Abstract
Although there have been some reports that hyperbaric oxygen therapy (HBOT) is effective in treating breast cancer-related lymphedema (BCRL), controversy regarding its therapeutic effects remains.
We sought to evaluate the efficacy of HBOT in addition to conventional complex decongestive therapy (CDT) for BCRL.
A prospective observational study was conducted on 10 patients with BCRL. After screening, the subjects were stratified into a CDT-only group and a CDT and HBOT combination (CDT–HBOT) group. All patients received a total of 10 treatments over 2 weeks. Changes in the circumference of the upper limbs, quality-of-life questionnaire results, and bioelectrical impedance values were compared between the 2 groups.
Between both groups, there were no significant differences in demographic or clinical characteristics and in the quality-of-life outcomes for lymphedema of the limbs. The parameters measured by bioimpedance spectroscopy showed more significant improvements in the CDT–HBOT group than in the CDT-only group.
In patients with BCRL, HBOT may be recommended as an adjunct treatment to the existing therapies.
Keywords: breast cancer, complex decongestive therapy, hyperbaric oxygen therapy, lymphedema
1. Introduction
Breast cancer-related lymphedema (BCRL) is a common complication after surgery, chemotherapy, and radiotherapy for breast cancer. Its incidence ranges from 3% to 65% depending on the treatment, mode of diagnosis, and length of follow-up.[1] Patients with BCRL may experience functional impairment, psychological morbidity, and diminished quality of life (QOL).[1,2] However, a lack of awareness regarding lymphedema and treatment options among patients, their families, and medical staff members continues to persist.
Traditional therapies for lymphedema fall under the category of complex decongestive therapy (CDT) and include manual lymphatic drainage, compression therapy, remedial exercise, and skin care.[3,4] These treatments can be expected to improve lymphedema. However, there are many inconveniences associated with such treatments that must be continuously considered after disease onset, and there are certain cases wherein the treatment effect is not as good as expected or wherein the condition deteriorates despite the application of treatment. Therefore, various other methods such as low-level laser therapy (LLLT), extracorporeal shock wave therapy, and hyperbaric oxygen therapy (HBOT) have been applied to treat lymphedema.[5–9]
HBOT is recognized as a treatment for carbon monoxide poisoning or decompression illness and is a safe treatment option that is effective in the recovery of injured bones and soft tissues affected in radiation therapy as well as in diabetic wound healing. Its mechanism of action involves supplying oxygen to damaged tissues to promote blood flow, generating new blood vessels, promoting wound healing, and reducing fibrosis.[10–13] Although there have been several reports stating that hyperbaric oxygen therapy (HBOT) is effective in managing lymphedema following cancer treatment, there is still some controversy regarding its therapeutic effect.[7–9] Gothard et al[8] found no evidence of a beneficial effect of HBOT in the treatment of chronic BCRL. Since then, there has been no HBOT study on lymphedema.
Patients with lymphedema have higher levels of functional impairment, poorer psychological adjustment, and more incidence of anxiety and depression than the general population. There have been some reports about the effects of lymphedema on the QOL of breast cancer survivors.[14–18] In addition, a lymphedema-specific QOL measuring tool is available.[19]
The severity of lymphedema can be examined using various methods such as circumference or volume measurement. While these methods are easy to perform, they have drawbacks such as inability to describe the tissue composition of affected limbs. On the other hand, bioimpedance spectroscopy (BIS) is a relatively new diagnostic tool for lymphedema and can be used to evaluate the severity of the condition.[20–22]
Therefore, the aims of this study were to evaluate the efficacy of HBOT in addition to conventional CDT for lymphedema and related QOL and to determine whether HBOT can be recommended as a new treatment option for BCRL.
