Highlights
-
•
Myocarditis in COVID-19 was relatively rare but can be severe and lead to mortality.
-
•
Cardiac MRI showing cardiac oedema and injury was valuable in diagnosing myocarditis.
-
•
Left ventricular dysfunction and hypokinesis was common and should be managed.
-
•
Steroids were often used but implications on viral clearance should be considered.
Keywords: Coronavirus, COVID-19, Myocarditis, Meta-summary
Abstract
Background
Myocarditis caused by SARS-CoV-2 infection was proposed to account for a proportion of cardiac injury in patients with COVID-19. However, reports of coronavirus-induced myocarditis were scarce. The aim of this review was to summarise the published cases of myocarditis and describe their presentations, diagnostic processes, clinical characteristics and outcomes.
Methods
A literature search of MEDLINE, EMBASE, Scopus, Web of Science, CENTRAL and OpenGrey on was performed on 3 June 2020. Studies of myocarditis in patients with COVID-19 were included, and those only reporting cardiac injury or heart failure were excluded. Cases were “confirmed” myocarditis if diagnosed on cardiac magnetic resonance imaging (CMR) or histopathology. Those without were grouped as “possible” myocarditis.
Results
A total of 31 studies on 51 patients were included; 12 cases were confirmed myocarditis while 39 had possible myocarditis. The median age was 55 and 69% were male. The most common presenting symptoms were fever, shortness of breath, cough and chest pain. Electrocardiogram changes included non-specific ST-segment and T-wave changes and ventricular tachycardia. Most patients had elevated cardiac and inflammatory biomarkers. Left ventricular dysfunction and hypokinesis was common. CMR established the diagnosis in 10 patients, with features of cardiac oedema and cardiac injury. Five patients had histopathological examination. Some cases required mechanical ventilation and extracoporeal membrane oxygenation, and 30% of patients recovered but 27% died.
Conclusions
COVID-19 myocarditis was associated with ECG, cardiac biomarker and echocardiographic changes, and the manifestation could be severe leading to mortality. Endomyocardial biopsy was not available in most cases but CMR was valuable.
Introduction
Recent studies on the Coronavirus Disease 2019 (COVID-19) pandemic caused by SARS-CoV-2 infection found that cardiac injury occurred in up to 30% of patients, and was associated with increased disease severity.1 Myocarditis, an inflammatory disease of the myocardium, has been proposed to account for a proportion of cardiac injury, in addition to systemic inflammation. Myocarditis is classified as acute, chronic or fulminant, the latter being a sudden, severe manifestation associated with acute heart failure, cardiogenic shock and life-threatening arrhythmias. Viral infections such as enteroviruses and adenoviruses are common causes of myocarditis, which can cause a combination of direct cellular injury and T-cell cytotoxic response.2 However, the evidence for COVID-19 myocarditis has been limited to case reports and case series, which is complicated by a difficulty in establishing a confirmed diagnosis.3 The aim of this review was to summarise and describe cases of myocarditis caused by COVID-19 in order to increase the understanding of the presentation, clinical characteristics, diagnostic process and outcomes of this relatively rare but possibly severe presentation.
Methods
We performed a search on MEDLINE, Embase, Scopus, Web of Science, CENTRAL and OpenGrey on 3 June 2020. The search terms were “coronavirus” or “COVID” or “SARS” or “MERS” and “myocarditis” or “myopericarditis” or ‘’heart failure” or “myocardial inflammation” or “myocardium” or “cardiomyopathy” or “myocardial injury” or “cardiac injury”. The search was not restricted in time, type or language of publication. The inclusion criteria were clinical studies on (1) laboratory-confirmed COVID-19 infection and (2) reported diagnosis of myocarditis. Additional articles were identified from hand searching of the references of reviews and included articles. Primary research studies including randomised trials, cohort studies, case series and case reports were included, and there were no limitations on publication type. Studies that did not report myocarditis e.g. cardiomyopathy or cardiac injury only and animal studies were excluded. Titles and abstracts were independently screened by two researchers (JSH and CHS) and discrepancies were resolved by discussion. Data from full texts were extracted onto a standardised form, which included the study type, viral infection, patient characteristics, investigations, management and outcomes.
