Abstract
As aspergillosis is a well-known complication of severe influenza, we suggest that SARS-CoV-2 might be a risk factor for invasive aspergillosis (IA). We report the case of an 87 year-old woman, with no history of immune deficit, admitted in our emergency room for severe respiratory distress. Coronavirus disease 2019 (COVID-19) diagnosis was confirmed by a SARS-CoV-2 reverse transcriptase polymerase chain reaction (PCR) on nasal swab. On day 14, pulmonary examination deteriorated with haemoptysis and a major increase of inflammatory response. A computed tomography (CT) scan revealed nodules highly suggestive of IA. Aspergillus antigen was found highly positive in sputum and blood, as was Aspergillusspp PCR on serum. Sputum cultures remained negative for Aspergillus. This patient died rapidly from severe respiratory failure, despite the addition of voriconazole. Considering SARS-CoV-2 acute respiratory distress syndrome (ARDS) as an acquired immunodeficiency, we report here a new case of “probable” IA based on clinical and biological arguments, in accordance with the last consensus definition of invasive fungal disease. On a routine basis, we have detected 30% of aspergillosis carriage (positive culture and antigen in tracheal secretions) in critically ill patients with COVID-19 in our centre. Further studies will have to determine whether sputum or tracheal secretions should be systematically screened for fungal investigations in intensive care unit (ICU) COVID-19 patients to early diagnose and treat aspergillosis.
Keywords: Invasive aspergillosis, Coronavirus Disease 2019 (COVID-19), Diagnosis, Antigen, Serology
Introduction
A novel coronavirus, SARS-CoV-2, was first identified in China in late 2019 as the cause of an outbreak of acute respiratory illness [1], [2]. This virus can affect individuals of any age, although adults of middle age and older are most commonly involved, and older adults are more likely to have severe presentation [3]. At first considered as an influenza-like disease, this acute respiratory syndrome may be much more severe and differs in its presentation [4]. If aspergillosis is a well-known complication of severe influenza [5], [6], until very recently this fungal infection had not been described in association with the Coronavirus disease 2019 (COVID-19), although Chinese publications reported co-infections in patients treated in intensive care unit (ICU) with acute respiratory distress syndrome (ARDS), among them Aspergillus infections [7], [8]. A case report [9] and a small case series [10] of aspergillosis associated with COVID-19 respiratory failure have recently been pre-published. We report here a new case of COVID-19 complicated by invasive aspergillosis (IA).
Case description
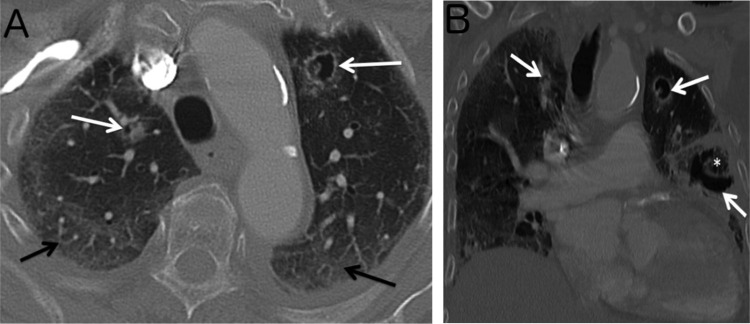
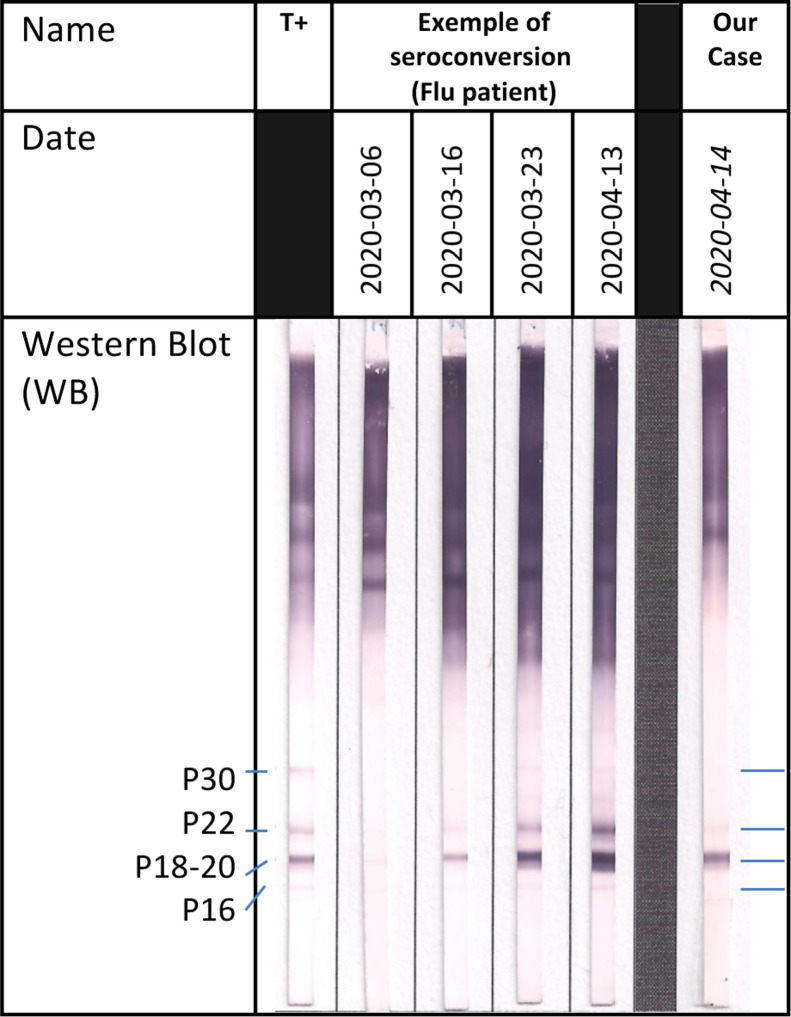
