU). The use of higher doses such as this would present supply difficulties given the current BCG vaccine shortages. However, these studies are proof of principle that alternative immunisations routes may enhance the protective efficacy of TB vaccines.
As infection with M. tuberculosis occurs through the respiratory tract, with early exposure of mucosa and the mucosa associated lymph nodes, more recent studies investigated the effect of mimicking the natural route of infection by oral vaccine administration. A study including 84 healthy adults administered BCG intradermally, orally or by both routes [79]. The intradermal route resulted in a more pronounced lymphoproliferation and higher mycobacteria-specific interferon-gamma release by T cells, whereas the oral route resulted in higher concentrations of mycobacteria-specific secretory IgA. The combination resulted in increased systemic and mucosal immune responses. These findings suggest that mucosal immunity can be stimulated by oral BCG immunisation, but it remains to be clarified whether immunological changes translate into enhanced protection.
New TB vaccines
WHO formulated preferred product characteristics for new TB vaccines which should provide protection against both TB infection and disease [80]. The new TB vaccine should have an efficacy of more than 50% with durable protection of at least 5–10 years. Mathematical models on the impact of new TB vaccines show that vaccines targeting adolescents and adults could have a greater impact than those targeting neonates and infants. In one model, a vaccine with an efficacy of 40% and a duration of protection of 10 years given to adolescents and adults could avert 40% of TB cases in a low-income country [81]. A systematic review of mathematical models suggests that a vaccine targeting adolescents and adults would have the most rapid and cost-effective outcome TB reduction [82].
Strategies for new TB vaccine use
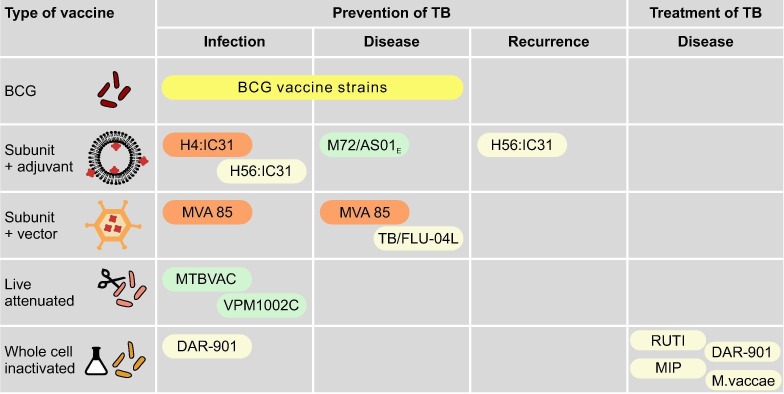
There are three main strategies for the design of new TB vaccines: prevention of infection (aimed mainly at infants and children without previous exposure to mycobacteria); prevention of disease (targeting individuals with TB infection to prevent progression to TB disease); and prevention of recurrence (focusing on individuals after completion of TB treatment to prevent reinfection or reactivation). Another concept is that of therapeutic immunisation which seeks to shorten or adapt treatment regimens with adjuvant vaccines which may be particularly valuable for drug-resistant TB (Fig. 2 ).
Fig. 2.
Novel TB vaccine strategies with example vaccines. Only vaccines that are in (or registered for) phase II or III trials are included. The colours around vaccines denote trials that have shown improved protection (green), no improvement (orange), or not discussed/no results available (yellow). H4:IC31: fusion protein of antigens 85B and TB10.4 with IC31 adjuvant H56:IC31: fusion protein of antigens 85B, ESAT-6 and Rv2660c with IC31 adjuvant M72/AS01E: fusion protein of antigen 32A and 39A with AS01E adjuvant MVA85: modified vaccinia Ankara 85A TB/FLU-04L: influenza A vectored vaccine expressing the antigens 85A and ESAT-6 MTBVAC: live attenuated M. tuberculosis strain (Euro-American lineage 4) with deletion mutations in the virulence genes phoP and fadD26 VPM1002C: live attenduated M. bovis BCG-Prague (rBCGΔureC:Hly) DAR-901: heat-killed, fragmented M. tuberculosis cultured under stress conditions MIP: heat-killed Mycobacterium indicus pranii a non-pathogenic, non-tuberculous mycobacterium approved in India as a leprosy vaccine M. vaccae: heat-killed Mycobacterium vaccae a non-pathogenic, non-tuberculous mycobacterium.
Examples of new subunit, viral vector and whole cell TB vaccines
There are currently over 20 vaccines in pre-clinical and clinical studies, and these can be classified into three groups: live-attenuated, inactivated whole cell and subunit vaccines [83]. For subunit vaccines, either an adjuvant or a viral vector is required to achieve sufficient immune stimulation [84]. A century later after the development of BCG, all new TB vaccines have to undergo a highly regulated process which includes preclinical testing in animal models, phase I trials in small groups of healthy adults, phase II trials with expanding numbers and clinical characteristics of individuals and, finally, phase III trials with large number of individuals in multiple geographic locations.
