Abstract
This study aimed to compare the sirtuin 2 (SIRT2) expression between tumor tissue and adjacent tissue, and to investigate the association of tumor SIRT2 expression with clinical characteristics and survival profiles in cervical cancer patients.
One hundred ninety-one cervical cancer patients were reviewed in this retrospective study. All patients underwent surgical resection and had well-preserved tumor tissue and adjacent tissue, which were obtained for SIRT2 expression detection by immunohistochemistry (IHC). Clinical parameters were obtained. Disease free survival (DFS) and overall survival (OS) were calculated.
Both SIRT2 expression by IHC score (P < .001) and the percentage of SIRT2 high expression (defined as IHC score >3) (P < .001) were declined in tumor tissue compared with paired adjacent tissue. In addition, SIRT2 expression in tumor tissue was negatively correlated with tumor size (P = .047), lymph node metastasis (P = .009) and FIGO stage (P = .001). And the DFS (P = .007) as well as OS (P = .008) were better in patients with SIRT2 high expression compared with patents with SIRT2 low expression. Univariate Cox's proportional hazards regression model analyses revealed that high SIRT2 expression in tumor tissue was a predictive factor for more prolonged DFS (P = .009) and OS (P = .011), while multivariate Cox's proportional hazards regression model analysis disclosed that it lacks independent predictive value for DFS (P = .084) or OS (P = .132).
SIRT2 expression exhibits potential to serve as a biomarker for disease surveillance and prognosis in the management of cervical cancer patients.
Keywords: cervical cancer, clinical characteristics, SIRT2, survival, tumor tissue
1. Introduction
Cervical cancer, the fourth most frequent cancer and the fourth leading cause for cancer deaths worldwide according to the most recent epidemiological report, is responsible for approximately 570,000 new cases and 311,000 cancer-related deaths in the world in 2018.[1] Although cervical cancer is a highly preventable cancer, effective screening and vaccination programs are only mature in most developed countries, therefore, the worldwide burden caused by cervical cancer is still not optimistic.[2–4] As for prognosis, clinical staging system has been the standard tool for guiding treatment and predicting survival; however, it could be inaccurate from time to time, and it is often months late until the discovery of metastasis after surgical resection in early-stage patients, in whom there are one-third of the patients develop recurrent disease post resections.[5–8] Thus, it is necessary to explore more novel and assistant biomarkers for diagnosis and prognosis of cervical cancer.
Sirtuin 2 (SIRT2), a member of the sirtuin family, is a deacetylase-dependent on NAD+ targeting histones and non-histone proteins and is correlated with possessing mono-ADP-ribosyl-transferase functions and lifespan extension by calorie restriction.[9–11] Recently, apart from its functions in metabolism, the role of SIRT2 in tumorigenesis has been found and extensively investigated, studies revealing that SIRT2 is involved in the regulation of tumor development and progression processes, including tumor angiogenesis and tumor cell functions.[12,13] However, the role of SIRT2 found in cancers is still controversial, and more importantly, the investigation of SIRT2 in cervical cancer is extremely lacking. In view of that SIRT2 might be a critical regulator in cancers, we hypothesized that SIRT2 may have the potential to be a biomarker in patients with cervical cancer.
Thus, this study aimed to compare the SIRT2 expression between tumor tissue and adjacent tissue, and to investigate the association of tumor SIRT2 expression with clinical characteristics and survival profiles in cervical cancer patients.
2. Materials and methods
2.1. Patients
From January 2010 to December 2014, 191 cervical cancer patients treated in our hospital were screened and reviewed in this retrospective study. All patients were diagnosed as primary cervical cancer confirmed by histopathological examination, clinically staged I–II by International Federation of Gynecology and Obstetrics (FIGO) staging system and underwent surgical resection with well-preserved tumor tissue and adjacent tissue. Patients were excluded if they were initially treated with chemotherapy or radiotherapy followed by resection for relapsed disease, had incomplete clinical data and follow-up records, underwent neoadjuvant therapy or complicated with other malignancies. Institutional Review Board of our hospital approved the present study. Written informed consent or verbal agreement (with recording) for the scientific use of the biological material was obtained from each patient.