Studies were grouped according to the method of diagnosis and certainty of myocarditis. Patients with myocarditis diagnosed on histopathological examination or cardiac magnetic resonance imaging (CMR) had “confirmed myocarditis”, while patients with reported myocarditis without the above investigations had “possible myocarditis”. The quality of the studies were assessed using validated scales such as the modified Pierson, Bradford Hills and Newcastle-Ottawa scale for case series and case reports.4
Statistical methods
Results were summarised in a results table and meta-summary, and statistical analysis were performed with SPSS (Version 25, Armonk, NY: IBM Corp.). Patients with confirmed cases were compared to possible cases in the meta-summary. Continuous variables were presented as mean and standard deviation (SD) or median and interquartile range (IQR). Categorical variables were presented as frequency and percentage.
Results
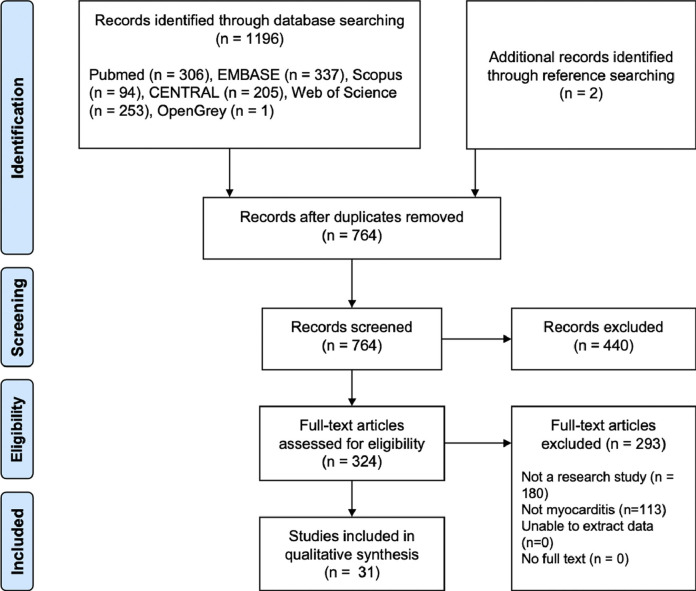
The primary search resulted in 764 studies after removal of duplicates from 1196 articles, 31 met the inclusion criteria and were included (Fig. 1 ). There were 24 case reports,5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28 5 case series29, 30, 31, 32, 33 and 2 cohort studies,34 , 35 on a total of 51 cases of myocarditis and SARS-CoV-2 infection. The studies were of poor to fair quality due to the high risk of selection bias in case reports and small case series (Supplementary Table 1). The demographics and clinical characteristics of the included cases were presented in Table 1 . Overall, the median age was 55 (IQR 34.3–67.8), and 69% were male. Three cases were under 18-years-old, with 2 cases of children under 13.15 , 19 , 24 The most common presenting symptoms were fever (62%), shortness of breath (48%), cough (48%), and chest pain (34%). Other reported symptoms include fatigue, nausea and vomiting, diarrhoea, myalgia, weakness and headache. Most patients had no reported cardiovascular co-morbidities, 8 (28%) had hypertension, 2 (7%) had diabetes mellitus, 2 (7%) had hyperlipidaemia.
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of the process of literature search, screening and inclusion of studies.
Table 1.
Characteristics of published cases of coronavirus-induced myocarditis.
| Variable | Valid n | Total cases (n = 30) | Confirmed myocarditis (n = 12) | Possible myocarditis (n = 18)* | P-value |
|---|---|---|---|---|---|
| Age (years) | 26 | 49.3 (21.6) | 43.3 (23.2) | 54.4 (19.5) | 0.20 |
| Male | 26 | 18 (69.2) | 3 (25.0) | 5 (35.7) | 0.68 |
| Region of case origin | 30 | 0.33 | |||
| Asia | 8 (26.7) | 3 (25.0) | 5 (27.8) | ||
| Europe | 13 (43.3) | 7 (58.3) | 6 (33.3) | ||
| North America | 9 (30.0) | 2 (16.7) | 7 (38.9) | ||
| Presenting symptoms | 28 | ||||
| Fever | 18 (64.3) | 7 (58.3) | 11 (68.8) | 0.70 | |
| Shortness of breath | 14 (50.0) | 5 (41.7) | 9 (56.3) | 0.45 | |
| Cough | 14 (50.0) | 5 (41.7) | 9 (56.3) | 0.45 | |