One of the first large phase II trials with a subunit-adjuvant TB vaccine compared H4:IC31 to BCG revaccination and placebo in 900 TB uninfected adolescents in South Africa [37]. H4 is a recombinant fusion protein of M. tuberculosis antigens 85B and TB10.4 and IC31, a synthetic adjuvant. Unfortunately, neither H4:IC31 nor BCG revaccination prevented TB infection (measured by early IGRA conversion). For H4:IC31, the efficacy to prevent sustained IGRA conversion was 31% but did not achieve statistical significance compared with placebo whereas BCG revaccination was associated with a significant efficacy of 46%, which has renewed interest in BCG revaccination. More successful was a different subunit-adjuvant vaccine, M72/AS01E with M72 being a fusion protein of M. tuberculosis antigens 32A and 39A combined with liposomal adjuvant (AS01E) which is also used in licensed zoster and malaria vaccines [85]. The final analysis of the phase IIb trial comprised 3575 HIV-negative participants in South Africa, Kenya and Zambia with TB infection (latent TB) who had two doses of M72/AS01E or placebo injected intramuscularly one month apart with the endpoint being prevention of bacteriologically confirmed pulmonary TB over three years. The vaccine efficacy was 54% (95% confidence interval: 2.9–74.2) at the three-year follow-up compared to placebo, which established proof of principle that prevention of disease by a new TB vaccine is possible. Further investigations on the correlates and duration of protection, the role of prior BCG immunisation and generalisability of the results will require analysis of biobanked samples and a phase III trial.
Another approach uses a viral vector gene encoding the M. tuberculosis antigenic target protein which results in intracellular uptake and stimulation of the innate immune response, meaning no adjuvant is needed. For viral vector vaccines, a potential problem is that an immune response against the vector itself might prevent efficient boosting. To overcome this, prime-boost regimes are proposed in which the same encoding gene is delivered for prime and boost vaccination, but in a different vector [83], [84]. The first trial, using a viral vector vaccine, used a modified Vaccinia Ankara virus expressing antigen 85A (MVA85A), which was given to HIV-uninfected healthy infants who had previously received BCG [86]. In the 2797 infants, followed for over 2 years, MVA85A did not show protection against TB infection (measured as IGRA conversion) or disease (based on the presence of clinical, radiological, and microbiological findings). The vaccine was used in further trials including in adults without success [87], and only few viral vector base TB vaccines have been developed and tested in trials since. One interesting new approach using cytomegalovirus (CMV) as the viral vector is still in the preclinical phase and was tested in macaques [88]. The idea behind this is to take advantage of the natural periodic reactivation of CMV, which leads to a recurring immune stimulus and thus makes boosters unnecessary. The CMV-based vaccine RhCMV/TB (which includes nine M. tuberculosis antigens) was subcutaneously administered in two doses three months apart and showed improved protection compared with BCG or controls one year after immunisation following challenge with M. tuberculosis Erdman [89]. This is therefore a promising approach and it will be important to determine if such a vaccine can safely be used in humans.
Among whole cell, live attenuated new TB vaccines there are two main representatives: the MTBVAC, a genetically attenuated M. tuberculosis vaccine and VPM1002, a recombinant BCG vaccine (i.e., originating from M. bovis). The advantage of the latter is the proof of concept and the knowledge and experience from BCG over the last century. In a phase I trial, MTBVAC (a live attenuated M. tuberculosis strain of the Euro-American lineage 4 with deletion mutations in the virulence genes phoP and fadD26) was used in 18 healthy HIV-uninfected adults with were IGRA negative and had no TB exposure and 36 newborns and showed durable CD4 T cell responses in both groups [90]. The vaccine has now moved on to phase II trials. The VPM1002 is a live attenuated M. bovis BCG-Prague, which was developed in the 1990s on the basis that genetic modification may lead to improved immunogenicity. This was done by including the listeriolysin-encoding gene from Listeria monocytogenes resulting in cytosol release of the BCG antigens. In a phase II clinical trial in South Africa, the immune response in 36 newborns immunised with intradermal VPM1002 was overall comparable to that in 12 newborns immunised with BCG. However, VPM1002 induced an increase in IL-17-producing CD8 T cells, which may contribute to protection [91].