2. Methods
2.1. Subjects
A prospective observational study was conducted on 10 patients with BCRL. After screening, they were allocated to a CDT-only group and a CDT and HBOT combination (CDT–HBOT) group. Subjects received a total of 10 treatments over 2 weeks. Changes in the circumference of the upper limbs and bioelectrical impedance values were compared between the 2 groups. The study and all procedures were approved by the Institutional Review Board of Gangneung Asan Hospital (2017-09-007).
This study was conducted between October 2017 and August 2019. After introducing the objectives and methods of this study to subjects, written informed consent was obtained from each subject before participating in this study. Patients who developed swelling of the arm after breast cancer treatment and who were diagnosed with lymphedema by a rehabilitation physician were recruited from the Rehabilitation Outpatient Clinic. The criteria for the diagnosis of lymphedema were a difference in arm circumference of >2 cm at any point or compatible findings of lymphedema as per lymphoscintigraphy.
Subjects were excluded if they had tumor recurrence within 6 months of treatment, bilateral involvement, other combined neurological diseases, or communication problems. Patients who were considered unfit to undergo HBOT such as patients with epilepsy, those who recently underwent ear surgery, or those with an obstructive phobia were excluded from assignment to the CDT–HBOT group.
2.2. Circumference measurement
Using a tape measure, circumference was measured and compared by a dedicated physiotherapist for lymphedema at both wrists, 10 cm below the elbow, at the elbow, and 10 cm above the elbow between before and after treatment.[4]
2.3. CDT
All patients underwent 10 CDT sessions lasting 1 hour each, which included all of the following components: manual lymphatic drainage, compression bandaging, and meticulous skin care. This intervention continued for 2 weeks (5 working d/wk). Manual lymphatic drainage was performed by a certified physiotherapist, followed by wrapping of the limbs with short-stretch compression bandages. During the 2 weeks of CDT, the patients were educated on the proper bandaging technique and medical remedial exercises to promote lymphatic drainage as well as on the essentials of skin care.
2.4. HBOT
Subjects in the HBOT group breathed 100% oxygen at an average atmospheric pressure of 2.4 atmospheres absolute (ATA). The total time spent at 2.4 ATA was 100 minutes including 2 5-minute air breaks. Each subject received a total of 10 pressure exposures (5 d/wk for 2 weeks).
2.5. Bioelectrical impedance analysis
We used the InBodyS10 body water analyzer (InBody Co., Seoul, South Korea), which provides comprehensive information on the water content of the body such as intracellular water, extracellular water to total body water ratio, and history of body water condition. It allows 30 impedance measurements using 6 different frequencies (1, 5, 50, 250, 500, and 1000 kHz) at 5 segments of the body (right arm, left arm, trunk, right leg, and left leg).
We obtained values for extracellular fluid (ECF) using BIS specific to ECF and more sensitive to localized lymphedema with multifrequency (1 kHz to 1 MHz) and single-frequency bioimpedance analyses (SFBIAs; values collected at 1 and 5 kHz) for both upper limbs. At low frequencies, currents flow selectively through the extracellular water compartments, which reflect the lymph volume. Conversely, at high frequencies, currents pass through both intracellular fluid and ECF. The calculated ECF ratio was defined as a ratio of the affected side to the unaffected side, whereas the SFBIA ratio was defined as the ratio of the unaffected side to the affected side.[23,24]
2.6. QOL measure for lymphedema of the limbs
QOL and health status were measured using the validated, self-administered quality-of-life measure for lymphedema of the limbs (LYMQOL) for the arms, which comprises 4 domains with corresponding questions on the topics: function, appearance, symptoms, and mood. Overall QOL is scored as the value marked by patients between 0 and 10 points.[19]
2.7. Statistical analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences version 24.0 (IBM Corp., Armonk, NY). Mann–Whitney U test was used to compare the variables in the 2 groups. Changes in arm circumference (circumference before intervention − circumference after intervention; Δ) were calculated after interventions. Wilcoxon signed-rank test was used to compare variables between before and after treatment in each group. Continuous variables are expressed as mean ± standard deviation or median with interquartile range based on the results of normality testing. A 2-tailed P-value of <.05 was considered statistically significant.