| Chest pain | 10 (35.7) | 3 (25.0) | 7 (43.8) | 0.43 | |
| Fatigue | 5 (17.9) | 3 (25.0) | 2 (12.5) | 0.62 | |
| Co-morbidities | 27 | ||||
| Hypertension | 7 (25.9) | 4 (33.3) | 3 (20.0) | 0.66 | |
| diabetes mellitus | 2 (7.4) | 2 (16.7) | 0 (0) | 0.19 | |
| Hyperlipidaemia | 2 (7.4) | 0 (0) | 2 (13.3) | 0.19 | |
| ECG | 22 | ||||
| Arrhythmia | 4 (18.2) | 3 (30.0) | 1 (8.3) | 0.29 | |
| ST-segment changes | 13 (59.1) | 3 (30.0) | 10 (83.3) | 0.03 | |
| T-wave changes | 3 (13.6) | 2 (20.0) | 1 (8.3) | 0.57 | |
| Serology | |||||
| High troponin | 25 | 22 (88.0) | 9 (90.0) | 13 (86.7) | 1.00 |
| High NT-pro-BNP | 13 | 12 (92.3) | 7 (100) | 5 (83.3) | 0.46 |
| WCC (x109/L) | 10 | 12.5 (6.4) | 9.4 (5.7) | 14.6 (6.5) | 0.23 |
| CRP (mg/L) | 13 | 75.6 (48.2) | 66.6 (52.8) | 86.1 (44.5) | 0.49 |
| Echocardiogram | 28 | 22 (78.6) | 9 (81.8) | 13 (76.5) | 1.00 |
| LV systolic dysfunction | 20 | 15 (75.0) | 6 (75.0) | 9 (75.0) | 1.00 |
| LVEF (%) | 13 | 38.5 (16.5) | 41.0 (1.7) | 37.8 (19.0) | 0.78 |
| LV hypokinesia | 14 | 8 (57.1) | 3 (60.0) | 5 (55.6) | 1.00 |
| RV hypokinesia | 7 | 2 (28.6) | 0 | 2 (50.0) | 0.43 |
| LV dilatation | 11 | 3 (27.3) | 0 | 3 (37.5) | 0.49 |
| Intraventricular septum (mm) | 3 | 13.7 (0.6) | 14.0 | 13.5 (0.7) | 0.67 |
| Pericardial effusion | 19 | 7 (36.8) | 2 (22.2) | 5 (50.0) | 0.35 |
| CMR | 30 | 11 (36.7) | 11 (91.7) | 0 | <0.001 |
| EMB or autopsy | 30 | 5 (16.7) | 2 (16.7) | 3 (16.7) | 1.00 |
| Management | 25 | ||||
| Hydroxychloroquine | 7 (28.0) | 3 (27.3) | 4 (28.6) | 1.00 | |
| Steroids | 9 (36.0) | 3 (27.3) | 6 (42.9) | 0.68 | |
| Colchicine | 2 (8.0) | 1 (9.1) | 1 (7.1) | 1.00 | |
| IVIG | 7 (28.0) | 1 (9.1) | 6 (42.9) | 0.09 | |
| Azithromycin | 5 (20.0) | 2 (18.2) | 3 (21.4) | 1.00 | |
| Tocilizumab | 5 (20.0) | 2 (18.2) | 3 (21.4) | 1.00 | |
| Lopinavir/ritonavir | 6 (24.0) | 2 (18.2) | 4 (28.6) | 0.66 | |
| Heart failure medication | 4 (16.0) | 3 (27.3) | 1 (7.1) | 0.29 | |
| Anti-arrhythmics | 1 (4.0) | 1 (9.1) | 0 | 0.44 | |
| ECMO | 25 | 5 (20.0) | 0 | 5 (35.7) | 0.046 |
| IMV | 23 | 8 (34.8) | 2 (25.0) | 6 (40.0) | 0.66 |
| Vasopressor | 23 | 14 (60.9) | 3 (37.5) | 11 (73.3) | 0.18 |
| ARDS | 23 | 5 (21.7) | 2 (25.0) | 3 (20.0) | 1.00 |
| Outcome | 30 | ||||
| Recovery or discharged | 9 (30.0) | 5 (41.7) | 4 (22.2) | 0.42 | |
| Death | 8 (26.7) | 2 (16.7) | 6 (33.3) | 0.42 | |
| Undetermined | 13 (43.3) | 5 (41.7) | 8 (44.4) | 0.88 |
Abbreviations: ARDS – acute respiratory distress syndrome; CMR – cardiac magnetic resonance imaging; CRP – C reactive protein; ECMO – extracorporeal membrane oxygenation; EMB – endomyocardial biopsy; IMV – invasive mechanical ventilation; IVIG – intravenous immunoglobulins; LV – left ventricular; LVEF – left ventricular ejection fraction; NT-pro-BNP – N-terminal pro B-type natriuretic peptide; RV – right ventricular; WCC – white cell count.
Studies where patient level data were not available were excluded.