2.2. Data collection and sample acquisition
Clinical parameters, such as age, histological type, pathological grade, tumor size, lymph node metastasis, FIGO stage, human papillomavirus (HPV) status and so on, were acquired from electronic medical records. Fresh tissue samples, including tumor tissues and adjacent cervical tissues, were obtained from surgical removal, then were formalin-fixed and paraffin-embedded and preserved under appropriate conditions. After approval by our hospital, samples were collected from the storage room, and the SIRT2 expression was detected by immunohistochemistry (IHC) staining.
2.3. IHC staining and assessment
Formalin-fixed, paraffin-embedded tissues were cut into 4 μm sections, then were deparaffinized in xylene and ethanol and hydrated in a methanol gradient. Next, sections were quenched with fresh 3% hydrogen peroxide to inhibit endogenous tissue peroxidase activity, followed by the heat induced antigen epitope retrieval using citrate buffer (pH 6.0). After blocked by normal serum solution, sections were incubated overnight with rabbit SIRT2 Polyclonal Antibody (1:400 dilution, Invitrogen, Carlsbad, CA), then were incubated with horseradish peroxidase (HRP)-conjugated Goat anti-Rabbit IgG Secondary Antibody (1:5000 dilution, Invitrogen, Carlsbad, CA). Finally, sections were treated with diaminobenzidine (DAB) and hematoxylin for coloration and counterstaining. Then sections were dehydrated in a graded series of ethanol and xylene, covered with coverslips, and were viewed under the light microscope (Olympus Corp, Tokyo, Japan). According to IHC staining intensity and staining density of positively stained cells, SIRT2 expression was assessed using a semiquantitative scoring method as previously reported.[14,15] Briefly, staining intensity was scored as 0 = negative, 1 = weak, 2 = moderate, 3 = strong, and staining density was scored as 0 = 0%, 1 = 1% to 25%, 2 = 26% to 50%, 3 = 51% to 75%, 4 = 76% to 100%. A final IHC score was calculated by multiplying the score of staining intensity and staining density. Based on the IHC score, SIRT2 expression was classified as high expression (IHC score >3) and low expression (IHC score ≤3) by a threshold value of 3 as described in a previous study.[16]
2.4. Survival evaluation
Patients’ survival data were collected from follow-up records. As of June 30, 2018 (last follow-up date), the shortest, longest, and median follow-up duration were 8.0 months, 95.0 months, and 68.0 months, respectively. According to the survival data, disease free survival (DFS) and overall survival (OS) were calculated, which were defined as follows:
-
(i)
DFS: the time from the surgery to the disease relapse, progression or death;
-
(ii)
OS: the time from the surgery to the death.
2.5. Data analysis
Data were expressed as number (percentage). Difference was determined by Chi-square test, McNemar test, or Wilcoxon rank sum test. Survival curves were plotted with the use of Kaplan–Meier method, and the differences of DFS and OS between two groups were determined by log-rank test. The factors with influence on DFS and OS were analyzed by the univariable and multivariable Cox's proportional hazard regression models, and the relevant P values, hazard ratios (HR) as well as confidence intervals (CI) were calculated in the models. All tests were 2-sided and P < .05 indicated a significant difference. SPSS 24.0 statistical software (SPSS Inc, Chicago, IL) was used for statistical data processing, and GraphPad Prism 7.02 (GraphPad Software Inc., San Diego, CA) was applied for graphs making.
3. Results
3.1. Study flow
At the beginning, 396 cervical cancer patients who underwent surgery were screened, and 161 patients were subsequently excluded due to unavailable tumor specimens (n = 97), incomplete medical records required for inclusion (n = 36), history of neoadjuvant therapy (n = 14), resection for relapsed disease (n = 9), and concomitance of other malignancies (n = 5) (Fig. 1). Then the remaining 235 patients were eligible; however, 44 patients were then excluded from our study, which included 39 patients who cannot be reached for informed consents and 5 patients who disagreed with the scientific use of the biological material without compensation. Finally, 191 patients were included in the study.