3. Results
Initially, a total of 14 patients were enrolled in this study who joined one of the 2 groups. Then, 4 patients dropped out during the study period due to personal reasons (2 in CDT-only group and 2 in CDT–HBOT group). The remaining 10 patients completed the 2-week treatment program and were followed up immediately after the end of the program (Fig. 1).
Figure 1.
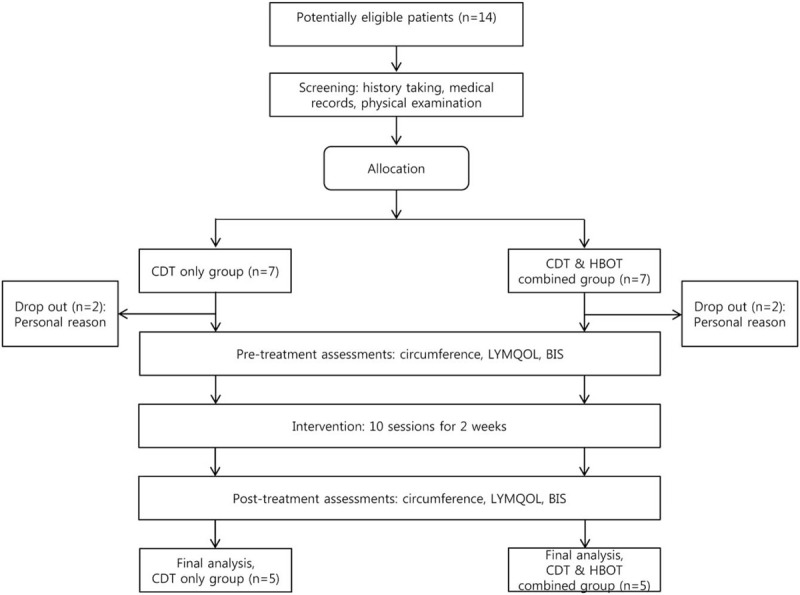
Flow chart showing subject recruitment and allocation. BIS = bioimpedance spectroscopy, CDT = complex decongestive therapy, HBOT = hyperbaric oxygen therapy, LYMQOL = quality-of-life measure for lymphedema of the limbs.
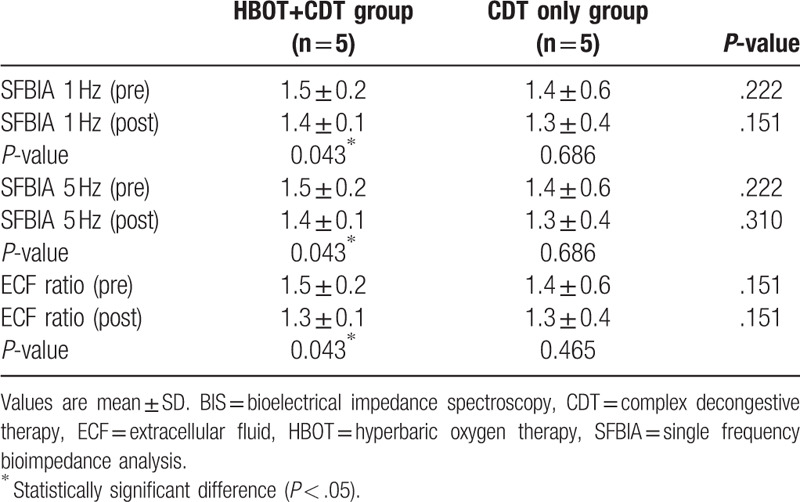
The demographic and clinical data of the study patients are summarized in Table 1. There were no significant differences between the 2 groups in baseline data or changes in arm circumference. Table 2 shows the LYMQOL scores of the 2 groups, which suggest a significant improvement in the function domain in the CDT–HBOT group (P < .05), whereas the other domains showed no significant changes in any group after treatment. The parameters measured by BIS before and after treatment were also not significantly different between both groups. However, there was a significant improvement in the CDT–HBOT group after treatment in this regard (Table 3; P < .05).
