Abstract
The enthesis is an organ that connects a soft, aligned tissue (tendon/ligament) to a hard, amorphous tissue (bone) via a fibrocartilage interface. Mechanically, the enthesis sustains a dynamic loading environment that includes tensile, compressive, and shear forces. The structural components of the enthesis act to minimize stress concentrations and control stretch at the interface. Current surgical repair of the enthesis, such as in rotator cuff repair and anterior cruciate ligament reconstruction, aim to bridge the gap between the injured ends via reattachment of soft-to-hard tissues or graft replacement. In this review, we discuss the multiscale, morphological, and mechanical characteristics of the fibrocartilage attachment. Additionally, we review historical and recent clinical approaches to treating enthesis injury. Lastly, we explore new technological advancements in tissue-engineered biomaterials that have shown promise in preclinical studies.
Keywords: enthesis, tendon, ligament, meniscus, bone, mechanics
Graphical Abstract
INTRODUCTION
The burden of musculoskeletal disorders on the economy is significant, resulting in lost wages, time away from work, and an ever-increasing duration of pain and disability.1 In 2014, 32% of lost work days were related to musculoskeletal injuries.2 Musculoskeletal injuries involving tendon or ligament rupture often require surgical repair for their functional reattachment back to bone.3–9 In such cases, the attachment of the connective tissue, known as the enthesis, rarely regenerates after surgical repair10–12 and can result in a high probability of failure.13–15 Unfortunately, some clinical cases of enthesis injury, such as rotator cuff tears and anterior cruciate ligament (ACL) rupture, are unresolved due to failure of the tendon/ligament to sufficiently integrate into the bone. The clear clinical problem associated with poor enthesis healing following injury requires understanding of the function, structure, and healing potential of the native attachment. Recapitulating the intact enthesis following injury is the “holy grail” in tendon and ligament reconstruction. Therefore, the development of strategies that guide the reattachment and bridging of soft tissue to bone, resulting in regeneration and return of function and strength of the attachment, are of high priority in the field of orthopedics.
Enthesis injuries can occur at practically any site of tendon/ligament attachment to bone, with the most common sites located at the rotator cuff of the shoulder16,17 and the cruciate ligaments of the knee.18,19 Upon surgical repair, the multiscale enthesis structure is difficult to regenerate without guided intervention, leaving the repaired tendon-bone attachment susceptible to failure at rates as high as 94%.13,14 Re-establishing a robust enthesis is important not only for the return-to-function for many athletes but also for daily function and independence for individuals of all ages.20–22 Unfortunately, following repair, pull-out or rupture of the graft-bone interface is the driving cause of failure. For example, nearly 25% of all ACL reconstructions fail due to laxity or failure at the graft–bone interface.23 Failure of the repaired ACL to integrate into bone can result in knee instability24,25 that has implications for the early onset of knee osteoarthritis (OA).23,26 Integration at the graft–bone interface is a problem for other musculoskeletal tissue analogues as well, such as the meniscus. Thus, successful regeneration of the native and strong enthesis is imperative for maintaining long-term joint health. Regenerating a functional and strong interface between graft and bone also presents critical design criteria for developing new biomaterials to treat tendon and ligament injuries that require engrafted replacement of the fibrous tissues. To accomplish congruent, integrated, and functional interfaces, we must understand the multiscale biological, mechanical, and structural incorporation of native tendon/ligament to bone.
The goal of this review is to highlight our current understanding of the multiscale morphology, mechanics, and pathology of the tendon/ligament enthesis, discuss current clinical strategies and obstacles of enthesis repair, and propose new and future directions for incorporating biomaterials in enthesis regeneration.
MORPHOLOGY AND MECHANICS OF THE MULTISCALE ENTHESIS
The enthesis is an organ that connects a soft, aligned tissue (tendon/ligament) to a hard, amorphous tissue (bone) (Figure 1). The morphology of the attachment is generally consistent across fibrocartilage entheses, as shown for ligament (Figure 1A), meniscus (Figure 1B), and tendon (Figure 1C) attachments. In this review, we will use “ligament” and “tendon” interchangeably in reference to enthesis morphology and mechanics. Additionally, we lead with the assumption that the ligament, meniscus, and tendon fibrocartilage enthesis are generally comparable in morphology and mechanical behavior. However, it should be noted that some variations in morphology and biochemistry exist among tendon, meniscus, and ligament as well as, presumably, their associated entheses. However, few have directly compared cross-anatomical entheses associated with ligament, meniscus, and tendon, and direct comparisons of morphological, biochemical, and mechanical properties of the ligament, meniscus, and tendon are not common in the literature. This may be a result in intraconnective tissue heterogeneity (i.e., tendon patterning is dependent on its anatomical and mechanical requirements). We do know that, in general, patellar and Achilles tendons have less DNA and glycosaminoglycan content, but have more collagen (type I), compared to several knee ligaments (e.g., ACL, posterior cruciate ligament, and medial collateral ligament).27 Historical work has shown that the patellar tendon is stronger and more stiff compared to the ACL, posterior cruciate ligament, and lateral collateral ligament.28 The differing characteristics noted previously between tendon and ligament are presumed to be related to (1) differences in the rate of maturation between tendon/ligament (e.g., proximal attachments may mature prior to distal attachments), (2) differences in metabolic/functional requirements (e.g., stabilizing ligaments versus large force-generation tendons), and (3) varying anatomical location/proximity to joints.27 Nonetheless, variability in tissue structure across tendons, let alone among tendon, meniscus, and ligament, exists; therefore, we aim to describe the fibrocartilage enthesis as it pertains to the connective tissue structures generally.27
Figure 1.
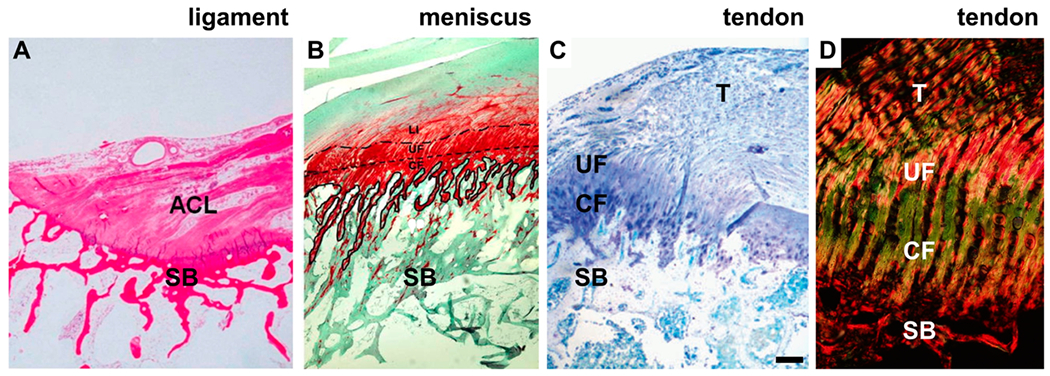
Enthesis of the anterior cruciate ligament (ACL), meniscus, and rotator cuff tendon, shown histologically. SB = subchondral bone, CF = calcified fibrocartilage, UF = unmineralized fibrocartilage, and T = tendon. (A) Histology of the ACL enthesis at the tibial attachment, stained using hematoxylin and eosin.43 (B) Histology of the meniscal enthesis, stained using Safranin O,44 highlights the fibrocartilage region rich in proteoglycan in red. The dashed line in B indicates the tidemark between SB and CF. (C and D) Histology of the supraspinatus enthesis of the rotator cuff tendon, stained using (C) toluidine blue45 for enhanced metachromatic stain of the fibrocartilage (purple color) and (D) picrosirius red for enhanced birefringence under polarized light. Scale bar in C = 200 μm. The image in A was adapted with permission from ref 43. Copyright 2014 Sage. The image in B was adapted with permission from ref 44. Copyright 2008 Springer. The image in C was modified with permission from 45. Copyright 2011 International Bone and Mineral Society.
Variation in the size, alignment, and footprint of the enthesis depends on the loading requirements of the attachment, as explained later in this review. Between the tendon and bone exists the transitional tissue known as the fibrocartilage, which maintains two distinct regions; the calcified fibrocartilage (CF, Figure 2, dark blue region) and the uncalcified fibrocartilage (UF, Figure 2, light blue region). The fibrocartilage forms during skeletal maturation, and the distinct regions are separated by a mineralized tidemark. The enthesis footprint typically forms on a bone eminence, which is defined as a projection, ridge, and tuberosity on the surface of bone that provides bone its topographical shape. The eminence is derived during embryonic development and forms modularly from cells that are descendants of a scleraxis+ and sox9+ cell population.29,30 The modular formation of the eminence has been suggested to follow a “segregation” model, which describes the initial establishment and subsequent separation of the common enthesis progenitor pool during embryonic growth.31 The segregation model does not require coordinated signaling for navigation of the tendon to the attachment site; rather, the tendon and enthesis form together and in situ. Currently, it is theorized that the pluripotency of the enthesis progenitors allows for the intricate assembly of its unique gradient cellular and extracellular morphology during development rather than coordinated, guided migration of tendon to the eminence.29,31–33
Figure 2.
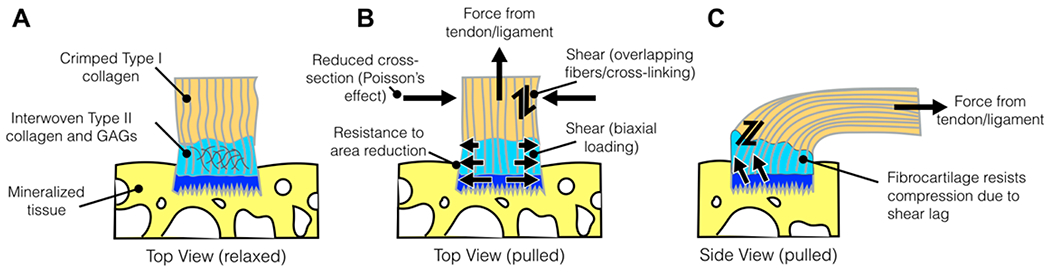
(A–C) Tan coloration represents the tendon, light blue represents the uncalcified fibrocartilage (UF), dark blue represents the calcified fibrocartilage (CF), and yellow represents the bone. Forces applied to the enthesis, illustrated in both B (top view) and C (side view), are derived from tensile tendon/ligament force, compression, and shear.