The vaccine is also being tested in trials in several other settings in adults for prevention of disease and recurrence, and also for its off-target effects on bladder cancer [83]. For any new TB vaccine the WHO suggests including studies on leprosy and Buruli ulcer and potentially other endpoints. It is also crucial that off-target effects are considered and included as outcomes in clinical trials to avoid an increase in all-cause mortality as a result of the loss of protection against other infections causing neonatal death.
Conclusion
BCG was developed over a century ago and became one of the most widely used vaccines worldwide. Despite not undergoing a modern vaccine development life cycle, the vaccine has protected many millions from severe and disseminated forms of TB as well as from infection caused by non-tuberculous mycobacteria. Off-target effects of BCG against other infections have potentially been more important in terms of reducing all-cause mortality in neonates. More recently, immunological studies, particularly relating to innate immunity, suggest that BCG may protect against viral respiratory diseases, potentially including SARS-CoV-2. New TB vaccines have been in development for over 30 years and now show progress, particularly in the prevention of progression from TB infection to disease in young adults. The role of BCG in the efficacy of new TB vaccines remains to be clarified as most participants included in trials were previously BCG immunised. BCG replacement vaccines are in trials and these may also have off-target effects which are an important consideration for any new vaccine.
Directions for future research
-
•
Alternative routes of administration of BCG vaccine that might enhance protective immunity, as well as provide easy and safe routes of administration.
-
•
Identification of biomarkers that provide correlates of protection for the short- and long-term protection provided by BCG or new TB vaccines.
-
•
Improved diagnostics for TB in children, including biomarkers and immune responses associated with disease progression.
-
•
Prospective immunological epidemiological studies and randomised controlled trials that investigate the off-target effects of BCG vaccination on non-TB infectious diseases, including COVID-19.
Funding
No funding was obtained for the writing of this review. NF is supported by a grant from H2020 (825579).
Competing interests
The authors declare that they have no competing interests.
Conflict of interest
The authors declare no conflict of interest.
Educational aims
The reader will be able to
-
•
Appreciate the history of the development of the BCG vaccine and how this influences current global use.
-
•
Be cognisant of the non-specific effects of BCG relevant in children which include cross-mycobacterial effects and off-target effects on all-cause mortality and respiratory infections.
-
•
Understand the concepts and strategies involved in the development of new TB vaccines.
CRediT authorship contribution statement
Nora Fritschi: Conceptualization, Writing - original draft, Visualization. Nigel Curtis: Writing - review & editing. Nicole Ritz: Conceptualization, Writing - original draft, Writing - review & editing.
References
- 1.Weill-Hallé B., Turpin R. Premières essay de vaccination antituberculeuse de l'enfent par le bacille Calmette-Guérin. Bulletin et mémoires de la société médicale des hôpitaux de Paris. 1925;49:1589–1601. [Google Scholar]
- 2.Petroff S.A., Branch A. Bacillus Calemette-Guérin (B.C.G.). Animal experimentation and prophylactic immunization of children. Am J Public Health. 1928;18(7):843–864. doi: 10.2105/ajph.18.7.843-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wallgren A. Intradermal vaccination by BCG virus. J Am Med Assoc. 1928;91(24):1876–1881. [Google Scholar]
- 4.Smith K.M., Orme I.M., Starke J.R. In: Vaccines. 5 ed. Plotkin S., Orenstein W., Offit P., editors. Saunders Elsevier; 2008. Tuberculosis vaccines. [Google Scholar]
- 5.Rodrigues L.C., Diwan V.K., Wheeler J.G. Protective effect of BCG against tuberculous meningitis and miliary tuberculosis: a meta-analysis. Int J Epidemiol. 1993;22(6):1154–1158. doi: 10.1093/ije/22.6.1154. [DOI] [PubMed] [Google Scholar]
- 6.Trial of BCG vaccines in south India for tuberculosis prevention: first report--Tuberculosis Prevention Trial. Bull World Health Organ. 1979;57(5):819-827. [PMC free article] [PubMed]