In the 51 patients, 12 were confirmed cases of myocarditis diagnosed by histopathological evidence or CMR features fulfilling the Lake Louise consensus criteria (Table 2 ).5, 6, 7 , 9 , 13, 14, 15 , 21, 22, 23, 24, 25 , 36 The remaining 39 were possible cases where authors reported a diagnosis of myocarditis, with or without supporting serological, electrocardiogram (ECG) and echocardiographic features. The age of confirmed cases was 39 (28.5–60) years, and 9 (75%) were male. Similarly, the most common presenting symptom was fever (58%), shortness of breath (42%), cough (42%), chest pain (25%), fatigue (25%) and diarrhoea (25%). In terms of comorbidities, 3 patients (25%) had hypertension, 1 had atrial fibrillation, 1 had diabetes mellitus and 1 was overweight. One patient recently recovered from COVID-19 pneumonia 3 weeks prior to presentation with myocarditis.
Table 2.
Patient characteristics of confirmed cases of myocarditis.
| Author | Age | Sex | Cardiac biomarker |
ECG | Echo | CMR |
Biopsy/PM | ||
|---|---|---|---|---|---|---|---|---|---|
| Trop (ng/ml) | NT-pro-BNP (pg/ml) | oedema | Injury | ||||||
| Coyle et al. | 57 | M | 7.33 (+) | 1300 (+) | – | Hypokinesia, LVEF 35–40% | + | + | NR |
| Sala et al. | 43 | F | 0.14 (+) | 512 (+) | ST | Hypokinesia, LVEF 43% | + | + | Immune cell infiltrate, oedema, necrosis |
| Yuan et al. | 33 | M | NR | NR | VT | NR | + | – | NR |
| Inciardi et al. | 53 | F | 0.24 (+) | 8465 (+) | ST, T | Hypokinesia, LVEF 40%, pericardial effusion | + | + | NR |
| Kim et al. | 21 | F | 1.26 (+) | 1929 (+) | VE | Severe LV systolic dysfunction | + | + | NR |
| Luetkens et al. | 79 | M | 63.5 (+) | 1178 (+) | – | – | + | + | NR |
| Craver et al. | 17 | M | NR | NR | NR | NR | NR | NR | Immune cell infiltrate, necrosis |
| Sardari et al. | 31 | M | <0.03 (-) | NR | – | Mild LV systolic dysfunction | + | + | NR |
| Doyen et al. | 69 | M | 9.00 (+) | NR | T, LVH | – | NR | + | NR |
| Paul et al. | 35 | M | 2.89 (+) | NR | NR | – | NR | + | NR |
| Oberweis et al. | 8 | M | 0.044 (+) | 5112 (+) | ST | LV systolic dysfunction, pericardial effusion | + | + | NR |
| Warchoł et al. | 74 | M | 0.10 (+) | 2451 (+) | VT | NR | – | + | NR |
Abbreviations: CMR – cardiac magnetic resonance imaging; ECG – electrocardiogram; Echo – echocardiogram; F – female; LVH – left ventricular hypertrophy; M – male; NR – not reported; NT-pro-BNP – N-terminal pro B-type natriuretic peptide; PM – post-mortem; ST – ST-segment changes; T – T-wave changes; Trop – troponin; VT – ventricular tachycardia; “+” – abnormal or elevated; “-” – normal.
On ECG, 2 patients had normal ECG, while 7 cases had ECG features consistent with myocarditis. Two patients had ventricular tachycardia, 1 had nonspecific intraventricular conduction delay and multiple premature ventricular complexes and 1 had a low atrial ectopic rhythm. Three patients showed ST-segment changes including elevation and depression, 2 had T wave inversions, 1 had diffuse U waves and 1 had low-voltage QRS complexes in limb leads. Troponin levels (troponin I or troponin T) were elevated in 9 patients, normal in 1 and not reported in 2. N-terminal pro B-type natriuretic peptide (NT-pro-BNP) was elevated in all 7 cases with reported values (median 1929pg/ml, range 512–8465). Inflammatory markers including white cell count, interleukin-6 levels and C-reactive protein levels were elevated in all patients.
Nine patients had echocardiography performed, and 6 had abnormalities consistent with myocarditis. Features included left ventricular (LV) systolic dysfunction (6 cases), with one reporting severe LV dysfunction. Pericardial effusion of up to 11 mm was observed in 2 patients. Other features include diffuse hypokinesis, dilated LV and increased wall thickness.