Table 1.
Demographic and clinical characteristics.
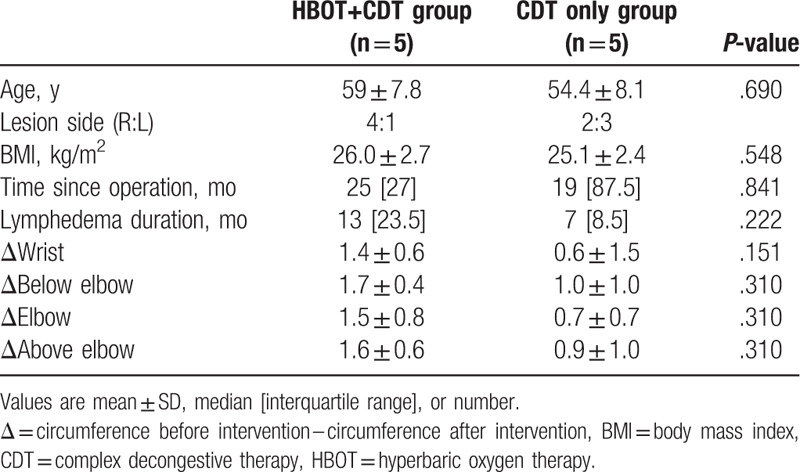
Table 2.
Changes in LYMQOL domains before and after treatment.
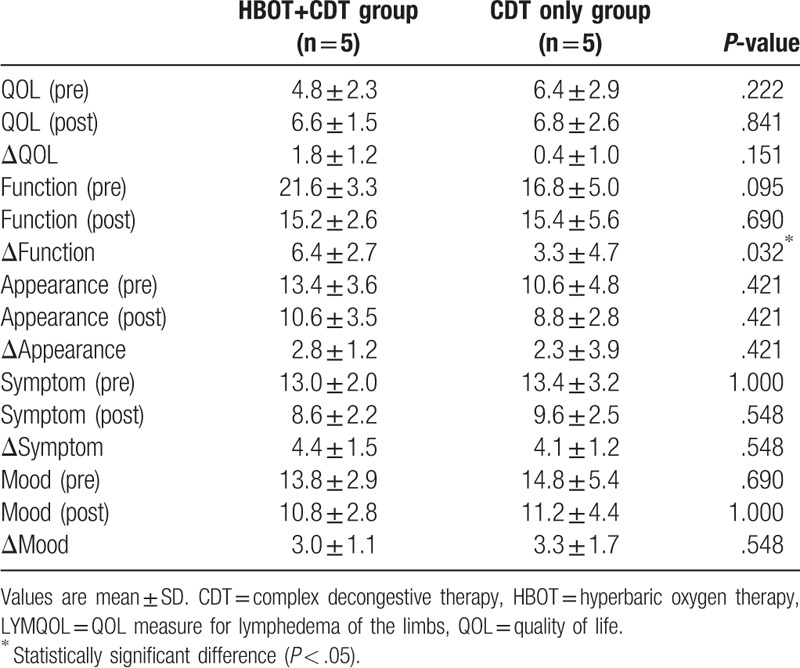
Table 3.
Changes in BIS parameters before and after treatment.
4. Discussion
The incidence of BCRL is decreasing faster than before due to the development of surgical techniques such as sentinel node biopsy and the increased awareness and treatment of lymphedema.[1,2] However, BCRL still occurs in a relatively large number of patients who experience psychological problems such as depression and subsequent functional discomfort.[15,16,18]
CDT is currently recognized as a standard treatment for lymphedema, and it focuses on reducing limb volume and maintaining healthy skin.[4,25] However, there are many inconveniences associated with this treatment approach, which should be considered continuously after the occurrence of lymphedema; thus, treatment compliance can be poor. There are also cases wherein the treatment effect is not as good as expected or wherein patient conditions deteriorate despite treatment.
