Abstract
Mutually exclusive expression of the var multigene family is key to immune evasion and pathogenesis in Plasmodium falciparum, but few factors have been shown to play a direct role. We adapted a CRISPR‐based proteomics approach to identify novel factors associated with var genes in their natural chromatin context. Catalytically inactive Cas9 (“dCas9”) was targeted to var gene regulatory elements, immunoprecipitated, and analyzed with mass spectrometry. Known and novel factors were enriched including structural proteins, DNA helicases, and chromatin remodelers. Functional characterization of Pf ISWI, an evolutionarily divergent putative chromatin remodeler enriched at the var gene promoter, revealed a role in transcriptional activation. Proteomics of Pf ISWI identified several proteins enriched at the var gene promoter such as acetyl‐CoA synthetase, a putative MORC protein, and an ApiAP2 transcription factor. These findings validate the CRISPR/dCas9 proteomics method and define a new var gene‐associated chromatin complex. This study establishes a tool for targeted chromatin purification of unaltered genomic loci and identifies novel chromatin‐associated factors potentially involved in transcriptional control and/or chromatin organization of virulence genes in the human malaria parasite.
Keywords: chromatin, CRISPR, epigenetics, Plasmodium falciparum, var genes
Subject Categories: Chromatin, Epigenetics, Genomics & Functional Genomics; Microbiology, Virology & Host Pathogen Interaction
CRISPR/dCas9‐based proteomics is used to purify specific DNA regulatory elements in their natural chromatin context and to identify novel chromatin factors associated with virulence genes in the human malaria parasite, Plasmodium falciparum.
Introduction
In the malaria parasite Plasmodium falciparum, antigenic variation is key to evasion of the immune system and persistence of infection in the human host. The P. falciparum variant surface antigen erythrocyte membrane protein 1 (PfEMP1) is a key component in this process and is encoded by the ~ 60‐member var gene family in the haploid genome (reviewed in (Scherf et al, 2008)). A system of mutually exclusive expression is used to activate only a single var gene at a time, but occasional switching occurs to facilitate host immune system evasion. Unlike that in other parasites, antigenic variation in P. falciparum is under epigenetic control (reviewed in (Cortés & Deitsch, 2017)). The single active var gene is associated with euchromatin while all other var genes are kept transcriptionally silent via heterochromatin. Functional studies of orthologous histone writers, readers, and erasers have implicated several chromatin‐associated proteins in mutually exclusive var gene transcription including heterochromatin protein 1 (HP1), the histone deacetylases HDA2 and silent information regulator 2 (SIR2a and b), and the histone methyltransferases SET2 and SET10 (Freitas‐Junior et al, 2005; Flueck et al, 2009; Pérez‐Toledo et al, 2009; Tonkin et al, 2009; Volz et al, 2012; Jiang et al, 2013; Coleman et al, 2014; Ukaegbu et al, 2014). Across the intraerythrocytic developmental cycle (IDC) and other stages, HP1 facilitates transcriptional silencing of all but one var gene via binding to trimethylation of histone H3 at lysine 9 (H3K9me3) (Flueck et al, 2009; Lopez‐Rubio et al, 2009; Pérez‐Toledo et al, 2009; Fraschka et al, 2018; Zanghì et al, 2018). The histone modifications H3K9ac and H3K4me2/3 and the histone variants H2A.Z and H2B.Z were shown to be enriched at the active var gene promoter (Lopez‐Rubio et al, 2007; Petter et al, 2013), but the molecular machinery involved in var gene activation, such as histone‐modifying enzymes or nucleosome remodelers, has yet to be elucidated.
These epigenetic regulatory proteins may be recruited via DNA regulatory elements. All var genes have the same basic genetic structure: a 5′ upstream promoter followed by exon I (encodes the polymorphic extracellular domain of the PfEMP1), a relatively conserved intron, and exon II (encodes the conserved intracellular domain; Fig 1A). Both the promoter and the intron have been implicated in mutually exclusive transcription control (reviewed in (Guizetti & Scherf, 2013)). Early studies suggested that the var gene promoter could drive transcription unless paired with a downstream var gene intron, which had a repressive effect that was purportedly due to its own bidirectional promoter activity (Calderwood et al, 2003; Gannoun‐Zaki et al, 2005; Dzikowski et al, 2006, 2007; Frank et al, 2006; Epp et al, 2009). Conversely, long non‐coding RNAs (lncRNA) originating from the var gene intron (Fig 1A) have been implicated in the transcriptional activation of var genes (Amit‐Avraham et al, 2015). While deletion of the endogenous var gene intron did not block transcriptional silencing or activation of the targeted var gene, it did lead to higher rates of var gene switching (Bryant et al, 2017). Thus, investigating the protein cohort that binds to either the var gene promoter or intron could provide new mechanistic insight into var gene regulation.
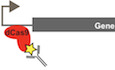
Figure 1. Immunoprecipitation of var gene‐targeted dCas9.
- Schematic of a representative var gene with two exons flanking an intron. Transcription originates from the promoter (sense) and intron (sense and antisense). Specific sgRNAs direct dCas9 to either the promoter region (red) or intron (blue). Antibodies were used to isolate the dCas9 and bound genomic regions via a 3xHA tag (yellow star).
- Schematic of var genes throughout the Plasmodium falciparum genome targeted with intron‐ (blue), promoter‐ (red), or both intron and promoter‐targeted (green) dCas9. Chromosomes are represented with gray bars, and chromosome numbers are indicated in roman numerals. var gene ups type is indicated on the chromosome, and var gene ID (excluding the preceding chromosome number) is listed to the left of its position on the chromosome. Direction of var gene transcription is indicated with an arrowhead.
- Western blot analysis of a dCas9 immunoprecipitation experiment in the promoter‐targeted strain at ring stage. Levels of dCas9 and histone H3 in the cytoplasmic (Cyt), nuclear (excluding chromatin, Nuc), and chromatin (Ch) fractions are revealed with anti‐HA and anti‐H3 antibodies, respectively. dCas9 is enriched in the immunoprecipitated fraction (IP) compared to the unbound supernatant (SN) and input (i.e., chromatin fraction) of the IP. Molecular weights are shown to the right.
Source data are available online for this figure.
Coordination of the var genes, located in subtelomeric (upsA and upsB type) or central chromosomal (upsC type) arrays across thirteen chromosomes (Fig 1B), is likely achieved via spatial positioning within the nucleus. Regardless of genome location, var genes form heterochromatic clusters at the nuclear periphery, with the single active var gene spatially separated from the rest (Duraisingh et al, 2005; Ralph et al, 2005; Lopez‐Rubio et al, 2009; Lemieux et al, 2013; Ay et al, 2014). This clustering may also facilitate the recombination observed among the polymorphic members of the var gene family during the asexual replicative cycle, which generates antigenic diversity via the formation of chimeric genes (Bopp et al, 2013; Claessens et al, 2014). While the RecQ DNA helicase PfWRN was shown to be important for promoting genome stability and preventing aberrant recombination between var genes, the molecular mechanism behind normal mitotic recombination of var gene members has not been elucidated (Claessens et al, 2018). The telomere repeat‐binding zinc finger protein (TRZ) and some members of the DNA/RNA‐binding Alba family of proteins or the Apetala2 (ApiAP2) family of transcription factors have been shown to bind to telomeres, subtelomeric regions, or potential var gene regulatory elements; however, a direct role for these proteins in transcriptional control, organization, or recombination of var genes has not been demonstrated (Flueck et al, 2010; Zhang et al, 2011; Chêne et al, 2012; Goyal et al, 2012; Bertschi et al, 2017; Martins et al, 2017; Sierra‐Miranda et al, 2017). In addition, actin has been linked to the nuclear positioning and thus transcriptional regulation of var genes via binding to the conserved var gene intron (Zhang et al, 2011). Importantly, proteins such as CCCTC binding factor (CTCF) or lamins, involved in higher‐order chromosome organization in metazoans, are absent in P. falciparum (Batsios et al, 2012; Heger et al, 2012).
Most studies of var gene biology have relied on in silico identification of factors found to be important for epigenetic regulation in other eukaryotic systems; however, P. falciparum has already shown important differences to other model organisms with regard to epigenetics (reviewed in (Cortés & Deitsch, 2017)). In addition, most studies investigating proteins that bind to specific DNA motifs have used oligo‐based in vitro approaches such as gel shift or immunoprecipitation. Thus, a new, unbiased approach is needed to identify novel var gene‐interacting factors that contribute to transcriptional regulation and organization of var genes.
To this end, we adapted a recently developed CRISPR technology for isolating specific genomic loci with a tagged, nuclease‐deficient Cas9 (dCas9) (Fujita & Fujii, 2013; Waldrip et al, 2014; Liu et al, 2017; Myers et al, 2018). Importantly, this technique identifies interactions taking place in a natural spatio‐temporal chromatin context and does not require genetic modification of the targeted locus. In this study, we show specific dCas9 enrichment at targeted var gene DNA regulatory elements with chromatin immunoprecipitation followed by sequencing (ChIP‐seq). dCas9 immunoprecipitation followed by liquid chromatography‐mass spectrometry (IP LC‐MS/MS) confirmed previously identified var gene interactors and revealed enrichment of several novel chromatin‐associated factors possibly involved in transcriptional control and/or chromatin organization of var genes. Using inducible knockdown, ChIP‐seq, and proteomics, we functionally characterize the promoter‐associated putative nucleosome remodeler, PfISWI, and demonstrate its role in var gene transcriptional activation.
Results
Targeting dCas9 to var genes
To identify factors associated with putative var gene DNA regulatory elements in an unbiased manner, we adapted the CRISPR/dCas9‐based engineered DNA‐binding molecule‐mediated chromatin immunoprecipitation (enChIP) method developed in Fujita and Fujii (2013) to P. falciparum. As both the var promoter and intron likely contain regulatory protein‐binding elements, a tagged dCas9 was directed by an optimized single guide RNA (sgRNA) to either feature (Fig 1A). As a consequence of (i) the need for a GC‐rich sequence and (ii) the homology of the promoter and intronic sequences across the var gene family, we designed an sgRNA for either genetic feature that targets multiple var loci. For the promoter sgRNA, we identified a 20‐base‐pair (bp) sequence flanking a protospacer adjacent motif (PAM) that was ~ 40 bp upstream of the translation start site of 19 var genes (Table 1, Table EV1, and Fig EV1A). For the intron sgRNA, we identified a 20‐bp sequence flanking a PAM that was in region III (Calderwood et al, 2003) of the intron of 13 var genes (Table 1, Table EV2, and Fig EV1C). For the promoter and intron sgRNAs, the target sequences were exclusive to upsA/B, upsB, upsB/C, and upsC type var genes (Fig 1B and [Link], [Link]). There was only a single off‐target sequence predicted for the intron sgRNA in the upstream region of PF3D7_0400200, which is a var pseudogene with a var exon II‐like sequence.
Table 1.
sgRNA characteristics for dCas9 targeting. Shown are the sequences (5′–3′), targeted DNA strand, GC content, and number of on‐target (intended) and off‐target sequences predicted for each sgRNA
| sgRNA | Sequence | Target strand | GC content | Predicted On‐target | Predicted Off‐target |
|---|---|---|---|---|---|
| Promoter | GGTTTATATCGTGGCATACA | Coding | 40% | 19 | 0 |
| Intron | GTAAATGTGTATGTAGTTAT | Coding | 25% | 16 | 1 |
| Control | GCTCGCGATGCTGCCCGACA | — | 70% | 0 | 0 |
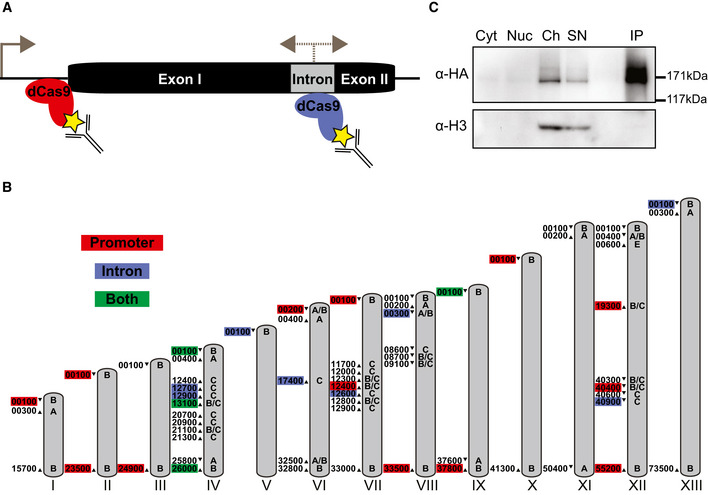
Figure EV1. Supplementary information for immunoprecipitation of non‐targeted and var gene‐targeted dCas9.
-
A–CAlignments of the var gene promoter (A) and intron (C) non‐coding strand sequences, with sgRNA sequences highlighted in red. The presumed off‐target sequence for the var promoter‐targeted dCas9 is shown in (B). Mismatches are highlighted in gray. Corresponding data for the promoter or intron sgRNAs can be found in [Link], [Link], respectively.
-
DWestern blot analysis of cell fractions from ring stage parasites of the non‐targeted dCas9 control strain. Levels of dCas9‐3HA and histone H3 in the cytoplasmic (Cyt), nuclear (excluding chromatin, Nuc), and chromatin (Ch) fractions are revealed with anti‐HA and anti‐H3 antibodies, respectively. Molecular weights are shown to the right.
Source data are available online for this figure.
To create P. falciparum strains expressing var gene‐targeted dCas9, we generated two types of plasmids—pUF1_dCas9‐3HA and pL7_var_IP—based on the two‐plasmid system originally designed for CRISPR/Cas9 in P. falciparum (Ghorbal et al, 2014). pUF1_dCas9‐3HA leads to expression of a hemagglutinin‐tagged (HA) catalytically inactive Cas9 containing the RuvC and HNH mutations originally described in Qi et al (2013). The pL7 plasmid encodes a sequence‐optimized sgRNA described in Dang et al (2015). A control plasmid (pL7_Control_IP) was used to determine background dCas9 binding to the genome, as it generates a non‐specific sgRNA that does not have a predicted target site in the P. falciparum 3D7 genome (Table 1). A bulk culture of 3D7 parasites was transfected with pUF_dCas9‐3HA and either pL7_Control_IP, pL7_varPromoter_IP, or pL7_varIntron_IP. Western blot analysis of the cytoplasmic, nuclear (non‐chromatin), and chromatin fractions showed that targeted dCas9 was most enriched in the chromatin fraction, and immunoprecipitation of dCas9 from the chromatin fraction with an anti‐HA antibody resulted in a robust enrichment of the tagged dCas9 (Fig 1C). The non‐targeted dCas9 was present in the nuclear and chromatin fractions (Fig EV1D).
Genome‐wide binding of intron‐ and promoter‐targeted dCas9 is specific and robust
To investigate the specificity of var gene‐targeted dCas9 binding, we performed ChIP‐seq in synchronized parasites cross‐linked at 14 h post‐invasion (hpi) of the host red blood cells, the ring stage during which var gene transcription is highest. Analysis of the ChIP‐seq data revealed that var promoter and intron sgRNAs led to specific and significant dCas9 enrichment at most intended target sequences (Fig 2A and [Link], [Link]) while the non‐targeted dCas9 control showed no specific enrichment (Fig EV2A). For the promoter sgRNA, a significant peak of dCas9 was found at 17 out of 19 predicted target sites, with only one major unpredicted off‐target binding event at PF3D7_1209900 (Figs 2A and EV1B, and Table EV1). For the intron sgRNA, a significant peak of dCas9 was found at 13 out of 16 predicted target sites and the predicted off‐target sequence PF3D7_0400200 (Fig 2A and Table EV2). Two additional peaks of dCas9 were detected with the var intron‐targeted sgRNA at unpredicted upsB var gene introns (PF3D7_0500100 and PF3D7_1300100) with similar sequences (one or two mismatches; Figs 2A and EV1C, and Table EV2).
Figure 2. ChIP sequencing shows specific enrichment of dCas9 at targeted var gene introns and promoters.
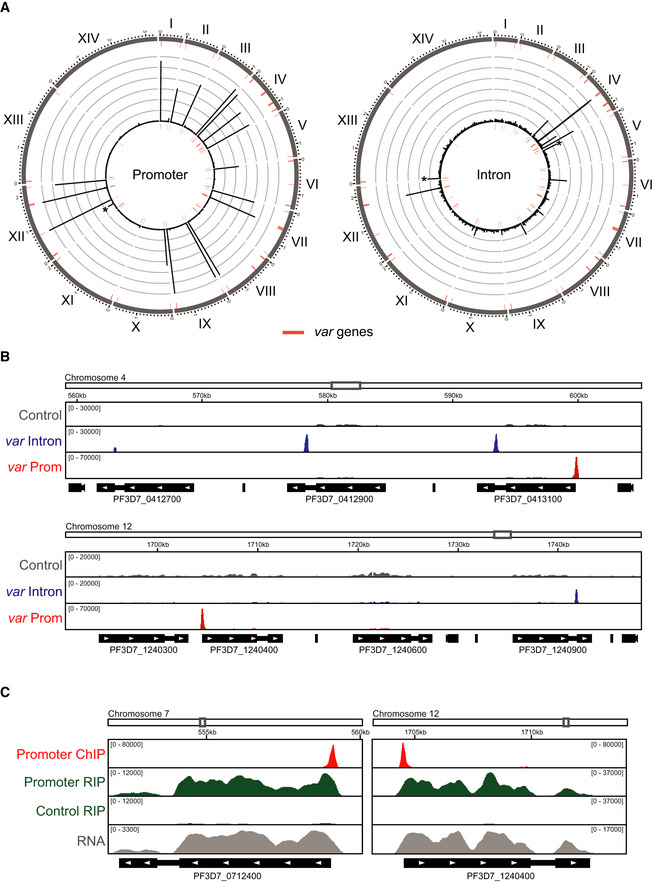
- Circos plots of ChIP‐seq data showing genome‐wide enrichment of dCas9 in ring stage parasites. The 14 chromosomes are represented circularly by the outer gray bars, with chromosome number indicated in roman numerals and chromosome distances indicated in Arabic numerals (Mbp). Enrichment for intron‐ or promoter‐targeted dCas9 (normalized to non‐targeted dCas9) is shown as average reads per million (RPM) over bins of 1,000 nt. The maximum y‐axis value is 3,000 RPM for the promoter‐targeted dCas9 (rings represent increments of 500) and 400 RPM for the intron‐targeted dCas9 (rings represent increments of 66.7). var genes are represented by red bars. An asterisk indicates an unintended binding event. One replicate was performed for each strain. Peak quantification for promoter‐ and intron‐targeted dCas9 ChIP‐seq can be found in [Link], [Link], respectively.
