Abstract
Kidney injury is a well-known complication in people with coronavirus disease 2019 (COVID-19). In kidney transplant recipients with COVID-19, presentation with nephrotic syndrome has not been well described. We report on a 49-year-old black female kidney transplant recipient who presented 25 years after transplant with clinical features of nephrotic syndrome following a diagnosis of COVID-19. Histologic examination showed acute tubular injury with unremarkable glomeruli on light microscopy and diffuse foot process effacement of podocytes on electron microscopy, consistent with minimal change–like podocyte injury. Apolipoprotein L1 (APOL1) genetic testing confirmed 2 high-risk APOL1 alleles in the kidney donor. We speculate that COVID-19–induced systemic or local cytokine release could serve as a second hit in the presence of APOL1 risk alleles and mediate a podocytopathy manifesting as nephrotic syndrome. The presented case with minimal change–like disease, occurring in the context of the donor high-risk APOL1 genotype, extends the spectrum of clinical manifestations in COVID-19–associated nephropathy.
Highlights
-
•
Kidney injury is common in coronavirus disease 2019 (COVID-19).
-
•
Minimal change disease can be seen as COVID-19–associated nephropathy.
-
•
High-risk apolipoprotein L1 may contribute to development of COVID-19–associated nephropathy.
Acute kidney disease can be seen with coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The kidney manifestations range from simple abnormalities on urinalysis to severe acute kidney injury (AKI) requiring renal replacement therapy. The development of AKI is associated with higher mortality in people with COVID-19 [1,2]. Initial presentation with nephrotic syndrome in COVID-19 is rare but has been reported in patients without a prior history of chronic kidney disease [[3], [4], [5]]. Although a number of kidney transplant recipients have developed COVID-19, there are scarce data on the occurrence of nephrotic syndrome in kidney transplantation [[6], [7], [8]].
We report a case of a long-term kidney transplant recipient with COVID-19 who presented with nephrotic syndrome attributable to minimal change–like podocytopathy in the context of donor homozygosity for apolipoprotein L1 (APOL1) risk allele, G1.
Case Report
The patient is a 49-year-old black woman with a history of pre-eclampsia who developed end-stage kidney disease. She underwent kidney transplant from a sibling donor in December 1995 at an outside hospital. Since 2003, when she relocated to our center, her maintenance immunosuppression has been tacrolimus 3 mg twice daily (target trough level, 3-8 ng/mL) and prednisone 5 mg once daily. She takes atenolol 25 mg twice daily and losartan 75 mg once daily. She had a baseline serum creatinine (SCr) ranging from 1.4 to 1.6 mg/dL (estimated glomerular filtration rate [eGFR] 45-50 mL/min/1.73 m2) and minimal proteinuria until her diagnosis of COVID-19 in April 2020 (Table 1 ).
Table 1.
Baseline and Admission Laboratory Tests
| Test | Reference Range | Baseline (Early April, 2020) | Diagnosis of COVID-19 (Late April, 2020) | Admission (Mid-May, 2020) |
|---|---|---|---|---|
| Days prior to admission | −37 d | −24 d | 0 | |
| WBC (× 103 cells/mm3) | 3.7-10.5 | 11.9 | 7.6 | 10.3 |
| % Neutrophil | 74 | |||
| % Lymphocyte | 20 | |||
| Hemoglobin, g/dL | 11.9-15.5 | 14.5 | 14.0 | 12.5 |
| Platelet, × 103 cells/mm3 | 150-400 | 243 | 196 | 301 |
| CRP (mg/dL) | < 0.5 | < 0.5 | ||
| PT-INR | 1.0 | |||
| Fibrinogen, mg/dL | 194-448 | 262 | ||
| Sodium, mEq/L | 135-145 | 145 | 135 | 138 |
| Potassium, mEq/L | 3.5-5.0 | 3.7 | 3.5 | 4.1 |
| Chloride, mEq/L | 95-107 | 110 | 98 | 107 |
| CO2, mEq/L | 22-29 | 21 | 19 | 16 |
| Urea nitrogen, mg/dL | 10-20 | 23 | 12 | 38 |
| Creatinine, mg/dL | 0.5-1.0 | 1.6 | 1.6 | 3.4 |
| eGFR, mL/min/1.73 m2 | 43 | 43 | 17 | |
| Calcium, mg/dL | 8.5-10.5 | 9.8 | 9.5 | 8.5 |
| Total protein, g/dL | 6.0-8.0 | 7.2 | 5.9 | |
| Albumin, g/dL | 3.4-4.8 | 3.8 | 2.5 (at admission) 2.4 (4 d later) |