The enthesis morphology is, in general, true for all fibrocartilage entheses, regardless of the soft-tissue derivative (i.e., tendon, ligament, or meniscus; Figure 1). The extracellular matrix of the UF includes a multitude of collagen types, including type I, II, III, IX, and X,12,34–36 as well as elastin fibers, aggrecan, glycosaminoglycans (GAGs), and proteoglycans (PGs).34 Alignment of the collagen fibrils from the soft tissue to bone (Figure 1D) has been shown to be dependent on the anatomical location,37 which is presumed to be a function of the loading requirements of the soft tissue. The cellular makeup of the enthesis is unique compared to the fibroblasts of the tendon and osteocytes/osteoblasts of the subchondral bone.38 The cell shape and organization spatially changes from tendon/ligament to bone in the mature fibrocartilage attachment. Aligned spindle cells reside in the tendon, with a mixture of spindle and round cells (or fibrochondrocytes) in the fibrocartilage and osteoblasts/osteocytes in the SB. During embryonic development, the enthesis cells modularly form the attachment29,30 and, in mice, have been recently shown to maintain residence throughout growth.39 Resident enthesis cells display a prehypertrophic, fibrochondrocyte phenotype akin to an arrested growth plate.39 The size and extent of the fibrocartilage and its degree of mineralization are dependent on the mechanical loading requirements of the attachment (Figure 2B and C).31,40–42
Mechanically, the enthesis sustains a dynamic loading environment that includes tensile, compressive, and shear forces (Figure 2B and C). This is accomplished through several structural and compositional elements, which act to minimize stress concentrations and control stretch at the interface. The complex, 3-dimensional fibrocartilage enthesis forms in response to the loading environment during development.10,12,31,46,47 Upon active loading from contracting skeletal muscle (tendon) or passively through joint motion (ligament), tensile forces from the tendon/ligament are transmitted along aligned collagen fibrils into the fibrocartilage and then bone (Figure 2B and C). The collagen fiber alignment splays outwardly near the interface and has a profound impact on the location of both maximum principle strain and maximum principle stress.47,48 The presence of GAGs within the fibrocartilage plays a role in resisting compression of the enthesis due to Poisson’s effect;49,50 interwoven collagen fibers and the accumulation of GAGs maintain an elevated swelling pressure by binding to and locking in water in the fibrocartilage and thus resisting compression. These structural features support the “stretching brake” theory, posited by Knese and Biermann,51 who described one functional role of the enthesis is to limit the narrowing of the interface under tension. However, tensile loads are not the only loads applied to the enthesis; fibrocartilage attachments develop at anatomical sites where tendons/ligaments function in multiaxis loading. In other words, the footprint of the fibrocartilage attachment is larger than the cross-section of the tendon/ligament itself, which aids in the redirection of forces away from the interface and distributes loads over a larger area (Figure 2).52 Macroscopically, fibrocartilage formation also experiences compression and shear at the articular surface, which occurs when the tendon wraps around bone rather than inserting directly into bone.46 The splayed fibrocartilage footprint also prevents the tendon from deforming around a sharp corner at the mineralized interface, acting as a grommet between bone and soft tissue.10 At the microscale, the tendon also experiences interfibrillar shear from fibril sliding due to load-sharing between fibrils.53
The fibrocartilage enthesis also establishes interdigitations at the CF/SB interface (Figure 1B and Figure 2).37,54 The frequency, size, and distribution of interdigitations at the ligament/bone interface are likely regulated by the mechanical loading environment.55 Recently, the interdigitation of the tendon–bone attachment has been demonstrated computationally to act as a toughening mechanism between the CF and SB.39 Deep-rooted interdigitations of ligamentous fibers (e.g., from the tibial ACL anteromedial bundle) derive their depth because of their need to resist multidirectional tensile forces.56,57 In the diseased state, such as OA, the tidemark at the meniscal UF/CF is disrupted, and interdigitation-like patterning forms in addition to soft-tissue calcium deposits, osteophyte formation, microcracks, fissuring, and duplication of the tidemark.54 Whether disruption of the interface is a result of or a driving factor in disease progression has yet to be determined.58
PATHOLOGICAL FACTORS THAT MODULATE ENTHESIS REGENERATION
Age-related changes can result in permanent changes to the structure and function of the enthesis.59 Disruption in the congruency of collagen fiber interdigitation,54 FC mineralization,54 and/or GAG and proteoglycan distribution58 across the enthesis are associated with enthesis disorders; however, it is unclear if these changes initiate or are the result of enthesis degeneration. Regardless, enthesis degeneration is prevalent in chronic disorders, such as OA54 and rotator cuff disease.60,61 Following injury, the residual stress in tendons and ligaments causes the soft tissues to retract from their bony insertions.62 Clinically, acute tears of the tendon/ligament have a higher capacity for healing compared to chronic, prolonged detached soft tissues.63–66 The inflammatory process involved in healing following acute injury has been studied in patients as well as using animal models. Acute injuries, such as following rotator cuff tears, follow the process of natural wound healing. Almost immediately following injury, vascularization and inflammation of the injured interface can occur.67–69 Chemotactic factors, such as vascular endothelial growth factor (VEGF), transforming growth factor-b (TGF-β), and platelet derived growth factor (PDGF), are secreted locally and encourage recruitment of fibroblasts to the injury site.70 Neutrophils, macrophages, and osteoclasts are then recruited to the injury site for remodeling of the damaged tendon, fibrocartilage, and bone.67,68,70–72 During repair, fibroblasts proliferate and produce ECM proteins including GAGs and collagen type III. Functionality of the tissue is typically restored within months during the last stage of healing, known as the remodeling phase, where deposited collagen type III is converted to highly organized collagen type I. However, the remodeling of enthesis following injury insufficiently recapitulates the native enthesis, and the healed structure is often structurally disorganized and mechanically inferior.1–3,31,48,63,73–75 Further, the morphology and organization of enthesis cells do not return to normal after injury.76 Additionally, chronic/large enthesis injuries, such as that observed with massive rotator cuff tears, have a high likelihood of failure to heal following repair.6,14 It is currently unknown what role the inflammatory process plays in these different healing scenarios. Along with potential variations in the inflammatory response, investigations have elucidated the potential role of mechanical cues in the healing response. Altered mechanical loading from the muscle or joint may differentially regulate the healing response following acute versus chronic tendon/ligament injury.63,64,68,77,78 While adaptations in bone and muscle structure are evident following chronic tendon-bone injury in several clinical and preclinical studies; the functional outcomes associated with these factors are nuanced.14,63,64,68,73,74,77,79,80 Additionally, associations with muscle and bone loss can have substantial implications in the health, maintenance, and healing of the enthesis, but these relationships have not yet been clearly defined.
CLINICAL STRATEGIES FOR REPAIRING THE INJURED ENTHESIS
Improvements in repair outcomes have recently relied on leveraging the technological advancements from tissue-engineered biomaterials. For example, mobilization of the tendon to the native bony footprint is difficult due to retraction forces and remodeling of tissue.74,81,82 Scaffolds that bridge the gap between the tendon stump and bone may provide reinforcement during healing.83,84 These scaffolds must be strong enough to withstand the physiological and pathophysiological loading of a normal joint. Many preclinical devices for repairing musculoskeletal interfaces, such as interpositional grafting85 and rotator cuff augmentation,86 have failed due to inadequate mechanical and structural properties of graft material (e.g., autograft, allograft, xenograft, or tissue engineered scaffold), which ultimately influence graft failure.87 Unfortunately, there have been few victories in the clinic with utilization of scaffolds for enthesis repair in human patients. Additionally, extracellular matrix scaffolds used clinically have had a limited number of follow-up studies in human patients.83 The use of cross-linking to stiffen biological scaffolds has been shown to elicit an immune response upon implantation, and several xenogenic patches have led to immunogenic responses.88,89 Nonetheless, some devices used for patch augmentation and restoration of the rotator cuff tendon-bone attachment, such as GraftJacket,90,91 have shown some promising results. The GraftJacket is a freeze-dried, human-derived, non-cross-linked, and decellularized dermal allograft that has been implemented clinically in small cohorts of patients.90,91 In human clinical trials, full matrix incorporation of the scaffold into the native tissue has been observed via magnetic resonance imaging.92 Additionally, following rotator cuff repair, patients with GraftJacket augmentation demonstrated improvements in external rotation strength compared to preoperative metrics.92 However, cadaveric93 and laboratory94 studies have demonstrated several potential failure mechanisms related to the GraftJacket, such as suture pull-through and breakage. These data suggest that a combination of improved scaffold properties (structural and mechanical) and suture techniques is important for the success of scaffold-based repair of the rotator cuff and other tendons/ligaments entheses. While these scaffolds act as a patch that physically connects the tendon to bone, their use has been primarily limited to patients with massive, otherwise irreparable tears.90,91 There exist several issues with the health of the rotator cuff in this distinct patient population, including fat atrophy, weakness of the cuff muscles, and localized subchondral bone loss at the attachment footprint. The verdict is still out as to whether or not patches and grafts, which behave as bridges between the tendon and bone, are capable of restoring the mechanical function of the interface as well as allowing for the patient to regain rotator cuff muscle and bone health. If the functional reattachment of the tendon to bone is enough to overcome the chronic, degenerative changes that occur in rotator cuff disease, then devices like the GraftJacket could revolutionize the way surgeons treat patients with otherwise irreparable tears.