- ChIP‐seq data show enrichment of dCas9 in strains at ring stage expressing non‐targeted “Control” (gray), var intron‐targeted (blue), or var promoter‐targeted (red) sgRNAs. Genome location is indicated at the top of each panel. The x‐axis is DNA sequence, with genes represented by black boxes indented to delineate introns and labeled with white arrowheads to indicate transcription direction. The y‐axis is input‐subtracted ChIP enrichment. q‐values for promoter‐targeted dCas9 peaks shown are 2.22 × 10−289 for PF3D7_0413100 and 7.07 × 10−257 for PF3D7_1240400. q‐values for intron‐targeted dCas9 peaks shown are 2.03 × 10−76 for PF3D7_412700, 6.81 × 10−46 for PF3D7_0412900, 1.53 × 10−62 for PF3D7_0413100, and 8.37 × 10−4 for PF3D7_1240900. One replicate was performed for each strain. Peak quantification for promoter‐ and intron‐targeted dCas9 ChIP‐seq can be found in [Link], [Link], respectively.
- ChIP‐seq (red) and RIP‐seq (green) data show enrichment of DNA and RNA, respectively, from var genes in the promoter‐targeted and non‐targeted “Control” dCas9 immunoprecipitation from non‐clonal bulk population of ring stage parasites. Genome location is indicated at the top of each panel. The x‐axis is DNA sequence, with genes represented by black boxes indented to delineate introns and labeled with white arrowheads to indicate transcription direction. The y‐axis is input‐subtracted dCas9 ChIP enrichment in the var gene promoter‐targeted dCas9 strain (red), dCas9 RIP enrichment normalized to IgG control in the var gene promoter‐targeted dCas9 strain or non‐targeted “Control” dCas9 strain (green), or var gene transcript levels in the promoter‐targeted dCas9 strain (RPKM, gray). One replicate was performed for ChIP‐seq and RNA‐seq, and one replicate was performed for HA and IgG control RIP‐seq. ChIP‐seq peak quantification can be found in Table EV1, and RIP‐seq quantification can be found in Table EV3.
Figure EV2. var gene transcription in non‐targeted and var gene‐targeted dCas9 strains.
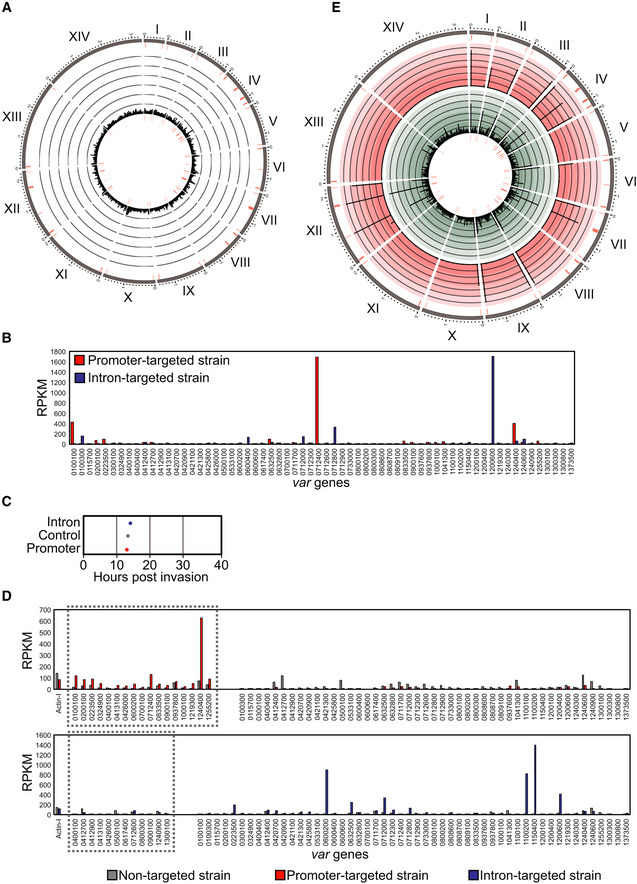
- Circos plot of ChIP‐seq data showing genome‐wide enrichment of non‐targeted dCas9 in ring stage parasites. The 14 chromosomes are represented circularly by the outer gray bars, with chromosome number indicated in roman numerals and chromosome distances indicated in Arabic numerals (Mbp). Enrichment (ChIP normalized to input) is shown as average reads per million (RPM) over bins of 1,000 nt. The maximum y‐axis value is 1,000 RPM (rings represent increments of 167). var genes are represented by red bars. One replicate was used for the dCas9 ChIP‐seq.
- RNA‐seq data from clonal parasite populations of promoter‐ (red) or intron‐targeted (blue) dCas9 strains show transcript abundance (y‐axis = RPKM) for all var genes (x‐axis) at 14 hpi. One replicate was performed for each clone.
- Cell cycle progression estimation of synchronized non‐clonal bulk parasite cultures of the promoter‐ (red), intron‐ (blue), and non‐targeted (gray) dCas9 strains at 14 hpi. RNA‐seq data from these parasites were compared to microarray data from Bozdech et al (2003) (Data ref: Bozdech et al, 2003) as in Lemieux et al (2009).
- RNA‐seq data from non‐clonal bulk parasite cultures of the promoter‐ (red), intron‐ (blue), and non‐targeted (gray) dCas9 strains at 14 hpi show transcript abundance (y‐axis = RPKM) for all var genes (x‐axis). The gray dotted boxes indicate var genes bound by dCas9 in the promoter‐targeted strain (top graph) or intron‐targeted strain (bottom graph). One replicate was performed for each strain.
- Circos plot of dCas9 ChIP‐seq (outer ring in red) and RIP‐seq (inner ring in green) data showing genome‐wide DNA and RNA enrichment, respectively, in var gene promoter‐targeted dCas9 immunoprecipitation at 14 hpi. The 14 chromosomes are represented circularly by the outer gray bars, with chromosome number indicated in roman numerals and chromosome distances indicated in Arabic numerals (Mbp). dCas9 ChIP enrichment (input‐subtracted and normalized to the corresponding value for the non‐targeted dCas9 control) is shown as average RPM over bins of 1,000 nt. dCas9 RIP enrichment (IgG‐subtracted and normalized to the corresponding value for the non‐targeted dCas9 control) is shown as average enrichment per gene. The maximum y‐axis value is 3,000 for ChIP‐seq, and 3 for RIP‐seq. var genes are represented by red bars. One replicate was performed for ChIP‐seq, and one replicate was performed for HA and IgG control and promoter‐targeted dCas9 RIP‐seq. ChIP‐seq peak quantification can be found in Table EV1, and RIP‐seq quantification can be found in Table EV3.
Comparison of the ChIP‐seq data of the var gene‐targeted dCas9 and non‐targeted dCas9 control highlights the specificity of the method from the most statistically significant dCas9 peak (enrichment ratio = 123.9, q = 2.22 × 10−289 at the promoter of PF3D7_0413100) to the least significant dCas9 peak (enrichment ratio = 42.5, q = 8.37 × 10−4 at the intron of PF3D7_1240900; Fig 2B and [Link], [Link]). dCas9 enrichment was approximately two‐fold higher at promoter sgRNA‐targeted sites (average ChIP/Input = 20.18) than intron sgRNA‐targeted sites (average ChIP/Input = 8.9; [Link], [Link]). However, because the var gene intronic sequence is AT‐rich relative to the var gene promoter sequence (Table 1 and Fig EV1A and C), it could be under‐represented in the sequencing data. Regardless, the dCas9 ChIP‐seq protocol we present here could be used to determine genome‐wide specificity and efficacy of sgRNAs for use in traditional CRISPR/Cas9 genome editing.
To determine the var gene transcriptional profile in the strains expressing dCas9, we performed sequencing of mRNA (RNA‐seq) from clones and non‐clonal bulk parasite cultures at 14 hpi. In clones of the intron‐ and promoter‐targeted dCas9 strains, a single var gene was predominantly transcribed, suggesting that mutually exclusive expression of the var gene family was unaffected by dCas9 binding (Fig EV2B). RNA‐seq of non‐clonal bulk parasite cultures of the promoter‐, intron‐, and non‐targeted dCas9 strains showed synchronicity at 14 hpi (Fig EV2C) and that multiple var genes, both targeted and untargeted, were transcribed in the population (Fig EV2D). mRNA from the var genes targeted at their promoters with dCas9 was, in general, present at higher levels in the promoter‐targeted dCas9 strain than in the non‐targeted dCas9 strain, suggesting that parasites in which dCas9 was able to more readily bind to the euchromatic promoter were selected for during transfection (Fig EV2D, top). To determine if mRNA was transcribed from var genes bound by dCas9 at their promoter, we performed RNA immunoprecipitation (RIP) with dCas9 in the promoter‐targeted dCas9 strain in ring stage parasites. Indeed, RNA transcribed from var genes bound by dCas9 at their promoters was highly enriched in the promoter‐targeted dCas9 RIP (Figs 2C and EV2E, Table EV3).
Proteomic analysis of dCas9 identifies known and new var gene‐associated factors
Validation of the enChIP method
Having demonstrated specificity of dCas9 binding with ChIP‐seq, we set out to identify proteins that associate with either the var gene promoter or intron. We performed dCas9‐3HA IP LC‐MS/MS with the var gene promoter‐ and intron‐targeted dCas9 and non‐targeted dCas9 strains at 14 hpi. A label‐free quantification approach allowed for comparison of protein enrichment between each sample using low amounts of input material. This led to the quantification of 1,413 total P. falciparum proteins (false discovery rate [FDR] of 1%, number of peptides used ≥ 3; Source Data for Tables EV5–8], with dCas9 being the most abundant protein in the LC‐MS/MS analysis for each immunoprecipitation. While dCas9 levels were similar in the var promoter‐ and intron‐targeted immunoprecipitations (promoter/intron ratio = 1.04, P = 0.15; Figs 3A and B, EV3A, Table EV4), dCas9 levels were significantly higher in the non‐targeted control when compared to the intron‐ (ratio = 3.16, P = 1.88 × 10−62) or promoter‐targeted dCas9 samples (ratio = 3.07, P = 5.64 × 10−86; Figs 3B and EV3A, Table EV4). Because we found that the number of dCas9 peptides correlated with total proteins identified (Fig EV3A, Table EV4), the non‐targeted dCas9 served as an extremely stringent control of background binding.
Figure 3. Proteomic analysis of dCas9‐purified var gene introns and promoters.
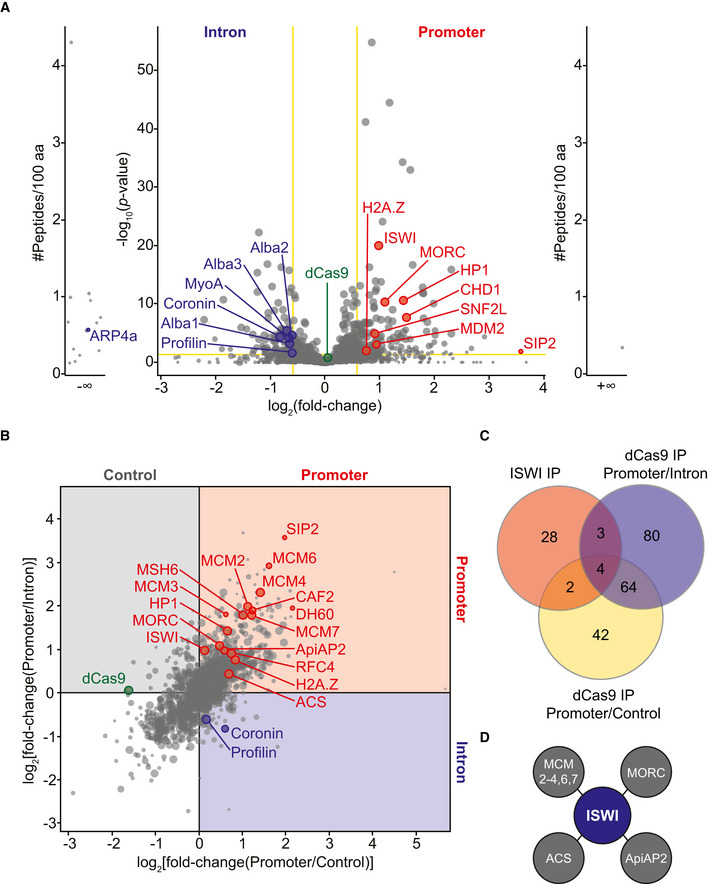
- Volcano plot representation of label‐free quantitative proteomic analysis of P. falciparum proteins present in intron‐ (left) and promoter‐targeted (right) dCas9 immunoprecipitations. Each dot represents a protein, and its size corresponds to the sum of peptides from both conditions used to quantify the ratio of enrichment. For the main volcano plot, x‐axis = log2(fold‐change), y‐axis = −log10(P‐value), horizontal yellow line indicates adjusted P‐value = 0.05, and vertical yellow lines indicate absolute fold‐change = 1.5. Side panels indicate proteins uniquely identified in either sample (y‐axis = number of peptides per 100 amino acids). dCas9 protein is highlighted in green for reference. Two replicates were used for the promoter‐targeted dCas9, and four replicates were used for the intron‐targeted dCas9. Fold enrichment and adjusted P‐values for each protein highlighted in red (promoter) and blue (intron) can be found in [Link], [Link], respectively.
- Correlation plot of label‐free quantitative proteomic analysis of P. falciparum proteins enriched in promoter‐targeted (top right quadrant in red) versus non‐targeted control (top left quadrant in gray) or intron (bottom right quadrant in blue) dCas9 immunoprecipitations. Each dot represents a protein, and its size corresponds to the sum of peptides from both conditions used to quantify the ratio of enrichment. The x‐axis = log2(fold‐change[promoter/non-targeted control]) and y‐axis = log2(fold‐change[promoter/intron]). dCas9 protein is highlighted in green for reference. Two replicates were used for the promoter‐targeted dCas9, and four replicates were used for the intron‐targeted and non‐targeted dCas9. Fold enrichment and adjusted P‐values can be found for each protein highlighted in red (promoter) in Tables EV5 (intron comparison) and EV7 (control comparison) and for each protein highlighted in blue (intron) in Tables EV6 (promoter comparison) and EV8 (control comparison).
- Venn diagram comparing proteins enriched in the ISWI IP LC‐MS/MS (red) and the promoter‐targeted dCas9 IP LC‐MS/MS compared to the intron‐targeted dCas9 (blue) or the non‐targeted dCas9 (yellow).
- Schematic of proteins shared between the ISWI IP LC‐MS/MS and the promoter‐targeted dCas9 IP LC‐MS/MS (overlapping regions shown in C): ISWI (PF3D7_0624600), MORC (PF3D7_1468100), ApiAP2 (PF3D7_1107800), MCM2‐4,6,7 (PF3D7_1417800, PF3D7_0527000, PF3D7_1317100, PF3D7_1355100, PF3D7_0705400), and acetyl‐CoA synthetase (ACS, PF3D7_0627800).
Figure EV3. Supplementary information for proteomic analysis of non‐targeted and var gene‐targeted dCas9 immunoprecipitation.
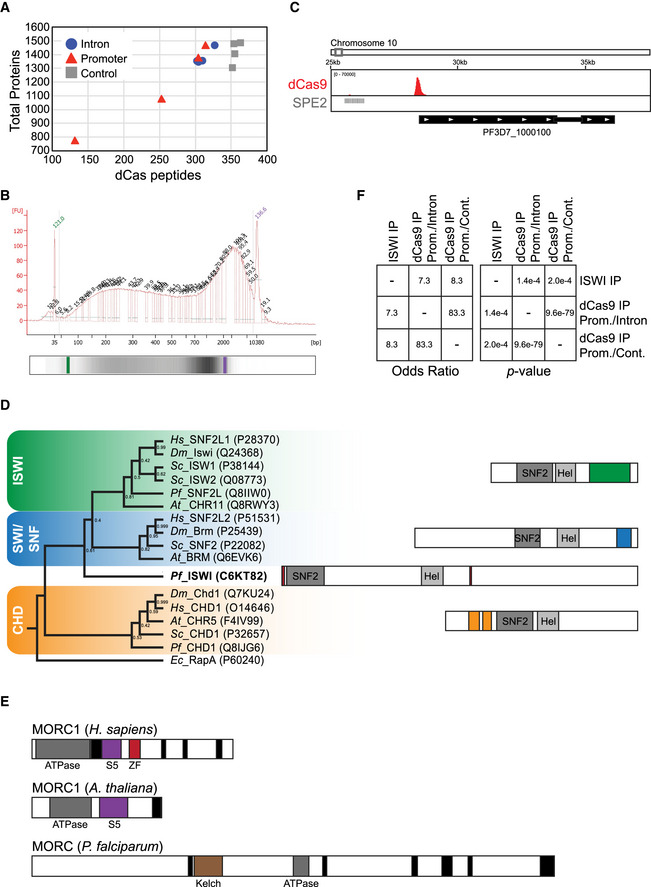
- Graph showing dCas9 peptide ions (x‐axis) versus total proteins (y‐axis) detected in the proteomic analysis for each of four replicates of the intron‐ (blue circles), promoter‐ (red triangles), or non‐targeted (gray squares) dCas9 immunoprecipitations. Corresponding data can be found in Table EV4 (top).