|
| Cholesterol, mg/dL | 261 | |||
| LDH, IU/L | 135-214 | 278 | ||
| Ferritin, ng/mL | 13-150 | 564 | ||
| C3, mg/dL | 90-180 | 81 | ||
| C4, mg/dL | 16-47 | 24 | ||
| ANA | < 1:80 | < 1:80 | ||
| DS-DNA, IU/mL | 1-4 | 1 | ||
| Cryoglobulin | negative | negative | ||
| SPEP | negative | negative | ||
| UPEP | negative | negative | ||
| Serum-free K/L LC ratio | 0.26-1.65 | 2.39 | ||
| Hepatitis B virus infection | negative | negative | ||
| Hepatitis C virus infection | negative | negative | ||
| HIV infection | negative | negative | ||
| BKV PCR | negative | negative | ||
| Tacrolimus, 12-h trough, ng/mL | 5.8 | 6.0 | ||
| Urinalysis with microscopy | ||||
| Occult blood | negative | trace | ||
| Protein | negative | 4+ | ||
| White blood cell, /HPF | < 5 | 0-2 | ||
| Red blood cell, /HPF | < 5 | 0-2 | ||
| Urine creatinine, mg/dL | 146 | 55 | ||
| Spot urine protein, mg/dL | 25 | 437 | ||
| UPCR, g/g creatinine | 0.2 | 8.0 | ||
| 24-h urine protein, g/24h | 6.3 |
Abbreviations: ANA, antinuclear antibody; BKV, BK polyomavirus; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; DS-DNA, double-stranded DNA antibody; eGFR, estimated glomerular filtration rate; HPF, high-power field; K/L LC, serum free kappa lambda light chain; LDH, lactate dehydrogenase; PCR, polymerase chain reaction; PT-INR, prothrombin time international normalization ratio; SPEP, serum protein electrophoresis; UPCR, urine protein creatinine; UPEP, urine protein electrophoresis; WBC, white blood cell count.
At presentation, she reported fever (up to 38.6°C), dry cough, myalgia, and anorexia in the context of exposure to SARS-CoV-2 (her spouse had been diagnosed as having COVID-19). Her results were positive for SARS-CoV-2 from a nasopharyngeal swab on reverse transcription-polymerase chain reaction (cycle threshold values of nucleocapsid protein 1 and 2 were 21 and 20, respectively, with a positive threshold of 40 or less). Initially, her renal function was stable, and she was treated conservatively with antipyretics. Her symptoms gradually improved except for an episode of low blood pressure with lightheadedness that prompted discontinuation of losartan. Repeat laboratory work 2 weeks later showed significant deterioration in renal function with a SCr of 4.4 mg/dL (eGFR, 13 mL/min/1.73 m2). She denied any use of nonsteroidal anti-inflammatory drugs. Prerenal AKI was suspected because of her poor oral intake, and her SCr improved to 3.7 mg/dL with intravenous fluids. Three weeks after the initial detection of SARS-CoV-2, she noticed bilateral pedal edema along with chest discomfort. She had persistent poor renal function with a SCr of 3.4 mg/dL (eGFR, 17 mL/min/1.73 m2), a urine protein dipstick test of 4+, and a urine protein creatinine ratio (UPCR) of 8.0 g/g Cr (Table 1). She was admitted to the hospital for further evaluation.
On admission, her body temperature was 36.9°C, heart rate 84 beats per minutes and regular, blood pressure 138/94 mm Hg, respiratory rate 15 beats per minute, pulse oximeter 99% on room air, height 165 cm, weight 79 kg, and body mass index 29 kg/m2. Her physical examination was notable for bilateral pedal edema but no periorbital edema. Admission laboratory studies revealed persistent positivity of a nasopharyngeal sample for SARS-CoV-2 by reverse transcription-polymerase chain reaction (cycle threshold values for nucleocapsid protein 1 and 2 were 36 and 37, respectively), She had a SCr of 3.4 mg/dL, carbon dioxide of 16 mg/dL, and albumin of 2.5 mg/dL but no leukocytosis, lymphopenia, or hypercholesterolemia (Table 1). Subsequently, nephrotic-range proteinuria of 6.3 g/d was confirmed by a 24-hour urine collection. Additional tests for an autoimmune process, other viral infection, and paraproteinemia were negative except for a reduced C3 level of 81 units/mL (normal reference, 90-180) (Table 1). Her chest radiograph at admission was unremarkable and no ultrasonographic evidence of hydronephrosis was seen.