- 7.Aronson N.E., Santosham M., Comstock G.W., et al. Long-term efficacy of BCG vaccine in American Indians and Alaska Natives: a 60-year follow-up study. JAMA. 2004;291(17):2086–2091. doi: 10.1001/jama.291.17.2086. [DOI] [PubMed] [Google Scholar]
- 8.Trunz B.B., Fine P., Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet. 2006;367(9517):1173–1180. doi: 10.1016/S0140-6736(06)68507-3. [DOI] [PubMed] [Google Scholar]
- 9.Abubakar I, Pimpin L, Ariti C, et al. Systematic review and meta-analysis of the current evidence on the duration of protection by bacillus Calmette-Guerin vaccination against tuberculosis. Health Technol Assess. 2013;17(37):1-372, v-vi. [DOI] [PMC free article] [PubMed]
- 10.Colditz G.A., Berkey C.S., Mosteller F., et al. The efficacy of bacillus Calmette-Guérin vaccination of newborns and infants in the prevention of tuberculosis: meta-analyses of the published literature. Pediatrics. 1995;96(1 Pt 1):29–35. [PubMed] [Google Scholar]
- 11.Roy A., Eisenhut M., Harris R.J., et al. Effect of BCG vaccination against Mycobacterium tuberculosis infection in children: systematic review and meta-analysis. BMJ. 2014;349 doi: 10.1136/bmj.g4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Setia M.S., Steinmaus C., Ho C.S., Rutherford G.W. The role of BCG in prevention of leprosy: a meta-analysis. Lancet Infect Dis. 2006;6(3):162–170. doi: 10.1016/S1473-3099(06)70412-1. [DOI] [PubMed] [Google Scholar]
- 13.Merle C.S., Cunha S.S., Rodrigues L.C. BCG vaccination and leprosy protection: review of current evidence and status of BCG in leprosy control. Expert Rev Vaccines. 2010;9(2):209–222. doi: 10.1586/erv.09.161. [DOI] [PubMed] [Google Scholar]
- 14.Zimmermann P., Finn A., Curtis N. Does BCG vaccination protect against nontuberculous Mycobacterial infection? A systematic review and meta-analysis. J Infect Dis. 2018;218(5):679–687. doi: 10.1093/infdis/jiy207. [DOI] [PubMed] [Google Scholar]
- 15.Kontturi A., Soini H., Ollgren J., Salo E. Increase in childhood nontuberculous Mycobacterial infections after Bacille Calmette-Guérin coverage drop: a nationwide, population-based retrospective study, Finland, 1995–2016. Clin Infect Dis. 2018;67(8):1256–1261. doi: 10.1093/cid/ciy241. [DOI] [PubMed] [Google Scholar]
- 16.Romanus V., Hallander H.O., Wåhlén P., Olinder-Nielsen A.M., Magnusson P.H., Juhlin I. Atypical mycobacteria in extrapulmonary disease among children. Incidence in Sweden from 1969 to 1990, related to changing BCG-vaccination coverage. Tuber Lung Dis. 1995;76(4):300–310. doi: 10.1016/s0962-8479(05)80028-0. [DOI] [PubMed] [Google Scholar]
- 17.Trnka L., Danková D., Svandová E. Six years' experience with the discontinuation of BCG vaccination. 4. Protective effect of BCG vaccination against the Mycobacterium avium intracellulare complex. Tuber Lung Dis. 1994;75(5):348–352. doi: 10.1016/0962-8479(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 18.Moorlag S., Arts R.J.W., van Crevel R., Netea M.G. Non-specific effects of BCG vaccine on viral infections. Clin Microbiol Infect. 2019;25(12):1473–1478. doi: 10.1016/j.cmi.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 19.Netea M.G., Giamarellos-Bourboulis E.J., Dominguez-Andres J., et al. Trained immunity: a tool for reducing susceptibility to and the severity of SARS-CoV-2 infection. Cell. 2020;181(5):969–977. doi: 10.1016/j.cell.2020.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freyne B., Marchant A., Curtis N. BCG-associated heterologous immunity, a historical perspective: intervention studies in animal models of infectious diseases. Trans R Soc Trop Med Hyg. 2015;109(4):287. doi: 10.1093/trstmh/trv021. [DOI] [PubMed] [Google Scholar]
- 21.Messina N.L., Zimmermann P., Curtis N. The impact of vaccines on heterologous adaptive immunity. Clin Microbiol Infect. 2019;25(12):1484–1493. doi: 10.1016/j.cmi.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 22.Calmette A. Preventive vaccination against tuberculosis with BCG. Proc R Soc Med. 1931;24(11):1481–1490. [PMC free article] [PubMed] [Google Scholar]
- 23.Naeslund C. Resultats Des Experiences de Vaccination Par le BCG Poursuivies Dans le Norrbotten (Suède) (Septembre 1927 ‐ Décembre 1931). In: Vaccination Préventive de la Tuberculose de l'homme et des animaux par le B C G Rapports et Documents Provenant Des Divers Pays (la France exceptée) transmis à l'Institut Pasteur en 1932. . Paris, France: Mason and Cie; 1932:274–281.