- Agilent DNA high sensitivity 2100 Bioanalyzer trace showing a representative distribution of DNA fragments after sonication of the chromatin samples used for dCas9 immunoprecipitation. x‐axis indicates DNA fragment size (bp), and y‐axis indicates abundance (fluorescence units). Lower DNA marker is shown in green, and upper DNA marker is shown in purple. A representative gel is shown at the bottom.
- ChIP‐seq data showing enrichment of dCas9 in the var gene promoter‐targeted strain. Genome location is indicated at the top. The x‐axis is DNA sequence, with a representative subtelomeric var gene (PF3D7_1000100) represented by black boxes indented to delineate introns and labeled with white arrowheads to indicate transcription direction. The y‐axis is input‐subtracted ChIP enrichment (peak q = 5.54 × 10−275). SPE2 DNA sequences identified in Flueck et al (2010) are indicated with gray lines. One replicate was performed for ChIP‐seq.
- Phylogenetic tree comparing PfISWI with SNF2 domain‐containing chromatin remodelers in P. falciparum and other organisms (indicated in italicized letters) based on the SNF2 domain sequence (left). Bootstrap values of 1,000 replicates are shown at nodes. General representations of protein compositions are shown on the right, with SNF2 and helicase (“Hel”) domains in gray. The ISWI family of chromatin remodelers contains HAND, SANT, and SLIDE domains (green), the SWI/SNF family contains a bromodomain (blue), and the CHD family contains chromodomains (orange). PfISWI contains zinc finger domains (red).
- Graphical representation of MORC family proteins in P. falciparum and other eukaryotes. S5 = S5 fold domain, ZF = zinc finger domain. Black box = coiled‐coil domain.
- Odds ratios and P‐values calculated with Fisher's exact test (with GeneOverlap R package) for protein overlap (Venn diagram in Fig 3C) between the ISWI IP LC‐MS/MS, var promoter‐targeted dCas9 LC‐MS/MS (compared to intron‐targeted dCas9), or var promoter‐targeted dCas9 LC‐MS/MS (compared to control dCas9). Odds ratio represents strength of association (≤ 1 indicates no association between two lists while > 1 indicates association).
Thus, we first compared the promoter‐ and intron‐targeted dCas9 data sets for a more unbiased analysis (see Materials and Methods for details of analysis). For most var genes, the promoter region is several thousand base pairs upstream of the intron. Thus, our method provides enough resolution to separate factors specifically bound to the promoter or intron, as the average DNA fragment length for the immunoprecipitation was 2 kilobases (Fig EV3B). There were 151 significantly enriched proteins (ratio ≥ 1.5, adjusted P‐value ≤ 0.05) in the var promoter‐targeted dCas9 immunoprecipitation (Table EV5) and 115 in the var intron‐targeted dCas9 immunoprecipitation (Table EV6). Providing strong validation that our method was successful, the AP2 domain‐containing SPE2‐interacting protein (SIP2, PF3D7_0604100) was the most highly enriched var promoter‐bound protein in the comparison with the intron‐targeted dCas9 (ratio = 11.94, P = 0.016, Fig 3A, Table EV5) and the second most highly enriched in the comparison with the non‐targeted dCas9 control (ratio = 3.93, P = 0.02, Fig 3B, Table EV7). SIP2 was shown to bind to SPE2 DNA motifs upstream of subtelomeric var genes, although the role of this protein has not been elucidated (Flueck et al, 2010). Indeed, the promoter‐targeted dCas9 binds to subtelomeric var genes with upstream SPE2/SIP2 sites (Fig EV3C, Table EV1).
When compared to the promoter‐targeted dCas9, the intron‐targeted dCas9 was enriched in the Alba DNA/RNA‐binding proteins 1–3 (ratio = 1.57, P = 6.6 × 10−4 for PF3D7_0814200; ratio = 1.52, P = 2.4 × 10−5 for PF3D7_1346300; ratio = 1.62, P = 4.22 × 10−6 for PF3D7_1006200; Fig 3A, Table EV6). The members of the Alba family have been shown to localize to the nuclear periphery and bind to subtelomeric regions as well as a var intronic sequence (Zhang et al, 2011; Chêne et al, 2012; Goyal et al, 2012). In addition to chromatin‐associated proteins, the intron‐targeted dCas9 sample was enriched in several cytoskeletal proteins such as a putative actin‐related protein ARP4a (PF3D7_1422800, ratio = ∞), myosin A (PF3D7_1342600, ratio = 1.58, P = 7.65 × 10−5), coronin (PF3D7_1251200, ratio = 1.78, P = 4.76 × 10−5), and profilin (PF3D7_0932200, ratio = 1.52, P = 2.57 × 10−2; Fig 3A, Table EV6). The same trend of cytoskeletal protein enrichment can be seen in the intron‐ versus non‐targeted dCas9 comparison (Table EV8). Gene Ontology (GO) analysis showed that proteins that were significantly enriched in the intron‐targeted dCas9 immunoprecipitation compared to both the promoter‐ and non‐targeted dCas9 immunoprecipitations are significantly represented by the biological function category of “cell motility” (P = 0.0018, Table EV9). Indeed, actin has been implicated in localizing var genes to the nuclear periphery by binding to the var gene intron (Zhang et al, 2011). Thus, our methodology was validated by the identification of specific proteins previously shown to bind to var gene upstream regions or introns such as SIP2 and the Alba family.
The var promoter is enriched in DNA replication and repair machinery
Gene Ontology analysis also showed that proteins that were significantly enriched in the promoter‐targeted dCas9 immunoprecipitation compared to both the intron‐ and non‐targeted dCas9 immunoprecipitations were significantly represented by the biological function categories of “DNA replication initiation” (P = 1.77 × 10−5) and “DNA replication” (P = 1.3 × 10−4; Table EV10). When compared to the intron‐ or non‐targeted dCas9 samples, the promoter‐targeted dCas9 sample was significantly enriched in proteins involved in DNA replication and repair such as DNA helicase 60 (PF3D7_1227100), minichromosome maintenance protein complex subunits MCM2,4,6 and 7 (PF3D7_1417800, 1317100, 1355100, 0705400), replication factor C subunit 4 (PF3D7_1241700), chromatin assembly complex subunit CAF2 (PF3D7_1329300), and MutS homologue proteins MSH2,6 (PF3D7_1427500 and 0505500; Fig 3B, [Link], [Link]). In P. falciparum, DNA helicase 60 was able to unwind double‐stranded DNA in both directions and has been implicated in DNA replication (Pradhan et al, 2005). In other eukaryotic systems, MCMs 2–7 form a hexameric DNA helicase and are loaded onto origins of replication by the origin recognition complex to form the basis of a prereplication complex in G1 phase of the cell cycle (Ansari & Tuteja, 2012). The RFC complex is also involved in DNA replication initiation while MSH2 and MSH6 form a heterodimer to recognize DNA mismatches (Kunkel & Erie, 2005; Ohashi & Tsurimoto, 2017). CAF2 is a putative subunit of the CAF‐1 chromatin assembly factor complex that assembles nucleosomes onto DNA during DNA replication and repair (Volk & Crispino, 2015).
The processes of DNA replication and repair have not been well characterized in P. falciparum, but as DNA replication has been reported to begin after 24 hpi (Matthews et al, 2018), it is unlikely that it would be taking place when the samples in this current study were collected (14 hpi). Protein components of the DNA replication and repair machinery were found to bind to the SPE2 DNA motif, but at a later stage of the IDC during DNA replication (Flueck et al, 2010). Thus, it is intriguing that these factors are associated with the var gene promoter during the G1 phase of the cell cycle.
Identification of novel var gene‐associated factors involved in chromatin structure
In addition to DNA replication and repair components, GO analysis showed that proteins that were significantly enriched in the promoter‐targeted dCas9 immunoprecipitation compared to both the intron‐ and non‐targeted dCas9 immunoprecipitations are significantly represented by the biological function categories of “chromatin assembly or disassembly” (P = 1.77 × 10−5) and “chromosome organization” (P = 1.94 × 10−5; Table EV10). These proteins include histone H2A.Z (PF3D7_0320900) and heterochromatin protein 1 (PF3D7_1220900), which have been shown to associate with the active and silent var gene promoters, respectively (Fig 3A and B) (Flueck et al, 2009; Petter et al, 2011). These data suggest that our method simultaneously purifies active and silent var gene promoters.
When compared to the intron‐targeted dCas9 immunoprecipitation, the promoter‐targeted dCas9 immunoprecipitation was enriched in several proteins involved in chromatin organization including a putative microrchidia (MORC) family protein (PF3D7_1468100, ratio = 2.13, P = 4.58 × 10−11), a SWIB/MDM2 domain‐containing protein (PF3D7_0518200, ratio = 1.91, P = 9.67 × 10−4), and three putative SNF2 domain‐containing ATP‐dependent chromatin remodelers: chromodomain‐helicase‐DNA‐binding protein 1 ([CHD1, PF3D7_1023900] ratio = 2.8, P = 1.93 × 10−8), SNF2L (PF3D7_1104200, ratio = 1.87, P = 1.21 × 10−5), and imitation switch ([ISWI, PF3D7_0624600] ratio = 1.97, P = 1.02 × 10−20; Fig 3A and Table EV5). In other eukaryotes, MORC proteins are able to topologically constrain DNA, which can lead to gene silencing via chromatin compaction (Koch et al, 2017; Kim et al, 2019). The SWIB domain can be found in proteins associated with the SWI/SNF chromatin‐remodeling complex (Brownlee et al, 2015). In other eukaryotes, SWI/SNF, CHD1, and ISWI are chromatin‐remodeling enzymes that use ATP hydrolysis to alter interactions between nucleosomes and DNA, leading to changes in chromatin organization/structure and accessibility to underlying DNA regulatory elements (reviewed in (Mueller‐Planitz et al, 2013)). As such, these remodelers play important roles in chromosome structure, transcription regulation, and DNA replication, recombination, and repair (reviewed in (Erdel & Rippe, 2011; Narlikar et al, 2013)).
While the domain compositions of PfCHD1 and PfSNF2L are fairly conserved compared to those in other eukaryotes, the putative PfISWI and PfMORC protein are more divergent (Fig EV3D and E). Comparison of the PfISWI amino acid sequence to those of other SNF2 domain‐containing chromatin remodelers revealed a divergence in its SNF2 domain as well as the rest of the protein (Fig EV3D). PfISWI contains several zinc finger motifs and the characteristic N‐terminal SNF2 ATPase and C‐terminal helicase domains, but does not contain chromo‐, bromo‐, SANT, or SLIDE domains (Fig EV3D). PfMORC contains a GHKL‐type ATPase/kinase domain and coiled‐coil domains similar to MORC proteins in other eukaryotes, but lacks the characteristic S5 fold domain believed to be important for its function in DNA compaction/loop formation (Fig EV3E) (Koch et al, 2017). However, PfMORC does contain an N‐terminal Kelch‐type beta propeller, which seems to be unique to apicomplexan MORC proteins (Iyer et al, 2008).
Using CRISPR/Cas9, we attempted to epitope tag and/or knock out several of the most highly/significantly enriched factors at the var gene promoter such as ISWI, CHD1, MORC, MSH6, and CAF2 (Ghorbal et al, 2014). We were only successful in achieving an inducible knockdown system with ISWI, where sequences encoding a 3× hemagglutinin (3HA) tag followed by a glmS ribozyme (Prommana et al, 2013) were inserted at the 3′ end of the corresponding gene, PF3D7_0624600. We performed immunoprecipitation followed by mass spectrometry of ISWI‐3HA and found significant overlap (Fig EV3F) between ISWI‐associated proteins and those enriched in the var gene promoter‐targeted dCas9 IP LC‐MS/MS analysis, including MCM2‐4,6,7, acetyl‐CoA synthetase (ACS, PF3D7_0627800), the putative MORC family protein, and an ApiAP2 protein (PF3D7_1107800) that was shown to strongly associate with PfMORC in a recent protein interaction screen (Hillier et al, 2019) (Fig 3C and D; [Link], [Link], [Link]).
These results provide further experimental support for the efficacy of the CRISPR/dCas9 chromatin purification method. ISWI also associates with various transcriptional co‐repressors and co‐activators such as histone deacetylase 1 (HDAC1) and GCN5, respectively (Table EV11). These data suggest that ISWI may perform specific chromatin‐related functions via different binding partners.
ISWI is a transcriptional activator involved in var gene regulation
Because our dCas9 immunoprecipitation‐proteomics approach did not distinguish between active and silent var gene promoters, functional characterization allowed us to unequivocally determine the role of ISWI in var gene regulation. As we were unable to achieve gene knockout, we used a gene knockdown (KD) approach, which allowed for the study of mutually exclusive expression in a clonal parasite population. Addition of glucosamine induced self‐cleavage of the ribozyme and KD of ISWI at the protein level to an extent that did not affect growth and cell cycle progression (Figs 4A and EV4A).
Figure 4. ISWI is a transcriptional activator involved in var gene regulation.
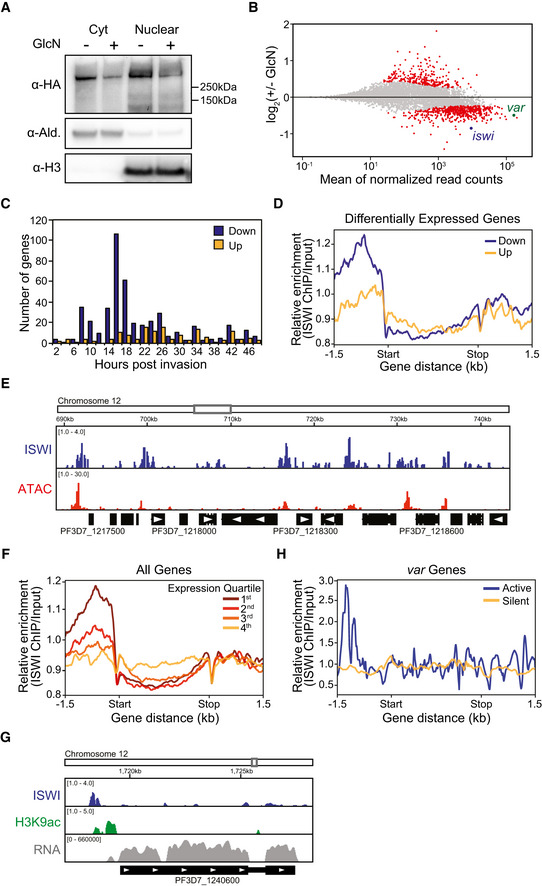
- Western blot analysis of cytoplasmic (Cty) and nuclear extracts from a bulk population of ISWI‐3HA-ribo parasites in the absence (−) or presence (+) of glucosamine (GlcN). ISWI‐3HA is detected with an anti‐HA antibody. Antibodies against aldolase (Ald.) and histone H3 are controls for the cytoplasmic and nuclear extracts, respectively. Molecular weights are shown to the right.
- MA plot of log2(glucosamine‐treated/untreated, M) plotted over the mean abundance of each gene (A) at 12 hpi. Transcripts with a significantly higher (above x‐axis) or lower (below x‐axis) abundance in the presence of glucosamine are highlighted in red (q ≤ 0.05). iswi is highlighted in blue (q = 6.71 × 10−10), and the active var gene is highlighted in green (q = 2.53 × 10−5). Two and three replicates were used for untreated and glucosamine‐treated parasites, respectively. P‐values were calculated with a Wald test for significance of coefficients in a negative binomial generalized linear model as implemented in DESeq2 (Love et al, 2014). q = Bonferroni corrected P‐value.
- Meta‐gene plot showing average ISWI enrichment (y‐axis = ChIP/Input) in clonal ISWI‐3HA parasites at 12 hpi from 1.5 kb upstream of the translation start site (“Start”) to 1.5 kb downstream of the translation stop site (“Stop”) for genes that are down‐ (blue) or up‐regulated (yellow) upon ISWI knockdown. One replicate was used for the ISWI ChIP‐seq.
- ChIP‐seq data show enrichment of ISWI (blue) in clonal ISWI‐3HA parasites at 12 hpi relative to regions of accessible chromatin (“ATAC,” red). Chromatin accessibility (ATAC‐seq) data were taken from the 15 hpi time point in Toenhake et al, 2018 (Data ref: Toenhake et al, 2018). Genome location is indicated at the top of the panel. The x‐axis is DNA sequence, with genes represented by black boxes indented to delineate introns and labeled with white arrowheads to indicate transcription direction. The y‐axis is enrichment (ChIP/Input or ATAC‐seq/genomic DNA). One replicate was used for the ISWI ChIP‐seq.
- Meta‐gene plot showing average ISWI enrichment (y‐axis = ChIP/Input) in clonal ISWI‐3HA parasites at 12 hpi from 1.5 kb upstream of the translation start site (“Start”) to 1.5 kb downstream of the translation stop site (“Stop”) for all genes, which are grouped into quartiles based on their transcript levels (RPKM) at 12 hpi. Dark red represents genes with the highest transcript levels (“1st”), light red represents genes with the second highest transcript levels (“2nd”), orange represents genes with the third highest transcript levels (“3rd”), and yellow represents genes with the lowest transcript levels (“4th”). One replicate was used for the ISWI ChIP‐seq, and the transcription data are an average from the two replicates of the untreated ISWI‐3HA clone used for the differential expression analysis.
