Abstract
Individuals infected with hepatitis B virus (HBV) are often coinfected with human immunodeficiency virus (HIV). However, individuals with chronic HBV infection living with acute HIV infection have a significantly lower HBV viral load, along with higher HBeAg and HBsAg loss than HBV-infected individuals alone. Here, we investigated the possible role of natural killer cells (NK cell) function in this progressive course to explore the relationship between phenotypic/functional changes in NK cells during acute HIV infection and HBV clearance in patients with HIV/HBV coinfection.
Peripheral blood NK cells from 38 patients with primary HIV infection, including 20 with untreated HIV infection and 18 treatment-naïve patients with HIV/HBV coinfection and 16 patients with chronic HBV infection, were enrolled in this study.
We found that the HIV/HBV-coinfected individuals had higher levels of NK cells than the HBV-infected individuals, due to expansion of the CD56neg NK cell population. The proportion of NK cells in CD56dim and CD56bri NK subsets was not found significant difference between HIV/HBV-coinfected and HBV-infected individuals. However, NKG2C levels on NK cells and subsets were significantly higher in HIV/HBV-coinfected individuals than in HBV-infected individuals, whereas NKG2A levels were unaffected or decreased. In addition, the levels of degranulation CD107a, cytotoxicity and IFN-γ production of NK cells were increased in HIV/HBV-coinfected individuals than in HBV-infected individuals. The level of IL-10 production of NK cells was decreased in HIV/HBV-coinfected individuals than in HBV-infected individuals. Furthermore, the level of HBV-DNA was inversely correlated with the proportion of NKG2C+ and NKG2C+NKG2A− NK cells, while positively correlated with the proportion of NKG2A+ and NKG2C-NKG2A+ NK cells. IFN-γ production was inversely correlated with levels of HBV-DNA, but the CD107a expression and IL-10 production of NK cells were not correlated with HBV-DNA levels.
These results demonstrate that the upregulation of NKG2C expression, but not of NKG2A expression on the surface of NK cells increases cytolytic capacity and the amounts of cytokines produced and may play a crucial role in HBV clearance during HIV/HBV-coinfection.
Keywords: hepatitis B virus, hepatitis B virus DNA, human immunodeficiency virus/hepatitis B virus coinfection, human immunodeficiency virus, natural killer cells, NKG2A/NKG2C
1. Introduction
Hepatitis B virus (HBV) infection is a leading cause of chronic hepatitis, liver fibrosis, cirrhosis, hepatocellular carcinoma and liver failure.[1,2] A recent report estimated that the global rate of HBV infection was 3.9% in 2016.[3] Since the introduction of antiviral drugs for the treatment of HBV infection, liver-related morbidity and mortality have been dramatically reduced. Antiviral drugs can completely abolish HBV replication, but cure or functional cure is only rarely achieved.
Both human immunodeficiency virus (HIV) and HBV are transmitted principally via the sexual and parenteral routes, and many patients infected with HIV are also infected with HBV.[4] About 2 to 4 million HIV-infected individuals worldwide are also positive for the hepatitis B surface antigen (HBsAg), corresponding to 5% to 20% of the population in various countries.[5] A meta-analysis exploring the progressive course of HBV infection in HIV-infected patients has indicated that chronic HIV infection contributes to the rapid development of end-stage liver disease, cirrhosis, hepatocellular carcinoma, and terminal liver failure.[6] The rates of hepatitis B e-antigen (HBeAg) and HBsAg seroclearance and seroconversion to anti-HBe and anti-HBs in untreated HIV/HBV-coinfected individuals are lower than those in treatment-naive chronic HBV-infected subjects.[7] However, data concerning the impact of acute HIV infection on individuals with chronic HBV infection are scarce. A previous study in our laboratory showed that HBV infection was more easily controlled in patients with acute HIV infection, due to higher rates of HBeAg and HBsAg loss and seroconversion to anti-HBe and anti-HBs, together with lower serum HBV DNA levels.[8] However, the mechanisms controlling HBV infection during acute HIV infection remain unknown.
Recent studies have highlighted the contribution of innate immune responses to HBV.[9] Natural killer (NK) cells, a key component of the innate immune system, play a crucial role in the containment and clearance of HBV.[10–14] It has also been suggested that Natural killer cells (NK cells) help to control HIV replication and limit viral spread.[15–18] Studies on HIV-exposed seronegative (HESN) subjects, such as men who have sex with men and infants born to HIV-infected mothers who remain uninfected despite long-term exposure to HIV, have shown an increase in NK cell effector capacity relative to seronegative individuals.[19,20]
NK cells are defined as the first barrier against various viral infections; they also take part in the regulation of adaptive immune responses.[21] NK cells are particularly abundant in the liver, accounting for 30% to 50% of liver lymphocytes and about 5% to 15% of peripheral blood mononuclear cells (PBMCs).[22] NK cells can be categorized into three main subsets on the basis of their expression of CD16 and CD56: CD56dimCD16+ (CD56dim), CD56briCD16−/+ (CD56bri) and CD56negCD16+ (CD56neg).[23] CD56bri NK cells are the main source of noncytolytic antiviral cytokines, such as interferon-γ (IFN-γ), transforming growth factor-β and interleukin-10 (IL-10), these cells make up the minority of peripheral blood NK cells. The majority of circulating NK cells that exert cytotoxic activity is restricted to the CD56dim subset. CD56neg NK cells are rarely detected in the peripheral blood of healthy individuals, while expanded in the presence of different viral infection including HIV and human cytomegalovirus (HCMV),[24] which display low cytotoxic activity and low production of IFN-γ.[25]
NK cell activation is tightly controlled by the balance between activation receptors and inhibitory receptors on the surface of NK cells, which determines whether or not non-cytolytic and cytotoxicity antiviral functions are exerted against infected target cells. In humans, latent HCMV infection is commonly widespread and is known to drive the increase of the specific NKG2C+ NK cells.[26] HIV was also responsible for the increase of NKG2C+ NK cells during HCMV infection.[27,28] Moreover, several studies showed that the increased NKG2C+ NK cells contributed to control of HIV viremia during primary infection, which displayed enhanced NK cell function.[17,28] It has been suggested that the decreased expression of inhibitory receptor NKG2A along with the increased level of activating receptor NKG2C correlated with enhanced NK cell function in response to viral infection such as HBV infection.[11,29,30] We therefore investigated NKG2A and NKG2C in this study. The NKG2A/CD94 heterodimers serve as an inhibitory receptor on NK cells. They bind to cell surface ligands such as HLA-E molecules in humans, and transmit inhibitory signals. NKG2C has a structure similar to that of NKG2A, but it transmits activation signals through the NKG2C/CD94 heterodimers binding to HLA-E molecules.[31,32] Several studies have reported that the frequency, phenotype and function of NK cells are modified during infection with HIV or HBV.[33] One previous study showed that strong NK cell function was related to the control of HBV after the cessation of nucleos(t)ide analog treatment in chronic hepatitis B (CHB)[34] and that there was a significant enhancement of NK cell during acute HIV-1 infection.[18] However, very little data are available for the estimation of NKG2C and NKG2A expression on NK cells, or to determine the role of NK cells in patients with acute HIV infection coinfected with HBV. We hypothesized that NK cell function was associated with virological events during acute HIV infection in individuals with CHB. In this study, we investigated the frequency, and function of NK cells, and the NKG2C and NKG2A expression of these cells in HIV/HBV-coinfected individuals, to explore the effects of such alterations on the mechanisms by which acute HIV infection controls HBV replication. These findings could be used in the development of immunotherapeutic strategies for improving viral control and clearance.