Knee ligament injuries, such as ACL tears, are common sources of musculoskeletal pain.95,96 ACL tears do not heal, leaving a gap between the injured ends,97 and surgical reconstruction is the current standard of care.98–103 Suturing the ends leads to failure rates as high as 17%,104 while reconstruction using allograft and xenograft has been shown to be limited by immune rejection.96,105–109 Trials using allografts, such as cadaveric patellar and hamstring tendons, have shown that failure rates following ACL reconstruction increase from 6 to 20%, compared to autografts, in adolescents.110 Surgical ACL reconstruction using autografts is the current standard of care.98,99,103 However, autograft ACL reconstruction surgery has been shown to be limited by donor-site morbidity, i.e., complications associated with the graft location.111 Donor-site morbidity may contribute to increased anterior knee pain and risk of repair failure after autograft reconstruction.103,110 Several factors have improved the outcomes of ACL reconstruction using autografts, including choice of autograft retrieval location and size, location and size of the bone tunnel, proper graft tension, anatomic graft fixation, and graft fixation strength.98 Clinical trials using patellar autografts have demonstrated reduced failure compared to hamstring tendon autografts;99,106 however, patellar autografts resulted in increased rate of anterior knee pain.99 Additionally, graft failure can occur due to mechanical factors, such as trauma-induced overloading, fatigue of the graft over time, and improper surgical technique.96,98,112 Potential biological modes of failure include effusion, infection, and bone tunnel widening, primarily at the site of graft-to-bone integration, producing mechanically weaker interfaces that can ultimately fail.106
Manufactured scaffolds to replace the ligament74,113,114 or to bridge the gap between the injured ends95,97,115 have several potential advantages, including obfuscating disease transmission, design control, sterilization, and biocompatibility.116 However, few have demonstrated success in clinical trials.117 While manufactured scaffolds for ligament replacement are numerous, the long-term disadvantages include fatigue of the material, indicating inadequate material properties for the mechanical loads found in vivo and decreased tissue ingrowth. Additionally, there are limited clinical trials using tissue-engineered ligaments. Approved devices, such as Intergraft (carbon fiber),118 Gore-Tex ACL (polytetrafluoroethylene),119 Stryker Dacron Ligament (polyethylene terephthalate),120 Leeds-Keio Artificial Ligament (polyethylene terephthalate),121 and Kennedy Ligament Augmentation Device (polypropylene),122 have reported negative results.114 Intergraft resulted in tunnel widening, synovitis, and high rupture rate.118 Patients with Gore-Tex and Kennedy ACL reconstructions presented with joint instability and a high rate of effusion and inflammation, and wear particles were found within the synovial fluid.122 Reconstruction with the Leeds-Keio Artificial Ligament, along with Stryker Dacron Ligament and Kennedy ligaments, resulted in excessive laxity and unacceptable rates of failure ranging from 19% to 80% at long-term follow-ups.118,119,121,122 These complications led to the removal of all of these devices from the market. The Ligament Augmentation and Reconstruction System (LARS)118,123 remains on the market and is showing promising results, but it is not available in the United States and lacks long-term follow-up. Current LARS outcomes demonstrate reduced incidence of infection and decreased failure rate compared to that of the previous synthetic grafts.123 Overall, mechanical and biological failure are the main limitations for synthetic grafts; thus, future grafts should have improved mechanical and biological properties more suited for the biomechanical environment within the injured knee.
Preclinical studies investigating tissue-engineered scaffolds suggest the potential benefit over traditional autograft reconstruction.95,113,115 Primarily, tissue-engineered scaffolds eliminate the risk of donor-site morbidity.111 These devices can be industrially manufactured to mimic the native structural and mechanical features of the enthesis and are processed for sterility and biocompatibility. Additionally, combinatorial approaches in tissue-engineered scaffold design can incorporate biological or chemical factors that encourage cellular infiltration, mineralization, and matrix deposition. For example, silk-collagen,113 silk-tricalcium phosphate-polyether ketone (silk-TCP),124 and poly-(lactic-co-glycolic acid) (PLGA) triphasic scaffolds125 have shown decent integration of scaffold-to-bone and may be potential alternatives to autograft reconstruction. Silk-collagen scaffolds induced a similar response to autograft reconstruction with abundant fibroblast-like cell proliferation and positive Tenascin-C staining, and improved trabecular bone growth in the scaffold group.113 Similarly, silk-TCP scaffolds created a transition of ligament-to-bone similar to native ACL bony insertions, with transitional zones of silk fibers, fibrous tissue, fibrocartilage, TCP, and bone.124 The PLGA triphasic scaffold maintained distinct cellular regions and phase-specific matrix deposition akin to the native ACL enthesis.125 Another alternative is bridge-enhanced ACL repair (BEAR), which was shown to have the same mechanical properties as autograft reconstruction and reduced post-traumatic OA in pigs.115 Future clinical trials with long-term follow-ups are necessary to validate these tissue-engineered scaffolds as alternatives to the current standard of ACL reconstruction.4
EMERGING STRATEGIES FOR ENHANCED ENTHESIS HEALING
Designing for success in enthesis augmentation is nuanced, with the mechanical environment an obvious factor to consider.126 Failures in the clinical and preclinical stages emphasize the need to develop new strategies to improve outcomes. Current approaches in tissue engineering have shown promising results to improve tendon-to-bone repair compared to the current, standard clinical treatments.127,128
The complex structure and function of the enthesis make it difficult to design appropriate devices and reliable strategies for augmentation and repair. Recent tissue-engineering approaches have attempted to recapitulate the local structural environment by capitalizing on the known alignment of collagen fibers at the interface. For example, scaffolds using biodegradable materials, such as silk129 and collagen,130 have been manufactured using electrospinning techniques, which result in spatially aligned, thin nanofiber sheets. Electrospun polymers have also been used to generate aligned fiber scaffolds showing potential in preclinical augmentation of the rotator cuff tendon–bone attachment and other musculoskeletal interfaces.131–150 Several advantages exist for using electrospinning techniques for interfacial scaffolds. The choice of material, pore space and connectivity, density, fibril alignment, mechanical properties, surface area, and functionalization can be controlled during or after electrospinning.145,151–154 Recent work has shown that the micromechanical properties of single electrospun PLGA fibers can be modified using crystalline coatings of hydroxyapatite.140 In addition, crimping of electrospun polylactic acid (PLA) nanofibers can be controlled using chemical modifications,155 airflow/heat,148,156 magnetic/electric fields157,158 or self-assembly.159 Aligned, electrospun fibers with gradations of mineral content160–162 also hold promise for recapitulating the microscale and nanoscale structure of the enthesis. Such scaffolds can provide a platform for fine control and patterning of cellular differentiation into osteoblasts163 and potentially chondrocytes and tenocytes as well.164 Stem cell differentiation can also be controlled by varying the alignment and diameter of electrospun PLGA fibers.144 The use of electrospinning techniques to create biomaterials that mimic the native structural and mechanical features of the multiscale enthesis holds promise for micropatterning and spatial deposition of biomolecules and scaffold materials to help guide regeneration of the microscale enthesis.
Additive manufacturing, also known as 3-dimensional printing, has become popular for personalized fabrication given its low cost, ease of use, and versatility.165–167 The use of additive manufacturing with hydrogel-based bioinks165 and/or selective laser/electron beam melting168 can aid in design specificity from computer-aided or image-based design.166,167 Additive manufacturing for medical implantation may hold promise for enthesis prostheses. Assembly of an artificial interface using 3-dimensional printing techniques that allows for controlled deposition of mineral and collagen, with spatially dependent mechanical reinforcement, could potentially revolutionize the current design of graft-bone replacements. Recently, printable hydrogels have been used for the manufacturing of organ-scale constructs of mechanically reinforced templates that promote bone formation, such as vertebral bodies.165 Additionally, the use of printing techniques to guide bone accrual at the bone/graft interface has shown promise in preclinical models of ACL replacement.166
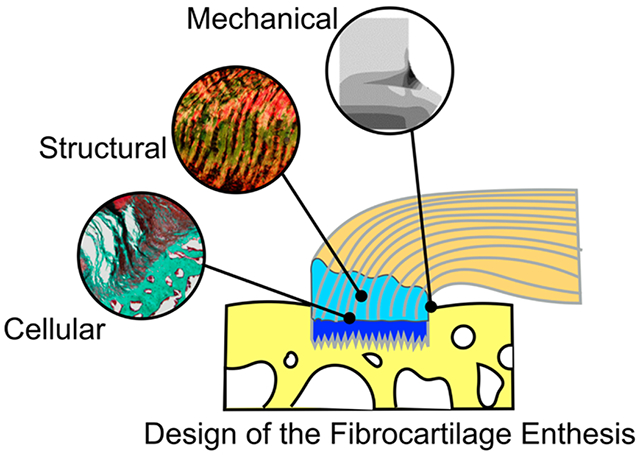
Examining the structural and functional elements of the enthesis reveals how nature overcomes the engineering feat of joining dissimilar materials. Engineering tools and principles have identified key features that contribute to this robust union. The clinical challenge now remains to reassemble these pieces and integrate them in the same fashion as they develop. The ongoing advancements in understanding the native enthesis continue to guide new and exciting approaches to improve repair strategies and long-term outcomes. Approaches to fine-tune the design of engineered materials will guide the cellular, mechanical, and structural regeneration of the fibrocartilage enthesis (Figure 3). Repairing the damaged enthesis using scaffolds that aid in spatially patterned cell differentiation (e.g., gradient in cell shape from tenocytes to fibrochondrocytes to osteoblasts) can enhance the stem cell differentiation from native or exogenously delivered cells. In order to recapitulate the mechanical environment, we can improve the design of scaffolds to address physiological design criteria. Potential directions include designing scaffolds with gradients in stiffness and mineral between soft tissue and bone, wrap-aligned fibers, spatial deposition of charged macromolecules for localized water storage, and/or fibril cross-linking (Figure 3). These design criteria must meet the needs of the in vivo loading environment, such that the scaffold can maintain multiaxis loading and act as a “stretching brake” (Figure 3). Although not exhaustive, such recommendations are currently being pursued in preclinical models and hold potential for bench-to-bedside therapies in the future.