- ChIP‐seq data show enrichment of ISWI (blue) and H3K9ac (green) in clonal ISWI‐3HA parasites at 12 hpi at the active var gene (PF3D7_1240600). RNA‐seq data from this clone at 12 hpi show transcript levels for this gene (gray). Genome location is indicated at the top of the panel. The x‐axis is DNA sequence, with genes represented by black boxes indented to delineate introns and labeled with white arrowheads to indicate transcription direction. The y‐axis is ChIP/Input for ChIP data and RPKM for RNA‐seq data. One replicate was used for each ChIP‐seq, and the RNA‐seq data are from a single replicate from the untreated ISWI‐3HA clone used for the differential expression analysis.
- Meta‐gene plot showing average ISWI enrichment (y‐axis = ChIP/Input) in clonal ISWI‐3HA parasites at 12 hpi from 1.5 kb upstream of the translation start site (“Start”) to 1.5 kb downstream of the translation stop site (“Stop”) for the active var gene (PF3D7_1240600, blue) or silent var genes (yellow). One replicate was used for the ISWI ChIP‐seq.
Source data are available online for this figure.
Figure EV4. Supplementary information for differential expression analysis of ISWI knockdown and ISWI ChIP sequencing.
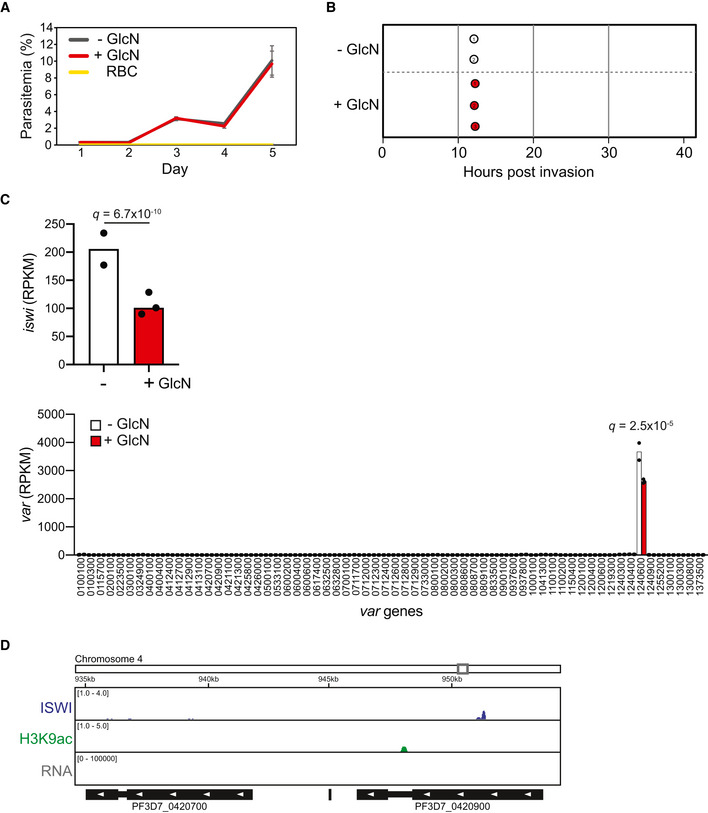
- Growth curve over 5 days of clonal ISWI‐3HA-ribo parasites in the absence (“− GlcN” in gray) or presence (“+ GlcN” in red) of glucosamine. Uninfected red blood cells (“RBC” in yellow) serve as reference of background. Error bars indicate standard deviation of three technical replicates in blood from two different donors (n = 6).
- Cell cycle progression estimation of an ISWI‐3HA-ribo parasite clone in the absence (“− GlcN” in white) or presence (“+ GlcN” in red) of glucosamine. RNA‐seq data from synchronized parasites harvested at 12 hpi were compared to microarray data from Bozdech et al (2003) (Data ref: Bozdech et al, 2003) as in Lemieux et al (2009). Replicates are represented with numbered circles.
- RNA‐seq of an ISWI‐3HA-ribo clone at 12 hpi shows iswi (top, q = 6.71 × 10−10) and var gene (bottom, active var gene q = 2.53 × 10−5) transcript levels in the absence (“−”, white) or presence (“+”, red) of glucosamine (GlcN). y‐axis is transcript level (RPKM). Bars represent averages of two and three replicates (individual values indicated with black dots) for untreated and glucosamine‐treated parasites, respectively. P‐values are calculated with a Wald test for significance of coefficients in a negative binomial generalized linear model as implemented in DESeq2 (Love et al, 2014). q = Bonferroni corrected P‐value. Corresponding data can be found in Source Data for Table EV12.
- ChIP‐seq data show enrichment of ISWI (blue) and H3K9ac (green) in clonal ISWI‐3HA parasites at 12 hpi at two silent var genes (PF3D7_0420700 and PF3D7_0420900). RNA‐seq data from this clone at 12 hpi show transcript levels for these genes (gray). Genome location is indicated at the top of the panel. The x‐axis is DNA sequence, with genes represented by black boxes indented to delineate introns and labeled with white arrowheads to indicate transcription direction. The y‐axis is ChIP/Input for ChIP data and RPKM for RNA‐seq data. One replicate was used for each ChIP‐seq, and the RNA‐seq data are from a single replicate from the untreated ISWI‐3HA clone used for the differential expression analysis.
A clone of ISWI‐3HA‐ribo was synchronized, glucosamine was added, and parasites were harvested 24 h later during ring stage, when var gene expression is at its highest. We performed RNA‐seq and compared the data to the microarray time course data in Bozdech et al (2003) (Data ref: Bozdech et al, 2003) as in Lemieux et al (2009), which provided a statistical estimation of cell cycle progression at 12 hpi in both control and glucosamine‐treated parasites (Fig EV4B). These data suggest that any differences in transcription were due to ISWI KD and not differences in cell cycle progression.
Indeed, glucosamine addition resulted in a significant knockdown (~ 50%, q = 6.71 × 10−10) of iswi transcript levels (Fig EV4C top, Table EV12) and a significant over‐representation of down‐regulated genes (562) compared to up‐regulated genes (261; chi‐square test P < 0.0001, Fig 4B, Table EV12). GO analysis showed that down‐regulated genes are most significantly represented by the molecular function category of “structural constituent of ribosome” (P = 2.82 × 10−31, Table EV13), but there was no strong pattern of enrichment for up‐regulated genes (Table EV14). Comparison of the RNA‐seq data to the transcription time course in Bozdech et al (2003) (Data ref: Bozdech et al, 2003) revealed that the majority of significantly down‐regulated genes normally reach their peak transcript level in ring stage parasites between 8 and 18 hpi; however, the same trend is not seen in significantly up‐regulated genes (Fig 4C).
To investigate the genome‐wide occupancy of ISWI‐3HA, we performed ChIP‐seq in ring stage parasites. These data showed ISWI enrichment in the promoter regions of genes that were down‐regulated upon ISWI KD (Fig 4D). When compared to published Assay for Transposase‐Accessible Chromatin using sequencing (ATAC‐seq) data from a similar time point in the IDC (Toenhake et al, 2018; Data ref: Toenhake et al, 2018), our ChIP‐seq data revealed an enrichment of ISWI that overlapped with accessible chromatin in intergenic regions (Fig 4E). Indeed, if all P. falciparum genes were divided into quartiles based on their transcript levels at 12 hpi, ISWI was most enriched in the promoter regions of genes with the highest transcript levels (Fig 4F). These data suggest that ISWI binding to promoter regions contributes to transcriptional activation in real time.
Among the genes that were significantly down‐regulated upon ISWI KD was the var gene that is active in this clone (PF3D7_1240600; q = 2.53 × 10−5, Figs 4B and EV4C bottom, Table EV12). However, mutually exclusive expression of the var gene family was maintained during ISWI KD (Fig EV4C bottom). Interestingly, ISWI and H3K9ac ChIP‐seq showed enrichment of both these features in the promoter region of the active var gene (Fig 4G and H). In comparison, ISWI and H3K9ac were largely absent from the promoters of the silent var genes (Figs 4H and EV4D). Taken together, these data suggest that ISWI plays a direct role in var gene activation, but not repression, in ring stage parasites.
Discussion
CRISPR/Cas9 continues to transform genetics in apicomplexan parasites, but the full range of CRISPR‐based tools has yet to be developed in Plasmodium. dCas9 has been used in mammalian systems to purify specific loci in the genome along with closely associated proteins, DNA, and RNA (Fujita & Fujii, 2013; Waldrip et al, 2014; Liu et al, 2017; Myers et al, 2018). Importantly, this method is able to capture chromatin complexes that may not be detected with in vitro affinity purification assays. In this study, we purified specific chromatin loci without the use of genome editing, which allowed us to identify factors bound in a biologically relevant spatio‐temporal context.
Traditionally, single‐locus dCas9 immunoprecipitation for proteomic analysis has proven challenging even in model systems due to the high amount of input material needed to overcome non‐specific background. The future development of an inducible, biotin‐based CRISPR/dCas9 IP LC‐MS/MS analysis system (Liu et al, 2017; Myers et al, 2018) in Plasmodium may allow for single‐locus purification. Many seminal studies attempting to purify a specific genomic sequence maximized the immunoprecipitated protein content per cell by targeting telomeric repeats, which are present in high numbers within the genome (Déjardin & Kingston, 2009; Fujita et al, 2013; Liu et al, 2017). For our analysis, we exploited the homology of targeted sequences within the var gene family and maximized protein content of the immunoprecipitation by targeting dCas9 to multiple var genes in the same strain with a single guide RNA (Figs 1 and EV1A and C). We used ChIP‐seq to demonstrate that dCas9 bound robustly to var gene regulatory elements (Fig 2). It is possible that we immunoprecipitated both silent and active var genes simultaneously from non‐clonal bulk cultured parasites, as they collectively did not express a single var gene (Fig EV2D). Importantly, RNA‐seq and dCas9 RIP‐seq of the var promoter‐targeted strains showed that dCas9‐targeted var genes are transcribed and that mutually exclusive expression is maintained (Figs 2C and EV2B–E). Thus, this technology could be used for investigation of other multigene families in P. falciparum and other parasites.
Label‐free quantitative proteomics and comparison of the promoter‐ and intron‐targeted dCas9 immunoprecipitations to each other and to the non‐targeted dCas9 immunoprecipitation allowed for the identification of proteins that were specific to each DNA element (Fig 3). Using a non‐targeted dCas9 that does not bind specifically to the genome for normalization, we identified var gene‐bound factors such as SIP2 and other proteins that were shown to bind to or near var genes like the Alba proteins, HP1, and H2A.Z (Flueck et al, 2009; Pérez‐Toledo et al, 2009; Petter et al, 2011; Chêne et al, 2012; Goyal et al, 2012). However, our analysis did not identify as significantly enriched several chromatin‐associated proteins that have been implicated in mutually exclusive var gene transcription including HDA2, SIR2, SET2, SET10, and RECQ1 (Freitas‐Junior et al, 2005; Tonkin et al, 2009; Volz et al, 2012; Jiang et al, 2013; Coleman et al, 2014; Ukaegbu et al, 2014; Li et al, 2019). SIR2, SET2, SET10, and RECQ1 were not detected in any sample, perhaps due to very low abundance or to weak/transient binding to var genes (Source Data for Tables EV5–EV8). It is also possible that these factors associate with var genes before or after the 14 hpi time point we analyzed. Indeed, SET10 was shown by IFA to associate with the active var gene at later stages, perhaps to facilitate gene poising (Volz et al, 2012).
Importantly, we identified novel var gene‐associated factors that have thus far not been implicated in var gene regulation, but provide important insight into var gene biology. The var gene promoter was enriched in members of the DNA prereplication complex. It is possible that the targeted var gene promoters are located near origins of replication where the prereplication complex is loaded during G1 phase (ring stage parasites), as in other eukaryotes (Ansari & Tuteja, 2012). However, the presence of DNA mismatch repair (MMR) components suggests that CAF2 and the MCM complex might be involved in DNA damage repair. Indeed, recent evidence showed that var genes frequently recombine during asexual replication, leading to crossover between different var sequences in a manner that preserves the reading frame (Claessens et al, 2014). These recombination events are often located near putative G‐quadruplex DNA motifs, which form secondary structures that can interfere with transcription or lead to DNA recombination by stalling DNA polymerase (Siddiqui‐Jain et al, 2002; Koole et al, 2014; Stanton et al, 2016). In P. falciparum, half of the total predicted G‐quadruplex motifs are within or upstream of var genes, especially of the upsB type, which we target with dCas9 in this study (Smargiasso et al, 2009). Thus, the MMR machinery, MCM helicases, and CAF‐1 complex could bind near var gene promoters to help maintain chromatin stability and DNA repair in the recombinogenic var gene family during asexual replication.
The var gene promoters were also enriched in chromatin remodelers and MORC, which indicates the importance of chromatin structure at this regulatory element. It has been demonstrated that chromatin accessibility and incorporation of histone variants H2A.Z and H2B.Z at P. falciparum gene promoters correlate with transcriptional activity, but the factors behind these phenomena are unknown (Hoeijmakers et al, 2013; Petter et al, 2013; Ruiz et al, 2018; Toenhake et al, 2018). Thus, our functional characterization of ISWI provides novel insight into transcriptional activation in P. falciparum. We showed that ISWI binds to the promoters of active genes and intergenic regions of high chromatin accessibility in ring stage parasites (Fig 4D–F). Although we showed that ISWI associates with proteins involved in transcriptional repression and activation, there was a significant over‐representation of down‐regulated genes upon ISWI knockdown, the majority of which normally reach peak transcription level in ring stage parasites (Fig 4B and C). One of these down‐regulated genes was the active var gene, which had an enrichment of ISWI at its promoter (Fig 4G and H). These data suggest that ISWI binds to upstream regions of genes to facilitate transcriptional activation, perhaps through changes in chromatin composition at the var gene promoter.
It is possible that ISWI achieves specificity and modulates nucleosome positioning/composition either directly or via binding partners, as has been shown for many chromatin remodelers in other eukaryotic systems (reviewed in (Erdel & Rippe, 2011)). Indeed, we show that the structurally divergent PfISWI contains zinc finger domains and interacts with diverse proteins such as MORC, an ApiAP2 transcription factor, HDAC1, and ACS (Fig 3D). Interestingly, recent studies have demonstrated that enzymes involved in acetyl‐CoA synthesis localize to specific genomic loci to effect changes in histone acetylation, which can enhance gene transcription or DNA repair (reviewed in (Li et al, 2018)). Likewise, localization of PfACS to the promoters of genes could have important implications for the relationship between gene expression and metabolism.
As the PfMORC sequence diverges from that of MORC proteins found in other eukaryotes, it is unclear what transcriptional role it might play with ISWI in P. falciparum. A recent study in another apicomplexan parasite, Toxoplasma gondii, showed that MORC associates with ApiAP2 transcription factors and a histone deacetylase (HDAC3) to transcriptionally silence specific cohorts of genes important for sexual differentiation (Farhat et al, 2020). This MORC/HDAC3 complex seems to modulate nucleosome positioning and/or histone acetylation to directly silence transcription or act as a barrier to neighboring euchromatin. A similar model could prove true in P. falciparum, where an ApiAP2 transcription factor (such as PF3D7_1107800) recruits ISWI and other cofactors such as MORC, HDAC1, or GCN5 to alter the local chromatin structure, which could lead to gene activation, repression, or insulation from neighboring HP1‐mediated heterochromatin. Future functional characterization of the PfISWI complex will elucidate specific roles in chromatin composition and transcription, especially with regard to var genes.
In addition, many proteins of unknown function without homologs in other organisms were enriched in our dCas9 IP LC‐MS/MS analysis. Because P. falciparum lacks CTCF and many conserved transcription factors found in other eukaryotes, the proteins identified in this study may prove to be missing links in var gene regulation and higher‐order chromatin organization (Aravind et al, 2003; Coulson et al, 2004; Templeton et al, 2004). Future functional characterization of these factors will reveal their roles in chromatin organization and unique transcriptional regulation of the var gene family.
