Editor—In a scenario of mass-scale respiratory failure, demand for reliable mechanical ventilators might exceed the combination of existing supply plus supplementation by relocation or new production.1 , 2 Commercial ventilators achieve essential reliability and precision by closed-loop control. Here, we show that simpler open-loop control can provide comparable reliability and acceptable precision. We developed a low-cost mandatory pressure-controlled mechanical ventilator constructed of hydraulic and electronic parts available essentially anywhere.
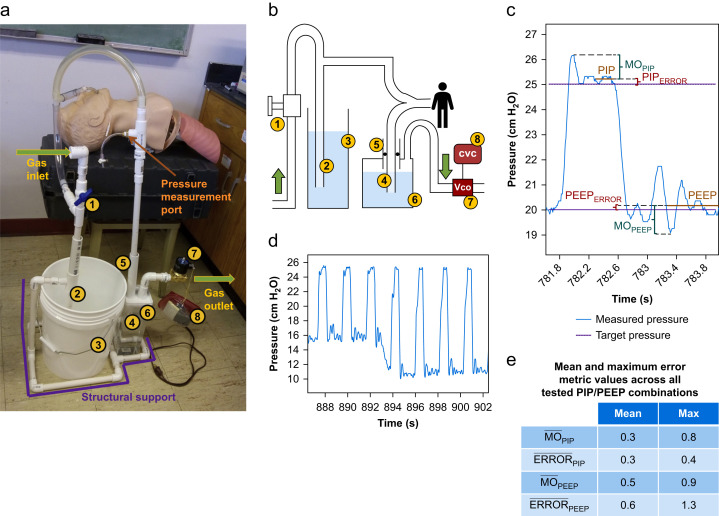
We built a prototype ventilator (Fig 1 a and b) that would be supplied by a combination of oxygen and medical air. Entering gas passes through an adjustable flow regulator. Next, a peak inspiratory pressure (PIP) control tube tees off the main gas-flow route. The downstream end of the PIP tube is immersed, to an adjustable depth, below the surface of a water reservoir open, at its surface, to atmospheric pressure. The main gas flow route continues to a junction connecting to the patient's tracheal tube, to which we connected model human lungs (Airway Management Trainer, Laerdal, Stavanger, Norway). Downstream of the junction, the expiratory circuit branch passes through an in-line PEEP control tube. The downstream end of the PEEP tube is immersed, to an adjustable depth, below the surface of a closed, partially-filled water reservoir. At the top of the PEEP reservoir, a tube leads to a solenoidal cycling outlet valve controlled by a square wave generating circuit.
Fig 1.
Low-cost mechanical ventilator design and operation. (a) Prototype constructed of polyvinyl chloride plumbing and common electrical components; (b) schematic with adjustable flow regulator: (1) 0.5 inch manually-controlled ball valve; peak inspiratory pressure (PIP) control assembly: (2) 57.5 cm long×0.5 inch diameter (15.8 mm ID) pipe, marked at 5 cm intervals, with open lower end submerged in (3) open reservoir; and PEEP control assembly: (4) 60 cm×0.5 inch pipe, marked at 5 cm intervals, inserted through (5) a concentric 25 cm×1 inch (26.6 mm ID) outer pipe attached with air-tight connection to the top of (6) transparent closed reservoir. In PEEP control assembly, an O-ring sealed the space between inner (#4) and outer (#5) pipes such that pipe #4 could be raised/lowered to adjust PEEP while the reservoir remained sealed. A tube from the top of the PEEP reservoir led to (6) the cycling outlet valve (Vco), 0.75 inch Brass Electric Solenoid Air Valve (U.S. Solid®, Cleveland, OH, USA) controlled by (7) a cycling valve circuit (CVC) that generated a square wave signal. (c) Variables of interest. (d) Pressure trace for the transition between two settings: PIP of 25 cm H2O and PEEP from 15 to 10 cm H2O. (e) Aggregated errors. MO, maximum overshoot.
Cycling the outlet valve with a frequency and duty cycle corresponding to the desired ventilatory frequency and inspiratory-to-expiratory ratio, respectively, controls ventilation. Outlet valve closure increases circuit pressure and inflates the lungs. Initially, gas is driven simultaneously into the PIP tube and the lungs. Once the circuit pressure causes gas to bubble out of the bottom of the PIP tube, the PIP tube acts as a pressure limiter,3 and inspiratory pressure plateaus. The submersion depth of the PIP tube, in centimetres, determines the PIP level, in cm H2O. Outlet valve opening releases the circuit pressure and deflates the lungs. Initially, gas flows through the PEEP tube, bubbles through the PEEP reservoir, and exits the circuit through the outlet valve. When circuit pressure decreases to equal PEEP tube submersion depth, gas stops flowing through the PEEP tube and expiratory pressure plateaus.
We tested the prototype by ventilating at 28 cycles min−1. We tested all combinations (10 cycles each) of PIP equal to 15, 20, 25, and 30 cm H2O and PEEP equal to 5, 10, 15, and 20 cm H2O, excluding those in which (PIP–PEEP) was ≤5 cm H2O. We used a single setting for the flow regulator that provided a flow rate appropriate for the full range of pressures tested. There was no need to alter flow rate in order to alter PIP or PEEP. PIP and PEEP were set by submerging the respective control tubes to depths equivalent to the desired values, without observing circuit pressure. To assess PIP and PEEP accuracy, we recorded the circuit pressure with a transducer (5 Hz low-pass analogue filter, 200 Hz sample rate) between the patient and the PEEP tube, where pressure differed from airway entrance pressure by <<0.5 cm H2O.