Thus, new treatment methods for lymphedema have been explored. Some reports have suggested that HBOT is effective for lymphedema, but there is still controversy regarding its therapeutic effect.[7–9] Baxter et al[5] reported, for the first time, that HBOT was effective in patients with chronic BCRL, which was later supported by Teas et al.[9] The present study also confirmed the therapeutic effect of HBOT on BCRL, but unlike the previous reports, it included a control group and the duration of lymphedema in the subjects was relatively short.
The mechanism of action of HBOT for the reduction of lymphedema remains unclear, but it has been reported to be the result of the stimulation of vascular endothelial growth factor.[9] It is believed that fibrosis contributes to the development of lymphedema, which may explain the positive effect of HBOT on lymphedema through the reduction of fibrosis.[7] This study revealed a significant improvement in the HBOT group when considering the BIS indices after treatment, probably due to the reduction of fibrosis. On the other hand, Gothard et al[8] failed to confirm the beneficial effect of HBOT in their follow-up randomized trial. They explained that the extended interval (almost 12 years) between radiotherapy and HBOT was extremely long to enable remodeling of mature fibrotic tissues.
Among other treatment options for lymphedema, LLLT or photobiomodulation therapy has been promoted and investigated for the management of BCRL. A previous study indicated that LLLT may be considered an effective treatment for women with BCRL.[5] However, a recent study that investigated the effectiveness of LLLT in addition to conventional CDT failed to show additional benefits of intervention with LLLT.[26] Future studies comparing the therapeutic effects of LLLT and HBOT might be interesting.
There have been some reports on the effects of lymphedema on the QOL of breast cancer survivors.[14–16,18] Women with lymphedema had lower physical and mental QOL scores than women without lymphedema. In addition, women with lymphedema reported a significantly higher Disabilities of the Arm, Shoulder and Hand score, suggesting the presence of limitations in physical activity and participation restrictions.[18] In this study, we assessed QOL using LYMQOL, a method designed for the assessment of QOL in patients with lymphedema that was validated in a previous study.[19] There was no significant difference between the 2 groups in this regard, but improvements in QOL were observed in both groups after treatment.
The severity of lymphedema can be evaluated by measuring limb circumference and volume or by using a perometer. However, although these methods are simple to apply, they cannot describe the tissue composition of affected limbs. On the other hand, BIS is a relatively new diagnostic tool for the detection and measurement of lymphedema. It attempts to calculate the amount of body fluid by measuring the amount of impedance to an electrical current that passes through a body segment, and it can be used to evaluate the severity of lymphedema and to predict prognosis after treatment.[20–22,24] In this study, we evaluated the changes in lymphedema before and after treatment using BIS. There was no significant difference in this regard between the 2 groups, but in the CDT–HBOT group, a significant improvement was observed after treatment.
There are some limitations to this study. First, the sample size was relatively small and that could have led to a selection bias, and the patients were not randomly allocated to the study groups. However, this study is possibly of clinical value as a preliminary study, and further studies that include more subjects and apply randomization are needed in the future. Second, the number of HBOT treatments in this study was low. HBOT is commonly used to address delayed wound healing, and evidence of improved angiogenesis frequently becomes apparent between 14 and 20 treatments, with many such patients beginning a visible healing process at this point.[9] In future studies, increasing the number of HBOT treatments may be helpful in better determining its effectiveness. Finally, we did not exclude factors such as comorbidities and drugs, which could affect the progression of lymphedema. These factors should be fully considered in future studies.
In conclusion, we have confirmed the effects of HBOT in combination with CDT, a conventional treatment for lymphedema, in this study. Therefore, HBOT may be recommended as an adjunct treatment to the existing therapies for patients with BCRL.