2. Methods
2.1. Study population
We conducted a cross-sectional study to investigate the effect of acute HIV infection on individuals with CHB. We recruited 38 individuals from the Primo cohort of men who have sex with men from Beijing Youan Hospital between 2011 and 2012. We defined four groups: individuals with primary HIV infection (PHI) (n = 38) including 20 treatment-naïve for HIV and 18 with HIV/HBV-coinfection, individuals with untreated CHB (n = 16) and healthy controls (HCs) negative for both HIV and HBV (n = 28). According to the definition proposed by Aurélia Henn and colleagues described,[35] PHI is defined as an interval of <12 weeks between HIV infection and the appearance of anti-HIV-antibodies following a negative test for anti-HIV-antibodies. We estimated the date of HIV infection as the time of the determinate HIV western blot bands minus 30 days or the mid-point between the positive and the negative anti-HIV-antibodies result according to the HIV-1 Fiebig laboratory stages for HIV-1. All of the HBV-infected individuals who were positive for HBeAg had been infected for at least one year and none received antiviral therapy before enrollment. All participants had been measured for CMV nucleic acids and anti-CMV IgG antibody. The exclusion criteria for all individuals were as follows:
-
(1)
positive for hepatitis C/hepatitis D virus antibody,
-
(2)
cirrhosis,
-
(3)
oncological diseases,
-
(4)
other autoimmune diseases.
The clinical characteristics of subjects are presented in Table 1. Cryopreserved PBMCs were collected at first HIV seroconversion point in treatment-naïve individuals with HIV or HIV/HBV coinfection. Serum alanine aminotransferase (ALT) levels and CD4+ T-cell counts were measured at erollment. Transaminase levels were considered high if >40 U/l.
Table 1.
Characteristics of all participants enrolled in this study.
2.2. Ethics statement
All the participants came from the Beijing Primo Clinical Cohort, which ran from 2011 to 2012. They provided written informed consent for participation in the study. This study and other related experiments were approved by the Beijing Youan Hospital Research Ethics Committee, and informed written consent was obtained in accordance with the Declaration of Helsinki. The study was carried out in accordance with approved guidelines and regulations.
2.3. Fluorescent antibodies
Monoclonal antibodies including Comp-BV510, anti-NKG2C-PE, anti-NKG2A-APC-Cy7, anti-CD3-PerCP, anti-CD16-FITC, anti-CD56-PE-Cy7 were obtained from BD Bioscience (San Jose, CA); anti-CD107a-APC, anti-IFN-γ-BV421, and anti-IL-10-APC were purchased from Biolegend (San Diego, CA). The isotype control mAbs were purchased from the corresponding companies.
2.4. Flow cytometry analysis
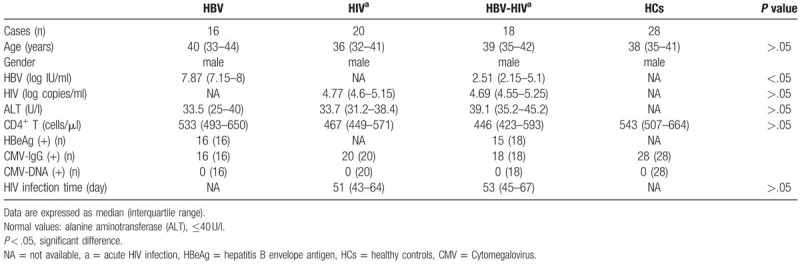
To determination of the frequency and function of different subsets of NK cells, cryopreserved PBMCs were thawed and 1 × 106 cells were mixed with fluorescent antibodies against CD3, CD56, CD16, NKG2A, and NKG2C and incubated at 4°C for 30 minutes in the dark. The cells were then washed three times with staining buffer and fixed in 1% paraformaldehyde (Sigma-Aldrich, USA). Thawed PBMCs (1 × 106) were resuspended in RPMI 1640 medium containing 10% fetal bovine serum (FBS), 50 IU penicillin, 2 mM L-glutamine and 50 μg/ml streptomycin. Brefeldin A (5 μg/ml; Sigma-Aldrich) and monensin (6 μg/ml [Golgi-Stop; BD Biosciences, Mississauga, ON, Canada]) were then added to the cells. They were then stimulated with phorbol 12-myristate 13-acetate (0.1 μg/ml) and ionomycin (5 μg/ml) for 6 hours at 37°C. PBMCs were stained for NK cell surface markers (CD3-PerCP, CD16-FITC, CD56-PE-Cy7, and CD107a-APC) for 30 min at 4°C in the dark. The cells were washed and stained with BV421-conjugated anti-IFN-γ antibody or APC-conjugated anti-IL-10 antibody for 30 minutes at 4°C, respectively. They were then fixed in 1% paraformaldehyde. NK cells were divided into three subsets: CD3−CD56neg, CD3−CD56dim, and CD3−CD56bri. The expression of NKG2A and/or NKG2C was analyzed on total NK cells and subsets (Fig. 1). Cytometer setup and tracking (CST) calibration particles were used to ensure that fluorescence intensity measurements were consistent in all experiments. Flow cytometry Comp-Beads kits (BD Bioscience, San Jose, CA) were used for compensation. Gating on forward scatter (FSC) and side scatter (SSC) light was used to exclude cell debris from the analysis; forward height and forward area were used to exclude doublet cells, and dead cells were excluded by staining with Live/Dead fixable viability stain 510 (BD Biosciences, San Jose, CA). Data was acquired by flow cytometry using BD FACS Canto II with Diva software (BD Biosciences, San Jose, CA) and analyzed with FlowJo 10.0.7 software (Tree Star Inc, Ashland, OR).
Figure 1.
Gating strategy to define cell populations. The lymphocytes were gated according to forward and side scatter dot plot (P1). Dead cells were excluded by staining with Live/Dead fixable viability stain 510 (P2). Single cells were gated according to forward height and side scatter forward area (P3). NK cells were defined from the CD3- gate (P4) on the basis of the expression of CD16 and CD56. NK cells were divided into 3 subsets: CD56briCD16−/+ (P5 and P6), CD56dimCD16+ (P7) and CD56negCD16+ (P8). NK cells and subsets were analyzed for surface expression of NKG2A and NKG2C (P9 and P10, respectively). Co-expression of NKG2A and NKG2C NK cells were indicated in P11 and P12 regions. NK = natural killer.
2.5. Viral load and other biochemical measurements
HIV-1 viral load tests were done by using an automated real-time PCR-based m2000 system (Abbott Molecular Inc, Des Plaines, IL) according to manufacturers’ instruction, and the sensitivity of detection was 40 copies/ml. Serological status for HBV (quantitative HBsAg, quantitative HBeAg, HBsAg/Anti-HBs and HBeAg/Anti-HBe) were determined by microparticle enzyme immunoassay (MEIA). The levels of serum ALT were detected by Biochemistry Automatic Analyzer (Roche Diagnostics, IN, USA).
2.6. CMV detection
Plasma HCMV-IgG of all subjects were detected by chemiluminescence immunoassay (LIAISONCMV IgG II, DiaSorin SpA, Saluggia, Italy). HCMV nucleic acids were measured by RT-PCR Kit (The Real-Q CMV DNA quantification kit, Liferiver, Shanghai, China).
2.7. NK cytotoxicity assay
To detect cytolytic killing in HIV/HBV-coinfected individuals and HBV-infected individuals, K562 target cells were labeled with carboxyfluorescein diacetate succinimidylester (CFSE-SE; Molecular Probes Inc, Eugene, OR). Cryopreserved PBMCs were thawed and NK cells were isolated based on the use of NK cell isolation kit (MACS Miltenyi Biotec Inc, CA, USA). NK effector cells were co-cultured with CFSE-labeled K562 target cells at (E:T) ratios of 10:1 for 6 hours. After 6 hours incubation, cells were stained with 7-aminoactinomycin D (7-AAD; BD Pharmingen, San Diego, CA) to detect lysed cells. Cytotoxicity against K562 cells was analyzed by flow cytometry using BD FACS Canto II with Diva software (BD Biosciences, San Jose, CA) and analyzed with FlowJo 10.0.7 software (Tree Star Inc., Ashland, OR).
2.8. Statistical analysis
Quantitative data were compared between study groups in nonparametric Mann–Whitney U or Wilcoxon signed-ranks tests, depending on the variable concerneds. P values for multiple comparisons were adjusted by “Bonferroni” method. Spearman rank correlation test was conducted to determine the correlation between two groups. P values of less than .05 (two-tailed test) were considered statistically significant. All data were analyzed with Prism version 6.0 (GraphPad software, CA, USA).