- 24.Benn C.S., Netea M.G., Selin L.K., Aaby P. A small jab - a big effect: nonspecific immunomodulation by vaccines. Trends Immunol. 2013;34(9):431–439. doi: 10.1016/j.it.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Higgins J.P., Soares-Weiser K., Lopez-Lopez J.A., et al. Association of BCG, DTP, and measles containing vaccines with childhood mortality: systematic review. BMJ. 2016;355 doi: 10.1136/bmj.i5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biering-Sorensen S., Aaby P., Lund N., et al. Early BCG-Denmark and neonatal mortality among infants weighing <2500 g: a randomized controlled trial. Clin Infect Dis. 2017;65(7):1183–1190. doi: 10.1093/cid/cix525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jayaraman K., Adhisivam B., Nallasivan S., et al. Two randomized trials of the effect of the Russian Strain of Bacillus Calmette-Guerin alone or with oral polio vaccine on neonatal mortality in infants weighing <2000 g in India. Pediatr Infect Dis J. 2019;38(2):198–202. doi: 10.1097/INF.0000000000002198. [DOI] [PubMed] [Google Scholar]
- 28.Curtis N. BCG vaccination and all-cause neonatal mortality. Pediatr Infect Dis J. 2019;38(2):195–197. doi: 10.1097/INF.0000000000002230. [DOI] [PubMed] [Google Scholar]
- 29.Stensballe L.G., Nante E., Jensen I.P., et al. Acute lower respiratory tract infections and respiratory syncytial virus in infants in Guinea-Bissau: a beneficial effect of BCG vaccination for girls community based case-control study. Vaccine. 2005;23(10):1251–1257. doi: 10.1016/j.vaccine.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 30.de Castro M.J., Pardo-Seco J., Martinon-Torres F. Nonspecific (heterologous) protection of neonatal BCG vaccination against hospitalization due to respiratory infection and sepsis. Clin Infect Dis. 2015;60(11):1611–1619. doi: 10.1093/cid/civ144. [DOI] [PubMed] [Google Scholar]
- 31.Haahr S., Michelsen S.W., Andersson M., et al. Non-specific effects of BCG vaccination on morbidity among children in Greenland: a population-based cohort study. Int J Epidemiol. 2016;45(6):2122–2130. doi: 10.1093/ije/dyw244. [DOI] [PubMed] [Google Scholar]
- 32.Benn C.S., Commentary S.S. BCG has no beneficial non-specific effects on Greenland. An answer to the wrong question? Int J Epidemiol. 2016;45(6):2131–2133. doi: 10.1093/ije/dyw299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hollm-Delgado M.G., Stuart E.A., Black R.E. Acute lower respiratory infection among Bacille Calmette-Guerin (BCG)-vaccinated children. Pediatrics. 2014;133(1):e73–81. doi: 10.1542/peds.2013-2218. [DOI] [PubMed] [Google Scholar]
- 34.Stensballe L.G., Ravn H., Birk N.M., et al. BCG vaccination at birth and rate of hospitalization for infection until 15 months of age in danish children: a randomized clinical multicenter trial. J Pediatric Infect Dis Soc. 2019;8(3):213–220. doi: 10.1093/jpids/piy029. [DOI] [PubMed] [Google Scholar]
- 35.Stensballe L.G., Sorup S., Aaby P., et al. BCG vaccination at birth and early childhood hospitalisation: a randomised clinical multicentre trial. Arch Dis Child. 2017;102(3):224–231. doi: 10.1136/archdischild-2016-310760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kjaergaard J., Birk N.M., Nissen T.N., et al. Nonspecific effect of BCG vaccination at birth on early childhood infections: a randomized, clinical multicenter trial. Pediatr Res. 2016;80(5):681–685. doi: 10.1038/pr.2016.142. [DOI] [PubMed] [Google Scholar]
- 37.Nemes E., Geldenhuys H., Rozot V., et al. Prevention of M. tuberculosis infection with H4:IC31 vaccine or BCG revaccination. N Engl J Med. 2018;379(2):138–149. doi: 10.1056/NEJMoa1714021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arts R.J.W., Moorlag S., Novakovic B., et al. BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe. 2018;23(1):89–100 e105. doi: 10.1016/j.chom.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 39.Zufferey C., Germano S., Dutta B., Curtis N., Ritz N. The contribution of non-conventional T cells and NK cells in the mycobacterial-specific IFNgamma response in Bacille Calmette-Guerin (BCG)-immunized infants. PLoS ONE. 2013;8(10) doi: 10.1371/journal.pone.0077334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kleinnijenhuis J., Quintin J., Preijers F., et al. BCG-induced trained immunity in NK cells: Role for non-specific protection to infection. Clin Immunol. 2014;155(2):213–219. doi: 10.1016/j.clim.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Curtis N., Sparrow A., Ghebreyesus T.A., Netea M.G. Considering BCG vaccination to reduce the impact of COVID-19. Lancet. 2020;395(10236):1545–1546. doi: 10.1016/S0140-6736(20)31025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ritz N., Strach M., Yau C., et al. A comparative analysis of polyfunctional T cells and secreted cytokines induced by Bacille Calmette-Guerin immunisation in children and adults. PLoS ONE. 2012;7(7) doi: 10.1371/journal.pone.0037535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller A, Reandelar MJ, Fasciglione K, Roumenova V, Li Y, Otazu GH. Correlation between universal BCG vaccination policy and reduced morbidity and mortality for COVID-19: an epidemiological study. medRxiv. 2020:2020.2003.2024.20042937.