An 87-year-old woman with no significant past medical history was admitted on March 30th, 2020 to the emergency room in a severely altered general status with cough, fever, severe dyspnoea and diarrhoea, which had been progressively worsening for 10 days. Initial medical examination showed a respiratory rate (RR) of 30/min, and a pulse oxymetry (SpO2) of 67% rising to 92% under supplemental oxygen at 15 L/min. Lung auscultation revealed diffuse crackles. Biological parameters showed thrombopenia at 105 G/L, high neutrophil count at 10.6 G/L, lymphopenia at 0.93 G/L, and C-reactive protein (CRP) at 233 mg/L. SARS-CoV-2 reverse transcriptase polymerase chain reaction test (PCR) was positive on nasal swab [11]. Lung X-ray showed bilateral pneumopathy with interstitial involvement. On day 0, the patient was admitted in our COVID-19 ward, as admission to ICU was refuted considering her age. Antibiotherapy with ceftriaxone 2000 mg/d and spiramycin 1.5 MUI/8 h was introduced for 5 days, and stopped because of lack of microbiological documentation. Methylprednisolone 1 mg/kg/d was used from day 0 to day 15, as commonly prescribed for most critically ill COVID-19 patients [12]. Hydroxychloroquine 200 mg twice daily was added under electrocardiogram monitoring, because of its approval at that date by most European health agencies for severely ill patients and its presumed safety. The respiratory state of our patient remained critical but stable under 15L/min O2, and CRP decreased to 65 mg/L at day 9. Despite thromboembolism prophylaxis with enoxaparin 6000UI AXA, a proximal deep venous thrombosis was diagnosed on day 13. On day 15 (day 25 from symptom onset), general status and pulmonary examination acutely deteriorated with expectorations of thick sputum and haemoptysis, but RR and SpO2 remained stable. Blood samples showed a major increase of CRP at 340 mg/L, white blood cells at 29 G/L (with 27 G/L neutrophils and 0.6 G/L lymphocytes). On day 16, a computed tomography revealed bilateral patchy ground glass opacities related to COVID-19 with an extensive damage estimated to 75% of lung parenchyma, and three nodules highly suggestive of IA (Fig. 1 ). There was no pulmonary embolism. Bronchoalveolar lavage (BAL) was not possible, however induced sputum was collected. A specific treatment with intravenous voriconazole was started (6 mg/kg/12 h first day, then 4 mg/kg/12 h), in association with an empiric antibiotic therapy with piperacillin/tazobactam. Galactomannan Aspergillus antigen in sputum (6.03 UA/mL) and blood (1.9 UA/mL) was found highly positive (Platelia™ ELISA Biorad, Cut-off = 0.5 UA/mL), as well as blood serology with Serion ELISA IgG-IgM assay (71 UA/mL) and with LDBioDiagnostics Western Blot (Fig. 2 ). Aspergillus spp PCR (MycoGENIE Ademtech® Real-Time PCR) performed in the serum, revealed a positive signal (Ct = 36). Despite these measures, the patient died from respiratory failure on day 17. After two weeks, microbiological sputum examination remained negative for Aspergillus spp.