In 10 patients, myocarditis was diagnosed with cardiac magnetic resonance imaging (CMR) based on the Lake Louise Criteria,36 which included signs of myocardial oedema on T2-weighted imaging and myocardial injury on T1-weighted imaging with late gadolinium enhancement (LGE) (Table 2). The CMR was performed between day 3 to 18 of the hospital stay. The mean LV ejection fraction (LVEF) measured on CMR was 49% (SD 20%), and one case showed a recovery of LVEF from 35 to 40% on echocardiogram to 82% on CMR after two weeks. On T1-weighted imaging, 3 patients showed diffuse hypokinesis in the left and/or right ventricles, and one patient had mild hypokinesis at basal and mid LV segments. On T2-weighted imaging, 6 patients had diffuse increase in signal uptake suggesting diffuse myocardial oedema, one patient had localised oedema in the apical region and another in the mid inferoseptal and inferior wall regions. Only one patient reported no oedema on initial CMR and 2 patients had resolution of oedema within 2 weeks. Imaging with LGE found nonischaemic patterns of enhancement in 8 patients. The inferolateral regions were most commonly affected, and LGE ranged from subepicardial to transmural patterns. In contrast, 3 patients had no LGE and one patient showed normalization of CMR on repeated scan one week after. Three patients had pericardial effusions without tamponade, which was a supporting criterion in the Lake Louise consensus criteria.
Histopathological evidence was found in two patients, one from endomyocardial biopsy (EMB) and the other from autopsy after sudden death. In a patient with hypokinesis and diffuse myocardial oedema at basal and mid left ventricular segments on CMR, EMB showed diffuse T-lymphocytic inflammatory infiltrates. There was huge interstitial oedema and limited foci of necrosis, fulfilling the Dallas criteria.37 However, SARS-CoV-2 genome was not identified within the myocardium, suggesting a diagnosis of acute virus-negative lymphocytic myocarditis. The second case was a previously healthy 17-year-old African American male who died on arrival to hospital after complaining of severe headaches, dizziness, nausea and vomiting for two days. Pathological examination of the heart found diffuse inflammatory infiltrates composed of lymphocytes, macrophages and prominent eosinophils, mainly in the interstitium and associated with multiple foci of myocyte necrosis. This was identified in both ventricles, and eosinophilic myocarditis was diagnosed.
Of the 12 patients with confirmed myocarditis, 3 were treated with hydroxychloroquine, 3 had steroids (methylprednisolone, hydrocortisone or oral prednisolone), 2 had azithromycin, 2 had lopinavir/ritonavir, 1 had colchicine, 1 had tocilizumab, 1 had oseltamivir and 1 had intravenous immunoglobulins. Heart failure was treated with diuretics, angiotensin converting enzyme inhibitors (ACEi) and beta-blockers. Although patients had complications such as heart failure, respiratory failure and multi-organ dysfunction, recovery of cardiac function occurred in 3 COVID-19 patients after severe disease requiring ICU admission. Six patients recovered or were discharged, one showed improvement and 2 patients died.
Discussion
Cardiac injury identified from an increase in cardiac biomarkers such as cardiac troponins has been shown to be associated with more severe disease in COVID-19 and was predictive of ICU admission and mortality.38 This cardiac injury may be caused by systemic inflammation and cytokine storm due to the viral infection and is associated with elevated inflammatory biomarkers interleukin-6 and C-reactive protein. Other proposed mechanisms include acute coronary syndrome (ACS) caused by increased thrombosis and pro-coagulative changes, plaque rupture, demand ischaemia or vasospasm.39 Direct viral infection of the myocardium may cause fulminant myocarditis. Fulminant myocarditis is characterised by sudden and severe diffuse inflammation of myocardium, leading to ventricular arrhythmias, cardiogenic shock and death.40
Patients with myocarditis often present with chest pain, fatigue and dyspnoea, which is similar to viral infections such as COVID-19, with or without myocarditis. Most of the cases identified in this meta-summary had one or more of these symptoms, as well as other symptoms such as myalgia, diarrhoea, nausea and vomiting and headaches. Myocarditis causes raised cardiac biomarkers, non-specific ST-segment and T-wave changes, and atrioventricular blocks on ECG. Echocardiograms are often performed, and typical features include global hypokinesis and pericardial effusion. Fulminant myocarditis is associated with increased septal thickness, possibly caused by myocardial oedema, but normal LV diastolic dimensions,41 which was observed in one confirmed case of myocarditis caused by COVID-19.9 Myocarditis presents similarly to ACS with ECG changes and cardiac injury, but coronary arteries are often normal on coronary angiography.