Figure 3.
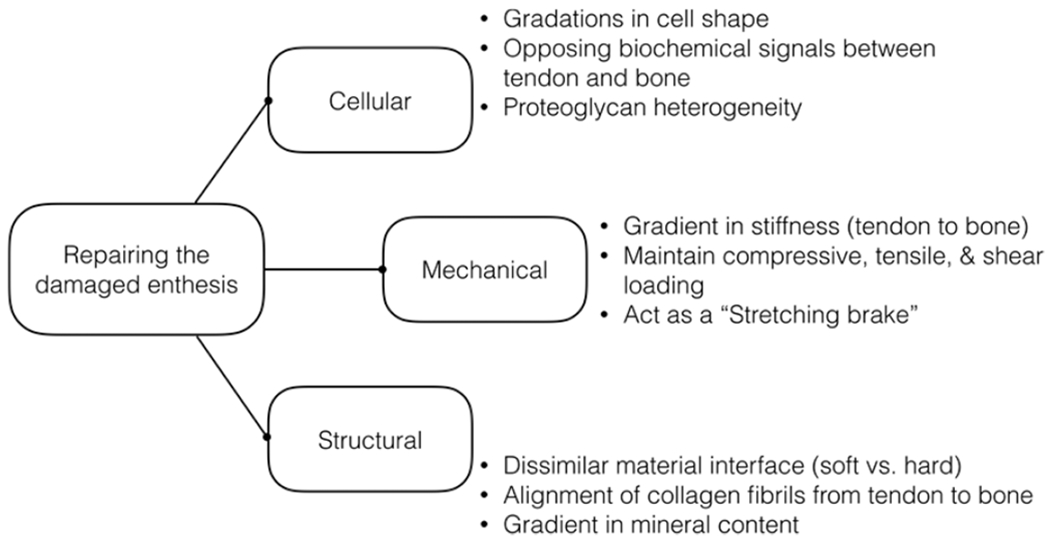
Repairing the damaged enthesis requires design criteria and strategies aimed to mimic the native cellular, mechanical, and structural features of the enthesis.
CONCLUSION
The native structural features of the enthesis play a crucial role in the mitigation of stress concentrations at the interface between tendon and bone. After injury, the tendon/ligament retracts from bone due to tensile forces and then undergoes typical wound healing, which produces a mechanically weaker and structurally disorganized tissue. Current clinical techniques used to repair the interface, such as bridging and graft reconstruction, can fail due to inadequate, weak graft-to-bone integration. Therefore, replicating the native biological, structural, and mechanical features of the tendon-to-bone interface is the idealized scenario for designing tissue engineered constructs for enthesis repair. Advanced techniques, for example, electrospinning and 3D printing, enable control of multiscale material properties and spatial deposition of mineral and biochemical patterning akin to the native enthesis. Development and investigation of materials that functionally restore the structural, cellular, and mechanical properties of the enthesis are needed to improve the repair of this unique musculoskeletal organ.
ACKNOWLEDGMENTS
This work was supported in part by funding from the Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health (P30GM103333) and from the University of Delaware Research Foundation. The Table of Contents and Abstract graphic was reproduced with permission from ref 47. Copyright 2006 Elsevier.
Footnotes
The authors declare no competing financial interest.
REFERENCES
- (1).US Burden of Disease Collaborators. 1990–2010: Burden of diseases, injuries, and risk factors. JAMA 2013, 310 (6), 591–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Nonfatal Occupational Injuries and Illnesses Requiring Days Away from Work, 2014. In USDL 15-2205; U.S. Department of Labor: Washington, DC, 2015. [Google Scholar]
- (3).Clancy JWG; Zoltan D Acute tears of the anterior cruciate ligament. Surgical versus conservative treatment. J, Bone Joint Surg. 1988, 70 (10), 1483–1488. [PubMed] [Google Scholar]
- (4).Herbst E; Hoser C; Göller P; Hepperger C; Abermann E; Neumayer K; Musahl V; Fink C Impact of surgical timing on the outcome of anterior cruciate ligament reconstruction. Knee Surgery Sports Traumatology, Arthroscopy 2016, DOI: 10.1007/s00167-016-4291-y. [DOI] [PubMed] [Google Scholar]
- (5).Sugaya H; Maeda K; Matsuki K; J M Functional and structural outcome after arthroscopic full-thickness rotator cuff repair: single-row versus dual-row fixation. Arthroscopy: J. Arthroscopic Relat. Surg 2005, 21, 1307–1316. [DOI] [PubMed] [Google Scholar]
- (6).Boileau P; Brassart N; Watkinson D; Carles M; Hatzidakis A; Krishnan S Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J. Bone Joint Surg 2005, 87, 1229–1240. [DOI] [PubMed] [Google Scholar]
- (7).Lo I; Burkhart S Double-row arthroscopic rotator cuff repair: re-establishing the footprint of the rotator cuff. Arthroscopy 2003, 19 (9), 1035–1042. [DOI] [PubMed] [Google Scholar]
- (8).Lafosse L; Brozska R; Toussaint B; Gobezie R The outcome and structural integrity of arthroscopic rotator cuff repair with use of the double-row suture anchor technique. J. Bone Joint Surg 2007, 89 (7), 1533–1541. [DOI] [PubMed] [Google Scholar]
- (9).Villegas DF; Maes JA; Magee SD; Donahue TL Failure properties and strain distribution analysis of meniscal attachments. J. Biomech 2007, 40 (12), 2655–2662. [DOI] [PubMed] [Google Scholar]
- (10).Benjamin M; Toumi H; Ralphs JR; Bydder G; Best TM; Milz S Where tendons and ligaments meet bone: attachment sites (’entheses’) in relation to exercise and/or mechanical load. J. Anat 2006, 208 (4), 471–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Lu HH; Subramony SD; Boushell MK; Zhang X Tissue engineering strategies for the regeneration of orthopedic interfaces. Ann. Biomed. Eng 2010, 38 (6), 2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Thomopoulos S; Genin GM; Galatz LM The development and morphogenesis of the tendon-to-bone insertion - what development can teach us about healing. J. Musculoskeletal Neuronal Interact 2010, 10 (1), 35–45. [PMC free article] [PubMed] [Google Scholar]
- (13).Harryman DT 2nd; Mack LA; Wang KY; Jackins SE; Richardson ML; Matsen FA 3rd Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. Journal of Bone & Joint Surgery 1991, 73 (7), 982–989. [PubMed] [Google Scholar]
- (14).Galatz LM; Ball CM; Teefey SA; Middleton WD; Yamaguchi K The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J. Bone Joint Surg 2004, 86 (2), 219–224. [DOI] [PubMed] [Google Scholar]
- (15).Galatz LM; Griggs S; Cameron BD; Iannotti JP Prospective longitudinal analysis of postoperative shoulder function: a ten-year follow-up study of full-thickness rotator cuff tears. J. Bone Joint Surg 2001, 83 (7), 1052–6. [PubMed] [Google Scholar]
- (16).Tempelhof S; Rupp S; Seil R Age-related prevalence of rotator cuff tears in asymptomatic shoulders. Journal of Shoulder and Elbow Surgery 1999, 8 (4), 296–299. [DOI] [PubMed] [Google Scholar]
- (17).Yamamoto A; Takagishi K.; Osawa T; Yanagawa T; Nakajima D; Shitara H; Kobayashi T Prevalence and risk factors of a rotator cuff tear in the general population. Journal of Shoulder and Elbow Surgery 2010, 19 (1), 116–120. [DOI] [PubMed] [Google Scholar]
- (18).Gianotti SM; Marshall SW; Hume PA; Bunt L Incidence of anterior cruciate ligament injury and other knee ligament injuries: a national population-based study. Journal of Science and Medicine in Sport 2009, 12 (6), 622–627. [DOI] [PubMed] [Google Scholar]
- (19).Prodromos CC; Han Y; Rogowski J; Joyce B; Shi K A meta-analysis of the incidence of anterior cruciate ligament tears as a function of gender, sport, and a knee injury—reduction regimen. Arthroscopy: The Journal of Arthroscopic & Related Surgery 2007, 23 (12), 1320–1325. [DOI] [PubMed] [Google Scholar]
- (20).Gardinier ES; Manal K; Buchanan TS; Snyder-Mackler L Clinically-relevant measures associated with altered contact forces in patients with anterior cruciate ligament deficiency. Clinical Biomechanics 2014, 29 (5), 531–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Lentz TA; Zeppieri G; George SZ; Tillman SM; Moser MW; Farmer KW; Chmielewski TL Comparison of physical impairment, functional, and psychosocial measures based on fear of reinjury/lack of confidence and return-to-sport status after ACL reconstruction. American Journal of Sports Medicine 2015, 43 (2), 345–353. [DOI] [PubMed] [Google Scholar]
- (22).MacLeod TD; Snyder-Mackler L; Axe MJ; Buchanan TS Early regeneration determines long-term graft site morphology and function after reconstruction of the anterior cruciate ligament with semitendinosus-gracilis autograft: a case series. Int. J. Sports Phys. Ther 2013, 8 (3), 256–268. [PMC free article] [PubMed] [Google Scholar]
- (23).Salmon LJ; Russell VJ; Refshauge K; Kader D; Connolly C; Linklater J; Pinczewski LA Long-term outcome of endoscopic anterior cruciate ligament reconstruction with patellar tendon autograft: minimum 13-year review. Am. J. Sports Med 2006, 34 (5), 721–32. [DOI] [PubMed] [Google Scholar]
- (24).Fleming BC; Spindler KP; Palmer MP; Magarian EM; Murray MM Collagen-platelet composites improve the biomechanical properties of healing anterior cruciate ligament grafts in a porcine model. American Journal of Sports Medicine 2009, 37 (8), 1554–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Beynnon BD; Johnson RJ; Toyama H; Renstrom PA; Arms SW; Fischer RA The relationship between anterior-posterior knee laxity and the structural properties of the patellar tendon graft. A study in canines. American Journal of Sports Medicine 1994, 22 (6), 812–820. [DOI] [PubMed] [Google Scholar]
- (26).Siebold R; Buelow JU; Boes L; Ellermann A Primary- and revision-reconstruction of the anterior cruciate ligament with allografts: a retrospective study including 325 patients. Zentralbl. Chir 2002, 127 (10), 850–4. [DOI] [PubMed] [Google Scholar]
- (27).Amiel D; Frank C; Harwood F; Fronek J; Akeson W Tendons and ligaments: a morphological and biochemical comparison. J. Orthop. Res 1984, 1 (3), 257–265. [DOI] [PubMed] [Google Scholar]