3. Results
3.1. Demographic characteristics and clinical features
As shown in Table 1, 16 individuals with chronic HBV infection (HBV-infected individuals), 20 acute HIV-infected individuals, 18 acute HIV-infected individuals coinfected with CHB (HIV/HBV-coinfected individuals) and 28 HCs were enrolled in the study. No significant differences were observed among the groups in terms of sex, age, ALT levels and CD4+ T cells. There was no difference in HIV viral load or HIV infection time between HIV-infected individuals and the HIV/HBV-coinfected individuals. The HBV load in HIV/HBV-coinfected individuals was lower than that in HBV-infected individuals (P = .001). HBV DNA levels were not available for 3 of the HIV/HBV-coinfected subjects. No significant differences on status of anti-HCMV IgG and nucleic acids of HCMV were found among the groups.
3.2. Differences in the frequency of circulating NK cells and NK subsets between groups
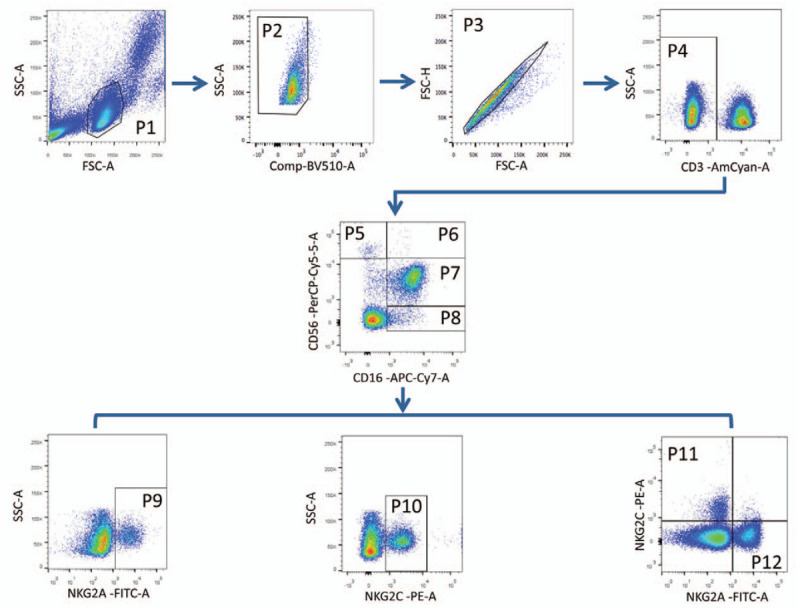
Flow cytometry analysis of the peripheral blood NK cells revealed an alteration in the frequency of NK cells in HIV/HBV-coinfected individuals. As shown in Figure 2A, the proportion of total NK cells was significantly elevated in these individuals compared to that in HBV-infected individuals and HCs (P = .01 and P < .001, respectively). In addition, the proportion of total NK cells in HIV-infected individuals was significantly higher than in HCs (P = .018).
Figure 2.
Alterations of proportion of circulating NK cells and subsets in study groups. (A) Proportion of total NK cells in PBMCs in four groups. (B–D) Proportion of CD56negCD16+, CD56dimCD16+, CD56briCD16−/+ in total NK cells in four groups, respectively. Mann–Whitney tests were used to compare groups, and P < .05 was considered statistically significant. Error bars indicate median and interquartile range. NK = natural killer.
Furthermore, we estimated the proportion of each NK cell subset for each group. The proportion of CD56neg NK cells was significantly higher in HIV/HBV-coinfected individuals than that in HBV-infected individuals and HCs (P = 0.004 and P < .001, respectively). The proportion of CD56neg NK cells in HIV-infected individuals displayed a similar trend as that in HIV/HBV-coinfected subjects (P = .001 and P < .001, respectively) (Fig. 2B). The frequency of CD56dim NK cells in HIV/HBV-coinfected and HIV-infected individuals was significantly lower than that in HCs (P = .01 and P = .016, respectively), while the difference among patients with HBV-infection, HIV-infection and HBV/HIV-coinfection was not significant (Fig. 2C). No significant difference in the proportion of CD56bri NK cells was observed among the four study groups (Fig. 2D).
3.3. NKG2A and NKG2C expression on the NK cells and subsets among the groups
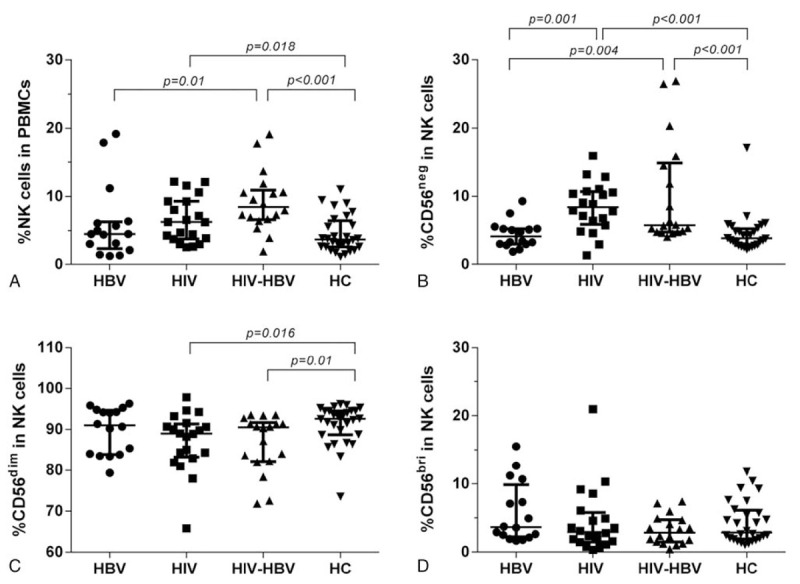
The activation of NK cells is tightly regulated through the integration of signals from activating receptors and inhibitory receptors on the surface of NK cells to exert antiviral functions. In this study, we investigated the expression of NKG2A and NKG2C to detect alterations in these receptors on the surface of NK cells. First, we investigated the expression of these receptors on NK cells from the PBMCs in each group. The results are shown in Figure 3. The expression of NKG2A on circulating NK cells was significantly elevated in HBV-infected individuals compared to HCs, HIV/HBV-coinfected and HIV-infected individuals (P = .048, P = 0.008 and P = .002, respectively) (Fig. 3A). The proportion of NKG2C+ NK cells was significantly higher in HIV/HBV-coinfected individuals or HIV-infected individuals than in HBV-infected individuals and HCs (P = .001 and P = .008; P < .001 and P = .005; Fig. 3B). The statistical analysis of the NKG2C/NKG2A ratio revealed a similar trend to the frequency of NKG2C+ NK cells among the groups (P < .001 and P = .013; P < .001 and P = .031; Fig. 3C).
Figure 3.
The proportion of NKG2A+ and NKG2C+ NK cells, as well as the ratio of NKG2C/NKG2A in total NK cells and subsets were compared among the four groups. (A–C) The expression of NGK2A and NGK2C, along with the ratio of NKG2C/NKG2A in total NK cells among the 4 groups, respectively. (D–F) The expression of NGK2A and NGK2C, along with the ratio of NKG2C/NKG2A in CD56negCD16+ NK cells among the four groups, respectively. (G-I) The expression of NGK2A and NGK2C, along with the ratio of NKG2C/NKG2A in CD56dimCD16+ NK cells among the 4 groups, respectively. (J–L) The expression of NGK2A and NGK2C, along with the ratio of NKG2C/NKG2A in CD56briCD16−/+ NK cells among the four groups, respectively. Mann–Whitney tests were used to compare groups and P < .05 was considered statistically significant. Error bars indicate median and interquartile range. NK = natural killer.