Materials and Methods
Reagents and Tools table
| Reagent/Resource | Reference or Source | Identifier or Catalog Number |
|---|---|---|
| Experimental Models | ||
| 3D7 strain (P. falciparum) | (Walliker et al, 1987) PMID 3299700 | |
| 3D7 ISWI‐3HA‐ribo (P. falciparum) | This study | |
| 3D7 pUF1‐dCas9‐3HA, pL7_varpromoter_IP | This study | |
| 3D7 pUF1‐dCas9‐3HA, pL7_varintron_IP | This study | |
| 3D7 pUF1‐dCas9‐3HA, pL7_Control_IP | This study | |
| Recombinant DNA | ||
| pL7_0624600_A | This study | |
| pL7_0624600_B | This study | |
| pL7_Control_IP | This study | |
| pL7_varintron_IP | This study | |
| pL7_varpromoter_IP | This study | |
| pUF1‐Cas9 | (Ghorbal et al, 2014) PMID 24880488 | |
| pUF1‐dCas9‐3HA | This study | |
| Antibodies | ||
| HRP‐conjugated donkey anti‐rabbit (1:5,000 for western blot) | Sigma | Cat # GENA934 |
| Rabbit IgG | Diagenode | Cat # C15410206 |
| Rabbit polyclonal anti‐H3 (1:1,000 for western blot) | Abcam | Cat # ab1791 |
| Rabbit polyclonal anti‐H3K9ac (1 μg/25uL Dynabeads for immunoprecipitation) | Millipore | Cat # 07‐352 |
| Rabbit polyclonal anti‐HA (1:1,000 for western blot, 1 μg/25 μl Dynabeads for immunoprecipitation) | Abcam | Cat # ab9110 |
| Rabbit polyclonal HRP‐conjugated anti‐aldolase (1:5,000 for western blot) | Abcam | Cat # ab38905 |
| Oligonucleotides and other sequence‐7based reagents | Sequence (5′–3′) | |
| pL7_0624600_A sgRNA | This study | ATCATTATTACTTCTGTCTG |
| pL7_0624600_B sgRNA | This study | ATGTCGTTTTAATTAATTTC |
| pL7_varintron_IP sgRNA | This study | GTAAATGTGTATGTAGTTAT |
| pL7_varpromoter_IP sgRNA | This study | GGTTTATATCGTGGCATACA |
| Chemicals, Enzymes and other reagents | ||
| 10% Albumax | Thermo Fisher | Cat # 11020 |
| 4–12% Bis‐Tris NuPage gel | Thermo Fisher | Cat # NP0321 |
| Anti‐HA Dynabeads | Thermo Fisher | Cat # 88836 |
| Benzonase | Merck | Cat # 71206 |
| C18 column (50 cm × 75 μm; nanoViper Acclaim PepMap™ RSLC, 2 μm, 100 Å) | Thermo Fisher | Cat # 164535 |
| C18 column (75 μm inner diameter × 2 cm; nanoViper Acclaim PepMap™ 100) | Thermo Fisher | Cat # 164942 |
| C18 StageTips | In house | |
| C18 ziptips (for ISWI‐HA IP/MS) | Merck | Cat # ZTC04S096 |
| d‐(+)‐Glucosamine hydrochloride | Sigma | Cat # G1514 |
| Dithiobissuccinimidyl propionate | Thermo Fisher | Cat # 22585 |
| Dithiothreitol | Sigma | Cat # D9779 |
| DNase Turbo | Thermo Fisher | Cat # AM2238 |
| DSM1 | MR4/BEI Resources | Cat # MRA‐1161 |
| Dulbecco's phosphate‐buffered saline | Thermo Fisher | Cat # 14190 |
| EASY‐Spray column, 50 cm × 75 μm ID, PepMap RSLC C18, 2 μm | Thermo Fisher | Cat # ES803A |
| Gentamicin | Sigma | Cat # G1397 |
| Glycine | Sigma | Cat # G8898 |
| Glycogen | Thermo Fisher | Cat # 10814 |
| Hypoxanthine | C.C.Pro | Cat # Z‐41‐M |
| In‐Fusion HD Cloning Kit | Clontech | Cat # 639649 |
| Iodoacetamide | Sigma | Cat # I1149 |
| KAPA HiFi DNA Polymerase | Roche | Cat # 07958846001 |
| Methanol‐free formaldehyde | Thermo Fisher | Cat # 28908 |
| NH4HCO3 | Sigma | Cat # 09830 |
| NuPage Reducing Agent | Thermo Fisher | Cat # NP0004 |
| NuPage Sample Buffer | Thermo Fisher | Cat # NP0008 |
| Plasmion | Fresenius Kabi | |
| Protease Inhibitor Cocktail | Roche | Cat # 11836170001 |
| Protein G Dynabeads | Invitrogen | Cat # 10004D |
| Proteinase K | New England Biolabs | Cat # P8107S |
| RNaseA | Thermo Fisher | Cat # EN0531 |
| RNasin RNase inhibitor | Promega | Cat # N2511 |
| RPMI‐1640 culture media | Thermo Fisher | Cat # 11875 |
| Saponin | Sigma | Cat # S7900 |
| Sorbitol | Sigma | Cat # S6021 |
| SuperSignal West Pico chemiluminescent substrate | Thermo Fisher | Cat # 34080 |
| SYBR Green I | Sigma | Cat # S9430 |
| Trizol LS | Thermo Fisher | Cat # 10296010 |
| Trypsin (for ISWI‐HA IP/MS) | Thermo Fisher | Cat # 90059 |
| Trypsin/LysC | Promega | Cat # V5071 |
| WR99210 | Jacobus Pharmaceuticals | |
| XL10‐Gold Ultracompetent Escherichia coli | Agilent Technologies | Cat # 200315 |
| Software | ||
| FigTree (v 1.4.3) | http://tree.bio.ed.ac.uk/software/figtree/ | |
| GraphPad Prism | www.graphpad.com | |
| Integrative Genomics Viewer | (Robinson et al, 2011) | |
| Mascot (v 2.4.1) | (Perkins et al, 1999) | |
| myProMS (v 3.9) | (Poullet et al, 2007) | |
| Proteome Discoverer | Thermo Fisher | |
| Other | ||
| 2100 Bioanalyzer | Agilent | |
| Bioruptor Pico | Diagenode | Cat # B01060010 |
| ChemiDoc XRS+ | Bio‐Rad | Cat # 1708265 |
| Dynabeads mRNA DIRECT Kit | Thermo Fisher | Cat # 61012 |
| DynaMag | Thermo Fisher | Cat # 12321D |
| Guava easyCyte Flow Cytometer | Merck Millipore | |
| HPLC | Thermo Fisher | Cat # Easy‐nLC1000 |
| MicroPlex library preparation kit (v2) | Diagenode | Cat # C05010014 |
| miRNeasy Mini Kit | Qiagen | Cat # 217004 |
| NextSeq 500 | Illumina | Cat # SY‐415‐1002 |
| Q Exactive HF‐X mass spectrometer | Thermo Fisher | |
| RNeasy MinElute Cleanup Kit | Qiagen | Cat # 74204 |
| Sonication tubes | Diagenode | Cat # C30010016 |
| TruSeq stranded mRNA LT library prep kit | Illumina | Cat # 20020594 |
Methods and Protocols
Parasite culture
Asexual blood stage 3D7 P. falciparum parasites were cultured as previously described in (Lopez‐Rubio et al, 2009). Briefly, parasites were cultured in human RBCs (obtained from the Etablissement Français du Sang with approval number HS 2016‐24803) in RPMI‐1640 culture medium (Thermo Fisher 11875) supplemented with 10% v/v Albumax I (Thermo Fisher 11020), hypoxanthine (0.1 mM final concentration, C.C.Pro Z‐41‐M) and 10 mg gentamicin (Sigma G1397) at 4% hematocrit and under 5% O2, 3% CO2 at 37 °C. Parasite development was monitored by Giemsa staining. Parasites were synchronized by sorbitol (5%, Sigma S6021) lysis at ring stage, plasmagel (Plasmion, Fresenius Kabi) enrichment of late stages 24 h later, and an additional sorbitol lysis 6 h after plasmagel enrichment. The 0 h time point was considered to be 3 h after plasmagel enrichment. Parasites were harvested at 1–5% parasitemia.
Generation of strains
All cloning was performed using KAPA HiFi DNA Polymerase (Roche 07958846001), In‐Fusion HD Cloning Kit (Clontech 639649), and XL10‐Gold Ultracompetent E. coli (Agilent Technologies 200315). Transgenic dCas9 parasites were generated using a two‐plasmid system (pUF1 and pL7) based on the CRISPR/Cas9 system previously described in (Ghorbal et al, 2014). The sequence encoding the 3xFLAG‐Cas9 in the pUF1 plasmid was replaced with a gene encoding a catalytically inactive Cas9 (with RuvC and HNH mutations developed in (Qi et al, 2013)), which was fused at its carboxy terminus with a 3× hemagglutinin (HA) tag. The pL7 sgRNA expression plasmid was modified to encode an optimized sgRNA (with duplex extension and thymine mutation as developed in (Dang et al, 2015)). For pL7_varPromoter_IP, the non‐coding strand sequence 5′‐GGTTTATATCGTGGCATACA‐3′ approximately 40 bp upstream of the translation start site of 19 var genes was ordered from Eurofins Genomics (Ebersberg, Germany) and inserted into the optimized pL7 sgRNA expression plasmid. For pL7_varIntron_IP, the non‐coding strand sequence 5′‐GTAAATGTGTATGTAGTTAT‐3′ in region III of the intron (as defined in (Calderwood et al, 2003)) of 13 var genes was ordered from Eurofins Genomics (Ebersberg, Germany) and inserted into the optimized pL7 sgRNA expression plasmid. The pL7_Control_IP plasmid contains the empty cloning site 5′‐CCTAGGAACTCATCGCTCGCGATGCTGCCCGACA‐3′ as the sgRNA. Short read alignment using bowtie analysis shows that neither this sequence nor the final 20 nucleotide (nt) sequence adjacent to the tracrRNA correspond to any sequence in the P. falciparum 3D7 genome with three or less mismatches (Fig EV2A). A 3D7 wild‐type bulk ring stage culture was transfected with 25 μg each of pUF1 and pL7, and continuous drug selection was applied with 1.33 nM WR99210 (Jacobus Pharmaceuticals) and 0.75 μM DSM1 (MR4/BEI Resources MRA‐1161).
The ISWI‐3HA‐ribo strain was generated with CRISPR/Cas9 as in (Ghorbal et al, 2014) by transfecting a 3D7 wild‐type bulk ring stage culture with 25 μg pUF1‐Cas9 and 12.5 μg each of two pL7 plasmids containing sgRNA‐encoding sequences 5′‐ATCATTATTACTTCTGTCTG‐3′ and 5′‐ATGTCGTTTTAATTAATTTC‐3′ (ordered from Eurofins Genomics [Ebersberg, Germany]) targeting the 3′ end of the PF3D7_0624600 coding sequence. Each pL7 plasmid contained the homology repair construct 5′‐ATGATAAGAGCAAAAATGTGAGGAATGATGACGAAGATGACGATGATGATGAAGATGATGATGAAGAGGATGACGAAGATAAAAATGAAAGTTCAAATTATAATAATAATAAGAAAAAAAAGACAAATACTTCAAGTAGAAATAGCAGTAATAATAATAGTAGTAATAAAAATAAAAATAATAAAAGTGGTAATGATATTCATCAAGCTAGTAACTTACTGTATCAAAATTTATTAAATAATCCGCAAAGTCTTTTACAGCATTTAAATTTGGAAGATGTAAAGAATTTCTTAAAAGCTGCTGACAGAAGTAATAATGATAACTTACCAGAAATTAATGGCGGTGGATACCCTTACGATGTGCCTGATTACGCGTATCCCTATGACGTACCAGACTATGCGTACCCTTATGACGTTCCGGATTATGCTCACGGGGTGTAAGCGGCCGCGGTCTTGTTCTTATTTTCTCAATAGGAAAAGAAGACGGGATTATTGCTTTACCTATAATTATAGCGCCCGAACTAAGCGCCCGGAAAAAGGCTTAGTTGACGAGGATGGAGGTTATCGAATTTTCGGCGGATGCCTCCCGGCTGAGTGTGCAGATCACAGCCGTAAGGATTTCTTCAAACCAAGGGGGTGACTCCTTGAACAAAGAGAAATCACATGATCTTCCAAAAAACATGTAGGAGGGGACAACGACATTTCTTTAATAAATAAATTAATATATATATATATATATATAGAGAGAGAGAGAGAAATATTATATTTGATATATGTAGCTGCGAATGTTTAATTTTTTAGTTTATATATTTTGGAAATGTGTCTTTTGAATTTTTTTTTATTTTGAAAAGATGAATGATATATGATATATGATATATGAGGATATAATAAGAAGGAAATATATCTATATGTATATATATATATATATATGTATATTTTAGTTTTTGAGAGAATTTTATTTTCAGAAGGATATAAAAAAGAAAAAAATGGATAA‐3′ (synthesized by GenScript Biotech [Piscataway, NJ, USA]). This repair construct contains a 3xHA‐encoding sequence followed by a glmS ribozyme sequence (Prommana et al, 2013), which are flanked by 300 bp homology repair regions upstream and downstream of the Cas9 cut sites. Two silent shield mutations were made in the homology repair region to prevent further Cas9 cutting. After transfection, drug selection was applied for 7 days at 2.67 nM WR99210 (Jacobus Pharmaceuticals) and 1.5 μM DSM1. Parasites reappeared ~ 3 weeks after transfection, and 5‐fluorocytosine was used to negatively select the pL7 plasmid. Parasites were cloned by limiting dilution, and the targeted genomic locus was sequenced to confirm tag and ribozyme integration.
Western blot analysis
For the Western blot in Fig 1C, extracts prepared during the dCas9 immunoprecipitation protocol (see below) for LC‐MS/MS were supplemented with NuPage Sample Buffer (Thermo Fisher NP0008) and NuPage Reducing Agent (Thermo Fisher NP0004) and denatured for 5 min at 95°C. For Fig 4A, 2 ml of iRBC (3% parasitemia) was synchronized and split into two 1 ml cultures at 3 hpi, and glucosamine (1.25 mM, Sigma G1514) was added to one culture. After 24 h, iRBCs were washed once with Dulbecco's phosphate‐buffered saline (DPBS, Thermo Fisher 14190) at 37°C and lysed with 0.075% saponin (Sigma S7900) in DPBS at 37°C. Parasites were washed once with DPBS, resuspended in 1 ml cytoplasmic lysis buffer (25 mM Tris–HCl pH 7.5, 10 mM NaCl, 1.5 mM MgCl2, 1% IGEPAL CA‐630, and 1× protease inhibitor cocktail [“PI”, Roche 11836170001]) at 4°C, and incubated on ice for 30 min. Cells were further homogenized with a chilled glass douncer, and the cytoplasmic lysate was cleared with centrifugation (13,500 g, 10 min, 4°C). The pellet (containing the nuclei) was resuspended in 100 μl nuclear extraction buffer (25 mM Tris–HCl pH 7.5, 600 mM NaCl, 1.5 mM MgCl2, 1% IGEPAL CA‐630, PI) at 4°C and sonicated for 10 cycles with 30 s (on/off) intervals (5 min total sonication time) in a Diagenode Pico Bioruptor at 4°C. This nuclear lysate was cleared with centrifugation (13,500 g, 10 min, 4°C). Protein samples were supplemented with NuPage Sample Buffer (Thermo Fisher NP0008) and NuPage Reducing Agent (Thermo Fisher NP0004) and denatured for 10 min at 70°C. Proteins were separated on a 4–12% Bis‐Tris NuPage gel (Thermo Fisher NP0321) and transferred to a PVDF membrane. The membrane was blocked for 1 h with 1% milk in PBST (PBS, 0.1% Tween 20) at 25°C. HA‐tagged proteins and histone H3 were detected with anti‐HA (Abcam ab9110, 1:1,000 in 1% milk‐PBST) and anti‐H3 (Abcam ab1791, 1:1,000 in 1% milk‐PBST) primary antibodies, respectively, followed by donkey anti‐rabbit secondary antibody conjugated to horseradish peroxidase (“HRP”, Sigma GENA934, 1:5,000 in 1% milk‐PBST). Aldolase was detected with anti‐aldolase‐HRP (Abcam ab38905, 1:5,000 in 1% milk‐PBST). HRP signal was developed with SuperSignal West Pico chemiluminescent substrate (Thermo Fisher 34080) and imaged with a ChemiDoc XRS+ (Bio‐Rad).
dCas9 chromatin immunoprecipitation
Using a DynaMag magnet (Thermo Fisher 12321D), wash 25 μl Protein G Dynabeads (Invitrogen 10004D) twice with 1 ml ChIP dilution buffer (16.7 mM Tris–HCl pH 8, 150 mM NaCl, 1.2 mM EDTA pH 8, 1% Triton X‐100, 0.01% SDS).
Resuspend beads in 1 ml ChIP dilution buffer and add 1 μg of anti‐HA antibody (Abcam ab9110). Incubate on a rotator at 4°C for ˜ 6 h.
Cross‐link synchronized parasites (˜ 2 × 108 parasites) by adding methanol‐free formaldehyde (Thermo Fisher 28908) to the culture (final concentration 1%) and incubating with gentle agitation for 10 min at 25°C.
Quench the cross‐linking reaction by adding glycine (final concentration 125 mM) and incubating with gentle agitation for 5 min at 25°C.
Centrifuge at 974 g for 2 min at 4°C and remove supernatant.
Resuspend and wash cross‐linked infected red blood cells with 10 ml DPBS at 4°C.
Centrifuge at 974 g for 2 min at 4°C and remove supernatant.
Lyse infected red blood cells in 10 ml 0.15% saponin (Sigma S7900) in DPBS at 4°C for 5–10 min (until liquid becomes transparent).
Centrifuge at 3,220 g for 5 min at 4°C and remove supernatant.
Wash the parasites with 12 ml DPBS at 4°C.
Centrifuge at 3,220 g for 5 min at 4°C and remove supernatant.
Repeat steps 10–11.
If the supernatant is clear and not red, remove it and proceed to the next step or snap freeze and store at −80°C. If it is not clear, wash the pellet again with DPBS.
Resuspended parasites in 2 ml of lysis buffer (10 mM HEPES pH 8, 10 mM KCl, 0.1 mM EDTA pH 8, PI) at 4°C and incubate with gentle agitation at 4°C for 30 min.
Add IGEPAL CA‐630 to a final concentration of 0.25% and lyse cells with a prechilled dounce homogenizer (usually 200 strokes are sufficient for ring stage parasites).
Transfer extracts to microcentrifuge tubes and centrifuge for 10 min at 13,500 g at 4°C.
Remove supernatant and resuspend the pellet in 300 μl SDS lysis buffer (50 mM Tris–HCl pH 8, 10 mM EDTA pH 8, 1% SDS, PI) at 4°C.