Acknowledgment
The authors are grateful to the statistician at Gangneung Asan Hospital for his contribution to the statistical area of this study.
Author contributions
Conceptualization: Jung Hoi Koo, Ho Suk Oh.
Data curation: Sun Hong Song, Ho Suk Oh, Se Hyun Oh.
Formal analysis: Jung Hoi Koo.
Investigation: Jung Hoi Koo, Se Hyun Oh.
Methodology: Jung Hoi Koo.
Resources: Sun Hong Song, Ho Suk Oh.
Supervision: Ho Suk Oh, Se Hyun Oh.
Validation: Se Hyun Oh.
Writing – original draft: Jung Hoi Koo.
Writing – review & editing: Jung Hoi Koo.
Jung Hoi Koo orcid: 0000-0002-4179-3217.
Sun Hong Song orcid: 0000-0002-0728-8997.
Footnotes
Abbreviations: BCRL = breast cancer-related lymphedema, BIS = bioimpedance spectroscopy, CDT = complex decongestive therapy, HBOT = hyperbaric oxygen therapy, QOL = quality of life.
How to cite this article: Koo JH, Song SH, Oh HS, Oh SH. Comparison of the short-term effects of hyperbaric oxygen therapy and complex decongestive therapy on breast cancer-related lymphedema: A pilot study. Medicine. 2020;99:11(e19564).
This research was supported by the Medical Research Promotion Program through the Gangneung Asan Hospital funded by the Asan Foundation (2017-C03).
No commercial party had a direct financial interest in the results of the research supporting this article or will confer a financial benefit upon the authors or upon any organization with which the authors are associated in relation to the publication of this article.
References
- [1].Nguyen TT, Hoskin TL, Habermann EB, et al. Breast cancer-related lymphedema risk is related to multidisciplinary treatment and not surgery alone: results from a large cohort study. Ann Surg Oncol 2017;24:2972–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zou L, Liu FH, Shen PP, et al. The incidence and risk factors of related lymphedema for breast cancer survivors post-operation: a 2-year follow-up prospective cohort study. Breast Cancer 2018;25:309–14. [DOI] [PubMed] [Google Scholar]
- [3].Yesil H, Eyigor S, Caramat I, et al. Effects of complex decongestive therapy on quality of life, depression, neuropathic pain, and fatigue in women with breast cancer-related lymphedema. Turk J Phys Med Rehabil 2017;63:329–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sezgin Ozcan D, Dalyan M, Unsal Delialioglu S, et al. Complex decongestive therapy enhances upper limb functions in patients with breast cancer-related lymphedema. Lymphat Res Biol 2018;16:446–52. [DOI] [PubMed] [Google Scholar]
- [5].Baxter GD, Liu L, Petrich S, et al. Low level laser therapy (Photobiomodulation therapy) for breast cancer-related lymphedema: a systematic review. BMC Cancer 2017;17:833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cebicci MA, Sutbeyaz ST, Goksu SS, et al. Extracorporeal shock wave therapy for breast cancer-related lymphedema: a pilot study. Arch Phys Med Rehabil 2016;97:1520–5. [DOI] [PubMed] [Google Scholar]
- [7].Gothard L, Stanton A, MacLaren J, et al. Non-randomised phase II trial of hyperbaric oxygen therapy in patients with chronic arm lymphoedema and tissue fibrosis after radiotherapy for early breast cancer. Radiother Oncol 2004;70:217–24. [DOI] [PubMed] [Google Scholar]
- [8].Gothard L, Haviland J, Bryson P, et al. Randomised phase II trial of hyperbaric oxygen therapy in patients with chronic arm lymphoedema after radiotherapy for cancer. Radiother Oncol 2010;97:101–7. [DOI] [PubMed] [Google Scholar]