Next, we compared the expression levels of NKG2A and NKG2C on the surface of the NK subsets for each group. A significant downregulation in the proportion of CD56neg NKG2A+ NK cells in HIV/HBV-coinfected individuals was observed relative to that in HIV-infected individuals (P = 0.008; Fig. 3D). The proportion of CD56neg NKG2C+ NK cells in HIV/HBV-coinfected individuals was increased significantly relative to that in HBV-infected individuals and HCs (P < .001 and P = .01, respectively). The proportion of CD56neg NKG2C+ NK cells in HIV-infected individuals was significantly enhanced than that in HBV-infected individuals, HIV/HBV-coinfected and HCs (P < .001, P = .028 and P < .001, respectively; Fig. 3E). In addition, there was a significant difference in this proportion between HBV-infected individuals and HCs (P < .001; Fig. 3E). The ratio of CD56neg NKG2C/NKG2A cells was significantly elevated in HIV/HBV-coinfected and HIV-infected individuals than that in HBV-infected individuals (P < .001 and P = .005, respectively; Fig. 3F). Moreover, the ratio of CD56neg NKG2C/NKG2A cells in HIV/HBV-coinfected individuals was significantly enhanced than that in HCs (P = .003; Fig. 3F). There was no difference in the ratio of CD56neg NKG2C/NKG2A cells between HIV/HBV-coinfected individuals and HIV-infected individuals or between HIV-infected individuals and HCs (Fig. 3F).
The proportion of CD56dim NKG2A+ NK cells in HIV/HBV-coinfected and HIV-infected individuals was significantly decreased compared with that in HBV-infected individuals (P = .004 and P = 0.003, respectively). There was no significant difference among HCs, HIV-infected and HIV/HBV-coinfected individuals (Fig. 3G). The proportion of CD56dim NKG2C+ NK cells in HIV/HBV-coinfected and HIV-infected individuals was significantly elevated than that in HBV-infected individuals or HCs (P < .001 and P = .001; P = .001 and P = .004, respectively; Fig. 3H). The statistical analysis of the ratio of CD56dim NKG2C/NKG2A cells revealed a trend that was similar to the proportion of CD56dim NKG2C+ NK cells among the groups (P < .001 and P = .002; P = .001 and P = .011, respectively; Fig. 3I).
The proportion of CD56bri NKG2A+ NK cells in HIV/HBV-infected individuals was no significant difference than that in the other three groups, while there was a significant difference in the proportion of CD56bri NKG2A+ NK cells in HBV-infected individuals compared to HCs (P = .01; Fig. 3J).
The proportion of CD56bri NKG2C+ NK cells in HIV/HBV-coinfected individuals was increased significantly relative to that in HBV-infected, HIV-infected individuals and HCs (P < .001, P = .01 and P = .02, respectively). The proportion of CD56bri NKG2C+ NK cells in HIV-infected individuals was significantly enhanced than that in HBV-infected individuals and HCs (P < .001 and P = .049, respectively; Fig. 3K). In addition, there was a significant difference between HBV-infected individuals and HCs (P = .045; Fig. 3K).
The ratio of CD56bri NKG2C/NKG2A cells in HIV/HBV-coinfected individuals was significantly increased compared with that in HIV-infected, HBV-infected individuals and HCs (P = .026, P < .001 and P = .003, respectively; Fig. 3L). The ratio of CD56bri NKG2C/NKG2A cells in HBV-infected individuals was significantly lower than that in HIV-infected individuals and HCs (P < .001 and P = .002, respectively; Fig. 3L).
3.4. Co-expression of NKG2A and NKG2C on the NK cells in the various groups
In this study, we further examined the change in NKG2C and NKG2A coexpression as a supplement of NKG2A+ and NKG2C+ expression on NK cells and the results were similar to those of single expression of NKG2A+ and NKG2C+ NK cells. The proportion of NKG2A+ NKG2C- NK cells was highest in HBV-infected individuals compared with that in HCs, HIV-infected and HIV/HBV-coinfected individuals, and the differences were significant (P = .014, P = .003 and P = .001, respectively; Fig. 4A). The proportion of NKG2A−NKG2C+ NK cells in HIV/HBV-coinfected or HIV-infected individuals was significantly increased compared with that in HBV-infected individuals and HCs (P < .001 and P = .01; P < .001 and P = .003; Fig. 4B). A significant lower proportion of NKG2A−NKG2C+ NK cells was found in HBV-infected individuals compared with that in HCs (P = .036; Fig. 4B), whereas no significant difference was detected between HIV/HBV-coinfected and HIV-infected individuals. These results were similar to those of the expression of NKG2C+ NK cells alone, except that there was no significant difference between the HBV-infected individuals and HCs.
Figure 4.
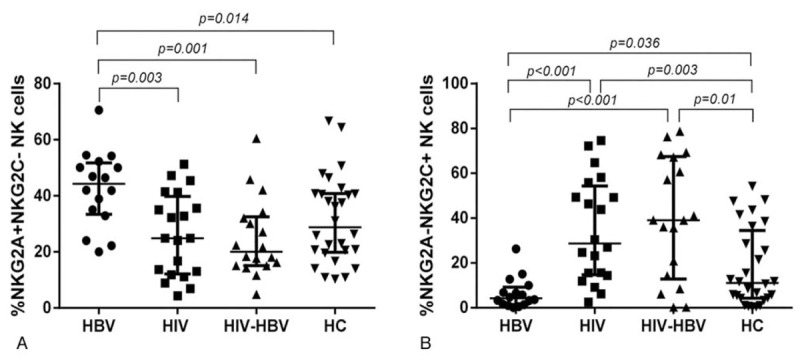
Alterations of NKG2A and NKG2C co-expression on NK cells among the 4 groups. (A-B) The proportion of NKG2A-NKG2C+ and NKG2A+NKG2C- expression on the surface of NK cells among the 4 groups, respectively. Mann–Whitney tests were used to compare groups and P < .05 was considered statistically significant. Error bars indicate median and interquartile range. NK = natural killer.
We also determined the mean fluorescence intensity (MFI) for NKG2C and NKG2A expression alone and together on NK cells and subsets. The results obtained were consistent with the differences in frequency observed between groups (data not shown).
3.5. Alteration of degranulation, IL-10 and IFN-γ secretion by NK cells between groups
To determine the functional capacity of NK cells, we analyzed degranulation assay (the expression of CD107a), which represents the cytotoxic activity, IL-10 and IFN-γ production of these cells. CD107a expression of NK cells in HIV/HBV-coinfected individuals was significantly higher than that in HCs, HIV-infected and HBV-infected individuals (P < .001, P = .002 and P < .001, respectively; Fig. 5A). Moreover, the expression level of CD107a in HIV-infected individuals was significantly enhanced compared with that in HBV-infected individuals and HCs (P = .027 and P < .001, respectively; Fig. 5A). There was no significant difference of CD107a expression between HBV-infected individuals and HCs (Fig. 5A). The production of IFN-γ by NK cells in HIV/HBV-coinfected individuals was obviously increased compared with that in HBV-infected individuals but significantly downregulated than that in HIV-infected individuals (Fig. 5B). The IFN-γ production by NK cells in HIV-infected individuals was also elevated compared with that in HCs and HBV-infected individuals (P = .006 and P < .001, respectively; Fig. 5B). The production of IFN-γ in HBV-infected individuals was statistically decreased than that in HCs (P < .001; Fig. 5B). No significant difference in IFN-γ secretion was observed between HIV/HBV-coinfected individuals and HCs (Fig. 5B). In addition, the production of IL-10 by NK cells in HIV/HBV-coinfected individuals was significantly reduced compared with that in HBV-infected individuals, but significantly increased than that in HIV-infected individuals and HCs (Fig. 5C). No significant difference in IL-10 secretion was observed between HIV-infected individuals and HCs (Fig. 5C).
Figure 5.
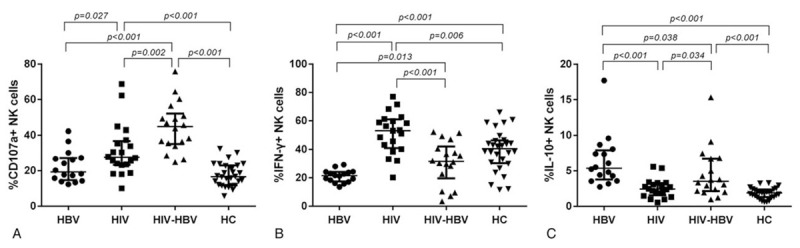
Functional activities of NK cells based on their capacity of IFN-γ and IL-10 production and degranulation (expressing CD107a) when stimulated by PMA together with ionomycin among the four groups. (A) The proportion of CD107a+ NK cells among the four groups. (B) The proportion of IFN-γ+ NK cells among the 4 groups. (C) The proportion of IL-10+ NK cells among the four groups. Mann–Whitney tests were used to compare groups, and P < .05 was considered statistically significant. Error bars indicate median and interquartile range. NK = natural killer.