Transfer liquid to a 1.5 ml sonication tube (Diagenode C30010016) and sonicate for 12 min total (24 cycles of 30 s on/off) in a Diagenode Pico Bioruptor at 4°C. After sonication, run a small amount of sample on a DNA gel or an Agilent 2100 Bioanalyzer to make sure the average DNA fragment size is 250 bp.
Centrifuge sonicated extracts for 10 min at 13,500 g at 4°C.
Using the DynaMag, wash antibody‐conjugated Dynabeads twice with 1 ml ChIP dilution buffer and resuspend in 100 μl of ChIP dilution buffer at 4°C.
Dilute 120 μl of supernatant 1:10 in ChIP dilution buffer at 4°C.
Add 30 μl of supernatant to 170 μl of elution buffer (50 mM Tris–HCl pH 8, 10 mM EDTA pH 8, 1% SDS) and keep as “Input” at −20°C.
Add washed, antibody‐conjugated Dynabeads to the diluted chromatin sample and incubate overnight with rotation at 4°C.
-
Collect beads on a DynaMag, remove supernatant, and wash for 5 min with gentle rotation with 1 ml of the following buffers, sequentially:
Low salt wash buffer (20 mM Tris–HCl pH 8, 150 mM NaCl, 2 mM EDTA pH 8, 1% Triton X‐100, 0.1% SDS) at 4°C.
High salt wash buffer (20 mM Tris–HCl pH 8, 500 mM NaCl, 2 mM EDTA pH 8, 1% Triton X‐100, 0.1% SDS) at 4°C.
LiCl wash buffer (10 mM Tris–HCl pH 8, 250 mM LiCl, 1 mM EDTA pH 8, 0.5% IGEPAL CA‐630, 0.5% sodium deoxycholate) at 4°C.
TE wash buffer (10 mM Tris–HCl pH 8, 1 mM EDTA pH 8) at room temperature.
Collect beads on a DynaMag, remove supernatant, and resuspend the beads in 205 μl of elution buffer.
Incubate the beads for 30 min at 65°C with agitation.
Collect beads on a DynaMag and transfer 200 μl of eluate to a different tube. This is the “ChIP” sample.
De‐cross‐link input and ChIP samples for ˜ 10 h at 65°C.
Add 200 μl of TE buffer to each sample.
Add 8 μl RNaseA (Thermo Fisher EN0531) to each sample (final concentration of 0.2 mg/ml) and incubate for 2 h at 37°C.
Add 4 μl Proteinase K (New England Biolabs P8107S) to each sample (final concentration of 0.2 mg/ml) and incubate for 2 h at 55°C.
Add 400 μl phenol:chloroform:isoamyl alcohol (25:24:1) to each sample, vortex, and separate phases by centrifugation for 10 min at 13,500 g at 4°C.
Keep the top (aqueous) layer, add 30 μg glycogen (Thermo Fisher 10814) and NaCl (200 mM final concentration), and mix.
Add 800 μl 100% EtOH at 4°C to each sample and incubate at −20°C for 30 min.
Pellet DNA by centrifugation for 10 min at 13,500 g at 4°C.
Wash pellet with 500 μl 80% EtOH at 4°C and centrifuge for 5 min at 13,500 g at 4°C.
Remove the EtOH and dry pellet at 25°C.
Resuspend dried pellet in 30 μl 10 mM Tris–HCl, pH 8.
dCas9 chromatin immunoprecipitation sequencing and analysis
Libraries for sequencing were made with the MicroPlex library preparation kit (Diagenode C05010014). Libraries were sequenced on the NextSeq 500 platform (Illumina). Sequenced reads (150 bp paired end) were mapped to the P. falciparum genome (Gardner et al, 2002) (plasmoDB.org, version 3, release 29) using “bwa mem” (Li & Durbin, 2009) allowing a read to align only once to the reference genome (option “–c 1”). Alignments were subsequently filtered for duplicates and a mapping quality ≥ 20 using samtools (Li et al, 2009). For ChIP‐seq coverage plots, deeptool's bamCompare (Ramírez et al, 2016) was used to normalize the read coverage per genome position (option “–bs 1”) in the respective input and ChIP samples to the total number of mapped reads in each library (option “–normalizeUsingRPKM”). Normalized input coverage per bin was subtracted from the ChIP values (option “–ratio subtract”), and coverage plots were visualized in the Integrative Genomics Viewer (Robinson et al, 2011). For genome‐wide coverage plots, the same approach was used as above but with a bin size of 1,000 nt. Background levels of unspecific dCas9 binding were further removed by subtracting normalized coverage of the non‐targeted dCas9 control sample from the normalized promoter or intron coverages and visualized using circos (Krzywinski et al, 2009).
Significant peaks of dCas9 binding sites were identified using macs2 (version 2.2.6) (Zhang et al, 2008) “callpeak” using default settings and options “–nomodel”, “–extsize150” and “–B” set. A Benjamini–Hochberg‐adjusted P‐value (i.e., q‐value) was calculated for each peak. To compare the ChIP enrichment of dCas9 in the var gene intron‐ or promoter‐targeted strains to the non‐targeted control strain, a likelihood ratio of enrichment comparing promoter/control (Table EV1) and intron/control (Table EV2) was calculated using the output pileup files from “callpeak” and the macs2 command “bdgdiff” with default settings. One replicate was performed per strain.
dCas9 RNA immunoprecipitation sequencing and analysis
dCas9 RIP‐seq was performed with the same protocol for dCas9 ChIP‐seq (above) with the following modifications. RNasin RNase inhibitor (Promega N2511) was added to all lysis and wash buffers. A control immunoprecipitation was performed using 1 μg of rabbit IgG (Diagenode C15410206) conjugated to 25 μl of Protein G Dynabeads (Invitrogen 10004D). After bead incubation, samples were washed and eluted as above and then incubated for 30 min at 70°C to reverse cross‐links. One microliter of DNase Turbo (Thermo Fisher AM2238) was added, and samples were incubated for 20 min at 37°C. Six hundred microliters of Trizol LS (Thermo Fisher 10296010) were mixed with the sample, followed by 160 μl chloroform. Samples were vortexed and phases were separated by centrifugation for 10 min at 13,500 g at 4°C. The aqueous (top) layer was then purified with the RNeasy MinElute Cleanup Kit (Qiagen 74204). RNA libraries were prepared with the TruSeq stranded mRNA LT library prep kit (Illumina 20020594) without mRNA selection, ribosomal RNA depletion, or fragmentation. Libraries were sequenced, and data were treated as above for ChIP. One replicate was performed for anti‐HA and IgG immunoprecipitations in non‐clonal bulk cultures at 12 hpi of non‐targeted dCas9 and var promoter‐targeted dCas9 strains.
For dCas9 RIP‐seq analysis of the var promoter‐targeted dCas9, read coverage in the anti‐HA and IgG samples was calculated using deepTools “bamCompare” (Ramírez et al, 2016) in 10 nt windows (option “–bs 10”) and normalized to the total number of mapped reads in each library (option “–normalizeUsingRPKM”). The normalized coverage in each bin of the IgG sample was then subtracted from the anti‐HA sample (option “–ratio subtract”). Promoter/control fold enrichment of each IgG‐normalized 10 nt window was calculated using macs2 “bdgcmp” (Zhang et al, 2008). For genome‐wide coverage plots, the fold enrichment as calculated by macs2 “bdgcmp” was averaged over the gene body and visualized using circos (Krzywinski et al, 2009).
Stranded RNA sequencing and analysis
Infected RBCs containing synchronized (14 hpi ± 3 h) parasites were lysed in 0.075% saponin (Sigma S7900) in DPBS at 37°C. The parasite cell pellet was washed once with DPBS and then resuspended in 700 μl QIAzol reagent (Qiagen 79306). Total RNA was extracted using the miRNeasy Mini Kit (Qiagen 217004), including an on‐column DNase I digestion according to the manufacturer's protocol. RNA samples were depleted of rRNA with the Dynabeads mRNA DIRECT Kit (Thermo Fisher 61012), and libraries were prepared with the TruSeq Stranded mRNA LT Sample Prep Kit (Illumina 20020594). Libraries were sequenced on the NextSeq 500 platform (Illumina). Sequenced reads (150 bp paired end) were mapped, aligned, and visualized as described above for ChIP‐seq.
Immunoprecipitation of dCas9 for mass spectrometry analysis
Wash and conjugate beads as described above for ChIP‐seq. Ten milligrams of Protein G Dynabeads (Invitrogen 10004D) conjugated to 10 μg of anti‐HA antibody (Abcam ab9110) are needed per immunoprecipitation.
Cross‐link and perform saponin lysis with synchronized parasites (14 hpi ± 3 h) as described above for ChIP‐seq. 3 × 109 cross‐linked parasites were collected for each immunoprecipitation, with four replicates per strain (non‐targeted, promoter‐targeted, or intron‐targeted dCas9).
Resuspend pellet (3 × 109 parasites) in 10 ml of lysis buffer (10 mM Tris–HCl pH 7.5, 1 mM EDTA, 0.5% IGEPAL CA‐630, PI) at 4°C and incubate with rotation for 30 min at 4°C.
Centrifuge extracts for 8 min at 380 g at 4°C and remove the supernatant.
Resuspend the pellet in 10 ml nuclear lysis buffer (10 mM Tris–HCl pH 7.5, 500 mM NaCl, 1 mM EDTA, 1% IGEPAL CA‐630, 0.5% sodium deoxycholate, PI) at 4°C and incubate for 10 min at 4°C, vortexing every ˜3 min.
Centrifuge lysates for 8 min at 380 g at 4°C and remove supernatant.
Resuspend the pellet in 1.8 ml chromatin shearing buffer (10 mM Tris–HCl pH 7.5, 150 mM NaCl, 1 mM EDTA, 0.1% sodium deoxycholate, 0.1% SDS, PI) at 4°C and transfer to 1.5 ml sonication tubes (300 μl per tube, Diagenode C30010016).
Sonicate for 12 min total (24 cycles of 30 s on/off) in a Diagenode Pico Bioruptor at 4°C.
Centrifuge lysate for 10 min at 13,500 g at 4°C and keep supernatant.
Dilute all supernatant of the chromatin lysate 1:10 in wash buffer (20 mM Tris–HCl pH 7.5, 150 mM NaCl, 1 mM EDTA, 0.1% IGEPAL CA‐630, 10% glycerol, PI) at 4°C.
Add antibody‐conjugated beads to the diluted chromatin sample and incubate overnight with rotation at 4°C.
For each immunoprecipitation, use three microcentrifuge tubes to collect the beads from the total volume, 1 ml at a time using the DynaMag. For example, add 1 ml to the tube on the magnet, collect the beads, remove supernatant, and repeat.
Wash the beads in each tube 3 × 1 ml wash buffer for 5 min with rotation at 4°C.
Wash the beads in each tube with 1 ml TE buffer for 5 min with rotation at 4°C.
Collect beads on the DynaMag and wash three times with 100 μl 25 mM ammonium bicarbonate (NH4HCO3) without removing the beads from the magnet.
Remove tube from the magnet and resuspend beads in 100 μl 25 mM NH4HCO3.
Perform on‐bead digestion for 1 h with 0.6 μg of trypsin/LysC (Promega V5071).
Load samples onto homemade C18 StageTips for desalting and elute peptides using 40/60 MeCN/H2O + 0.1% formic acid.
Vacuum concentrate samples to dryness.
Perform online chromatography with an RSLCnano system (Thermo Fisher, Ultimate 3000) coupled online to a Q Exactive HF‐X with a Nanospray Flex ion source (Thermo Fisher). Peptides were first trapped on a C18 column (75 μm inner diameter × 2 cm; nanoViper Acclaim PepMap™ 100, Thermo Fisher 164942) with buffer A (2/98 MeCN/H2O in 0.1% formic acid) at a flow rate of 2.5 μl/min over 4 min. Separation was then performed on a 50 cm × 75 μm C18 column (nanoViper Acclaim PepMap™ RSLC, 2 μm, 100 Å, Thermo Scientific 164535) regulated to a temperature of 50°C with a linear gradient of 2–30% buffer B (100% MeCN in 0.1% formic acid) at a flow rate of 300 nl/min over 91 min.
Perform MS full scans in an ultrahigh‐field Orbitrap mass analyzer in ranges m/z 375–1,500 with a resolution of 120,000 at m/z 200. The top 20 intense ions were subjected to Orbitrap for further fragmentation via high energy collision dissociation (HCD) activation and a resolution of 15,000 with the intensity threshold kept at 1.3 × 105. Ions with charge state from 2+ to 6+ were selected for screening. Normalized collision energy (NCE) was set at 27 with a dynamic exclusion of 40 s.
-
Perform analysis.
For identification, the data were searched against the P. falciparum FASTA database (PlasmoDB‐36 Pfaciparum3D7 AnnotatedProtein containing Cas9 and the common contaminants) using Sequest HF through proteome discoverer (Thermo Fisher version 2.2). Enzyme specificity was set to trypsin, and a maximum of two missed cleavage sites were allowed. Oxidized methionine and N‐terminal acetylation were set as variable modifications. Maximum allowed mass deviation was set to 10 ppm for monoisotopic precursor ions and 0.02 Da for MS/MS peaks.
The resulting files were further processed using myProMS (version 3.9) (Poullet et al, 2007). For identification, FDR was computed using Percolator (Spivak et al, 2009) and was set to 1% at the peptide‐level for the whole study. The label‐free quantification was performed by peptide Extracted Ion Chromatograms (XICs) computed with MassChroQ version 2.2 (Valot et al, 2011). For protein quantification, XICs from proteotypic peptides shared between compared conditions (TopN matching) with two missed cleavages were used. Median and scale normalization was applied on the total signal to correct the XICs for each replicate (n = 4). Two replicates of the promoter‐targeted dCas9 sample were excluded from the analysis due to significantly lower amounts of dCas9 and total protein detected, which would lead to a biased analysis (Fig EV3A and Table EV4).
To estimate the significance of the change in protein abundance, a linear model (adjusted on peptides and replicates) based on two‐tailed t‐test was performed and P‐values were adjusted using Benjamini–Hochberg FDR procedure. Proteins with at least 1.5‐fold enrichment, P‐value ≤ 0.05, and at least three total peptides in all replicates were considered significantly enriched in sample comparisons.
ISWI immunoprecipitation and mass spectrometry
An ISWI‐3HA‐ribo clone (n = 3 technical replicates) and wild‐type culture (as a negative control) were synchronized. At 36 hpi, each culture (1.5 × 109 parasites) was centrifuged and RBCs were lysed with six volumes of 0.15% saponin in DPBS for 5 min at 4°C. Parasites were centrifuged at 4,000 g for 5 min at 4°C, and the pellet was washed twice with DPBS at 4°C. Parasites were then cross‐linked with 1 ml 0.5 mM dithiobissuccinimidyl propionate (DSP; Thermo Fisher 22585) in DPBS for 60 min at 37°C (as in (Mesén‐Ramírez et al, 2016)). Cross‐linked parasites were washed once with DPBS and then lysed with 10 volumes of RIPA buffer (10 mM Tris–HCl pH 7.5, 150 mM NaCl, 0.1% SDS, 1% Triton) containing protease and phosphatase inhibitor cocktail (Thermo Fisher 78440) and 1 U/μl of Benzonase (Merck 71206). The lysates were cleared by centrifugation at 16,000 g for 10 min at 4°C. Supernatants were incubated with 25 μl of anti‐HA Dynabeads (Thermo Fisher 88836) overnight with rotation at 4°C. Beads were collected with a magnet and washed five times with 1 ml RIPA buffer, then five times with 1 ml DPBS, and then once with 1 ml 25 mM NH4HCO3 (Sigma 09830). The beads were reduced with 100 mM dithiothreitol (Sigma D9779), alkylated with 55 mM iodoacetamide (Sigma I1149), and subjected to on‐bead digestion using 1 μg of trypsin (Thermo Fisher 90059). The resulting peptides were desalted using C18 ziptips (Merck ZTC04S096) and sent for MS analysis.
Peptides were separated by reverse phase HPLC (Thermo Fisher Easy‐nLC1000) using an EASY‐Spray column, 50 cm × 75 μm ID, PepMap RSLC C18, 2 μm (Thermo Fisher ES803A) over a 90‐min gradient before nanoelectrospray using a Q Exactive HF‐X mass spectrometer (Thermo Fisher). The mass spectrometer was operated in a data‐dependent mode. The parameters for the full scan MS were as follows: resolution of 60,000 across 350–1,500 m/z, AGC 1e3 (as in (Kensche et al, 2016)), and maximum injection time (IT) 50 ms. The full MS scan was followed by MS/MS for the top 15 precursor ions in each cycle with an NCE of 28 and dynamic exclusion of 30 s and maximum IT of 300 ms. Raw mass spectral data files (.raw) were searched using Proteome Discoverer (Thermo Fisher) and Mascot (version 2.4.1) (Perkins et al, 1999). Mascot search parameters were as follows: 10 ppm mass tolerance for precursor ions; 0.8 Da for fragment ion mass tolerance; two missed cleavages of trypsin; fixed modification was carbamidomethylation of cysteine; and variable modifications were methionine oxidation, CAMthiopropanoyl on lysine or protein N‐terminal, and serine, threonine, and tyrosine phosphorylation. Only peptides with a Mascot score greater than or equal to 25 and an isolation interference less than or equal to 30 were included in the data analysis.