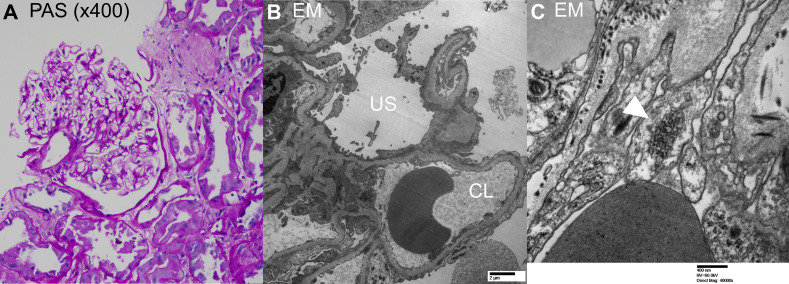
A transplanted kidney biopsy performed 5 days after admission revealed acute tubular injury and diffuse foot process effacement of podocytes in the absence of segmental glomerular sclerosis. Light microscopy showed renal cortex with a total of 9 glomeruli of which 4 were globally sclerosed in the single focal area of scar. The remaining nonsclerosed glomeruli were normocellular with no crescents or podocyte hypertrophy (Fig 1A). No segmental lesion was identified. Tubules showed evidence of acute tubular injury with luminal cell exfoliation and dilatation. There was no tubulitis. There was moderate interstitial fibrosis and tubular atrophy, and moderate arteriosclerosis and arteriolar hyalinosis. C4d stain was negative in the peritubular capillaries; SV-40 was negative by immunohistochemistry, and the Epstein-Barr encoding region was negative by in situ hybridization. Electron microscopy showed glomerular capillary loop basement membrane with preserved trilaminar architecture. There was extensive foot process effacement (up to 90%) with microvillus transformation (Fig 1B). Multiple tubuloreticular inclusions were noted in endothelial cells (Fig 1C), indicative of a viral etiology. The sample for immunofluorescence examination did not contain any glomeruli. However, electron microscopy examination did not show any deposits, essentially ruling out the possibility of immune-complex glomerulonephritis.
Fig 1.
Kidney biopsy. (A) Periodic acid–Schiff stain (× 400), normal glomerulus without crescent, spikes and holes, or basement membrane reduplication and acute tubular injury. No tubulitis was seen. (B) Ultrastructural examination shows extensive foot process effacement with microvillous transformation. (C) Tubuloreticular inclusion noted in capillary loop endothelial cells (white arrowhead). CL, capillary lumen; EM, electron microscopy; PAS, periodic acid–Schiff stain; US, urinary space.
A sample from her kidney tissue tested negative by immunohistochemistry and in situ hybridization for SARS-CoV-2 (Arkana Laboratories, Little Rock, Ark, United States). Testing of her serum sample 5 weeks after her initial presentation showed positive SARS-CoV-2 qualitative antibody by 2 assays (Elecsys Anti-SARS-CoV-2, Roche Diagnostics, Indianapolis, Ind; LIAISON SARS-CoV-2 S1/S2 IgG, DiaSorin Inc, Stillwater, Minn, United States). Genetic testing (Iowa Institute of Human Genetics, Iowa City, Iowa, United States) confirmed that both the recipient and her donor were homozygous for the APOL1 renal risk allele, G1.
Pertinent findings in this patient were acute tubular injury and diffuse foot process effacement of podocytes with endothelial cell tubuloreticular inclusions without evidence for an immune complex–mediated process, graft rejection, or other infectious process. While her AKI was likely secondary to acute tubular injury, her nephrotic syndrome was probably secondary to a viral podocytopathy, manifesting as minimal change disease (MCD).
On day 6 after admission, the patient was discharged home with her maintenance immunosuppression. Because of lack of improvement in proteinuria at 3 weeks after the discharge, oral glucocorticoids (prednisone 60 mg once daily) and angiotensin-converting enzyme inhibitor (lisinopril 10 mg once daily) were added. At her last follow-up, 9 weeks since discharge and after 6 weeks of oral glucocorticoid therapy, she has clinically improved with a SCr of 3.4 mg/dL and UPCR of 2.6 g/g Cr (cf. SCr of 4.4 mg/dL and UPCR of 8.0 g/g Cr at admission). Despite her improvement in proteinuria, her glucocorticoid therapy was weaned down earlier than planned because of its adverse effects.