- (28).Butler DL; Kay MD; Stouffer DC Comparison of material properties in fascicle-bone units from human patellar tendon and knee ligaments. J. Biomech 1986, 19 (6), 425–432. [DOI] [PubMed] [Google Scholar]
- (29).Blitz E; Sharir A; Akiyama H; Zelzer E Tendon-bone attachment unit is formed modularly by a distinct pool of Scx- and Sox9-positive progenitors. Development 2013, 140 (13), 2680–2690. [DOI] [PubMed] [Google Scholar]
- (30).Sugimoto Y; Takimoto A; Akiyama H; Kist R; Scherer G; Nakamura T; Hiraki Y; Shukunami C Scx+/Sox9+ progenitors contribute to the establishment of the junction between cartilage and tendon/ligament. Development 2013, 140 (11), 2280–8. [DOI] [PubMed] [Google Scholar]
- (31).Zelzer E; Blitz E; Killian M; Thomopoulos S Tendon-to-bone attachment: from development to maturity. Birth Defects Res., Part C 2014, 102 (1), 101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Schweitzer R; Chyung JH; Murtaugh LC; Brent AE; Rosen V; Olson EN; Lassar A; Tabin CJ Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development 2001, 128 (19), 3855–66. [DOI] [PubMed] [Google Scholar]
- (33).Huang AH; Riordan TJ; Pryce B; Weibel JL; Watson SS; Long F; Lefebvre V; Harfe BD; Stadler HS; Akiyama H; Tufa SF; Keene DR; Schweitzer R Musculoskeletal integration at the wrist underlies the modular development of limb tendons. Development 2015, 142 (14), 2431–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Galatz L; Rothermich S; VanderPloeg K; Petersen B; Sandell L; Thomopoulos S Development of the supraspinatus tendon-to-bone insertion: localized expression of extracellular matrix and growth factor genes. J. Orthop. Res 2007, 25 (12), 1621–8. [DOI] [PubMed] [Google Scholar]
- (35).Thomopoulos S; Williams GR; Gimbel JA; Favata M; Soslowsky LJ Variation of biomechanical, structural, and compositional properties along the tendon to bone insertion site. J. Orthop. Res 2003, 21 (3), 413–419. [DOI] [PubMed] [Google Scholar]
- (36).Thomopoulos S; Hattersley G; Rosen V; Mertens M; Galatz L; Williams GR; Soslowsky LJ The localized expression of extracellular matrix components in healing tendon insertion sites: an in situ hybridization study. J. Orthop. Res 2002, 20 (3), 454–463. [DOI] [PubMed] [Google Scholar]
- (37).Abraham AC; Haut Donahue TL From meniscus to bone: A quantitative evaluation of structure and function of the human meniscal attachments. Acta Biomater. 2013, 9 (5), 6322–6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Moffat KL; Sun W-HS; Pena PE; Chahine NO; Doty SB; Ateshian GA; Hung CT; Lu HH Characterization of the structure-function relationship at the ligament-to-bone interface. Proc. Natl. Acad. Sci U. S. A 2008, 105, 7947–7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Hu Y; Birman V; Deymier-Black A; Schwartz A; Thomopoulos S; Genin G Stochastic interdigitation as a toughening mechanism at the interface between tendon and bone. Biophys. J 2015, 108 (2), 431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Schwartz A; Lipner J; Pasteris J; Genin G; Thomopoulos S Muscle loading is necessary for the formation of a functional tendon enthesis. Bone 2013, 55 (1), 44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Thomopoulos S; Kim HM; Rothermich SY; Biederstadt C; Das R; Galatz LM Decreased muscle loading delays maturation of the tendon enthesis during postnatal development. J. Orthop. Res 2007, 25 (9), 1154–1163. [DOI] [PubMed] [Google Scholar]
- (42).Killian M; Thomopoulos S Scleraxis is required for the development of a functional tendon enthesis. FASEB J. 2016, 30 (1), 301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Tensho K; Shimodaira H; Aoki T; Narita N; Kato H; Kakegawa A; Fukushima N; Moriizumi T; Fujii M; Fujinaga Y; Saito N Bony Landmarks of the Anterior Cruciate Ligament Tibial Footprint: A Detailed Analysis Comparing 3-Dimensional Computed Tomography Images to Visual and Histological Evaluations. American Journal of Sports Medicine 2014, 42 (6), 1433–1440. [DOI] [PubMed] [Google Scholar]
- (44).Villegas DF; Hansen TA; Liu DF; Donahue TL A quantitative study of the microstructure and biochemistry of the medial meniscal horn attachments. Ann. Biomed. Eng 2008, 36 (1), 123–131. [DOI] [PubMed] [Google Scholar]
- (45).Thomopoulos S The role of mechanobiology in the attachment of tendon to bone. IBMS BoneKEy 2011, 8, 271–285. [Google Scholar]
- (46).Benjamin M; Ralphs JR Fibrocartilage in tendons and ligaments-an adaptation to compressive load. J. Anat. 1998, 193 (4), 481–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Thomopoulos S; Marquez JP; Weinberger B; Birman V; Genin GM Collagen fiber orientation at the tendon to bone insertion and its influence on stress concentrations. J. Biomech 2006, 39 (10), 1842–1851. [DOI] [PubMed] [Google Scholar]
- (48).Thomopoulos S; Williams G; Soslowsky L Tendon to bone healing: differences in biomechanical, structural, and compositional properties due to a range of activity levels. J. Biomech. Eng 2003, 125 (1), 106–113. [DOI] [PubMed] [Google Scholar]
- (49).Vogel K; Ordog A; Pogany G; Olah J Proteoglycans in the compressed region of human tibialis posterior tendon and in ligaments. J. Orthop. Res 1993, 11 (1), 68–77. [DOI] [PubMed] [Google Scholar]
- (50).Berenson M; Blevins F; Plaas A; Vogel K Proteoglycans of human rotator cuff tendons. J. Orthop. Res 1996, 14 (4), 518–525. [DOI] [PubMed] [Google Scholar]
- (51).Knese K-H; Biermann H Die Knochenbildung an Sehnenund Bandsatzen im Bereich ursprunglich chondraler Apophysen. Cell Tissue Res. 1958, 49, 142–187. [PubMed] [Google Scholar]
- (52).Schneider H Zeitschrift für Anatomie und Entwicklungsge-schichte (Structure of tendon attachments). Anat. Embryol 1956, 119, 431–456. [PubMed] [Google Scholar]
- (53).Szczesny SE; Elliott DM Interfibrillar shear stress is the loading mechanism of collagen fibrils in tendon. Acta Biomater. 2014, 10 (6), 2582–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Abraham A; Pauly H; Haut Donahue T Deleterious effects of osteoarthritis on the structure and function of the meniscal enthesis. Osteoarthritis and Cartilage 2014, 22 (2), 275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Gao J; Messner K Quantitative comparison of soft tissue-bone interface at chondral ligament insertions in the rabbit knee joint. J. Anat 1996, 188 (Pt 2), 367–372. [PMC free article] [PubMed] [Google Scholar]
- (56).Zhao L; Thambyah A; Broom ND A multi-scale structural study of the porcine anterior cruciate ligament tibial enthesis. J. Anat 2014, 224, 624–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Gao J; Rasanen T; Persliden J; Messner K The morphology of ligament insertions after failure at low strain velocity: an evaluation of ligament entheses in the rabbit knee. J. Anat 1996, 189 (Pt 1), 127–133. [PMC free article] [PubMed] [Google Scholar]
- (58).Benjamin M; McGonagle D The anatomical basis for disease localisation in seronegative spondyloarthropathy at entheses and related sites. J. Anat 2001, 199 (5), 503–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Wang IE; Mitroo S; Chen FH; Lu HH; Doty SB Age-dependent changes in matrix composition and organization at the ligament-to-bone insertion. J. Orthop. Res 2006, 24 (8), 1745–1755. [DOI] [PubMed] [Google Scholar]
- (60).Fukuda H; Hamada K; Nakajima T; Tomonaga A Pathology and pathogenesis of the intratendinous tearing of the rotator cuff viewed from en bloc histologic sections. Clin. Orthop. Relat. Res 1994, 304, 60–7. [PubMed] [Google Scholar]
- (61).Gotoh M; Hamada K; Yamakawa H; Tomonaga A; Inoue A; Fukuda H Significance of granulation tissue in torn supraspinatus insertions: an immunohistochemical study with antibodies against interleukin-1 beta, cathepsin D, and matrix metalloprotease-1. J. Orthop. Res 1997, 15 (1), 33–9. [DOI] [PubMed] [Google Scholar]
- (62).Meyer DC; Farshad M; Amacker NA; Gerber C; Wieser K Quantitative analysis of muscle and tendon retraction in chronic rotator cuff tears. American Journal of Sports Medicine 2012, 40 (3), 606–10. [DOI] [PubMed] [Google Scholar]
- (63).Killian M; Cavinatto L; Shah S; Sato E; Ward S; Havlioglu N; Galatz L; Thomopoulos S The effects of chronic unloading and gap formation on tendon-to-bone healing in a rat model of massive rotator cuff tears. J. Orthop. Res 2014, 32 (3), 439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Killian M; Cavinatto L; Ward S; Havlioglu N; Thomopoulos S; Galatz L Chronic degeneration leads to poor healing of repaired massive rotator cuff tears in rats. American Journal of Sports Medicine 2015, 43 (10), 2401–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Cadet ER; Hsu JW; Levine WN; Bigliani LU; Ahmad CS The relationship between greater tuberosity osteopenia and the chronicity of rotator cuff tears. Journal of Shoulder and Elbow Surgery 2008, 17 (1), 73–7. [DOI] [PubMed] [Google Scholar]