Figure 1.
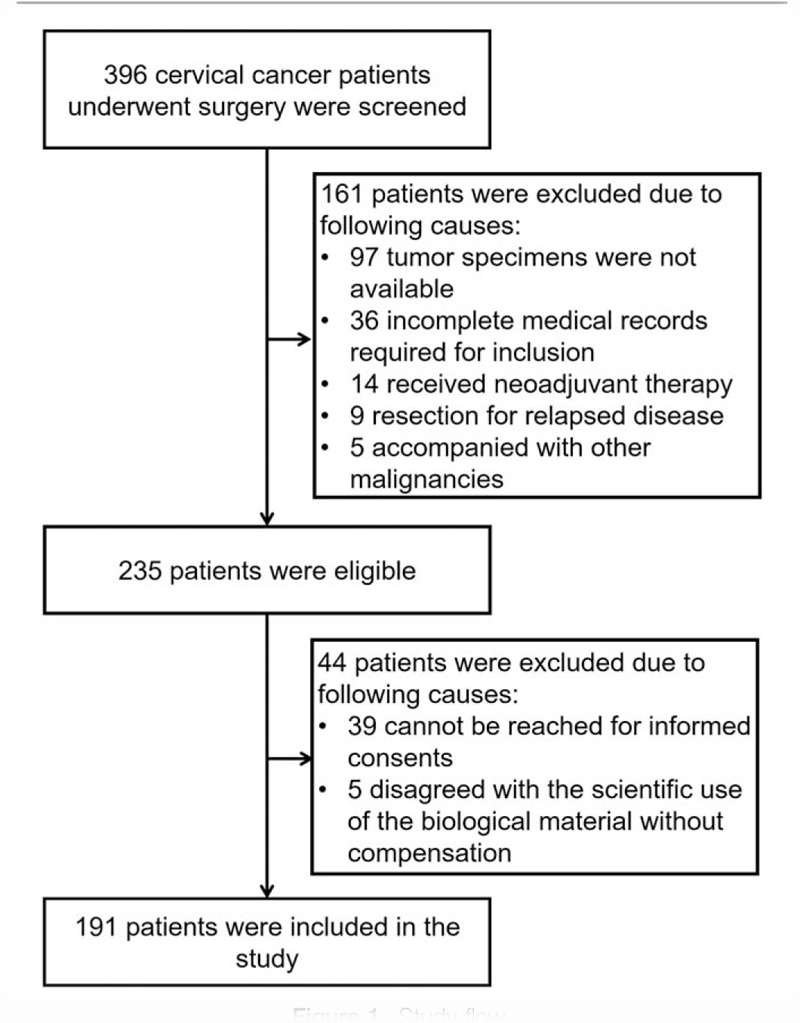
Study flow.
3.2. Baseline characteristics
In the 191 cervical cancer patients in our study, the number of patients with age <45 years was 90, and the others had an age ≥45 years (Table 1). The numbers of patients with histological type of SCC, ADC, and ADSC were 142, 40, and 9, and there were 55, 71, and 65 patients who were in pathological grade of G1, G2, and G3, respectively. In addition, 107 patients had tumor size <4 cm and 84 patients had tumor size ≥4 cm. The number of patients without lymph node metastasis and patients with lymph node metastasis were 151 and 40, respectively. And the number of patients who were in FIGO stage I and II were 112 and 79, respectively. As for the HPV status, 47 patients had a negative HPV stage and 144 patients were HPV positive.
Table 1.
Correlation of SIRT2 in tumor with clinical characteristics.