- 44.Berg MK, Yu Q, Salvador CE, Melani I, Kitayama S. Mandated Bacillus Calmette-Guérin (BCG) vaccination predicts flattened curves for the spread of COVID-19. medRxiv. 2020:2020.2004.2005.20054163. [DOI] [PMC free article] [PubMed]
- 45.Riccò M., Gualerzi G., Ranzieri S., Bragazzi N. Stop playing with data: there is no sound evidence that Bacille Calmette-Guérin may avoid SARS-CoV-2 infection (for now) Acta bio-medica: Atenei Parmensis. 2020;91 doi: 10.23750/abm.v91i2.9700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hamiel U., Kozer E., Youngster I. SARS-CoV-2 rates in BCG-vaccinated and unvaccinated young adults. JAMA. 2020 doi: 10.1001/jama.2020.8189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schaaf H.S., du Preez K., Kruger M., et al. Bacille Calmette-Guérin (BCG) vaccine and the COVID-19 pandemic: responsible stewardship is needed. Int J Tuberc Lung Dis. 2020 doi: 10.5588/ijtld.20.0267. [DOI] [PubMed] [Google Scholar]
- 48.Leentjens J., Kox M., Stokman R., et al. BCG vaccination enhances the immunogenicity of subsequent influenza vaccination in healthy volunteers: a randomized, placebo-controlled pilot study. J Infect Dis. 2015;212(12):1930–1938. doi: 10.1093/infdis/jiv332. [DOI] [PubMed] [Google Scholar]
- 49.Ritz N., Mui M., Balloch A., Curtis N. Non-specific effect of Bacille Calmette-Guerin vaccine on the immune response to routine immunisations. Vaccine. 2013;31(30):3098–3103. doi: 10.1016/j.vaccine.2013.03.059. [DOI] [PubMed] [Google Scholar]
- 50.Ota M.O., Vekemans J., Schlegel-Haueter S.E., et al. Influence of Mycobacterium bovis bacillus Calmette-Guerin on antibody and cytokine responses to human neonatal vaccination. J Immunol. 2002;168(2):919–925. doi: 10.4049/jimmunol.168.2.919. [DOI] [PubMed] [Google Scholar]
- 51.Zimmermann P., Donath S., Perrett K.P., et al. The influence of neonatal Bacille Calmette-Guerin (BCG) immunisation on heterologous vaccine responses in infants. Vaccine. 2019;37(28):3735–3744. doi: 10.1016/j.vaccine.2019.03.016. [DOI] [PubMed] [Google Scholar]
- 52.Nissen T.N., Birk N.M., Smits G., et al. Bacille Calmette-Guerin (BCG) vaccination at birth and antibody responses to childhood vaccines. A randomised clinical trial. Vaccine. 2017;35(16):2084–2091. doi: 10.1016/j.vaccine.2017.02.048. [DOI] [PubMed] [Google Scholar]
- 53.Yamazaki-Nakashimada M.A., Unzueta A., Berenise Gamez-Gonzalez L., Gonzalez-Saldana N., Sorensen R.U. BCG: a vaccine with multiple faces. Hum Vaccin Immunother. 2020:1–10. doi: 10.1080/21645515.2019.1706930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Freyne B., Curtis N. Does neonatal BCG vaccination prevent allergic disease in later life? Arch Dis Child. 2014;99(2):182–184. doi: 10.1136/archdischild-2013-305655. [DOI] [PubMed] [Google Scholar]
- 55.Gwee A., Rodrigo R., Casalaz D., Ritz N., Curtis N. Infants born in Australia to mothers from countries with a high prevalence of tuberculosis: to BCG or not to BCG? Med J Aust. 2013;199(5):324–326. doi: 10.5694/mja13.10107. [DOI] [PubMed] [Google Scholar]
- 56.Dierig A., Tebruegge M., Krivec U., Heininger U., Ritz N. Paediatric Tuberculosis Network European Trials g. Current status of Bacille Calmette Guerin (BCG) immunisation in Europe - A ptbnet survey and review of current guidelines. Vaccine. 2015;33(38):4994–4999. doi: 10.1016/j.vaccine.2015.06.097. [DOI] [PubMed] [Google Scholar]
- 57.Kontturi A., Santiago B., Tebruegge M., von Both U., Salo E., Ritz N. The impact of Bacille Calmette-Guérin shortage on immunisation practice and policies in Europe - A Paediatric Tuberculosis Network European Trials Group (ptbnet) survey. Tuberculosis (Edinb) 2016;101:125–129. doi: 10.1016/j.tube.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 58.World Health Organization. Global Tuberculosis Report 2019. 2019.