3.6. NK cell cytotoxic activity
As shown in Figure 6A, NK cell cytotoxicity was evaluated by the analysis of E:T ratio of 10:1 in HIV/HBV-coinfected individuals and HBV-infected individuals. The cytotoxic activity of NK cells was increased in HIV/HBV-coinfected individuals compared to HBV-infected individuals at E:T ratio of 10:1 (P = .018; Fig. 6B).
Figure 6.
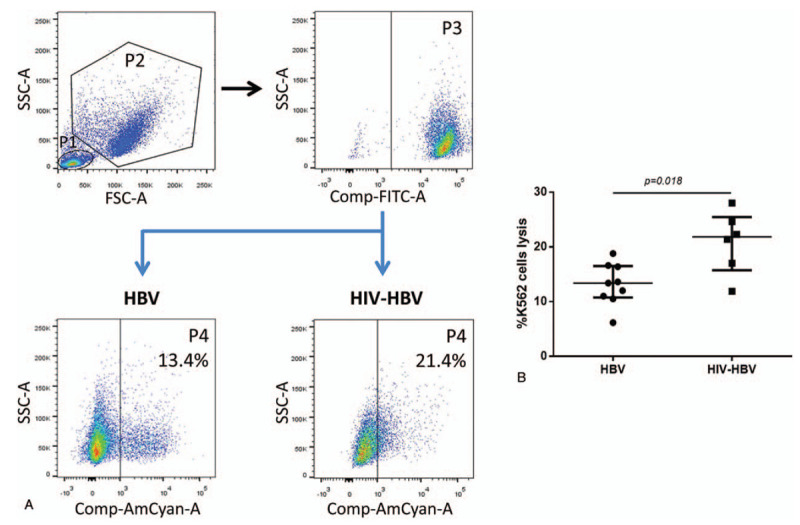
Cytotoxicity of peripheral NK cells in HBV-infected and HIV/HBV-coinfected individuals. (A) The NK cells and K562 cells were gated according to forward and side scatter dot plot (P1 and P2). CFSE-labeled K562 cells (P3) were defined by side scatter dot plot and CFSE. K562 cells lysis (P4) was defined by side scatter dot plot and 7AAD. (B) The proportions of K562 lysis at the E:T ratio of 10:1 between 2 groups. Mann–Whitney tests were used to compare groups, and P < .05 was considered statistically significant. Error bars indicate median and interquartile range. NK = natural killer.
3.7. Correlation between the expression phenotype (NKG2C and NKG2A) or function of NK cells and plasma levels of HBV DNA in HIV/HBV-coinfected individuals
We further analyzed the association between the expression levels of NKG2C and NKG2A on NK cells and the plasma HBV load in HIV/HBV-coinfected individuals. The results revealed that the proportions of NKG2A+ NK cells and NKG2A+NKG2C- NK cells were positively correlated with HBV load (r = 0.4552, P = .0882; r = 0.5821, P = .0228; Fig. 7A and C); the proportions of NKG2C+ NK cells and NKG2A−NKG2C+ NK cells were inversely correlated with HBV load (r = −0.6666, P = .0067; r = −0.5622, P = .0291; Fig. 7B and D). We also analyzed the relationship between NK function and plasma levels of HBV DNA. The results showed that the proportion of CD107a+ NK cells was not correlated with HBV load (r = −0.05, P = .8594; Fig. 7E); however, the proportion of IFN-γ+ NK cells was inversely correlated with HBV load (r = −0.6299, P = .0118; Fig. 7F). Moreover, the proportion of IL-10+ NK cells was not correlated with HBV load (data not shown).
Figure 7.
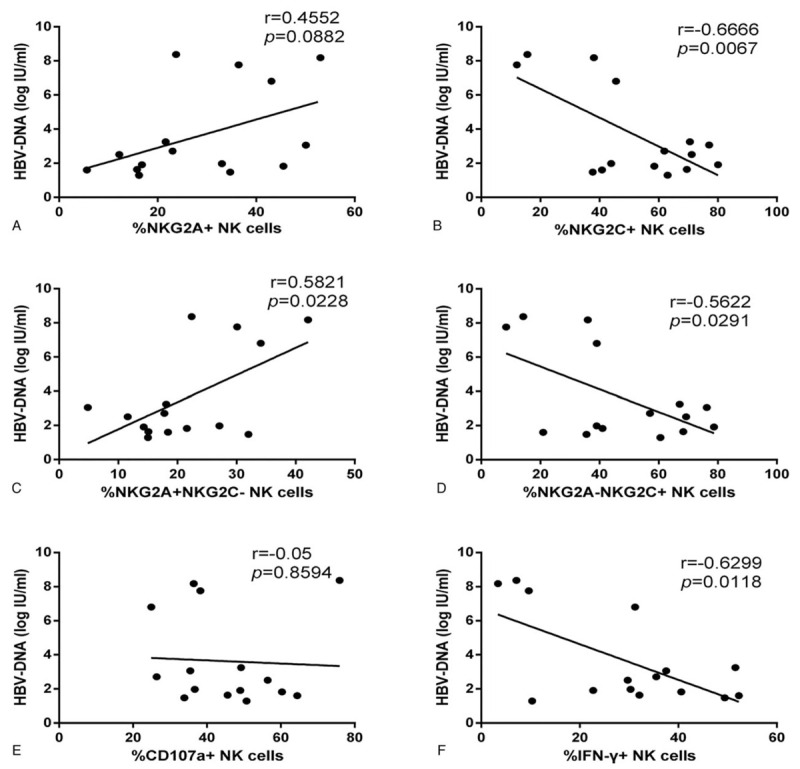
Associations between the expression of NKG2C or NKG2A or function of NK cells and HBV load in HIV/HBV-coinfected individuals. (A-B) Correlation analysis between the expression of NKG2A or NKG2C on NK cells and the plasma levels of HBV-DNA. (C-D) Correlation analysis between the proportion of NKG2A-NKG2C+ or NKG2A+NKG2C- NK cells and the plasma levels of HBV DNA. (E-F) Correlation analysis between the proportion of CD107a+ or IFN-γ+ NK cells and the plasma levels of HBV-DNA. Spearman rank test was used to correlate the data, and P < .05 was considered significant. NK = natural killer.
4. Discussion
Few data are available concerning the impact of acute HIV infection on individuals with chronic HBV infection. Based on our previous study,[8] our main objective in this study was to explore the role of NK cells in controlling HBV replication during acute HIV infection in HIV/HBV-coinfected individuals. NK cells are an important component of innate immunity, serving as the first line of defense against viral infections. NK cell frequency and its function are closely correlated. We therefore first assessed changes in the proportions of these cells in patients with PHI, CHB or HIV/HBV coinfection and HCs.
The proportion of total NK cells was greater in HIV/HBV-coinfected individuals, due to higher levels of CD56neg NK cells were observed than that in the HBV group, whereas CD56dim and CD56bri NK cell levels did not differ between the two groups, implying that there was not difference between the groups in the frequency of effective NK cells. Since, we observed the increased frequency of CD56neg NK cells both in HIV/HBV-coinfected individuals and HIV-infected subjects; it is possible to infer that acute HIV-infection but not HBV can affect NK cell subsets distribution. Studies of NK cell phenotypes therefore are required further investigation to explore the NK cell functions.
NK cell function is mediated by the balance between several inhibitory (NKG2A, NKR-P1A etc) and activating (NKG2C, NKG2D etc) receptors, the expression profiles of which are altered during infections with many pathogenic microorganisms, such as HBV and HIV in our case. NKG2A and NKG2C, both of which belong to the NKG2 receptor family and bind HLA-E, despite their opposite functions, are known to be involved in the activation of NK cells against viral infections.[11,29,30] We therefore investigated whether changes in their expression were related to the progression and outcome of disease in HIV/HBV-coinfected patients.