- (66).Accousti KJ; Flatow EL Technical pearls on how to maximize healing of the rotator cuff. Instructional Course Lectures 2007, 56, 3–12. [PubMed] [Google Scholar]
- (67).Lin TW; Cardenas L; Soslowsky LJ Biomechanics of tendon injury and repair. J. Biomech 2004, 37, 865–877. [DOI] [PubMed] [Google Scholar]
- (68).Killian M; Cavinatto L; Galatz L; Thomopoulos S The role of mechanobiology in tendon healing. Journal of Shoulder and Elbow Surgery 2012, 21 (2), 228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Fenwick SA; Hazleman BL; Riley GP The vasculature and its role in the damaged and healing tendon. Arthritis Research 2002, 4 (4), 252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Docheva D; Müller SA; Majewski M; Evans CH Biologics for tendon repair. Adv. Drug Delivery Rev 2015, 84, 222–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Bunker DLJ; Ilie V; Ilie V; Nicklin S Tendon to bone healing and its implications for surgery. Muscles, Ligaments and Tendons Journal 2014, 4 (3), 343–350. [PMC free article] [PubMed] [Google Scholar]
- (72).Yang G; Rothrauff BB; Tuan RS Tendon and Ligament Regeneration and Repair: Clinical Relevance and Developmental Paradigm. Birth Defects Res., Part C 2013, 99, 203–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Killian M; Cavinatto L; Galatz L; Thomopoulos S Recent advances in shoulder research. Arth. Res. Ther 2012, 14 (3), 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Sato E; Killian M; Choi A; Lin E; Esparza M; Galatz L; Thomopoulos S; Ward S Skeletal muscle fibrosis and stiffness increase after rotator cuff tendon injury and neuromuscular compromise in a rat model. J. Orthop. Res 2014, 32 (9), 1111–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Sato EJ; Killian ML; Choi AJ; Lin E; Choo AD; Rodriguez-Soto AE; Lim CT; Thomopoulos S; Galatz LM; Ward SR Architectural and Biochemical Adaptations in Skeletal Muscle and Bone Following Rotator Cuff Injury in a Rat Model. Journal of Bone & Joint Surgery 2015, 97, 565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Kanazawa T; Gotoh M; Ohta K; Honda H; Ohzono H; Shimokobe H; Shiba N; Nakamura K.-i. Histomorphometric and ultrastructural analysis of the tendon-bone interface after rotator cuff repair in a rat model. Sci. Rep 2016, 6, 33800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Killian M; Lim C; Thomopoulos S; Charlton N; Kim H-M; Galatz L The effect of unloading on gene expression of healthy and injured rotator cuffs. J. Orthop. Res 2013, 31 (8), 1240–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Galatz LM; Charlton N; Das R; Kim HM; Havlioglu N; Thomopoulos S Complete removal of load is detrimental to rotator cuff healing. Journal of Shoulder and Elbow Surgery 2009, 18 (5), 669–675. [DOI] [PubMed] [Google Scholar]
- (79).Tashjian R; Hollins A; Kim H; Teefey S; Middleton W; Steger-May K; Galatz L; Yamaguchi K Factors affecting healing rates after arthroscopic double-row rotator cuff repair. American Journal of Sports Medicine 2010, 38 (12), 2435–2442. [DOI] [PubMed] [Google Scholar]
- (80).Provencher M; Kercher J; Galatz L; ElAttrache N; Frank R; Cole B Evolution of rotator cuff repair technologies: Are our patients really benefiting? AAOS Instructional Course Lectures 2011, 60, 123–136. [PubMed] [Google Scholar]
- (81).Gimbel JA; Mehta S; Van Kleunen JP; Williams GR; Soslowsky LJ The tension required at repair to reappose the supraspinatus tendon to bone rapidly increases after injury. Clin. Orthop. Relat. Res 2004, 426 (426), 258–65. [DOI] [PubMed] [Google Scholar]
- (82).Gimbel JA; Van Kleunen JP; Lake SP; Williams GR; Soslowsky LJ The role of repair tension on tendon to bone healing in an animal model of chronic rotator cuff tears. J. Biomech 2007, 40 (3), 561–568. [DOI] [PubMed] [Google Scholar]
- (83).Derwin KA; Badylak S; Steinmann SP; Iannotti JP Extracellular matrix scaffold devices for rotator cuff repair. Journal of Shoulder and Elbow Surgery 2010, 19 (3), 467–476. [DOI] [PubMed] [Google Scholar]
- (84).Derwin KA; Baker AR; Spragg RK; Leigh DR; Iannotti JP Commercial extracellular matrix scaffolds for rotator cuff tendon repair. J. Bone Joint Surg 2006, 88 (12), 2665–2672. [DOI] [PubMed] [Google Scholar]
- (85).Omi R; Gingery A; Steinmann SP; Amadio PC; An K-N; Zhao C Rotator cuff repair augmentation in a rat model that combines a multilayer xenograft tendon scaffold with bone marrow stromal cells. Journal of Shoulder and Elbow Surgery 2016, 25 (3), 469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Peterson DR; Ohashi KL; Aberman HM; Piza PA; Crockett HC; Fernandez JI; Lund PJ; Funk KA; Hawes ML; Parks BG; Mattern R-H Evaluation of a collagen-coated, resorbable fiber scaffold loaded with a peptide basic fibroblast growth factor mimetic in a sheep model of rotator cuff repair. Journal of Shoulder and Elbow Surgery 2015, 24 (11), 1764–1773. [DOI] [PubMed] [Google Scholar]
- (87).Karande TS; Ong JL; Agrawal CM Diffusion in musculoskeletal tissue engineering scaffolds: design issues related to porosity, permeability, architecture, and nutrient mixing. Ann. Biomed. Eng 2004, 32, 1728–1743. [DOI] [PubMed] [Google Scholar]
- (88).Soler J; Gidwani S; Curtis M Early complications from the use of porcine dermal collagen implants (Permacol) as bridging constructs in the repair of massive rotator cuff tears. A report of 4 cases. Acta Orthopaedica Belgica 2007, 73 (4), 432–436. [PubMed] [Google Scholar]
- (89).Walton J; Bowman N; Khatib Y; Linklater J; Murrell G Restore Orthobiologic Implant: Not Recommended for Augmentation of Rotator Cuff Repairs. J. Bone Joint Surg 2007, 89 (4), 786–791. [DOI] [PubMed] [Google Scholar]
- (90).Bond J; Dopirak R; Higgins J; Burns J; Snyder S Arthroscopic replacement of massive, irreparable rotator cuff tears using a GraftJacket Allograft: Technique and preliminary results. J. Arthroscopic Relat. Surg 2008, 24 (4), 403–409. [DOI] [PubMed] [Google Scholar]
- (91).Wong I; Burns J; Snyder S Arthroscopic GraftJacket repair of rotator cuff tears. Journal of Shoulder and Elbow Surgery 2010, 19 (2), 104–109. [DOI] [PubMed] [Google Scholar]
- (92).Bond JL; Dopirak RM; Higgins J; Burns J; Snyder SJ Arthroscopic replacement of massive, irreparable rotator cuff tears using a GraftJacket allograft: technique and preliminary results. Arthroscopy 2008, 24 (4), 403.e1–403.e8. [DOI] [PubMed] [Google Scholar]
- (93).Omae H; Steinmann SP; Zhao C; Zobitz ME; Wongtriratanachai P; Sperling JW; An KN Biomechanical effect of rotator cuff augmentation with an acellular dermal matrix graft: a cadaver study. Clinical biomechanics (Bristol Avon) 2012, 27 (8), 789–92. [DOI] [PubMed] [Google Scholar]
- (94).Barber FA; Herbert MA; Coons DA Tendon augmentation grafts: biomechanical failure loads and failure patterns. Arthroscopy 2006, 22 (5), 534–8. [DOI] [PubMed] [Google Scholar]
- (95).Kiapour AM; Fleming BC; Murray MM Biomechanical Outcomes of Bridge-enhanced Anterior Cruciate Ligament Repair Are Influenced by Sex in a Preclinical Model. Clin. Orthop. Relat. Res 2015, 473, 2599–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (96).Adams D; Logerstedt D; Hunter-Giordano A; Axe MJ; Snyder-Mackler L Current Concepts for Anterior Cruciate Ligament Reconstruction: A Criterion-Based Rehabilitation Progression. Journal of Orthopaedic & Sports Physical Therapy 2012, 42, 601–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (97).Haslauer CM; Proffen BL; Johnson VM; Murray MM Expression of modulators of extracellular matrix structure after anterior cruciate ligament injury: Ligament ECM after ACL injury. Wound Repair and Regeneration 2014, 22, 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (98).Dargel J; Gotter M; Mader K; Pennig D; Koebke J; Schmidt-Wiethoff R Biomechanics of the anterior cruciate ligament and implications for surgical reconstruction. Strategies in Trauma and Limb Reconstruction 2007, 2, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (99).Freedman KB; D’Amato MJ; Nedeff DD; Kaz A; Bach BR Arthroscopic anterior cruciate ligament reconstruction A metaanalysis comparing patellar tendon and hamstring tendon autografts. Am. J. Sports Med 2003, 31 (1), 2–11. [DOI] [PubMed] [Google Scholar]