Fig. 1.
Transverse (A) and coronal (B) CT images of the thorax showing three nodular opacities in the upper lobes, and the lower left lobe (white arrows) with a partial circumferential rim of radiolucent airspace, with air crescent sign, formed as a result of separation of devitalised necrotic centre (star) from the surrounding opaque rim. Bilateral posterior ground-glass opacities (black arrows) are one of the radiological patterns of infection due to Sars-CoV-2.
Fig. 2.
Serological profile with LDBIO Diagnostics WB assay: a two-specific bands pattern, compared to a seroconversion profile in a patient with influenza-induced respiratory failure complicated by invasive aspergillosis. T+ = positive control from patients with a chronic serological profile.
Discussion
We report here a new case of probable IA according to the last consensus definition of invasive fungal disease [13], considering severe SARS-CoV-2 ARDS as a host criterion (acquired immunodeficiency) [10]. Despite progressive biological improvement, oxygen needs remained high, and the patient experienced a secondary clinical worsening which led to a diagnosis of probable IA based on a set of arguments summarized in Table 1 : highly suggestive radiographic abnormalities, highly positive sputum antigen (not included in EORTC criteria), positive blood antigen (with excellent positive predictive value [14]) and a single positive serum PCR. Furthermore, the patient was in excellent previous general status, with no pre-existing vulnerability factors except advanced age (absence of chronic pulmonary disease), no pre-existent respiratory symptoms and indeed no suspicion of semi-invasive aspergillosis. In addition, we retain a serological argument for IA with a characteristic seroconversion profile similar to IA in a flu-patient (Fig. 2). Indeed, it is interesting to note that this serological tool (associated with the use of specific techniques such as the Western Blot [15]) has never been used for the diagnosis of invasive aspergillosis in ICU (EORTC criteria) although the prescription and use of serology is not consensual in this setting [16], [17], [18], [19]. There is no indication for serology for patients with aplasia (neutropenic patients, haematological diseases) but serology may have an interest in the context of non-neutropenic patients (such as critically ill patients with flu or COVID-19). In our opinion, it would be therefore particularly interesting to evaluate serological tests on different co-infected flu or COVID-19 and aspergillosis patients to assess their reliability. These results seem to be offsetting the lack of a positive Aspergillus sputum culture (a single sample) related to the important yeast colonization (107 UFC/mL on sputum) and the impossibility to collect a bronchoalveolar lavage sample.
Table 1.
Diagnosis criterion of probable aspergillosis according EORTC classification.
| Predisposing hosts factors | Use of corticosteroids 1 mg/kg/d Severe infection by SARS-CoV-2 |
| Clinical and radiological features | Cough Dyspnea Fever Haemoptysia CT images of nodular opacities with radiolucent airspace, with air crescent sign, associated with ground-glass opacities |
| Mycological criteria | Galactomannan antigen and Aspergillus PCR positive on serum |
CT: computed tomography; PCR: polymerase chain reaction.
The patient had no history of immuno-deficit with normal to high neutrophils count and negative HIV serology. IA may have been favoured by severe illness and corticosteroid therapy [20], [21], in a person who lived on a farm in a rural area with probable previous community contamination. To our knowledge, no cases of IA have been described with hydroxychloroquine treatment alone.