We first assessed the expression of NKG2A and NKG2C on the total NK cells. We investigated the relationship between the expression levels of NKG2C and NKG2A, by determining the NKG2C/NKG2A ratio. The level of NKG2C+ NK cells was significantly higher, and that of NKG2A+ NK cells was significantly lower in HIV/HBV-coinfected or HIV-infected individuals than in HBV-infected individuals. Furthermore, the differences between HIV/HBV-coinfected and HCs and between HIV-infected individuals and HCs were similar, except for the expression of NKG2A. No significant difference in NKG2A or NKG2C expressions was observed between HIV/HBV-coinfected and HIV-infected individuals. These results suggest that the activation of NK cells, leading to signaling via the NKG2C pathway, was probably stronger in patients with acute HIV infection, regardless of whether they were infected with HBV, than in patients infected with HBV alone and HCs.
Secondly, we further explored which subset of NK cells contributed to the increased expression of NKG2C and decreased expression of NKG2A among the groups. The ratio of NKG2C/NKG2A cells truly reflected the alteration of NKG2C and NKG2A expressions; hence we discussed the differences of this parameter among the groups. As shown in Figure 3, we found that the NKG2C/NKG2A ratio of each NK subset in HIV/HBV-coinfected and HIV-infected individuals was obviously enhanced compared with that in HBV-infected individuals. Furthermore, the NKG2C/NKG2A ratio of NK subsets in HIV/HBV-coinfected individuals was also increased compared with that in HCs; there was no significant difference in NK cell subsets between HBV-infected patients and HCs except for the CD56bri subset. Thus, all subsets of NK cells contributed to the increased expression of NKG2C in HIV/HBV-coinfected and HIV-infected individuals, while the elevated expression of NKG2A in HBV-infected individuals was largely caused by CD56dim NK cells (Fig. 3). On the other hand, the expression of NKG2C (increased) and NKG2A (normal or decreased) was mainly affected by acute HIV infection instead of HBV infection in HIV/HBV-coinfected individuals, which may imply that the progression and outcome of acute HIV-infected patients living with HBV are determined more by HIV rather than HBV. Many studies have demonstrated alterations to the proportions of NKG2A+ and NKG2C+ NK cells in patients with single HIV or HBV infections,[17,18,28,36–41] but our present study is the first to report changes in the levels of these receptors in the HIV/HBV-coinfected individuals.
In addition to analyzing the expression of NKG2A and NKG2C separately in NK cells, we also evaluated the co-expression of these receptors. The co-expression of NKG2A and NKG2C has been little studied. NKG2A+ NK cells can be split into 2 populations: NKG2A+NKG2C- NK cells and NKG2A+NKG2C+ NK cells. NKG2C+ NK cells can, similarly, be split into 2 populations defined in terms of their NKG2A expression status. NKG2A and NKG2C have opposite effects in NK cells, so the functions of total NKG2A+ NK cells and NKG2A+NKG2C- NK cells are likely to be different. We found higher levels of NKG2A−NKG2C+ NK cells and lower levels of NKG2A+NKG2C- NK cells in HIV/HBV-coinfected and HIV-infected individuals than in HBV-infected individuals. The levels of NKG2A and NKG2C obtained in analysis of the expression of a single receptor at a time and in analyses of co-expression were consistent. Moreover, it further demonstrates that the proportion of NKG2C+ NK cells among total NK cells increases whereas that of NKG2A+ NK cells decreases, potentially increasing NK cell activation and improving the control of HBV replication and HBeAg conversion in HIV/HBV-coinfected individuals. On the other hand, levels of NKG2A and NKG2C expression were similar in HIV/HBV-coinfected and HIV-infected individuals. Thus, acute HIV infection is the predominant factor contributing to progression and prognosis in HIV/HBV-coinfected individuals, with HBV infection having little or no impact on NK cells expressing NKG2C and NKG2A and on the progression and outcome of HIV in HIV/HBV-coinfected individuals, as reported by Thornton et al.[6] It is now generally believed that HCMV infection is common and the expression of NKG2C is increased during HCMV infection. HIV/HCMV-coinfected individuals in the absence of CMV reactivation have been showed that the driving force of NKG2C+ NK cell expansion by HIV infection,[28] our findings with HIV infection alone and HIV/HBV-coinfected individuals are consistent with previous studies.[28] Whereas, the alteration of NKG2C induced by HCMV was not affected in HBV-infected individuals compared to HCs. NKG2C+ NK cells were also showed higher expression of CD107a and IFN-γ production than those of NKG2C– NK cells, which displayed enhanced NK cell function.[17] Therefore, our results suggest that increasing the expression of activating receptors (NKG2C) and decreasing or preserved that of inhibitory receptors (NKG2A) enhances NK cell function in HIV/HBV-coinfected individuals.
NK cells can kill infected targets directly via cytolysis, which is dependent on the release of granules containing perforins and granzymes. We assessed the cytotoxic capacity of NK cells by assessing CD107a expression as an indicator of target cell degranulation and cytotoxicity against K562 cells. In addition to their direct cytotoxic capacity, NK cells make a major contribution to antiviral immunity by producing cytokines via indirect pathways. We assessed IFN-γ and IL-10 levels, to evaluate the antiviral functions of NK cells. IL-10 has been shown to be an immunosuppressive cytokine, which inhibit NK cell functions.[42] In CHB patients, elevated IL-10 production was perceived to cause impaired secretion of IFN-γ by NK cells but without altering cytotoxicity.[43–45] IL-10 production was significantly reduced in subjects with HIV/HBV-coinfection than in subjects infected with HBV alone. Another major finding of this study was that IFN-γ production levels are higher and CD107a expression is stronger in subjects with HIV/HBV-coinfection than in subjects infected with HBV alone. Similar results of NK cytotoxicity against K562 cells were found in HIV/HBV-coinfection individuals compared to HBV alone. The antiviral effect induced by cytokines such as IFN-γ is more effective than direct target cell lysis for patients with HBV infection; the impaired IFN-γ production may be responsible for viral persistence.[43,46] Significance of IFN-γ for controlling viral infection in several studies has been shown that it maybe a more efficient non-cytolytic mechanism of viral control from infected hepatocyte.[47–49] Thus, the direct and indirect functions of NK cells are enhanced in HIV/HBV-coinfected populations relative to HBV-infected subjects. In our study, NK cells retain their greater capacities for viral clearance and HBeAg seroconversion to HBeAb in HIV/HBV-coinfected patients compared to HBV-infected patients. These findings suggest that enhanced NK cell function favors viral clearance or functional cure for HBV infection and opens up possibilities for immunotherapy to treat CHB.
We found that NK cells displayed sustained degranulation activity and increased IL-10 production but impaired IFN-γ production in HBV-infected individuals than in HCs, our data are consistent with those of a previous report.[43,50] In short, NK cells display a functional dichotomy in CHB patients, characterized by impaired noncytolytic antiviral capacity such as IFN-γ production and enhanced or preserved cytotoxic function such as the degranulation capacity.[51,52] Furthermore, HIV-infected individuals exhibit significantly enhanced degranulation and IFN-γ production but no difference in IL-10 level compared with HCs. Our results are consistent with previous reports,[18,53] suggesting that NK cell functions are greatly enhanced in individuals with PHI relative to HCs. In HIV/HBV-coinfected individuals, NK cells displayed enhanced degranulation activity, and produced lower IFN-γ but higher IL-10 level than that in HIV-infected individuals. These discrepancies may result from HBV infection causing NK cells to retain a normal cytotoxic capacity but with an impaired capacity to produce non-cytolytic cytokines in response to activating signals.