Phylogenetic analysis of SNF2 domain‐containing chromatin remodelers
SNF2 domains were extracted from the full protein sequences following the “SNF2 family N‐terminal domain” Pfam annotation (Pfam ID: PF00176) using bedtools getfasta (Quinlan & Hall, 2010). Multiple sequence alignments were performed using MAFFT with default settings. Gaps were removed with trimAl (Capella‐Gutiérrez et al, 2009), and the best phylogenetic model (i.e., Le Gascuel [LG]) was calculated using ProtTest3 (Darriba et al, 2011). A maximum likelihood phylogenetic tree was constructed using MEGA (v7) (Kumar et al, 2016) with the LG model and 1,000 bootstrap replicates. The bootstrap consensus trees were visualized in FigTree (v1.4.3, http://tree.bio.ed.ac.uk/software/figtree/).
Parasite growth assay
Parasite growth was measured as described previously (Vembar et al, 2015). Briefly, a clone of ISWI‐3HA‐ribo was tightly synchronized and diluted to 0.2% parasitemia (5% hematocrit) at ring stage using the blood of two different donors separately. Each culture was split, and glucosamine (Sigma G1514) was added (2.5 mM final concentration) to one half. The growth curve was performed in a 96‐well plate (200 μl culture per well) with three technical replicates per condition per blood. Parasitemia was measured every 24 h by staining parasite nuclei with SYBR Green I (Sigma S9430) and quantifying infected red blood cells with a Guava easyCyte flow cytometer (Merck Millipore).
Estimation of cell cycle progression
RNA‐seq‐based cell cycle progression was estimated in R by comparing the normalized expression values (i.e., RPKM, reads per kilobase per exon per one million mapped reads) of each sample to the microarray data from Bozdech et al (2003) (Data ref: Bozdech et al, 2003) using the statistical model as in Lemieux et al (2009).
Differential gene expression analysis
A clone of ISWI‐3HA‐ribo was synchronized and split into two cultures. Glucosamine (2.5 mM final concentration, Sigma G1514) was added to one culture at 36 hpi, and parasites were harvested 24 h later during ring stage (12 hpi). RNA sequencing reads for three technical replicates of the glucosamine‐treated and two technical replicates of the untreated ISWI‐3HA‐ribo clone were mapped to the P. falciparum genome and quality filtered as described above for ChIP‐seq and RNA‐seq. Strand‐specific gene counts were calculated using htseq‐count (Anders et al, 2015). Differential gene expression analysis was performed using DESeq2 (Love et al, 2014) with significantly differentially expressed genes featuring a Benjamini–Hochberg‐adjusted P‐value (i.e., q) ≤ 0.05. MA plots were generated using the “baseMean” (mean normalized read count over all replicates and conditions) and “log2FoldChange” values (glucosamine‐treated over control) as determined by DESeq2. RPKM values were calculated in R using the command rpkm() from the package edgeR (Robinson et al, 2010). Gene Ontology enrichments were calculated using the built‐in tool at PlasmoDB.org (Aurrecoechea et al, 2017).
ISWI and H3K9ac ChIP sequencing and analysis
Synchronous parasites (12 hpi ± 3) were treated as above for the dCas9 ChIP with minor differences. 5 × 108 parasites were cross‐linked and lysed in 4 ml ChIP lysis buffer. Chromatin was sonicated in 3.6 ml total ChIP SDS lysis buffer. For the ISWI‐HA ChIP, 8 μg DNA (chromatin) was diluted and incubated with 8 μg anti‐HA antibody (Abcam ab9110) conjugated to 200 μl Protein G Dynabeads (Invitrogen 10004D). For the H3K9ac ChIP, 2 μg DNA (chromatin) was diluted and incubated with 2 μg anti‐H3K9ac antibody (Millipore 07‐352) conjugated to 50 μl Protein G Dynabeads (Invitrogen 10004D).
Sequencing libraries were prepared, sequenced, and processed as with the dCas9 ChIP samples. Fold enrichment over input was calculated using deeptool's bamCompare (Ramírez et al, 2016) (option “–ratio ratio”) in windows of 1 nt (option “–bs 1”), and coverage plots were visualized in the Integrative Genomics Viewer (Robinson et al, 2011). Meta‐gene plots were visualized using deeptool's plotProfile over a region 1.5 kb upstream and downstream of the translation start and stop site, respectively, using the quality‐filtered bam files as input.
Author contributions
JMB, SB, and ASc conceptualized the project and conceived experiments. AC cloned the optimized pL7 plasmid. FD carried out the mass spectrometry experimental work for dCas9 enChIP, and DL supervised mass spectrometry and data analysis. SB performed DNA/RNA sequencing bioinformatic analysis. ASi performed the ISWI immunoprecipitation mass spectrometry and analysis. PRP, PCD, and ASc supervised and helped interpret analyses. JMB performed all other experiments and wrote the manuscript. All authors discussed and approved the manuscript.
Conflict of interest
The funders had no role in the study design, data collection and interpretation, or the decision to submit the work for publication. The authors declare that they have no conflict of interest.
Supporting information
Expanded View Figures PDF
Table EV1
Table EV2
Table EV3
Table EV4
Table EV5
Table EV6
Table EV7
Table EV8
Table EV9
Table EV10
Table EV11
Table EV12
Table EV13
Table EV14
Source Data for Expanded View
Review Process File
Source Data for Figure 1
Source Data for Figure 4
Acknowledgements
This work was supported by the European Research Council (grant PlasmoSilencing 670301 to A.Sc.); the Agence Nationale de la Recherche (grant ANR‐11‐LABEX‐0024‐01 ParaFrap to A.Sc.); the “Région Ile‐de‐France” (grants 2013‐2‐EML‐02‐ICR‐1 and 2014‐2‐INV‐04‐ICR‐1 to D.L.); the Fondation pour la Recherche Médicale (grant DGE20121125630 to D.L.); the Academic Research Fund (Tier 2) of the Ministry of Education, Singapore (MOE2018‐T2‐2‐131 to P.R.P.); and the Merlion Project (6.11.18 to P.R.P). Work in the laboratories of P.R.P. and P.C.D. was funded by the National Research Foundation of Singapore through the Singapore‐MIT Alliance for Research and Technology Antimicrobial Resistance Interdisciplinary Research Group. Proteomics work was performed in part in the Center for Environmental Health Sciences BioCore, which is supported by Center grant P30‐ES002109 from the National Institute of Environmental Health Sciences. J.M.B. was supported by a European Molecular Biology Organization long‐term postdoctoral fellowship (EMBO ALTF 180‐2015), the Institut Pasteur Roux‐Cantarini postdoctoral fellowship, and a ParaFrap fellowship. S.B. was supported by a European Molecular Biology Organization long‐term postdoctoral fellowship (EMBO ALTF 1444‐2016) and advanced fellowship (EMBO aALTF 632‐2018). A.Si. was supported by the Singapore‐MIT Alliance (SMA) Graduate Fellowship from the Ministry of Education of Singapore. The authors would like to acknowledge the use of mass spectrometer facilities at A*STAR Institute of Molecular and Cell Biology in the laboratory of Dr. Radoslaw Sabota with the aid of Dr. Wint Wint Phoo. The authors would like to thank Valentin Sabatet at the Institut Curie for his help with the interpretation of the dCas9 proteomics data.
Mol Syst Biol. (2020) 16: e9569
Data availability
The data sets generated in this study are available in the following databases:
ChIP‐seq data: NCBI BioProject accession #PRJNA529754 (http://ncbi.nlm.nih.gov/bioproject/PRJNA529754)
RNA‐seq data: NCBI BioProject accession #PRJNA529754 (http://ncbi.nlm.nih.gov/bioproject/PRJNA529754)
dCas9 Proteomics data: PRIDE (http://www.ebi.ac.uk/pride/archive/projects/PXD013131)
ISWI‐3HA Proteomics data: https://chorusproject.org/pages/dashboard.html#/projects/all/1645/experiments
References
- Amit‐Avraham I, Pozner G, Eshar S, Fastman Y, Kolevzon N, Yavin E, Dzikowski R (2015) Antisense long noncoding RNAs regulate var gene activation in the malaria parasite Plasmodium falciparum . Proc Natl Acad Sci USA 112: e982–e991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S, Pyl PT, Huber W (2015) HTSeq–a Python framework to work with high‐throughput sequencing data. Bioinformatics 31: 166–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari A, Tuteja R (2012) Genome wide comparative comprehensive analysis of Plasmodium falciparum MCM family with human host. Commun Integr Biol 5: 607–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravind L, Iyer LM, Wellems TE, Miller LH (2003) Plasmodium biology: genomic gleanings. Cell 115: 771–785 [DOI] [PubMed] [Google Scholar]
- Aurrecoechea C, Barreto A, Basenko EY, Brestelli J, Brunk BP, Cade S, Crouch K, Doherty R, Falke D, Fischer S et al (2017) EuPathDB: the eukaryotic pathogen genomics database resource. Nucleic Acids Res 45: D581–D591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ay F, Bunnik EM, Varoquaux N, Bol SM, Prudhomme J, Vert JP, Noble WS, Le Roch KG (2014) Three‐dimensional modeling of the P. falciparum genome during the erythrocytic cycle reveals a strong connection between genome architecture and gene expression. Genome Res 24: 974–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batsios P, Peter T, Baumann O, Stick R, Meyer I, Gräf R (2012) A lamin in lower eukaryotes. Nucleus 3: 237–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertschi NL, Toenhake CG, Zou A, Niederwieser I, Henderson R, Moes S, Jenoe P, Parkinson J, Bartfai R, Voss TS (2017) Malaria parasites possess a telomere repeat‐binding protein that shares ancestry with transcription factor IIIA. Nat Microbiol 2: 17033 [DOI] [PubMed] [Google Scholar]
- Bopp SE, Manary MJ, Bright AT, Johnston GL, Dharia NV, Luna FL, McCormack S, Plouffe D, McNamara CW, Walker JR et al (2013) Mitotic evolution of Plasmodium falciparum shows a stable core genome but recombination in antigen families. PLoS Genet 9: e1003293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozdech Z, Llinás M, Pulliam BL, Wong ED, Zhu J, DeRisi JL (2003). https://figshare.com/articles/The_Transcriptome_of_the_Intraerythrocytic_Developmental_Cycle_of_Plasmodium_falciparum_/153399 [DATASET] [DOI] [PMC free article] [PubMed]
- Bozdech Z, Llinás M, Pulliam BL, Wong ED, Zhu J, DeRisi JL (2003) The transcriptome of the intraerythrocytic developmental cycle of Plasmodium falciparum . PLoS Biol 1: E5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee PM, Meisenberg C, Downs JA (2015) The SWI/SNF chromatin remodelling complex: its role in maintaining genome stability and preventing tumourigenesis. DNA Repair (Amst) 32: 127–133 [DOI] [PubMed] [Google Scholar]
- Bryant JM, Regnault C, Scheidig‐Benatar C, Baumgarten S, Guizetti J, Scherf A (2017) CRISPR/Cas9 genome editing reveals that the intron is not essential for var2csa gene activation or silencing in Plasmodium falciparum . mBio 8: e00729‐17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood MS, Gannoun‐Zaki L, Wellems TE, Deitsch KW (2003) Plasmodium falciparum var genes are regulated by two regions with separate promoters, one upstream of the coding region and a second within the intron. J Biol Chem 278: 34125–34132 [DOI] [PubMed] [Google Scholar]
- Capella‐Gutiérrez S, Silla‐Martínez JM, Gabaldón T (2009) trimAl: a tool for automated alignment trimming in large‐scale phylogenetic analyses. Bioinformatics 25: 1972–1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chêne A, Vembar SS, Riviere L, Lopez‐Rubio JJ, Claes A, Siegel TN, Sakamoto H, Scheidig‐Benatar C, Hernandez‐Rivas R, Scherf A (2012) PfAlbas constitute a new eukaryotic DNA/RNA‐binding protein family in malaria parasites. Nucleic Acids Res 40: 3066–3077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claessens A, Hamilton WL, Kekre M, Otto TD, Faizullabhoy A, Rayner JC, Kwiatkowski D (2014) Generation of antigenic diversity in Plasmodium falciparum by structured rearrangement of Var genes during mitosis. PLoS Genet 10: e1004812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claessens A, Harris LM, Stanojcic S, Chappell L, Stanton A, Kuk N, Veneziano‐Broccia P, Sterkers Y, Rayner JC, Merrick CJ (2018) RecQ helicases in the malaria parasite Plasmodium falciparum affect genome stability, gene expression patterns and DNA replication dynamics. PLoS Genet 14: e1007490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman BI, Skillman KM, Jiang RHY, Childs LM, Altenhofen LM, Ganter M, Leung Y, Goldowitz I, Kafsack BFC, Marti M et al (2014) A Plasmodium falciparum histone deacetylase regulates antigenic variation and gametocyte conversion. Cell Host Microbe 16: 177–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortés A, Deitsch KW (2017) Malaria epigenetics. Cold Spring Harb Perspect Med 7: a025528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson RM, Hall N, Ouzounis CA (2004) Comparative genomics of transcriptional control in the human malaria parasite Plasmodium falciparum . Genome Res 14: 1548–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang Y, Jia G, Choi J, Ma H, Anaya E, Ye C, Shankar P, Wu H (2015) Optimizing sgRNA structure to improve CRISPR‐Cas9 knockout efficiency. Genome Biol 16: 280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darriba D, Taboada GL, Doallo R, Posada D (2011) ProtTest 3: fast selection of best‐fit models of protein evolution. Bioinformatics 27: 1164–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Déjardin J, Kingston RE (2009) Purification of proteins associated with specific genomic loci. Cell 136: 175–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duraisingh MT, Voss TS, Marty AJ, Duffy MF, Good RT, Thompson JK, Freitas‐Junior LH, Scherf A, Crabb BS, Cowman AF (2005) Heterochromatin silencing and locus repositioning linked to regulation of virulence genes in Plasmodium falciparum . Cell 121: 13–24 [DOI] [PubMed] [Google Scholar]
- Dzikowski R, Frank M, Deitsch K (2006) Mutually exclusive expression of virulence genes by malaria parasites is regulated independently of antigen production. PLoS Pathog 2: e22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzikowski R, Li F, Amulic B, Eisberg A, Frank M, Patel S, Wellems TE, Deitsch KW (2007) Mechanisms underlying mutually exclusive expression of virulence genes by malaria parasites. EMBO Rep 8: 959–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epp C, Li F, Howitt CA, Chookajorn T, Deitsch KW (2009) Chromatin associated sense and antisense noncoding RNAs are transcribed from the var gene family of virulence genes of the malaria parasite Plasmodium falciparum . RNA 15: 116–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdel F, Rippe K (2011) Chromatin remodelling in mammalian cells by ISWI‐type complexes–where, when and why. FEBS J 278: 3608–3618 [DOI] [PubMed] [Google Scholar]
- Farhat DC, Swale C, Dard C, Cannella D, Ortet P, Barakat M, Sindikubwabo F, Belmudes L, De Bock PJ, Couté Y et al (2020) A MORC‐driven transcriptional switch controls Toxoplasma developmental trajectories and sexual commitment. Nat Microbiol 5: 570–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flueck C, Bartfai R, Volz J, Niederwieser I, Salcedo‐Amaya AM, Alako BT, Ehlgen F, Ralph SA, Cowman AF, Bozdech Z et al (2009) Plasmodium falciparum heterochromatin protein 1 marks genomic loci linked to phenotypic variation of exported virulence factors. PLoS Pathog 5: e1000569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flueck C, Bartfai R, Niederwieser I, Witmer K, Alako BT, Moes S, Bozdech Z, Jenoe P, Stunnenberg HG, Voss TS (2010) A major role for the Plasmodium falciparum ApiAP2 protein PfSIP2 in chromosome end biology. PLoS Pathog 6: e1000784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank M, Dzikowski R, Costantini D, Amulic B, Berdougo E, Deitsch K (2006) Strict pairing of var promoters and introns is required for var gene silencing in the malaria parasite Plasmodium falciparum . J Biol Chem 281: 9942–9952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraschka SA, Filarsky M, Hoo R, Niederwieser I, Yam XY, Brancucci NMB, Mohring F, Mushunje AT, Huang X, Christensen PR et al (2018) Comparative heterochromatin profiling reveals conserved and unique epigenome signatures linked to adaptation and development of malaria parasites. Cell Host Microbe 23: 407–420.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas‐Junior LH, Hernandez‐Rivas R, Ralph SA, Montiel‐Condado D, Ruvalcaba‐Salazar OK, Rojas‐Meza AP, Mancio‐Silva L, Leal‐Silvestre RJ, Gontijo AM, Shorte S et al (2005) Telomeric heterochromatin propagation and histone acetylation control mutually exclusive expression of antigenic variation genes in malaria parasites. Cell 121: 25–36 [DOI] [PubMed] [Google Scholar]
- Fujita T, Asano Y, Ohtsuka J, Takada Y, Saito K, Ohki R, Fujii H (2013) Identification of telomere‐associated molecules by engineered DNA‐binding molecule‐mediated chromatin immunoprecipitation (enChIP). Sci Rep 3: 3171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T, Fujii H (2013) Efficient isolation of specific genomic regions and identification of associated proteins by engineered DNA‐binding molecule‐mediated chromatin immunoprecipitation (enChIP) using CRISPR. Biochem Biophys Res Commun 439: 132–136 [DOI] [PubMed] [Google Scholar]