As early diagnosis is mandatory for effective IA treatment, we think that it is important to alert clinicians caring for patients with COVID-19 related respiratory distress on this severe co-infection. In a recent case series, Koehler et al. described 5 cases classified as putative IA among 19 consecutive severe patients admitted in two separate ICUs with a lethality of 60% [10]. Among these patients, three were under inhaled steroids for chronic obstructive pulmonary disease and one received intravenous corticosteroid therapy for 13 days, but none fulfilled the criterion for an underlying condition of immunosuppression [21]. Blaize et al. [9] reported the case of a 74-year-old patient with worsening respiratory condition on the fourth day of the onset of the COVID-19, which fulfilled criteria of “putative IA” according to the algorithm of Blot et al. [22], and positive A. fumigatus PCR in sputum and culture; this patient died from severe respiratory failure.
On a routine basis, as Alanio et al. stated in their study [23], we have detected approximatively 30% (13/42) of aspergillosis carriage (positive culture and positive antigen in tracheal secretions) in critically ill patients with COVID-19 in our centre, although no other patient have been diagnosed with IA. However, and by analogy with severe influenza and SARS-CoV-1 illness [24], we suggest that SARS-CoV-2 might be a risk factor for IA and other invasive fungal diseases, including pneumocystosis and mucormycosis [25].
Conclusion
Invasive aspergillosis should be suspected in case of clinical deterioration in patients with severe SARS-CoV-2 related acute respiratory illness, even in immunocompetent hosts. Prospective studies are needed to determine whether sputum or tracheal aspirate antigen as well as serum antigen or antibodies should be systematically screened for mycosis investigations in COVID-19 ICU patients. That is among of the objectives of MY-CO-VID study (prevalence/incidence of fungal diseases during COVID-19 pandemic) [14].
Disclosure of interest
The authors declare that they have no competing interest.
References
- 1.Stop the Wuhan virus. Nature. 2020;577:450. doi: 10.1038/d41586-020-00153-x. https://www.nature.com/articles/d41586-020-00153-x. [DOI] [PubMed] [Google Scholar]
- 2.Munster V.J., Koopmans M., van Doremalen N., van Riel D., de Wit E. A novel coronavirus emerging in China – key questions for impact assessment. N Engl J Med. 2020;382:692–694. doi: 10.1056/NEJMp2000929. [DOI] [PubMed] [Google Scholar]
- 3.Verity R., Okell L.C., Dorigatti I., Winskill P., Whittaker C., Imai N., et al. Estimates of the severity of Coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. 2020;20:669–677. doi: 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kobayashi T., Jung S.-M., Linton N.M., Kinoshita R., Hayashi K., Miyama T., et al. Communicating the risk of death from novel Coronavirus disease (COVID-19) J Clin Med. 2020;9:580. doi: 10.3390/jcm9020580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schauwvlieghe A.F.A.D., Rijnders B.J.A., Philips N., Verwijs R., Vanderbeke L., Van Tienen C., et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir Med. 2018;6:782–792. doi: 10.1016/S2213-2600(18)30274-1. [DOI] [PubMed] [Google Scholar]
- 6.Van de Veerdonk F.L., Kolwijck E., Lestrade P.P.A., Hodiamont C.J., Rijnders B.J.A., van Paassen J., et al. Influenza-associated aspergillosis in critically ill patients. Am J Respir Crit Care Med. 2017;196:524–527. doi: 10.1164/rccm.201612-2540LE. [DOI] [PubMed] [Google Scholar]