The gold standard in the diagnosis of myocarditis is histopathological evidence by EMB, but as it is an invasive procedure, it is often not clinically performed especially in a COVID-19 positive patient due to infection risk. Alternatively, a clinical diagnosis can be established with CMR which was performed in 10 patients in this study. Although CMR is indicated in patients presenting with acute chest pain with normal coronary arteries and recent systemic viral infection,42 most cases had no CMR despite suggestive features of myocarditis on serology, ECG and echocardiogram due to difficulties of obtaining the scan in an infectious disease outbreak.33
Guidelines from European Society of Cardiology Working Group on Myocardial and Pericardial Diseases43 recommended that heart failure and arrhythmias caused by myocarditis should be optimally managed, including use of diuretics, ACE inhibitors, beta-adrenergic blockade, anti-arrhythmics, temporary pacing and extracoporeal membrane oxygenation. Immunomodulatory therapy using anti-viral medications and intravenous immunoglobulins has been suggested but evidence of benefit is limited. Immunosuppression using steroids, azathioprine and ciclosporin is often attempted but guidelines recommend that they should be started only after ruling active infections by polymerase chain reaction. Although steroid therapy was initially thought to impair clearance of the SARS-CoV-2 virus,44 the recent RECOVERY trial demonstrated that dexamethasone reduced mortality in patients receiving invasive mechanical ventilation and other oxygen therapy, but not in those without respiratory support.45 The role of steroids in myocarditis caused by COVID-19 is therefore unclear and needs to be investigated further.
Recent studies identified a Kawasaki-like multisystem inflammatory syndrome in children, despite children previously thought to mainly present with mild respiratory symptoms unlike adults.46 , 47 The prospective observational study found that 76% of the 21 children and adolescents with features of Kawasaki disease had myocarditis diagnosed on cardiac troponins, ECG and echocardiogram. All the patients in the case series, as well as two case reports included in this study, recovered,19 , 24 suggesting that prognosis is good.
Study limitations
The studies included in this meta-summary were mostly case reports and case series, which were low on the hierarchy of evidence and the cases may not be representative or generalizable. The reporting of data in case reports were also incomplete, therefore risk of information bias is high. The diagnostic uncertainty of included cases was a major limitation, and there may be mimics such as stress and septic cardiomyopathy, although this was reduced by only including cases with confirmed myocarditis using pathological or CMR evidence at the expense of increased selection bias. EMB was not available in most cases but CMR was valuable to confirm myocarditis in coronavirus infections.
Conclusion
In this meta-summary of cases of myocarditis associated with COVID-19, we identified 12 cases with myocarditis diagnosed on CMR or histopathology and 39 cases with reported myocarditis based on cardiac biomarkers, ECG and/or echocardiogram. Patients with myocarditis often showed typical symptoms of fever, cough, shortness of breath and chest pain. In general, myocarditis is a rare presentation, but fulminant myocarditis can lead to severe disease and mortality in patients with COVID-19. Future studies should focus on reliable diagnosis of myocarditis in an outbreak scenario, and characterising these patients in larger, prospective studies.
Funding
None.
Declaration of Competing Interest
None declared
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.hrtlng.2020.08.013.
Appendix. Supplementary materials
References
- 1.Guo T., Fan Y., Chen M. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (covid-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esfandiarei M., McManus B.M. Molecular biology and pathogenesis of viral myocarditis. Annu Rev Pathol. 2008;3:127–155. doi: 10.1146/annurev.pathmechdis.3.121806.151534. [DOI] [PubMed] [Google Scholar]