3.3. SIRT2 expression in tumor tissue and adjacent tissue
The SIRT2 expression in tumor tissue was detected using IHC, which showed that SIRT2 expression IHC score was decreased in tumor tissue compared with paired adjacent tissue (3.2 ± 2.0 vs 4.7 ± 3.0, P < .001) (Fig. 2A and B). Moreover, the percentage of SIRT2 high expression by IHC score >3 was declined in tumor tissues than that in paired adjacent tissues (26.2% vs 50.3%, P < .001) (Fig. 2C). These implied SIRT2 was downregulated in tumor tissue compared to adjacent tissue in cervical cancer patients.
Figure 2.
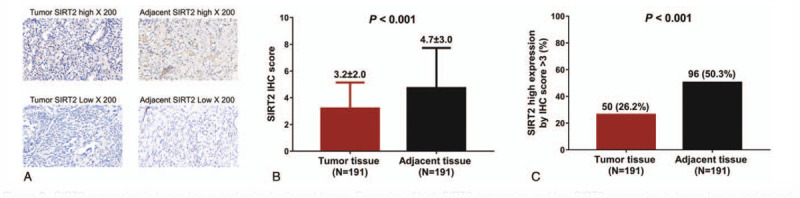
SIRT2 expression in tumor tissue and paired adjacent tissue. Examples of high SIRT2 expression and low SIRT2 expression in tumor tissue and paired adjacent tissue (A). SIRT2 was downregulated in tumor tissue compared with paired adjacent tissue (B), and the percentage of SIRT2 high expression was declined in tumor tissues than paired adjacent tissues (C). Comparisons between the two groups were determined by Wilcoxon rank sum test. P < .05 indicated a significant difference. SIRT2, sirtuin 2.
3.4. Association of tumor SIRT2 expression with clinical characteristics
The SIRT2 expression in tumor tissue was negatively correlated with tumor size (P = .047), lymph node metastasis (P = .009), and FIGO stage (P = .001) in cervical cancer patients (Table 2). However, SIRT2 expression was not associated with age (P = .885), histological type (P = .547), pathological grade (P = .162), or HPV status (P = .790).
Table 2.
Univariate and multivariate Cox's proportional hazards regression model analyses of factors affecting DFS.

3.5. Association of tumor SIRT2 expression with survival profiles
The K–M curve analyses and log-rank test revealed that the DFS was more prolonged in patients who had SIRT2 high expression compared with patients who had SIRT2 low expression (P = .007) (Fig. 3A), and the OS was also elevated in patients with SIRT2 high expression than that in patients with SIRT2 low expression (P = .008) (Fig. 3B).
Figure 3.

Correlation of SIRT2 with survival. DFS (A) and OS (B) were both worse in patients with low SIRT2 tumor tissue expression compared with patients with high SIRT2 tumor tissue expression. Survival curves were plotted with the use of Kaplan–Meier method, and the differences of DFS and OS between two groups were determined by log-rank test. P < .05 indicated a significant difference. DFS, disease free survival; OS, overall survival; SIRT2, sirtuin 2.
3.6. Analyses of factors affecting DFS and OS
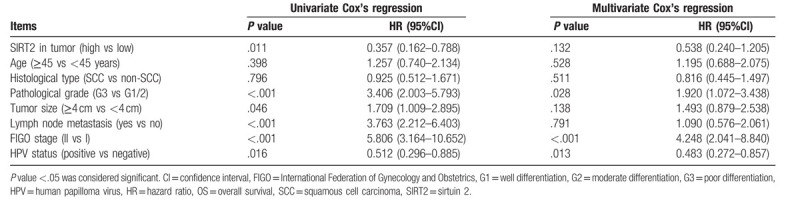
Univariate Cox's proportional hazards regression model analysis displayed that SIRT2 expression in tumor (high vs low) (P = .009) predicted better DFS, while pathological grade (G3 vs G1/2) (P < .001), tumor size (≥4 cm vs <4 cm) (P = .026), lymph node metastasis (yes vs no) (P < .001), and FIGO stage (II vs I) (P < .01) predicted worse DFS in cervical cancer patients (Table 2). Then all the factors were included in the multivariate Cox's proportional hazards regression model analysis, which elucidated that SIRT2 in tumor (high vs low) (P = .084) was not an independent predictive factor for DFS in cervical cancer patients, while pathological grade (G3 vs G1/2) (P = .001) and FIGO stage (II vs I) (P < .001) were independent predictive factors for worse DFS. Furthermore, univariate and multivariate Cox's proportional hazards regression model analyses were also performed for analyzing the factors affecting OS, which revealed that SIRT2 expression in tumor (high vs low) was a predictive factor for more prolonged OS in cervical cancer patients (P = .011), while was not an independent factor for predicting OS (P = .132) (Table 3). In addition, pathological grade (G3 vs G1/2) (P < .001), tumor size (≥4 cm vs <4 cm) (P = .046), lymph node metastasis (yes vs no) (P < .001), FIGO stage (II vs I) (P < .001), and HPV status (positive vs negative) (P = .016) predicted worse OS by univariate Cox's proportional hazards regression model analysis, and pathological grade (G3 vs G1/2) (P = .028), FIGO stage (II vs I) (P < .001) as well as HPV status (positive vs negative) (P = .013) independently predicted shorter OS by multivariate Cox's proportional hazards regression model analysis.