- 59.Ritz N., Connell T.G., Curtis N. To BCG or not to BCG? Preventing travel-associated tuberculosis in children. Vaccine. 2008;26(47):5905–5910. doi: 10.1016/j.vaccine.2008.08.061. [DOI] [PubMed] [Google Scholar]
- 60.Ritz N., Curtis N. Mapping the global use of different BCG vaccine strains. Tuberculosis (Edinb) 2009;89(4):248–251. doi: 10.1016/j.tube.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 61.Brosch R., Gordon S.V., Garnier T., et al. Genome plasticity of BCG and impact on vaccine efficacy. PNAS. 2007;104(13):5596–5601. doi: 10.1073/pnas.0700869104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abdallah A.M., Hill-Cawthorne G.A., Otto T.D., et al. Genomic expression catalogue of a global collection of BCG vaccine strains show evidence for highly diverged metabolic and cell-wall adaptations. Sci Rep. 2015;5:15443. doi: 10.1038/srep15443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Behr M.A. BCG — different strains, different vaccines? Lancet Infect Dis. 2002;2(2):86–92. doi: 10.1016/s1473-3099(02)00182-2. [DOI] [PubMed] [Google Scholar]
- 64.Ritz N., Dutta B., Donath S., et al. The influence of bacille Calmette-Guerin vaccine strain on the immune response against tuberculosis: a randomized trial. Am J Respir Crit Care Med. 2012;185(2):213–222. doi: 10.1164/rccm.201104-0714OC. [DOI] [PubMed] [Google Scholar]
- 65.Kiravu A., Osawe S., Happel A.U., et al. Bacille Calmette-Guérin vaccine strain modulates the ontogeny of both mycobacterial-specific and heterologous T cell immunity to vaccination in infants. Front Immunol. 2019;10:2307. doi: 10.3389/fimmu.2019.02307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Favorov M., Ali M., Tursunbayeva A., et al. Comparative tuberculosis (TB) prevention effectiveness in children of Bacillus Calmette-Guerin (BCG) vaccines from different sources, Kazakhstan. PLoS ONE. 2012;7(3) doi: 10.1371/journal.pone.0032567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ritz N., Hanekom W.A., Robins-Browne R., Britton W.J., Curtis N. Influence of BCG vaccine strain on the immune response and protection against tuberculosis. FEMS Microbiol Rev. 2008;32(5):821–841. doi: 10.1111/j.1574-6976.2008.00118.x. [DOI] [PubMed] [Google Scholar]
- 68.Schaltz-Buchholzer F., Bjerregaard-Andersen M., Oland C.B., et al. Early vaccination with BCG-Denmark or BCG-Japan versus BCG-Russia to healthy newborns in Guinea-Bissau: a randomized controlled trial. Clin Infect Dis. 2019 doi: 10.1093/cid/ciz1080. [DOI] [PubMed] [Google Scholar]
- 69.UNICEF Supply Division. Bacillus Calmette-Guérin Vaccine: Supply and Demand Update. 2019.
- 70.Harris RC, Dodd PJ, White RG. The potential impact of BCG vaccine supply shortages on global paediatric tuberculosis mortality. BMC Med. 2016;14(1):138-138. [DOI] [PMC free article] [PubMed]
- 71.du Preez K., Seddon J.A., Schaaf H.S., et al. Global shortages of BCG vaccine and tuberculous meningitis in children. Lancet Global Health. 2019;7(1):e28–e29. doi: 10.1016/S2214-109X(18)30474-1. [DOI] [PubMed] [Google Scholar]
- 72.World Health Organization. BCG vaccines: WHO position paper – February 2018. Weekly epidemiological record. 2018;No 8, 93,:73–96.
- 73.Mahasha P.W., Ndwandwe D.E., Mavundza E.J., Shey M., Wiysonge C.S. Systematic review protocol on Bacillus Calmette-Guerin (BCG) revaccination and protection against tuberculosis. BMJ Open. 2019;9(10) doi: 10.1136/bmjopen-2018-027033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ahmad NA, Abd Hamid HA, Sahril N, Yusoff M, BM N, Aris T. Bacille Calmette-Guerin (BCG) revaccination: is it beneficial for tuberculosis control? Open access scientific reports 2013; https://www.omicsonline.org/scientific-reports/2161-1068-SR-656.pdf.