- 7.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel Coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blaize M., Mayaux J., Nabet C., Lampros A., Marcelin A.-G., Thellier M., et al. Fatal invasive aspergillosis and coronavirus disease in an immunocompetent patient. Emerg Infect Dis. 2020;26:1636–1637. doi: 10.3201/eid2607.201603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koehler P., Cornely O.A., Böttiger B.W., Dusse F., Eichenauer D.A., Fuchs F., et al. COVID-19 associated pulmonary aspergillosis. Mycoses. 2020;63:528–534. doi: 10.1111/myc.13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25:2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shang L., Zhao J., Hu Y., Du R., Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020;395:683–684. doi: 10.1016/S0140-6736(20)30361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donnelly J.P., Chen S.C., Kauffman C.A., Steinbach W.J., Baddley J.W., Verweij P.E., et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organisation for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis. 2019 doi: 10.1093/cid/ciz1008. [ciz1008] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verweij P.E., Gangneux J.P., Bassetti M., Brüggemann R.J.M., Cornely O.A., et al. Diagnosing COVID-19-associated pulmonary aspergillosis. Lancet Microbe. 2020;1:e53–e55. doi: 10.1016/S2666-5247(20)30027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oliva A., Flori P., Hennequin C., Dubus J.C., Reynaud-Gaubert M., Charpin D., et al. Evaluation of the Aspergillus Western Blot IgG Kit for diagnosis of chronic aspergillosis. J Clin Microbiol. 2015;53:248–254. doi: 10.1128/JCM.02690-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Persat F. Aspergillus serology, from yesterday to today for tomorrow. J Med Mycol. 2012;22:72–82. doi: 10.1016/j.mycmed.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Richardson M.D., Page lain D. Aspergillus serology: have we arrived yet? Med Mycol. 2017;55:48–55. doi: 10.1093/mmy/myw116. [DOI] [PubMed] [Google Scholar]
- 18.Hage C.A., Carmona E.M., Epelbaum O., Evans S.E., Gabe L.M., Haydour Q., et al. Microbiological laboratory testing in the diagnosis of fungal infections in pulmonary and critical care practice. An Official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2019;200:535–550. doi: 10.1164/rccm.201906-1185ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verweij P.E., Rijnders B.J.A., Brüggemann R.J.M., Azoulay E., Bassetti M., Blot S., et al. Review of influenza-associated pulmonary aspergillosis in ICU patients and proposal for a case definition: an expert opinion. Intensive Care Med. 2020;22:1–12. doi: 10.1007/s00134-020-06091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tejerina E.E., Abril E., Padilla R., Rodríguez Ruíz C., Ballen A., Frutos-Vivar F., et al. Invasive aspergillosis in critically ill patients: an autopsy study. Mycoses. 2019;62:673–679. doi: 10.1111/myc.12927. [DOI] [PubMed] [Google Scholar]
- 21.Koehler P., Salmanton-García J., Gräfe S.K., Koehler F.C., Mellinghoff S.C., Seidel D., et al. Baseline predictors influencing the prognosis of invasive aspergillosis in adults. Mycoses. 2019;62:651–658. doi: 10.1111/myc.12926. [DOI] [PubMed] [Google Scholar]
- 22.Blot S.I., Taccone F.S., Van den Abeele A.-M., Bulpa P., Meersseman W., Brusselaers N., et al. A clinical algorithm to diagnose invasive pulmonary aspergillosis in critically ill patients. Am J Respir Crit Care Med. 2012;186:56–64. doi: 10.1164/rccm.201111-1978OC. [DOI] [PubMed] [Google Scholar]
- 23.Alanio A., Delliere S., Fodil S., Bretagne S., Megarbane B. High prevalence of putative invasive pulmonary aspergillosis in critically ill COVID-19 patients. SSRN. 2020 doi: 10.1101/2020.04.21.20064915. [published online April 15; 3575581 (abstr)] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hwang D.M., Chamberlain D.W., Poutanen S.M., Low D.E., Asa S.L., Butany J. Pulmonary pathology of severe acute respiratory syndrome in Toronto. Mod Pathol. 2005;18:1–10. doi: 10.1038/modpathol.3800247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gangneux J.-P., Bougnoux M.-E., Dannaoui E., Cornet M., Zahar J.R. Invasive fungal diseases during COVID-19: we should be prepared. J Mycol Med. 2020 doi: 10.1016/j.mycmed.2020.100971. [DOI] [PMC free article] [PubMed] [Google Scholar]