For inspiration, we calculated maximum overshoot (MOPIP) and PIP error (ERRORPIP) as follows (Fig 1c). Over the second half of the inspiratory plateau in a given cycle we calculated measured PIP as mean plateau pressure. We calculated MOPIP for a single cycle as maximum pressure during the cycle minus measured PIP, and averaged those values across the 10 cycles for given pressure settings to obtain . Similarly, we calculated ERRORPIP for a single cycle as the absolute value of the difference between measured PIP and target PIP, and averaged ERRORPIP across 10 cycles to obtain PIP. For expiratory pressures, we performed the same analysis excepting that we used the expiratory rather than inspiratory plateau, and maximum overshoot equalled measured PEEP minus minimum pressure during the cycle. Again averaging over 10 cycles, we obtained PEEP and PEEP.
Circuit pressure for transition between two representative PIP/PEEP combinations is shown in Figure 1d. Across all PIP/PEEP combinations, we determined the mean and maximum values of each error metric. These aggregated errors are shown in Figure 1e. In ad hoc tests over an extreme range of alternative gas flow rates, error was similarly acceptable (data not shown).
The ventilator delivers mandatory ventilation, thus is designed for patients without significant ventilatory effort. Nonetheless, concerned about unintended spontaneous breathing, we applied continuous −30 cm H2O ‘inspiratory effort’ over more than 10 cycles with each of two extreme circuit flow rates. With minimal flow, the simulated inspiratory effort decreased PIP ∼5 cm H2O without altering PEEP. With very high flow, the reduction in PIP was <0.5 cm H2O. There was no significant risk of circuit water ‘inhalation’.
The proposed ventilator is no substitute for a commercial ventilator with assist ventilation, curve monitoring, alarms, and other functions. However, for a ventilator of last resort, as for other applications,3 , 4 water columns appear to offer a safe and reliable alternative for pressure limitation, even with a very high flow rate. Pressure sensor use should further reduce error. The possibility of a patient inhaling water was shown to be highly unlikely. Placement of the water reservoirs below the patient's bed should further decrease the chance of liquid inhalation; a more negative inspiratory effort would be required to raise reservoir water to the patient.
The potential for escaping gas bubbles to contaminate the environment with exhaled pathogens is an important consideration.5 However the PIP bubbles released from the open reservoir comprise fresh gas and the PEEP bubbles pass through a closed reservoir to the circuit outlet, where a low resistance filter could be attached. The likelihood of contamination is low.
This ventilator can be constructed for less than $50 US by personnel with minimal training. Likewise, minimal training would be required for maintenance and operation. This and similar3 , 6 , 7 ventilators could be valuable in scenarios of mass-scale respiratory failure. Although our tests performed on a physical model suggest safety and reliability, in vivo animal testing is needed before clinical use of this and similar devices.
Declarations of interest
The authors declare that they have no conflicts of interest.
Funding
US National Institutes of Health (NIH) grant R01 HL113577 (Bethesda, MD, USA).
Acknowledgements
We thank Tam L. Nguyen for kindly providing operational support during prototype fabrication and testing.
References
- 1.Branson R.D., Johannigman J.A., Daugherty E.L., Rubinson L. Surge capacity mechanical ventilation. Respir Care. 2008;53:78–88. [PubMed] [Google Scholar]
- 2.Daugherty E.L., Branson R., Rubinson L. Mass casualty respiratory failure. Curr Opin Crit Care. 2007;13:51–56. doi: 10.1097/MCC.0b013e3280129979. [DOI] [PubMed] [Google Scholar]
- 3.Mestriner R.G., Fernandes R.O., Steffen L.C., Donadio M.V.F. Optimum design parameters for a therapist-constructed positive-expiratory-pressure therapy bottle device. Respir Care. 2009;54:504–508. [PubMed] [Google Scholar]
- 4.Isgrò S., Zanella A., Giani M., Abd El Aziz El Sayed Deab S., Presenti A., Patroniti N. Performance of different PEEP valves and helmet outlets at increasing gas flow rates: a bench top study. Minerva Anestesiol. 2012;78:1095–1100. [PubMed] [Google Scholar]
- 5.Faulkner W.B., Memarzadeh F., Riskowski G., Kalbasi A., Chang A.C.Z. Effects of air exchange rate, particle size and injection place on particle concentrations within a reduced-scale room. Build Environ. 2015;92:246–255. [Google Scholar]
- 6.Pereira A., Lopes L., Fonte P. Prototype of an affordable pressure-controlled emergency mechanical ventilator for COVID-19. https://arxiv.org/abs/2004.00310v2 (preprint)
- 7.Cristiano G., Bonivento W., Caravati M. Mechanical Ventilator Milano (MVM):A novel mechanical ventilator designed for mass scale production in response to the COVID-19 pandemics. 2020. (preprint) [DOI]