- 75.Benévolo-de-Andrade T.C., Monteiro-Maia R., Cosgrove C., Castello-Branco L.R. BCG Moreau Rio de Janeiro: an oral vaccine against tuberculosis–review. Mem Inst Oswaldo Cruz. 2005;100(5):459–465. doi: 10.1590/s0074-02762005000500002. [DOI] [PubMed] [Google Scholar]
- 76.Barclay W.R., Busey W.M., Dalgard D.W., et al. Protection of monkeys against airborne tuberculosis by aerosol vaccination with bacillus Calmette-Guerin. Am Rev Respir Dis. 1973;107(3):351–358. doi: 10.1164/arrd.1973.107.3.351. [DOI] [PubMed] [Google Scholar]
- 77.Darrah P.A., Zeppa J.J., Maiello P., et al. Prevention of tuberculosis in macaques after intravenous BCG immunization. Nature. 2020;577(7788):95–102. doi: 10.1038/s41586-019-1817-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sharpe S., White A., Sarfas C., et al. Alternative BCG delivery strategies improve protection against Mycobacterium tuberculosis in non-human primates: Protection associated with mycobacterial antigen-specific CD4 effector memory T-cell populations. Tuberculosis (Edinb) 2016;101:174–190. doi: 10.1016/j.tube.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hoft D.F., Xia M., Zhang G.L., et al. PO and ID BCG vaccination in humans induce distinct mucosal and systemic immune responses and CD4(+) T cell transcriptomal molecular signatures. Mucosal Immunol. 2018;11(2):486–495. doi: 10.1038/mi.2017.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.World Halth Organization. WHO preferred product characteristics for new tuberculosis vaccines. 2018.
- 81.Knight G.M., Griffiths U.K., Sumner T., et al. Impact and cost-effectiveness of new tuberculosis vaccines in low- and middle-income countries. Proc Natl Acad Sci U S A. 2014;111(43):15520–15525. doi: 10.1073/pnas.1404386111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Harris R.C., Sumner T., Knight G.M., White R.G. Systematic review of mathematical models exploring the epidemiological impact of future TB vaccines. Hum Vaccin Immunother. 2016;12(11):2813–2832. doi: 10.1080/21645515.2016.1205769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kaufmann S.H.E. Vaccination against tuberculosis: revamping BCG by molecular genetics guided by immunology. Front Immunol. 2020;11:316. doi: 10.3389/fimmu.2020.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schrager L.K., Vekemens J., Drager N., Lewinsohn D.M., Olesen O.F. The status of tuberculosis vaccine development. Lancet Infect Dis. 2020;20(3):e28–e37. doi: 10.1016/S1473-3099(19)30625-5. [DOI] [PubMed] [Google Scholar]
- 85.Tait D.R., Hatherill M., Van Der Meeren O., et al. Final analysis of a trial of M72/AS01(E) vaccine to prevent tuberculosis. N Engl J Med. 2019;381(25):2429–2439. doi: 10.1056/NEJMoa1909953. [DOI] [PubMed] [Google Scholar]
- 86.Tameris M.D., Hatherill M., Landry B.S., et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet. 2013 doi: 10.1016/S0140-6736(13)60177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kashangura R., Jullien S., Garner P., Johnson S. MVA85A vaccine to enhance BCG for preventing tuberculosis. Cochrane Database Syst Rev. 2019;4:CD02915. doi: 10.1002/14651858.CD012915.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Carpenter S.M., Behar S.M. A new vaccine for tuberculosis in rhesus macaques. Nat Med. 2018;24(2):124–126. doi: 10.1038/nm.4488. [DOI] [PubMed] [Google Scholar]
- 89.Hansen S.G., Zak D.E., Xu G., et al. Prevention of tuberculosis in rhesus macaques by a cytomegalovirus-based vaccine. Nat Med. 2018;24(2):130–143. doi: 10.1038/nm.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tameris M., Mearns H., Penn-Nicholson A., et al. Live-attenuated Mycobacterium tuberculosis vaccine MTBVAC versus BCG in adults and neonates: a randomised controlled, double-blind dose-escalation trial. Lancet Respiratory Med. 2019;7(9):757–770. doi: 10.1016/S2213-2600(19)30251-6. [DOI] [PubMed] [Google Scholar]
- 91.Loxton A.G., Knaul J.K., Grode L., et al. Safety and immunogenicity of the recombinant Mycobacterium bovis BCG vaccine VPM1002 in HIV-unexposed newborn infants in South Africa. Clin Vaccine Immunol. 2017;24(2) doi: 10.1128/CVI.00439-16. [DOI] [PMC free article] [PubMed] [Google Scholar]