In addition to NK cell function, we also explored the correlation between the phenotypic expression (NKG2C and NKG2A) or function of NK cells and HBV load in HIV/HBV-coinfected individuals. We found that HBV-DNA levels was inversely correlated with the proportions of NKG2C+ and NKG2C+NKG2A− NK cells, but positively correlated with the proportions of NKG2A+ and NKG2C-NKG2A+ NK cells. IFN-γ production was inversely correlated with levels of HBV-DNA levels, but the IL-10 production and CD107a expression of NK cells was not correlated with HBV-DNA levels, consistent with previous reports.[49,54] Furthermore, no significant correlation was found between the phenotypic expression (NKG2C and NKG2A) or function of NK cells and HBV load in HBV-infected individuals (data no shown), because the impaired function of NK cells in HBV-infected individuals may have contributed to viral persistence.[52,55] However, the HBV replication could be controlled due to acute HIV infection in HIV/HBV-coinfected individuals through changing expressions of NK cell surface receptor, which resulting in enhanced NK cell function. As previous studies have shown, the expansion of NKG2C+ NK cells in acute HIV infection contributes to the enhancement of NK cell function.[17] Moreover, other studies have demonstrated that enhanced IFN-γ production by NK cells in HIV/HBV-coinfected individuals may contribute to the clearance of HBV.[43,56,57] Our findings clearly show that HBV replication was controlled more effectively in individuals with CHB who had acquired acute HIV infection, due to an increase in IFN-γ production by NK cells which in turn results in enhancement of an antiviral immune response.
This study was subject to several limitations. Firstly, we performed a cross-sectional study, and longitudinal changes in the frequency, phenotype and function of NK cells may be more meaningful in HIV/HBV-coinfected individuals. Secondly, we investigated the response of peripheral blood NK cells, but not that of intrahepatic NK cells in vivo. Therefore, further investigation will be required to elucidate the precise mechanisms.
5. Conclusions
In summary, acute HIV infection enhances the cytolytic capacity of NK cells and their cytokine production, through increases in NKG2C expression without a corresponding increase in NKG2A expression, resulting in enhanced HBV clearance in HIV/HBV-coinfected individuals. These findings highlight the new immunotherapeutic strategies, which could be developed targeting NK cell activation, for the definitive clearance of the virus.
Acknowledgments
We thank Drs Wei Xia and Yuefang Zhou for patients recruiting, blood and information collecting; Yunxia Ji and Rui Wang for cell counting and viral load detecting, and the patients participated in our study.
Author contributions
Conceptualization: Li Li, Bin Su, Guizhen Sun, Hao Wu.
Data curation: Ting Song, Li Li, Bin Su, Yan Liu,
Investigation: Ting Song, Li Li, Lifeng Liu, Xiaodong Yang, Qiuyue Zhang, Hao Wu.
Methodology: Li Li, Bin Su, Lifeng Liu, Na Guo.
Project administration: Bin Su, Tong Zhang, Guizhen Sun, Hao Wu.
Resources: Bin Su, Lifeng Liu, Tong Zhang, Hao Wu.
Software: Ting Song, Li Li.
Writing – original draft: Ting Song, Li Li, Bin Su, Yan Liu.
Writing – review & editing: Ting Song, Bin Su, Guizhen Sun, Hao Wu.
Footnotes
Abbreviations: ALT = serum alanine aminotransferase, CHB = chronic hepatitis B, HBeAg = hepatitis B e-antigen, HBsAg = hepatitis B surface antigen, HBV = hepatitis B virus, HCMV = human cytomegalovirus, HCs = healthy controls, HESN = HIV-exposed seronegative, HIV = human immunodeficiency virus, IFN-γ = interferon-γ, IL-10 = interleukin-10, NK cells = natural killer cells, PHI = primary HIV infection.
How to cite this article: Song T, Li L, Su B, Liu L, Liu Y, Yang X, Zhang Q, Guo N, Zhang T, Sun G, Wu H. NKG2C+ natural killer cell function improves the control of HBV replication in individuals with acute HIV infection coinfected with HBV. Medicine. 2020;99:18(e20073).
The authors have no conflicts of interest to disclose.
This work was supported by the National Natural Science Foundation of China (NSFC, 81571973 to HW; 81772165 to BS), the NSFC-NIH Biomedical collaborative research program (81761128001 to HW), the National 13th Five-Year Grand Program on Key Infectious Disease Control (2017ZX10202102-005-003 to BS, 2017ZX10202101-004-001 to TZ), the Beijing Municipal of Science and Technology Major Project (D161100000416003 to HW: Z161100000516148 to BS), and the Beijing Key Laboratory for HIV/AIDS Research (BZ0089). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- [1].Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat 2004;11:97–107. [DOI] [PubMed] [Google Scholar]
- [2].Te HS, Jensen DM. Epidemiology of hepatitis B and C viruses: a global overview. Clin Liver Dis 2010;14:1–21. [DOI] [PubMed] [Google Scholar]
- [3].Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol 2018;3:383–403. [DOI] [PubMed] [Google Scholar]
- [4].Konopnicki D, Mocroft A, de Wit S, et al. Hepatitis B and HIV: prevalence, AIDS progression, response to highly active antiretroviral therapy and increased mortality in the EuroSIDA cohort. AIDS 2005;19:593–601. [DOI] [PubMed] [Google Scholar]
- [5].Singh KP, Crane M, Audsley J, et al. HIV-hepatitis B virus coinfection: epidemiology, pathogenesis, and treatment. AIDS 2017;31:2035–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Thornton AC, Jose S, Bhagani S, et al. Hepatitis B, hepatitis C, and mortality among HIV-positive individuals. AIDS 2017;31:2525–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Qadir MI. Hepatitis in AIDS patients. Rev Med Virol 2018;28:1–7. [DOI] [PubMed] [Google Scholar]
- [8].Jiao Y, Li N, Chen X, et al. Acute HIV infection is beneficial for controlling chronic hepatitis B. Clin Infect Dis 2015;60:128–34. [DOI] [PubMed] [Google Scholar]
- [9].Yu WH, Cosgrove C, Berger CT, et al. ADCC-mediated CD56(DIM) NK cell responses are associated with early HBsAg clearance in acute HBV infection. Pathog Immun 2018;3:2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fisicaro P, Valdatta C, Boni C, et al. Early kinetics of innate and adaptive immune responses during hepatitis B virus infection. Gut 2009;58:974–82. [DOI] [PubMed] [Google Scholar]
- [11].Li F, Wei H, Gao Y, et al. Blocking the natural killer cell inhibitory receptor NKG2A increases activity of human natural killer cells and clears hepatitis B virus infection in mice. Gastroenterology 2013;144:392–401. [DOI] [PubMed] [Google Scholar]
- [12].Peppa D, Gill US, Reynolds G, et al. Up-regulation of a death receptor renders antiviral T cells susceptible to NK cell-mediated deletion. J Exp Med 2013;210:99–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tseng TC, Huang LR. Immunopathogenesis of hepatitis B virus. J Infect Dis 2017;216:S765–70. [DOI] [PubMed] [Google Scholar]
- [14].Huang WC, Easom NJ, Tang XZ, et al. T Cells infiltrating diseased liver express ligands for the NKG2D stress surveillance system. J Immunol 2017;198:1172–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Quillay H, El Costa H, Duriez M, et al. NK cells control HIV-1 infection of macrophages through soluble factors and cellular contacts in the human decidua. Retrovirology 2016;13:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Florez-Alvarez L, Hernandez JC, Zapata W. NK Cells in HIV-1 Infection: From Basic Science to Vaccine Strategies. Front Immunol 2018;9:2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ma M, Wang Z, Chen X, et al. NKG2C(+)NKG2A(-) natural killer cells are associated with a lower viral set point and may predict disease progression in individuals with primary HIV infection. Front Immunol 2017;8:1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Alter G, Teigen N, Ahern R, et al. Evolution of innate and adaptive effector cell functions during acute HIV-1 infection. J Infect Dis 2007;195:1452–60. [DOI] [PubMed] [Google Scholar]