Table 3.
Univariate and multivariate Cox's proportional hazards regression model analyses of factors affecting OS.
4. Discussion
In this study, we found that
-
(1)
SIRT2 expression was downregulated in tumor tissue compared with that in paired adjacent tissue.
-
(2)
SIRT2 expression in tumor tissue was negatively correlated with tumor size, lymph node metastasis, and FIGO stage.
-
(3)
Both the DFS and OS were more satisfactory in patients with SIRT2 high expression in tumor tissue compared with patients with low SIRT2 expression in tumor tissue.
SIRT2, implicated in the regulation of many intracellular functions, exerts its capacities in the interactions of proteins responsible for membrane trafficking, secretion processes, transcriptional regulation, and so on.[17] Since that sirtuins, including SIRT2, have a very broad-spectrum functions, the mediating roles of SIRT2 found in cancers are becoming increasingly abundant. An in vivo experiment reports that the deletion of SIRT2 elevates the tumorigenesis induced by KRAS via mediating K147 acetylation status.[18] And another experiment reveals that SIRT2 is capable of stabilizing slug, which subsequently reduces the malignancy in basal-like breast cancer in vitro.[19] In addition, decreased SIRT2 expression enhances cell proliferation in serious ovarian carcinoma by disinhibiting CDK4 level.[20] And an in vitro experiment shows that SIRT2 diminishes the actin polymerization and cell migration via deacetylating and degrading heat-shock protein 90 (HSP90) in cancer cells.[21] These studies all indicate a tumor suppressive function of SIRT2 in cancers. However, the present knowledge of how SIRT2 functions in cervical cancer are still scarce. In our study, we found that SIRT2 expression in tumor was decreased compared with paired adjacent tissue, and was negatively associated with tumor size, lymph node metastasis, and FIGO stage in cervical cancer patients. Based on the functions of SIRT2 in cancers found by the previous studies, we may conclude that SIRT2 might inhibit tumor progression in cervical cancer via repressing cancer cell proliferation and migration by mediating various carcinogenetic proteins, such as KRAS and HSP90, thus, decreased SIRT2 expression in tumor tissue was correlated with smaller tumor size, no lymph node metastasis, and decreased FIGO stage in cervical cancer in our study.[17–21]
Multiple genes and proteins have been introduced as prognostic biomarkers in cervical cancer; however, the investigation of SIRT2 as biomarker in cervical cancer has not been reported. SIRT2 has been implicated in other carcinomas as prognostic biomarkers, nonetheless, the results of the other studies are controversial. A previous cohort study reveals that the proportion of tumor tissue with positive SIRT2 expression is higher compared with paired adjacent tissue, and SIRT2 expression in tumor tissue is positively associated with tumor invasion, lymph node metastasis, advanced clinical stage, poor DFS, and short OS in esophageal squamous cell carcinoma patients.[22] In contrast, another study elucidates that reduced SIRT2 expression in tumor tissue is correlated with worse clinical outcome in prostate cancer patients.[23] In addition, there is a study reporting that high nuclear SIRT2 protein level is correlated with decreased time to progression in ER+ breast cancer patients in Grade 3; however, in ER+ breast cancer patients in Grade 2, high SIRT2 protein level in nuclear is correlated with elevated time to progression.[24] In our study, patients with SIRT2 high expression in tumor tissue had both longer DFS and OS, which may be resulted from that SIRT2 is capable of delaying the progression of cancer through regulating multiple oncogenic processes, such as cancer cell migration and proliferation, via mediating various proteins, for instance the KRAS.[17–21] However, the multivariate Cox's proportional hazards regression model analysis revealed that SIRT2 expression in tumor tissue was not an independent predictive factor for DFS or OS. This result indicated that SIRT2 might impact on the patients’ survival through implementing other independent predictive factors, such as tumor size and lymph node metastasis, furthermore, our study also revealed that SIRT2 expression was negatively correlated with the level of tumor size and lymph node metastasis, which may also provide explanations to this result.