- 3.Driggin E., Madhavan M.V., Bikdeli B. Cardiovascular considerations for patients, health care workers, and health systems during the coronavirus disease 2019 (covid-19) pandemic. J Am Coll Cardiol. 2020;18 doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murad M.H., Sultan S., Haffar S., Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid-Base Med. 2018;23:60. doi: 10.1136/bmjebm-2017-110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coyle J., Igbinomwanhia E., Sanchez-Nadales A., Danciu S., Chu C., Shah N. A recovered case of covid-19 myocarditis and ards treated with corticosteroids, tocilizumab, and experimental at-001. JACC Case Rep. 2020 doi: 10.1016/j.jaccas.2020.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sala S., Peretto G., Gramegna M. Acute myocarditis presenting as a reverse tako-tsubo syndrome in a patient with sars-cov-2 respiratory infection. Eur Heart J. 2020;41:1861–1862. doi: 10.1093/eurheartj/ehaa286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuan W.-.F., Tang X., Zhao X.-.X. An “asymptomatic” driver with covid-19: atypical suspected myocarditis by sars-cov-2. Cardiovasc Diagn The. 2020;10:242–243. doi: 10.21037/cdt.2020.03.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beri A., Kotak K. Cardiac injury, arrhythmia, and sudden death in a covid-19 patient. HeartRhythm Case Rep. 2020 doi: 10.1016/j.hrcr.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inciardi R.M., Lupi L., Zaccone G. Cardiac involvement in a patient with coronavirus disease 2019 (covid-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y., Yang Y., Zhang C. Clinical and biochemical indexes from 2019-ncov infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang S.W., Gao H., Wu L. Clinical feature changes of a covid-19 patient from mild to critical condition and cardiopulmonary pathological results. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48:E018. doi: 10.3760/cma.j.cn112148-20200304-00155. [DOI] [PubMed] [Google Scholar]
- 12.Hu H., Ma F., Wei X., Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. 2020 doi: 10.1093/eurheartj/ehaa190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim I.C., Kim J.Y., Kim H.A., Han S. Covid-19-related myocarditis in a 21-year-old female patient. Eur Heart J. 2020;41:1859. doi: 10.1093/eurheartj/ehaa288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luetkens J.A., Isaak A., Zimmer S. Diffuse myocardial inflammation in covid-19 associated myocarditis detected by multiparametric cardiac magnetic resonance imaging. Circ Cardiovasc Imaging. 2020;13 doi: 10.1161/CIRCIMAGING.120.010897. [DOI] [PubMed] [Google Scholar]
- 15.Craver R., Huber S., Sandomirsky M., McKenna D., Schieffelin J., Finger L. Fatal eosinophilic myocarditis in a healthy 17-year-old male with severe acute respiratory syndrome coronavirus 2 (sars-cov-2c) Fetal Pediatr Pathol. 2020 doi: 10.1080/15513815.2020.1761491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeng J.-.H., Liu Y.-.X., Yuan J. First case of covid-19 complicated with fulminant myocarditis: a case report and insights. Infection. 2020 doi: 10.1007/s15010-020-01424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Irabien-Ortiz A., Carreras-Mora J., Sionis A., Pamies J., Montiel J., Tauron M. Fulminant myocarditis due to covid-19. Rev Esp Cardiol. 2020 doi: 10.1016/j.rec.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hua A., O'Gallagher K., Sado D., Byrne J. Life-threatening cardiac tamponade complicating myo-pericarditis in covid-19. Eur Heart J. 2020 doi: 10.1093/eurheartj/ehaa253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rauf A., Vijayan A., John S.T., Krishnan R., Latheef A. Multisystem inflammatory syndrome with features of atypical kawasaki disease during covid-19 pandemic. Indian J Pediatr. 2020 doi: 10.1007/s12098-020-03357-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tavazzi G., Pellegrini C., Maurelli M. Myocardial localization of coronavirus in covid-19 cardiogenic shock. Eur J Heart Fail. 2020 doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sardari A., Tabarsi P., Borhany H., Mohiaddin R., Houshmand G. Myocarditis detected after covid-19 recovery. Eur Heart J Cardiovasc Imaging. 2020 doi: 10.1093/ehjci/jeaa166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doyen D., Moceri P., Ducreux D., Dellamonica J. Myocarditis in a patient with covid-19: a cause of raised troponin and ecg changes. Lancet. 2020;395:1516. doi: 10.1016/S0140-6736(20)30912-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paul J.F., Charles P., Richaud C., Caussin C., Diakov C. Myocarditis revealing covid-19 infection in a young patient. Eur Heart J Cardiovasc Imaging. 2020 doi: 10.1093/ehjci/jeaa107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oberweis M.-.L., Codreanu A., Boehm W. Pediatric life-threatening coronavirus disease 2019 with myocarditis. Pediatr Infect Dis J. 2020 doi: 10.1097/INF.0000000000002744. [DOI] [PubMed] [Google Scholar]
- 25.Warchol I., Debska-Kozlowska A., Karcz-Socha I., Ksiazczyk M., Szymanska K., Lubinski A. Terra incognita: clinically suspected myocarditis in a sars-cov-2 positive patient. Pol Arch Intern Med. 2020 doi: 10.20452/pamw.15309. [DOI] [PubMed] [Google Scholar]