- [19].Horton RE, McLaren PJ, Fowke K, et al. Cohorts for the study of HIV-1-exposed but uninfected individuals: benefits and limitations. J Infect Dis 2010;202: Suppl 3: S377–81. [DOI] [PubMed] [Google Scholar]
- [20].Smith C, Jalbert E, de Almeida V, et al. Altered natural killer cell function in HIV-exposed uninfected infants. Front Immunol 2017;8:470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Crouse J, Xu HC, Lang PA, et al. NK cells regulating T cell responses: mechanisms and outcome. Trends Immunol 2015;36:49–58. [DOI] [PubMed] [Google Scholar]
- [22].Mackay IR. Hepatoimmunology: a perspective. Immunol Cell Biol 2002;80:36–44. [DOI] [PubMed] [Google Scholar]
- [23].Alter G, Teigen N, Davis BT, et al. Sequential deregulation of NK cell subset distribution and function starting in acute HIV-1 infection. Blood 2005;106:3366–9. [DOI] [PubMed] [Google Scholar]
- [24].Lugli E, Marcenaro E, Mavilio D. NK cell subset redistribution during the course of viral infections. Front Immunol 2014;5:390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bjorkstrom NK, Ljunggren HG, Sandberg JK. CD56 negative NK cells: origin, function, and role in chronic viral disease. Trends Immunol 2010;31:401–6. [DOI] [PubMed] [Google Scholar]
- [26].Guma M, Angulo A, Vilches C, et al. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood 2004;104:3664–71. [DOI] [PubMed] [Google Scholar]
- [27].Mela CM, Goodier MR. The contribution of cytomegalovirus to changes in NK cell receptor expression in HIV-1-infected individuals. J Infect Dis 2007;195:158–9. author reply 159-160. [DOI] [PubMed] [Google Scholar]
- [28].Gondois-Rey F, Cheret A, Granjeaud S, et al. NKG2C(+) memory-like NK cells contribute to the control of HIV viremia during primary infection: Optiprim-ANRS 147. Clin Transl Immunology 2017;6:e150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yan W, Wu D, Wang X, et al. Upregulation of NKG2C+ natural killer cells, TLR-2 expression on monocytes and downregulation of regulatory T-cells influence PEG-IFN treatment efficacy in entecavir-suppressed patients with CHB. Antivir Ther 2015;20:591–602. [DOI] [PubMed] [Google Scholar]
- [30].Bastidas-Legarda LY, Khakoo SI. Conserved and variable NK cell receptors: diverse approaches to viral infections. Immunology 2019;156:319–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lanier LL. NK cell receptors. Annu Rev Immunol 1998;16:359–93. [DOI] [PubMed] [Google Scholar]
- [32].Lee N, Llano M, Carretero M, et al. HLA-E is a major ligand for the natural killer inhibitory receptor CD94/NKG2A. Proc Natl Acad Sci U S A 1998;95:5199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Li W, Jiang Y, Wang X, et al. Natural Killer p46 controls hepatitis B virus replication and modulates liver inflammation. PLoS One 2015;10:e0135874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zimmer CL, Rinker F, Honer Zu Siederdissen C, et al. Increased NK cell function after cessation of long-term Nucleos(t)ide analogue treatment in chronic hepatitis B is associated with liver damage and HBsAg loss. J Infect Dis 2018;217:1656–66. [DOI] [PubMed] [Google Scholar]
- [35].Henn A, Flateau C, Gallien S. Primary HIV infection: clinical presentation, testing, and treatment. Curr Infect Dis Rep 2017;19:37. [DOI] [PubMed] [Google Scholar]
- [36].Brunetta E, Fogli M, Varchetta S, et al. Chronic HIV-1 viremia reverses NKG2A/NKG2C ratio on natural killer cells in patients with human cytomegalovirus co-infection. AIDS 2010;24:27–34. [DOI] [PubMed] [Google Scholar]
- [37].Schuch A, Zecher BF, Muller PA, et al. NK-cell responses are biased towards CD16-mediated effector functions in chronic hepatitis B virus infection. J Hepatol 2019;70:351–60. [DOI] [PubMed] [Google Scholar]
- [38].Lunemann S, Malone DF, Hengst J, et al. Compromised function of natural killer cells in acute and chronic viral hepatitis. J Infect Dis 2014;209:1362–73. [DOI] [PubMed] [Google Scholar]
- [39].Kulkarni AG, Paranjape RS, Thakar MR. Higher expression of activating receptors on cytotoxic NK cells is associated with early control on HIV-1C multiplication. Front Immunol 2014;5:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Mela CM, Burton CT, Imami N, et al. Switch from inhibitory to activating NKG2 receptor expression in HIV-1 infection: lack of reversion with highly active antiretroviral therapy. AIDS 2005;19:1761–9. [DOI] [PubMed] [Google Scholar]
- [41].Zhang QF, Shao JY, Yin WW, et al. Altered immune profiles of natural killer cells in chronic hepatitis B patients: a systematic review and meta-analysis. PLoS One 2016;11:e0160171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Li H, Zhai N, Wang Z, et al. Regulatory NK cells mediated between immunosuppressive monocytes and dysfunctional T cells in chronic HBV infection. Gut 2018;67:2035–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Peng H, Tian Z. NK cells in liver homeostasis and viral hepatitis. Sci China Life Sci 2018;61:1477–85. [DOI] [PubMed] [Google Scholar]
- [44].Maini MK, Peppa D. NK cells: a double-edged sword in chronic hepatitis B virus infection. Front Immunol 2013;4:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Li TY, Yang Y, Zhou G, et al. Immune suppression in chronic hepatitis B infection associated liver disease: a review. World J Gastroenterol 2019;25:3527–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Conroy MJ, Mac Nicholas R, Grealy R, et al. Circulating CD56dim natural killer cells and CD56+ T cells that produce interferon-gamma or interleukin-10 are expanded in asymptomatic, E antigen-negative patients with persistent hepatitis B virus infection. J Viral Hepat 2015;22:335–45. [DOI] [PubMed] [Google Scholar]
- [47].Guidotti LG, Chisari FV. Noncytolytic control of viral infections by the innate and adaptive immune response. Annu Rev Immunol 2001;19:65–91. [DOI] [PubMed] [Google Scholar]
- [48].Tong S, Liu G, Li M, et al. Natural killer cell activation contributes to hepatitis B viral control in a mouse model. Sci Rep 2017;7:314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Li Y, Wang JJ, Gao S, et al. Decreased peripheral natural killer cells activity in the immune activated stage of chronic hepatitis B. PLoS One 2014;9:e86927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Peppa D, Micco L, Javaid A, et al. Blockade of immunosuppressive cytokines restores NK cell antiviral function in chronic hepatitis B virus infection. PLoS Pathog 2010;6:e1001227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Schuch A, Zecher BF, Muller PA, et al. NK-cell responses are biased towards CD16-mediated effector functions in chronic hepatitis B virus infection. J Hepatol 2018;70:351–60. [DOI] [PubMed] [Google Scholar]
- [52].Mondelli MU, Oliviero B, Mele D, et al. Natural killer cell functional dichotomy: a feature of chronic viral hepatitis? Front Immunol 2012;3:351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Eller MA, Eller LA, Ouma BJ, et al. Elevated natural killer cell activity despite altered functional and phenotypic profile in Ugandans with HIV-1 clade A or clade D infection. J Acquir Immune Defic Syndr 2009;51:380–9. [DOI] [PubMed] [Google Scholar]
- [54].Tan AT, Koh S, Goh W, et al. A longitudinal analysis of innate and adaptive immune profile during hepatic flares in chronic hepatitis B. J Hepatol 2010;52:330–9. [DOI] [PubMed] [Google Scholar]
- [55].Schuch A, Hoh A, Thimme R. The role of natural killer cells and CD8(+) T cells in hepatitis B virus infection. Front Immunol 2014;5:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Zhang Z, Zhang S, Zou Z, et al. Hypercytolytic activity of hepatic natural killer cells correlates with liver injury in chronic hepatitis B patients. Hepatology 2011;53:73–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Shi A, Zhang X, Xiao F, et al. CD56(bright) natural killer cells induce HBsAg reduction via cytolysis and cccDNA decay in long-term entecavir-treated patients switching to peginterferon alfa-2a. J Viral Hepat 2018;25:1352–62. [DOI] [PubMed] [Google Scholar]