There were some limitations in this study
-
(1)
The sample size was relatively small, which may impair the statistical power.
-
(2)
As a retrospective cohort study, there might exist some bias and confounding factors, for instance, the selection bias.
-
(3)
The molecular function of SIRT2 in cervical cancer is not investigated in our study. Thus, a prospective study with larger sample size, and molecule mechanism experiments about SIRT2 in cervical cancer should be done in the future.
In conclusion, SIRT2 expression exhibits potential to serve as a biomarker for disease surveillance and prognosis in the management of cervical cancer patients.
Author contributions
Conceptualization: Jiancai Ma, Junde Hou.
Data curation: Jiancai Ma, Hong Wu.
Formal analysis: Liping Yang, Cairu Liu, Jun-de Hou.
Methodology: Hong Wu, Cairu Liu.
Project administration: Liping Yang, Jiancai Ma.
Writing – original draft: Liping Yang, Haiqin Feng, Jiancai Ma, Hong Wu, Cairu Liu, Jun-de Hou.
Writing – review & editing: Liping Yang, Haiqin Feng, Jiancai Ma, Hong Wu, Cairu Liu, Jun-de Hou.
Junde Hou orcid: 0000-0003-4974-9234.
Footnotes
Abbreviations: DAB = diaminobenzidine, DFS = disease free survival, HPV = human papillomavirus, HRP = horseradish peroxidase, IHC = immunohistochemistry, OS = overall survival, SIRT2 = sirtuin 2.
How to cite this article: Yang Lp, Feng Hq, Ma Jc, Wu H, Liu Cr, Hou Jd. SIRT2 expression exhibits potential to serve as a biomarker for disease surveillance and prognosis in the management of cervical cancer patients. Medicine. 2020;99:11(e18668).
The authors have no funding and conflicts of interest to disclose.