- (100).Hamido F; Al Harran H; Al Misfer AR; El Khadrawe T; Morsy MG; Talaat A; Elias A; Nagi A Augmented short undersized hamstring tendon graft with LARS® artificial ligament versus four-strand hamstring tendon in anterior cruciate ligament reconstruction: preliminary results. Orthopaedics & Traumatology: Surgery & Research 2015, 101, 535–538. [DOI] [PubMed] [Google Scholar]
- (101).Murray MM Current Status and Potential of Primary ACL Repair. Clinics in Sports Medicine 2009, 28, 51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (102).Murray MM, History of ACL Treatment and Current Gold Standard of Care In The ACL Handbook; Murray MM, Vavken P, Fleming B, Eds.; Springer New York: New York, NY, 2013; pp 19–28. [Google Scholar]
- (103).Yasuda K; Kondo E; Ichiyama H; Tanabe Y; Tohyama H Clinical Evaluation of Anatomic Double-Bundle Anterior Cruciate Ligament Reconstruction Procedure Using Hamstring Tendon Grafts: Comparisons Among 3 Different Procedures. Arthroscopy: The Journal of Arthroscopic & Related Surgery 2006, 22, 240–251. [DOI] [PubMed] [Google Scholar]
- (104).Kaplan N; Wickiewicz TL; Warren RF Primary surgical treatment of anterior cruciate ligament ruptures A long-term follow-up study. American Journal of Sports Medicine 1990, 18 (4), 354–358. [DOI] [PubMed] [Google Scholar]
- (105).Dashe J; Parisien RL; Cusano A; Curry EJ; Bedi A; Li X Allograft tissue irradiation and failure rate after anterior cruciate ligament reconstruction: A systematic review. World Journal of Orthopedics 2016, 7, 392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (106).Irvine JN; Arner JW; Thorhauer E; Abebe ES; Dauria J; Schreiber VM; Harner CD; Tashman S Is There a Difference in Graft Motion for Bone-Tendon-Bone and Hamstring Autograft ACL Reconstruction at 6 Weeks and 1 Year? American Journal of Sports Medicine 2016, 44, 2599. [DOI] [PubMed] [Google Scholar]
- (107).Shaerf DA Anterior cruciate ligament reconstruction best practice: A review of graft choice. World Journal of Orthopedics 2014, 5, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (108).Tejwani SG; Chen J; Funahashi TT; Love R; Maletis GA Revision Risk After Allograft Anterior Cruciate Ligament Reconstruction: Association With Graft Processing Techniques, Patient Characteristics, and Graft Type. American Journal of Sports Medicine 2015, 43, 2696–2705. [DOI] [PubMed] [Google Scholar]
- (109).Zaffagnini S; Grassi A; Marcheggiani Muccioli GM; Roberti Di Sarsina T; Raggi F; Benzi A; Marcacci M Anterior cruciate ligament reconstruction with a novel porcine xenograft: the initial Italian experience. Joints 2015, 3 (2), 85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (110).Spindler KP; Parker RD; Andrish JT; Kaeding CC; Wright RW; Marx RG; McCarty EC; Amendola A; Dunn WR; Huston LJ; Harrell FE; Group M Prognosis and predictors of ACL reconstructions using the MOON cohort: A model for comparative effectiveness studies. J. Orthop. Res 2013, 31, 2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (111).Kartus J; Movin T; Karlsson J Donor-site morbidity and anterior knee problems after anterior cruciate ligament reconstruction using autografts. Arthroscopy 2001, 17 (9), 971–80. [DOI] [PubMed] [Google Scholar]
- (112).Mascarenhas R; MacDonald PB; et al. Anterior cruciate ligament reconstruction: a look at prosthetics-past, present and possible future. McGill J. Med 2008, 11, 29. [PMC free article] [PubMed] [Google Scholar]
- (113).Bi F; Shi Z; Liu A; Guo P; Yan S Anterior Cruciate Ligament Reconstruction in a Rabbit Model Using Silk-Collagen Scaffold and Comparison with Autograft. PLoS One 2015, 10, e0125900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (114).Ratcliffe A; Butler DL; Dyment NA; Cagle PJ; Proctor CS; Ratcliffe SS; Flatow EL Scaffolds for Tendon and Ligament Repair and Regeneration. Ann. Biomed. Eng 2015, 43, 819–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (115).Murray MM; Fleming BC Use of a bioactive scaffold to stimulate anterior cruciate ligament healing also minimizes post-traumatic osteoarthritis after surgery. American Journal of Sports Medicine 2013, 41 (8), 1762–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (116).Proffen BL; Perrone GS; Fleming BC; Sieker JT; Kramer J; Hawes ML; Badger GJ; Murray MM Electron beam sterilization does not have a detrimental effect on the ability of extracellular matrix scaffolds to support in vivo ligament healing. J. Orthop. Res 2015, 33 (7), 1015–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (117).Batty LM; Norsworthy CJ; Lash NJ; Wasiak J; Richmond AK; Feller JA Synthetic devices for reconstructive surgery of the cruciate ligaments: a systematic review. Arthroscopy 2015, 31 (5), 957–68. [DOI] [PubMed] [Google Scholar]
- (118).Smith C; Ajuied A; Wong F; Norris M; Back D; Davies A The Use of the Ligament Augmentation and Reconstruction System (LARS) for Posterior Cruciate Reconstruction. Arthroscopy: The Journal of Arthroscopic & Related Surgery 2014, 30, 111–120. [DOI] [PubMed] [Google Scholar]
- (119).Indelicato PA; Pascale MS; Huegel MO Early experience with the GORE-TEX polytetrafluoroethylene anterior cruciate ligament prosthesis. American Journal of Sports Medicine 1989, 17 (1), 55–62. [DOI] [PubMed] [Google Scholar]
- (120).Wredmark T; Engstrom B Five-year results of anterior cruciate ligament reconstruction with the Stryker Dacron high-strength ligament. Knee Surgery, Sports Traumatology, Arthroscopy 1993, 1, 71–75. [DOI] [PubMed] [Google Scholar]
- (121).Murray AW; Macnicol MF 10–16 year results of Leeds-Keio anterior cruciate ligament reconstruction. Knee 2004, 11 (1), 9–14. [DOI] [PubMed] [Google Scholar]
- (122).Kdolsky RK; Gibbons DF; Kwasny O; Schabus R; Plenk H Braided polypropylene augmentation device in reconstructive surgery of the anterior cruciate ligament: Long-term clinical performance of 594 patients and short-term arthroscopic results, failure analysis by scanning electron microscopy, and synovial histomorphology. J. Orthop. Res 1997, 15 (1), 1–10. [DOI] [PubMed] [Google Scholar]
- (123).Nau T; Lavoie P; Duval N A new generation of artificial ligaments in reconstruction of the anterior cruciate ligament. J. Bone Jt. Surg., Br. Vol 2002, 84, 356–360. [DOI] [PubMed] [Google Scholar]
- (124).Li X; He J; Bian W; Li Z; Li D; Snedeker JG A novel silk-TCP-PEEK construct for anterior cruciate ligament reconstruction: an off-the shelf alternative to a bone-tendon-bone autograft. Biofabrication 2014, 6 (1), 015010. [DOI] [PubMed] [Google Scholar]
- (125).Spalazzi JP; Doty SB; Moffat KL; Levine WN; Lu HH Development of controlled matrix heterogeneity on a triphasic scaffold for orthopedic interface tissue engineering. Tissue Eng. 2006, 12 (12), 3497–3508. [DOI] [PubMed] [Google Scholar]
- (126).Nossov S; Dines J; Murrell G; Rodeo S; Bedi A Biologic augmentation of tendon-to-bone healing: scaffolds, mechanical load, vitamin D, and diabetes. Instructional Course Lectures 2014, 63, 451–462. [PubMed] [Google Scholar]
- (127).Zhang W; Pan W; Zhang M; Wei Y In vivo evaluation of two types of bioactive scaffold used for tendon-bone interface healing in the reconstruction of anterior cruciate ligament. Biotechnol. Lett 2011, 33, 837–844. [DOI] [PubMed] [Google Scholar]
- (128).Ji X; Chen Q; Thoreson AR; Qu J; An KN; Amadio PC; Steinmann SP; Zhao C Rotator cuff repair with a tendonfibrocartilage-bone composite bridging patch. Clinical Biomechanics 2015, 30, 976–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (129).Shi P; Teh TKH; Toh SL; Goh JCH Variation of the effect of calcium phosphate enhancement of implanted silk fibroin ligament bone integration. Biomaterials 2013, 34 (24), 5947–5957. [DOI] [PubMed] [Google Scholar]
- (130).Matthews JA; Wnek GE; Simpson DG; Bowlin GL Electrospinning of Collagen Nanofibers. Biomacromolecules 2002, 3, 232–238. [DOI] [PubMed] [Google Scholar]