- Gannoun‐Zaki L, Jost A, Mu J, Deitsch KW, Wellems TE (2005) A silenced Plasmodium falciparum var promoter can be activated in vivo through spontaneous deletion of a silencing element in the intron. Eukaryot Cell 4: 490–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner MJ, Hall N, Fung E, White O, Berriman M, Hyman RW, Carlton JM, Pain A, Nelson KE, Bowman S et al (2002) Genome sequence of the human malaria parasite Plasmodium falciparum . Nature 419: 498–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghorbal M, Gorman M, Macpherson CR, Martins RM, Scherf A, Lopez‐Rubio JJ (2014) Genome editing in the human malaria parasite Plasmodium falciparum using the CRISPR‐Cas9 system. Nat Biotechnol 32: 819–821 [DOI] [PubMed] [Google Scholar]
- Goyal M, Alam A, Iqbal MS, Dey S, Bindu S, Pal C, Banerjee A, Chakrabarti S, Bandyopadhyay U (2012) Identification and molecular characterization of an Alba‐family protein from human malaria parasite Plasmodium falciparum . Nucleic Acids Res 40: 1174–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guizetti J, Scherf A (2013) Silence, activate, poise and switch! Mechanisms of antigenic variation in Plasmodium falciparum . Cell Microbiol 15: 718–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heger P, Marin B, Bartkuhn M, Schierenberg E, Wiehe T (2012) The chromatin insulator CTCF and the emergence of metazoan diversity. Proc Natl Acad Sci USA 109: 17507–17512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillier C, Pardo M, Yu L, Bushell E, Sanderson T, Metcalf T, Herd C, Anar B, Rayner JC, Billker O et al (2019) Landscape of the Plasmodium interactome reveals both conserved and species‐specific functionality. Cell Rep 28: 1635–1647.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeijmakers WA, Salcedo‐Amaya AM, Smits AH, Françoijs KJ, Treeck M, Gilberger TW, Stunnenberg HG, Bártfai R (2013) H2A.Z/H2B.Z double‐variant nucleosomes inhabit the AT‐rich promoter regions of the Plasmodium falciparum genome. Mol Microbiol 87: 1061–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer LM, Anantharaman V, Wolf MY, Aravind L (2008) Comparative genomics of transcription factors and chromatin proteins in parasitic protists and other eukaryotes. Int J Parasitol 38: 1–31 [DOI] [PubMed] [Google Scholar]
- Jiang L, Mu J, Zhang Q, Ni T, Srinivasan P, Rayavara K, Yang W, Turner L, Lavstsen T, Theander TG et al (2013) PfSETvs methylation of histone H3K36 represses virulence genes in Plasmodium falciparum . Nature 499: 223–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensche PR, Hoeijmakers WA, Toenhake CG, Bras M, Chappell L, Berriman M, Bártfai R (2016) The nucleosome landscape of Plasmodium falciparum reveals chromatin architecture and dynamics of regulatory sequences. Nucleic Acids Res 44: 2110–2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Yen L, Wongpalee SP, Kirshner JA, Mehta N, Xue Y, Johnston JB, Burlingame AL, Kim JK, Loparo JJ et al (2019) The gene‐silencing protein MORC‐1 topologically entraps DNA and forms multimeric assemblies to cause DNA compaction. Mol Cell 75: 700–710.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A, Kang HG, Steinbrenner J, Dempsey DA, Klessig DF, Kogel KH (2017) MORC proteins: novel players in plant and animal health. Front Plant Sci 8: 1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koole W, van Schendel R, Karambelas AE, van Heteren JT, Okihara KL, Tijsterman M (2014) A Polymerase Theta‐dependent repair pathway suppresses extensive genomic instability at endogenous G4 DNA sites. Nat Commun 5: 3216 [DOI] [PubMed] [Google Scholar]
- Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, Jones SJ, Marra MA (2009) Circos: an information aesthetic for comparative genomics. Genome Res 19: 1639–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33: 1870–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel TA, Erie DA (2005) DNA mismatch repair. Annu Rev Biochem 74: 681–710 [DOI] [PubMed] [Google Scholar]
- Lavstsen T, Salanti A, Jensen AT, Arnot DE, Theander TG (2003) Sub‐grouping of Plasmodium falciparum 3D7 var genes based on sequence analysis of coding and non‐coding regions. Malar J 2: 27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux JE, Gomez‐Escobar N, Feller A, Carret C, Amambua‐Ngwa A, Pinches R, Day F, Kyes SA, Conway DJ, Holmes CC et al (2009) Statistical estimation of cell‐cycle progression and lineage commitment in Plasmodium falciparum reveals a homogeneous pattern of transcription in ex vivo culture. Proc Natl Acad Sci USA 106: 7559–7564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux JE, Kyes SA, Otto TD, Feller AI, Eastman RT, Pinches RA, Berriman M, Su XZ, Newbold CI (2013) Genome‐wide profiling of chromosome interactions in Plasmodium falciparum characterizes nuclear architecture and reconfigurations associated with antigenic variation. Mol Microbiol 90: 519–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Durbin R (2009) Fast and accurate short read alignment with Burrows‐Wheeler transform. Bioinformatics 25: 1754–1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, 1000 GPDPS (2009) The sequence alignment/map format and SAMtools. Bioinformatics 25: 2078–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Egervari G, Wang Y, Berger SL, Lu Z (2018) Regulation of chromatin and gene expression by metabolic enzymes and metabolites. Nat Rev Mol Cell Biol 19: 563–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Yin S, Sun M, Cheng X, Wei J, Gilbert N, Miao J, Cui L, Huang Z, Dai X et al (2019) DNA helicase RecQ1 regulates mutually exclusive expression of virulence genes in Plasmodium falciparum via heterochromatin alteration. Proc Natl Acad Sci USA 116: 3177–3182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhang Y, Chen Y, Li M, Zhou F, Li K, Cao H, Ni M, Liu Y, Gu Z et al (2017) In situ capture of chromatin interactions by biotinylated dCas9. Cell 170: 1028–1043.e19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez‐Rubio JJ, Gontijo AM, Nunes MC, Issar N, Hernandez‐Rivas R, Scherf A (2007) 5’ flanking region of var genes nucleate histone modification patterns linked to phenotypic inheritance of virulence traits in malaria parasites. Mol Microbiol 66: 1296–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez‐Rubio JJ, Mancio‐Silva L, Scherf A (2009) Genome‐wide analysis of heterochromatin associates clonally variant gene regulation with perinuclear repressive centers in malaria parasites. Cell Host Microbe 5: 179–190 [DOI] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA‐seq data with DESeq2. Genome Biol 15: 550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins RM, Macpherson CR, Claes A, Scheidig‐Benatar C, Sakamoto H, Yam XY, Preiser P, Goel S, Wahlgren M, Sismeiro O et al (2017) An ApiAP2 member regulates expression of clonally variant genes of the human malaria parasite Plasmodium falciparum . Sci Rep 7: 14042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews H, Duffy CW, Merrick CJ (2018) Checks and balances? DNA replication and the cell cycle in Plasmodium . Parasit Vectors 11: 216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesén‐Ramírez P, Reinsch F, Blancke Soares A, Bergmann B, Ullrich AK, Tenzer S, Spielmann T (2016) Stable translocation intermediates jam global protein export in Plasmodium falciparum parasites and link the PTEX component EXP2 with translocation activity. PLoS Pathog 12: e1005618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller‐Planitz F, Klinker H, Becker PB (2013) Nucleosome sliding mechanisms: new twists in a looped history. Nat Struct Mol Biol 20: 1026–1032 [DOI] [PubMed] [Google Scholar]
- Myers SA, Wright J, Peckner R, Kalish BT, Zhang F, Carr SA (2018) Discovery of proteins associated with a predefined genomic locus via dCas9‐APEX‐mediated proximity labeling. Nat Methods 15: 437–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narlikar GJ, Sundaramoorthy R, Owen‐Hughes T (2013) Mechanisms and functions of ATP‐dependent chromatin‐remodeling enzymes. Cell 154: 490–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi E, Tsurimoto T (2017) Functions of multiple clamp and clamp‐loader complexes in eukaryotic DNA replication. Adv Exp Med Biol 1042: 135–162 [DOI] [PubMed] [Google Scholar]
- Pérez‐Toledo K, Rojas‐Meza AP, Mancio‐Silva L, Hernández‐Cuevas NA, Delgadillo DM, Vargas M, Martínez‐Calvillo S, Scherf A, Hernandez‐Rivas R (2009) Plasmodium falciparum heterochromatin protein 1 binds to tri‐methylated histone 3 lysine 9 and is linked to mutually exclusive expression of var genes. Nucleic Acids Res 37: 2596–2606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins DN, Pappin DJ, Creasy DM, Cottrell JS (1999) Probability‐based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 20: 3551–3567 [DOI] [PubMed] [Google Scholar]
- Petter M, Lee CC, Byrne TJ, Boysen KE, Volz J, Ralph SA, Cowman AF, Brown GV, Duffy MF (2011) Expression of P. falciparum var genes involves exchange of the histone variant H2A.Z at the promoter. PLoS Pathog 7: e1001292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petter M, Selvarajah SA, Lee CC, Chin WH, Gupta AP, Bozdech Z, Brown GV, Duffy MF (2013) H2A.Z and H2B.Z double‐variant nucleosomes define intergenic regions and dynamically occupy var gene promoters in the malaria parasite Plasmodium falciparum . Mol Microbiol 87: 1167–1182 [DOI] [PubMed] [Google Scholar]
- Poullet P, Carpentier S, Barillot E (2007) myProMS, a web server for management and validation of mass spectrometry‐based proteomic data. Proteomics 7: 2553–2556 [DOI] [PubMed] [Google Scholar]
- Pradhan A, Chauhan VS, Tuteja R (2005) Plasmodium falciparum DNA helicase 60 is a schizont stage specific, bipolar and dual helicase stimulated by PKC phosphorylation. Mol Biochem Parasitol 144: 133–141 [DOI] [PubMed] [Google Scholar]
- Prommana P, Uthaipibull C, Wongsombat C, Kamchonwongpaisan S, Yuthavong Y, Knuepfer E, Holder AA, Shaw PJ (2013) Inducible knockdown of Plasmodium gene expression using the glmS ribozyme. PLoS ONE 8: e73783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi LS, Larson MH, Gilbert LA, Doudna JA, Weissman JS, Arkin AP, Lim WA (2013) Repurposing CRISPR as an RNA‐guided platform for sequence‐specific control of gene expression. Cell 152: 1173–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan AR, Hall IM (2010) BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26: 841–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph SA, Scheidig‐Benatar C, Scherf A (2005) Antigenic variation in Plasmodium falciparum is associated with movement of var loci between subnuclear locations. Proc Natl Acad Sci USA 102: 5414–5419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez F, Ryan DP, Grüning B, Bhardwaj V, Kilpert F, Richter AS, Heyne S, Dündar F, Manke T (2016) deepTools2: a next generation web server for deep‐sequencing data analysis. Nucleic Acids Res 44: W160–W165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK (2010) edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26: 139–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JT, Thorvaldsdóttir H, Winckler W, Guttman M, Lander ES, Getz G, Mesirov JP (2011) Integrative genomics viewer. Nat Biotechnol 29: 24–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz JL, Tena JJ, Bancells C, Cortés A, Gómez‐Skarmeta JL, Gómez‐Díaz E (2018) Characterization of the accessible genome in the human malaria parasite Plasmodium falciparum . Nucleic Acids Res 46: 9414–9431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherf A, Lopez‐Rubio JJ, Riviere L (2008) Antigenic variation in Plasmodium falciparum . Annu Rev Microbiol 62: 445–470 [DOI] [PubMed] [Google Scholar]
- Siddiqui‐Jain A, Grand CL, Bearss DJ, Hurley LH (2002) Direct evidence for a G‐quadruplex in a promoter region and its targeting with a small molecule to repress c‐MYC transcription. Proc Natl Acad Sci USA 99: 11593–11598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra‐Miranda M, Vembar SS, Delgadillo DM, Ávila‐López PA, Herrera‐Solorio AM, Lozano Amado D, Vargas M, Hernandez‐Rivas R (2017) PfAP2Tel, harbouring a non‐canonical DNA‐binding AP2 domain, binds to Plasmodium falciparum telomeres. Cell Microbiol 19: e12742 [DOI] [PubMed] [Google Scholar]
- Smargiasso N, Gabelica V, Damblon C, Rosu F, De Pauw E, Teulade‐Fichou MP, Rowe JA, Claessens A (2009) Putative DNA G‐quadruplex formation within the promoters of Plasmodium falciparum var genes. BMC Genom 10: 362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivak M, Weston J, Bottou L, Käll L, Noble WS (2009) Improvements to the percolator algorithm for Peptide identification from shotgun proteomics data sets. J Proteome Res 8: 3737–3745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton A, Harris LM, Graham G, Merrick CJ (2016) Recombination events among virulence genes in malaria parasites are associated with G‐quadruplex‐forming DNA motifs. BMC Genom 17: 859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templeton TJ, Iyer LM, Anantharaman V, Enomoto S, Abrahante JE, Subramanian GM, Hoffman SL, Abrahamsen MS, Aravind L (2004) Comparative analysis of apicomplexa and genomic diversity in eukaryotes. Genome Res 14: 1686–1695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toenhake CG, Fraschka SA, Vijayabaskar MS, Westhead DR, van Heeringen SJ, Bártfai R (2018) Gene Expression Omnibus GSE104075, “Pf‐3D7-t15, ATACseq” (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE104075) [DATASET] [DOI] [PMC free article] [PubMed]
- Toenhake CG, Fraschka SA, Vijayabaskar MS, Westhead DR, van Heeringen SJ, Bártfai R (2018) Chromatin accessibility‐based characterization of the gene regulatory network underlying Plasmodium falciparum blood‐stage development. Cell Host Microbe 23: 557–569.e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonkin CJ, Carret CK, Duraisingh MT, Voss TS, Ralph SA, Hommel M, Duffy MF, Silva LM, Scherf A, Ivens A et al (2009) Sir2 paralogues cooperate to regulate virulence genes and antigenic variation in Plasmodium falciparum . PLoS Biol 7: e84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukaegbu UE, Kishore SP, Kwiatkowski DL, Pandarinath C, Dahan‐Pasternak N, Dzikowski R, Deitsch KW (2014) Recruitment of PfSET2 by RNA polymerase II to variant antigen encoding loci contributes to antigenic variation in P. falciparum . PLoS Pathog 10: e1003854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valot B, Langella O, Nano E, Zivy M (2011) MassChroQ: a versatile tool for mass spectrometry quantification. Proteomics 11: 3572–3577 [DOI] [PubMed] [Google Scholar]
- Vembar SS, Macpherson CR, Sismeiro O, Coppée JY, Scherf A (2015) The PfAlba1 RNA‐binding protein is an important regulator of translational timing in Plasmodium falciparum blood stages. Genome Biol 16: 212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk A, Crispino JD (2015) The role of the chromatin assembly complex (CAF‐1) and its p60 subunit (CHAF1b) in homeostasis and disease. Biochim Biophys Acta 1849: 979–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz JC, Bártfai R, Petter M, Langer C, Josling GA, Tsuboi T, Schwach F, Baum J, Rayner JC, Stunnenberg HG et al (2012) PfSET10, a Plasmodium falciparum methyltransferase, maintains the active var gene in a poised state during parasite division. Cell Host Microbe 11: 7–18 [DOI] [PubMed] [Google Scholar]
- Waldrip ZJ, Byrum SD, Storey AJ, Gao J, Byrd AK, Mackintosh SG, Wahls WP, Taverna SD, Raney KD, Tackett AJ (2014) A CRISPR‐based approach for proteomic analysis of a single genomic locus. Epigenetics 9: 1207–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walliker D, Quakyi IA, Wellems TE, McCutchan TF, Szarfman A, London WT, Corcoran LM, Burkot TR, Carter R (1987) Genetic analysis of the human malaria parasite Plasmodium falciparum . Science 236: 1661–1666 [DOI] [PubMed] [Google Scholar]
- Zanghì G, Vembar SS, Baumgarten S, Ding S, Guizetti J, Bryant JM, Mattei D, Jensen ATR, Rénia L, Goh YS et al (2018) A specific PfEMP1 is expressed in P. falciparum sporozoites and plays a role in hepatocyte infection. Cell Rep 22: 2951–2963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, Nusbaum C, Myers RM, Brown M, Li W et al (2008) Model‐based analysis of ChIP‐Seq (MACS). Genome Biol 9: R137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Huang Y, Zhang Y, Fang X, Claes A, Duchateau M, Namane A, Lopez‐Rubio JJ, Pan W, Scherf A (2011) A critical role of perinuclear filamentous actin in spatial repositioning and mutually exclusive expression of virulence genes in malaria parasites. Cell Host Microbe 10: 451–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Toenhake CG, Fraschka SA, Vijayabaskar MS, Westhead DR, van Heeringen SJ, Bártfai R (2018) Gene Expression Omnibus GSE104075, “Pf‐3D7-t15, ATACseq” (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE104075) [DATASET] [DOI] [PMC free article] [PubMed]
Supplementary Materials
Expanded View Figures PDF
Table EV1
Table EV2
Table EV3
Table EV4
Table EV5
Table EV6
Table EV7
Table EV8
Table EV9
Table EV10
Table EV11
Table EV12
Table EV13
Table EV14
Source Data for Expanded View
Review Process File
Source Data for Figure 1
Source Data for Figure 4
Data Availability Statement
The data sets generated in this study are available in the following databases:
ChIP‐seq data: NCBI BioProject accession #PRJNA529754 (http://ncbi.nlm.nih.gov/bioproject/PRJNA529754)
RNA‐seq data: NCBI BioProject accession #PRJNA529754 (http://ncbi.nlm.nih.gov/bioproject/PRJNA529754)
dCas9 Proteomics data: PRIDE (http://www.ebi.ac.uk/pride/archive/projects/PXD013131)
ISWI‐3HA Proteomics data: https://chorusproject.org/pages/dashboard.html#/projects/all/1645/experiments