References
- [1].Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [2].Brotherton JM, Fridman M, May CL, et al. Early effect of the HPV vaccination programme on cervical abnormalities in Victoria, Australia: an ecological study. Lancet 2011;377:2085–92. [DOI] [PubMed] [Google Scholar]
- [3].Drolet M, Benard E, Boily MC, et al. Population-level impact and herd effects following human papillomavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis 2015;15:565–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gargano JW, Zhou F, Stokley S, et al. Human papillomavirus vaccination in commercially-insured vaccine-eligible males and females, United States, 2007–2014. Vaccine 2018;36:3381–6. [DOI] [PubMed] [Google Scholar]
- [5].Mackay HJ, Wenzel L, Mileshkin L. Nonsurgical management of cervical cancer: locally advanced, recurrent, and metastatic disease, survivorship, and beyond. Am Soc Clin Oncol Educ Book, American Society of Clinical Oncology Annual Meeting 2015;e299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].O’Connor JP, Rose CJ, Waterton JC, et al. Imaging intratumor heterogeneity: role in therapy response, resistance, and clinical outcome. Clin Cancer Res: Off J Am Assoc Cancer Res 2015;21:249–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lee YY, Choi CH, Kim HJ, et al. Pretreatment neutrophil:lymphocyte ratio as a prognostic factor in cervical carcinoma. Anticancer Res 2012;32:1555–61. [PubMed] [Google Scholar]
- [8].Tangjitgamol S, Katanyoo K, Laopaiboon M, et al. Adjuvant chemotherapy after concurrent chemoradiation for locally advanced cervical cancer. Cochrane Database Syst Rev 2014;CD010401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Imai S, Armstrong CM, Kaeberlein M, et al. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 2000;403:795–800. [DOI] [PubMed] [Google Scholar]
- [10].Tanny JC, Dowd GJ, Huang J, et al. An enzymatic activity in the yeast Sir2 protein that is essential for gene silencing. Cell 1999;99:735–45. [DOI] [PubMed] [Google Scholar]
- [11].Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science 2000;289:2126–8. [DOI] [PubMed] [Google Scholar]
- [12].Hu F, Sun X, Li G, et al. Inhibition of SIRT2 limits tumour angiogenesis via inactivation of the STAT3/VEGFA signalling pathway. Cell Death Dis 2018;10:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sun F, Jiang X, Wang X, et al. Vincristine ablation of Sirt2 induces cell apoptosis and mitophagy via Hsp70 acetylation in MDA-MB-231 cells. Biochem Pharmacol 2019;162:142–53. [DOI] [PubMed] [Google Scholar]
- [14].Hu Z, Gu X, Zhong R, et al. Tumor-infiltrating CD45RO(+) memory cells correlate with favorable prognosis in patients with lung adenocarcinoma. J Thorac Dis 2018;10:2089–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ye SL, Li XY, Zhao K, et al. High expression of CD8 predicts favorable prognosis in patients with lung adenocarcinoma: a cohort study. Medicine 2017;96:e6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Shi P, Zhou M, Yang Y. Upregulated tumor sirtuin 2 expression correlates with reduced TNM stage and better overall survival in surgical breast cancer patients. Irish J Med Sci 2019. [DOI] [PubMed] [Google Scholar]
- [17].Budayeva HG, Cristea IM. Human sirtuin 2 localization, transient interactions, and impact on the proteome point to its role in intracellular trafficking. Mol Cell Proteomics 2016;15:3107–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Song HY, Biancucci M, Kang HJ, et al. SIRT2 deletion enhances KRAS-induced tumorigenesis in vivo by regulating K147 acetylation status. Oncotarget 2016;7:80336–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zhou W, Ni TK, Wronski A, et al. The SIRT2 deacetylase stabilizes slug to control malignancy of basal-like breast cancer. Cell Rep 2016;17:1302–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Du Y, Wu J, Zhang H, et al. Reduced expression of SIRT2 in serous ovarian carcinoma promotes cell proliferation through disinhibition of CDK4 expression. Mol Med Rep 2017;15:1638–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Min JS, Kim JC, Kim JA, et al. SIRT2 reduces actin polymerization and cell migration through deacetylation and degradation of HSP90. Biochim Biophys Acta Mol Cell Res 2018;1865:1230–8. [DOI] [PubMed] [Google Scholar]
- [22].Yan L, Zhan X, Jia Z, et al. Sirtuin 2 (Sirt2) expression predicts lymph node metastasis and poor overall survival of patients with esophageal squamous cell carcinoma. Clin Lab 2018;64:669–75. [DOI] [PubMed] [Google Scholar]
- [23].Damodaran S, Damaschke N, Gawdzik J, et al. Dysregulation of sirtuin 2 (SIRT2) and histone H3K18 acetylation pathways associates with adverse prostate cancer outcomes. BMC Cancer 2017;17:874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].McGlynn LM, Zino S, MacDonald AI, et al. SIRT2: tumour suppressor or tumour promoter in operable breast cancer? Eur J Cancer 2014;50:290–301. [DOI] [PubMed] [Google Scholar]