- [9].Teas J, Cunningham JE, Cone L, et al. Can hyperbaric oxygen therapy reduce breast cancer treatment-related lymphedema? A pilot study. J Womens Health (Larchmt) 2004;13:1008–18. [DOI] [PubMed] [Google Scholar]
- [10].Gill AL, Bell CN. Hyperbaric oxygen: its uses, mechanisms of action and outcomes. QJM 2004;97:385–95. [DOI] [PubMed] [Google Scholar]
- [11].Carl UM, Feldmeier JJ, Schmitt G, et al. Hyperbaric oxygen therapy for late sequelae in women receiving radiation after breast-conserving surgery. Int J Radiat Oncol Biol Phys 2001;49:1029–31. [DOI] [PubMed] [Google Scholar]
- [12].Teguh DN, Bol Raap R, Struikmans H, et al. Hyperbaric oxygen therapy for late radiation-induced tissue toxicity: prospectively patient-reported outcome measures in breast cancer patients. Radiat Oncol 2016;11:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hoggan BL, Cameron AL. Systematic review of hyperbaric oxygen therapy for the treatment of non-neurological soft tissue radiation-related injuries. Support Care Cancer 2014;22:1715–26. [DOI] [PubMed] [Google Scholar]
- [14].Kornblith AB, Ligibel J. Psychosocial and sexual functioning of survivors of breast cancer. Semin Oncol 2003;30:799–813. [DOI] [PubMed] [Google Scholar]
- [15].McWayne J, Heiney SP. Psychologic and social sequelae of secondary lymphedema: a review. Cancer 2005;104:457–66. [DOI] [PubMed] [Google Scholar]
- [16].Ahmed RL, Prizment A, Lazovich D, et al. Lymphedema and quality of life in breast cancer survivors: the Iowa Women's Health Study. J Clin Oncol 2008;26:5689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Morgan PA, Franks PJ, Moffatt CJ. Health-related quality of life with lymphoedema: a review of the literature. Int Wound J 2005;2:47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dawes DJ, Meterissian S, Goldberg M, et al. Impact of lymphoedema on arm function and health-related quality of life in women following breast cancer surgery. J Rehabil Med 2008;40:651–8. [DOI] [PubMed] [Google Scholar]
- [19].Keeley V, Crooks S, Locke J, et al. A quality of life measure for limb lymphoedema (LYMQOL). J Lymphoedema 2010;5:26–37. [Google Scholar]
- [20].Kaufman DI, Shah C, Vicini FA, et al. Utilization of bioimpedance spectroscopy in the prevention of chronic breast cancer-related lymphedema. Breast Cancer Res Treat 2017;166:809–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Shah C, Vicini FA, Arthur D. Bioimpedance spectroscopy for breast cancer related lymphedema assessment: Clinical Practice Guidelines. Breast J 2016;22:645–50. [DOI] [PubMed] [Google Scholar]
- [22].Seward C, Skolny M, Brunelle C, et al. A comprehensive review of bioimpedance spectroscopy as a diagnostic tool for the detection and measurement of breast cancer-related lymphedema. J Surg Oncol 2016;114:537–42. [DOI] [PubMed] [Google Scholar]
- [23].Warren AG, Janz BA, Slavin SA, et al. The use of bioimpedance analysis to evaluate lymphedema. Ann Plast Surg 2007;58:541–3. [DOI] [PubMed] [Google Scholar]
- [24].Jung M, Jeon JY, Yun GJ, et al. Reference values of bioelectrical impedance analysis for detecting breast cancer-related lymphedema. Medicine (Baltimore) 2018;97:e12945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cheville A, McGarvey C, Petrek J, et al. Lymphedema management. Semin Radiat Oncol 2003;13:290–301. [DOI] [PubMed] [Google Scholar]
- [26].Baxter GD, Liu L, Tumilty S, et al. Laser Lymphedema Trial Team. Low level laser therapy for the management of breast cancer-related lymphedema: a randomized controlled feasibility study. Lasers Surg Med 2018;50:924–32. [DOI] [PubMed] [Google Scholar]