- 26.Fried J.A., Ramasubbu K., Bhatt R. The variety of cardiovascular presentations of covid-19. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.047164. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asif T., Ali Z. Transient st segment elevation in two patients with covid-19 and a normal transthoracic echocardiogram. Eur J Case Rep Intern Med. 2020;7 doi: 10.12890/2020_001672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radbel J., Narayanan N., Bhatt P.J. Use of tocilizumab for covid-19-induced cytokine release syndrome: a cautionary case report. Chest. 2020 doi: 10.1016/j.chest.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khalid N., Chen Y., Case B.C. Covid-19 (sars-cov-2) and the heart - an ominous association. Cardiovasc Revasc. 2020 doi: 10.1016/j.carrev.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fernandez-Ruiz M., Andres A., Loinaz C. Covid-19 in solid organ transplant recipients: a single-center case series from Spain. Am J Transplant. 2020 doi: 10.1111/ajt.15929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nunes Duarte-Neto A., de Almeida Monteiro R.A., da Silva L.F.F. Pulmonary and systemic involvement of covid-19 assessed by ultrasound-guided minimally invasive autopsy. Histopathology. 2020 doi: 10.1111/his.14160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buja L.M., Wolf D., Zhao B. The emerging spectrum of cardiopulmonary pathology of the coronavirus disease 2019 (covid-19): report of 3 autopsies from houston, texas, and review of autopsy findings from other United States cities. Cardiovasc Pathol. 2020;48 doi: 10.1016/j.carpath.2020.107233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Secco G.G., Tarantini G., Mazzarotto P. Invasive strategy for covid patients presenting with acute coronary syndrome: the first multicenter Italian experience. Catheter Cardiovasc Interv. 2020 doi: 10.1002/ccd.28959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to covid-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deng Q., Hu B., Zhang Y. Suspected myocardial injury in patients with covid-19: evidence from front-line clinical observation in Wuhan, China. Int J Cardiol. 2020 doi: 10.1016/j.ijcard.2020.03.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Friedrich M.G., Sechtem U., Schulz-Menger J. Cardiovascular magnetic resonance in myocarditis: a jacc white paper. J Am Coll Cardiol. 2009;53:1475. doi: 10.1016/j.jacc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baughman Kenneth L. Diagnosis of myocarditis. Circulation. 2006;113:593–595. doi: 10.1161/CIRCULATIONAHA.105.589663. [DOI] [PubMed] [Google Scholar]
- 38.Santoso A., Pranata R., Wibowo A., Al-Farabi M.J., Huang I., Antariksa B. Cardiac injury is associated with mortality and critically ill pneumonia in covid-19: a meta-analysis. Am J Emerg Med. 2020 [DOI] [PMC free article] [PubMed]
- 39.Ho J.S., Tambyah P.A., Ho A.F., Chan M.Y., Sia C.-.H. Effect of coronavirus infection on the human heart: a scoping review. Eur J Prev Cardiol. 2020 doi: 10.1177/2047487320925965. 2047487320925965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Veronese G., Ammirati E., Cipriani M., Frigerio M. Fulminant myocarditis: characteristics, treatment, and outcomes. Anatol J Cardiol. 2018;19:279–286. doi: 10.14744/AnatolJCardiol.2017.8170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Felker G.M., Boehmer J.P., Hruban R.H. Echocardiographic findings in fulminant and acute myocarditis. J Am Coll Cardiol. 2000;36:227–232. doi: 10.1016/s0735-1097(00)00690-2. [DOI] [PubMed] [Google Scholar]
- 42.Friedrich M.G., Marcotte F. Cardiac magnetic resonance assessment of myocarditis. Circ Cardiovasc Imaging. 2013;6:833–839. doi: 10.1161/CIRCIMAGING.113.000416. [DOI] [PubMed] [Google Scholar]
- 43.Caforio A.L.P., Pankuweit S., Arbustini E. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the european society of cardiology working group on myocardial and pericardial diseases. Eur Heart J. 2013;34:2636–2648. doi: 10.1093/eurheartj/eht210. [DOI] [PubMed] [Google Scholar]
- 44.Yang Z., Liu J., Zhou Y., Zhao X., Zhao Q., Liu J. The effect of corticosteroid treatment on patients with coronavirus infection: a systematic review and meta-analysis. J Infect. 2020 doi: 10.1016/j.jinf.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Horby P., Lim W.S., Emberson J., et al. Effect of dexamethasone in hospitalized patients with covid-19: preliminary report. medRxiv. 2020:2020. 2006.2022.20137273
- 46.Toubiana J., Poirault C., Corsia A. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ. 2020;369:m2094. doi: 10.1136/bmj.m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Viner R.M., Whittaker E. Kawasaki-like disease: emerging complication during the covid-19 pandemic. Lancet. 2020;395:1741–1743. doi: 10.1016/S0140-6736(20)31129-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.