- (131).Meimandi-Parizi A; Oryan A; Moshiri A Tendon tissue engineering and its role on healing of the experimentally induced large tendon defect model in rabbits: A comprehensive in vivo study. PLoS One 2013, 8, e73016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (132).Oryan A; Moshiri A; Parizi AM; Maffulli N Implantation of a Novel Biologic and Hybridized Tissue Engineered Bioimplant in Large Tendon Defect: An In Vivo Investigation. Tissue Eng., Part A 2014, 20, 447–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (133).Xie J; Li X; Lipner J; Manning CN; Schwartz AG; Thomopoulos S; Xia Y ‘Aligned-to-random’ nanofiber scaffolds for mimicking the structure of the tendon-to-bone insertion site. Nanoscale 2010, 2, 923–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (134).Orr SB; Chainani A; Hippensteel KJ; Kishan A; Gilchrist C; Garrigues NW; Ruch DS; Guilak F; Little D Aligned multilayered electrospun scaffolds for rotator cuff tendon tissue engineering. Acta Biomater. 2015, 24, 117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (135).Moffat KL; Kwei ASP; Spalazzi JP; Doty SB; Levine WN; Lu HH Novel nanofiber-based scaffold for rotator cuff repair and augmentation. Tissue Eng. Part A 2009, 15 (1), 115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (136).Chainani A; Hippensteel KJ; Kishan A; Garrigues NW; Ruch DS; Guilak F; Little D Multilayered electrospun scaffolds for tendon tissue engineering. Tissue Eng. Part A 2013, 19, 2594–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (137).Erisken C; Kalyon DM; Wang H; Ornek-Ballanco C; Xu J Osteochondral tissue formation through adipose-derived stromal cell differentiation on biomimetic polycaprolactone nanofibrous scaffolds with graded insulin and Beta-glycerophosphate concentrations. Tissue Eng. Part A 2011, 17 (9–10), 1239–52. [DOI] [PubMed] [Google Scholar]
- (138).Inui A; Kokubu T; Mifune Y; Sakata R; Nishimoto H; Nishida K; Akisue T; Kuroda R; Satake M; Kaneko H; Fujioka H Regeneration of rotator cuff tear using electrospun poly(d,l-Lactide-Co-Glycolide) scaffolds in a rabbit model. Arthroscopy: The Journal of Arthroscopic & Related Surgery 2012, 28, 1790–1799. [DOI] [PubMed] [Google Scholar]
- (139).Katta P; Alessandro M; Ramsier RD Continuous electrospinning of aligned polymer nanofibers onto a wire drum collector. Nano Lett. 2004, 4 (11), 2215–2218. [Google Scholar]
- (140).Kolluru PV; Lipner J; Liu W; Xia Y; Thomopoulos S; Genin GM; Chasiotis I Strong and tough mineralized PLGA nanofibers for tendon-to-bone scaffolds. Acta Biomater. 2013, 9, 9442–9450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (141).Li D; McCann JT; Marquez M; Xia Y Electrospinning: a simple and versatile technique for producing ceramic nanofibers and nanotubes. J. Am. Ceram. Soc 2006, 89 (6), 1861–1869. [Google Scholar]
- (142).Li D; Wang Y; Xia Y Electrospinning of polymeric and ceramic nanofibers as uniaxially aligned arrays. Nano Lett. 2003, 3 (8), 1167–1171. [Google Scholar]
- (143).Li D; Wang Y; Xia Y Electrospinning nanofibers as uniaxially aligned arrays and layer-by-layer stacked films. Adv. Mater 2004, 16 (4), 361–366. [Google Scholar]
- (144).Lyu S; Huang C; Yang H; Zhang X Electrospun fibers as a scaffolding platform for bone tissue repair. J. Orthop. Res 2013, 31 (9), 1382–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (145).Ma B; Xie J; Jiang J; Shuler FDF; Bartlett DDE Rational design of nanofiber scaffolds for orthopedic tissue repair and regeneration. Nanomedicine (London, U. K.) 2013, 8 (9), 1459–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (146).Ma Z; Kotaki M; Inai R; Ramakrishna S Potential of nanofiber matrix as tissue-engineering scaffolds. Tissue Eng. 2005, 11 (1–2), 101–9. [DOI] [PubMed] [Google Scholar]
- (147).Narayanan N; Jiang C; Uzunalli G; Thankappan SK; Laurencin CT; Deng M Polymeric Electrospinning for Musculoskeletal Regenerative Engineering. Regenerative Engineering and Translational Medicine 2016, 2, 69. [Google Scholar]
- (148).Varesano A; Montarsolo A; Tonin C Crimped polymer nanofibres by air-driven electrospinning. Eur. Polym. J 2007, 43 (7), 2792–2798. [Google Scholar]
- (149).Visser J; Melchels FPW; Jeon JE; van Bussel EM; Kimpton LS; Byrne HM; Dhert W. J. a.; Dalton PD; Hutmacher DW; Malda J Reinforcement of hydrogels using three-dimensionally printed microfibres. Nat. Commun 2015, 6, 6933. [DOI] [PubMed] [Google Scholar]
- (150).Zhao S; Zhao J; Dong S; Huangfu X; Li B; Yang H; Zhao J; Cui W Biological augmentation of rotator cuff repair using bFGF-loaded electrospun poly (lactide-co-glycolide) fibrous membranes. Int. J. Nanomed 2014, 9, 2373–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (151).Zhu J; Ge Y; Jasper S; Zhang X Physical Characterization of Electrospun Nanofibers In Electrospun Nanofibers; Afshari M, Ed.; Woodhead Publishing: Cambridge, U.K., 2017; Vol. 1, pp 207–238. [Google Scholar]
- (152).Pham QP; Sharma U; Mikos AG Electrospinning of polymeric nanofibers for tissue engineering applications: a review. Tissue Eng. 2006, 12 (5), 1197–1211. [DOI] [PubMed] [Google Scholar]
- (153).Kumbar SG; James R; Nukavarapu SP; Laurencin CT Electrospun nanofiber scaffolds: engineering soft tissues. Biomed. Mater 2008, 3, 034002. [DOI] [PubMed] [Google Scholar]
- (154).Ashammakhi N; Ndreu A; Piras AM; Nikkola L; Sindelar T; Ylikauppila H; Harlin A; Gomes ME; Neves NM; Chiellini E; Chiellini F; Hasirci V; Redl H; Reis RL Biodegradable nanomats produced by electrospinning: expanding multifunctionality and potential for tissue engineering. J. Nanosci. Nanotechnol 2007, 7 (3), 862–882. [DOI] [PubMed] [Google Scholar]
- (155).Liu W; Lipner J; Moran CH; Feng L; Li X; Thomopoulos S; Xia Y Generation of electrospun nanofibers with controllable degrees of crimping through a simple, plasticizer-based treatment. Adv. Mater 2015, 27 (16), 2583–2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (156).Chen F; Hayami JWS; Amsden BG Electrospun poly(l-lactide-co-acryloyl carbonate) fiber scaffolds with a mechanically stable crimp structure for ligament tissue engineering. Biomacromolecules 2014, 15 (5), 1593–1601. [DOI] [PubMed] [Google Scholar]
- (157).Liu Y; Zhang X; Xia Y; Yang H Magnetic Field-Assisted Electrospinning of Aligned Straight and Wavy Polymeric Nanofibers. Adv. Mater 2010, 22 (11), 2454–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (158).Markx GH The use of electric fields in tissue engineering. Organogenesis 2008, 4, 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (159).Surrao DC; Hayami JWS; Waldman SD; Amsden B Self-Crimping, Biodegradable, Electrospun Polymer Microfibers. Biomacromolecules 2010, 11 (12), 3624–3629. [DOI] [PubMed] [Google Scholar]
- (160).Lipner J; Liu W; Liu Y; Boyle J; Genin GM; Xia Y; Thomopoulos S The mechanics of PLGA nanofiber scaffolds with biomimetic gradients in mineral for tendon-to-bone repair. Journal of the Mechanical Behavior of Biomedical Materials 2014, 40, 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (161).Liu W; Yeh YC; Lipner J; Xie J; Sung HW; Thomopoulos S; Xia Y Enhancing the stiffness of electrospun nanofiber scaffolds with a controlled surface coating and mineralization. Langmuir 2011, 27 (15), 9088–9093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (162).Li X; Xie J; Lipner J; Yuan X; Thomopoulos S; Xia Y Nanofiber scaffolds with gradations in mineral content for mimicking the tendon-to-bone insertion site. Nano Lett. 2009, 9 (7), 2763–2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (163).Liu W; Lipner J; Xie J; Manning CN; Thomopoulos S; Xia Y Nanofiber scaffolds with gradients in mineral content for spatial control of osteogenesis. ACS Appl Mater. Interfaces 2014, 6 (4), 2842–2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (164).Baker BM; Nathan AS; Gee AO; Mauck R L. The influence of an aligned nanofibrous topography on human mesenchymal stem cell fibrochondrogenesis. Biomaterials 2010, 31, 6190–6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (165).Daly AC; Cunniffe GM; Sathy BN; Jeon O; Alsberg E; Kelly DJ 3D bioprinting of developmentally inspired templates for whole bone organ engineering. Adv. Healthcare Mater 2016, 5 (18), 2353–2362. [DOI] [PubMed] [Google Scholar]
- (166).Liu A; Xue GH; Sun M; Shao HF; Ma CY; Gao Q; Gou ZR; Yan SG; Liu YM; He Y 3D printing surgical implants at the clinic: A experimental study on anterior cruciate ligament reconstruction. Sci. Rep 2016, 6, 21704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (167).Zadpoor AA; Malda J Additive Manufacturing of Biomaterials, Tissues, and Organs. Ann. Biomed. Eng 2016, 1–11. [DOI] [PubMed] [Google Scholar]
- (168).Murr LE; Gaytan SM; Martinez E; Medina F; Wicker RB Next generation orthopaedic implants by additive manufacturing using electron beam melting. Int. J. Biomater 2012, 2012, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]