Discussion
Based on a thorough literature review, the presented case, to our knowledge, is the first to demonstrate MCD-type podocyte injury with nephrotic syndrome in a kidney transplant recipient with COVID-19. Our case with the podocytopathy, occurring in the context of the donor high-risk APOL1 genotype, extends the spectrum of COVID-19–associated nephropathy.
Nephrotic syndrome refers to a constellation of clinical features that are usually seen with a variety of glomerular diseases including the podocytopathies [9]. MCD is the most common cause of nephrotic syndrome in children, whereas it is a less common cause in adults, and conditions like focal and segmental glomerulosclerosis (FSGS) predominate in those patients [10]. It is pertinent to differentiate MCD from FSGS to determine a therapeutic option and its prognosis; however, it is not always an easy task in clinical practice because early FSGS can only present with diffuse foot process effacement. In the presented case, the possibility of early FSGS cannot be completely excluded this time, given the low number of glomeruli obtained and the limited follow-up. However, her prompt response to glucocorticoids in reduction of proteinuria could support MCD-type podocyte injury, whereas her eGFR remains poor because of chronic scarring in the renal interstitium.
Although the exact mechanism of MCD is not known, there could be a pathogenic association between certain viral infections and the resulting T-lymphocyte activation with cytokine release that may contribute to its pathogenesis [11,12]. In some patients with nephrotic syndrome in remission, respiratory infections including SARS-CoV-2 are associated with a relapse of proteinuria [13,14]. On the other hand, in hospitalized patients with moderate to severe COVID-19, about 5% exhibited AKI on admission and 10% had proteinuria defined by a urine protein dipstick of 2+ or greater [15]. A higher rate of proteinuria, up to 42%, was observed in patients with COVID-19 who developed AKI during hospitalization [2]. AKI in critically ill patients with COVID-19 may be secondary to acute tubular injury resulting from hemodynamic instability, although the cause of the reported proteinuria is not well described.
Direct involvement of renal tubules and glomeruli by SARS-CoV-2 is supported by postmortem analyses of fatal cases with COVID-19. Of those, coronavirus-like particles with distinctive spikes were recognized by electron microscopy; viral RNA and protein of SARS-CoV-2 were identified by in situ hybridization and indirect immunofluorescence, respectively [16,17]. This virus binds to the transmembrane protein, angiotensin-converting enzyme 2 (ACE2), during the fusion process [18]. In the kidney, ACE2 is present in podocytes, parietal epithelium of the Bowman capsule, and proximal tubules [18]. This expression pattern and upregulation of ACE2 correlate with the patterns of renal disease in COVID-19, namely, acute tubular injury and proteinuria [16,17]. However, we could not confirm the presence of SARS-CoV-2 in kidney parenchyma by immunohistochemistry or by in situ hybridization, although virus-like particles were seen on electron microscopy (data not shown). Our failure to prove the direct evidence of viral infection in podocytes are actually in line with other studies that speculates cytokine milieu in response to SARS-CoV-2 infection, rather than direct viral involvement in podocytes, as a culprit of COVID-19–associated nephropathy [5,19].
The high-risk APOL1 genotype may contribute to the podocytopathy seen in COVID-19, perhaps by acting in concert with local or systemic cytokine release [5,19]. In viral- or immune-mediated podocytopathies, tubuloreticular inclusions can be seen and these are considered to be interferon footprints because they are induced by both endogenous and exogenous interferon [20]. Analyses of patients with COVID-19 demonstrate an elevation of plasma cytokines including interferon ɣ and induction of interferon-stimulated genes in the respiratory system [21,22]. Similar elevations in cytokines may occur locally in podocytes with COVID-19. Cytokines are known to promote APOL1 gene expression in podocytes, although it is undetermined if the induction of APOL1 gene contributes to podocyte injury [23,24]. Nevertheless, the APOL1 risk alleles, G1 and G2, are well-established risk factors for a number of other kidney diseases including HIV-associated nephropathy, end-stage kidney disease from hypertension, lupus nephritis, sickle cell nephropathy, and FSGS [23,24].
Collapsing glomerulopathy, characterized by podocyte hyperplasia and hypertrophy, is considered the most severe form of podocytopathy and one of variants of FSGS. This collapsing glomerulopathy is the histologic hallmark of HIV-associated nephropathy that is primarily seen in individuals carrying the high-risk APOL1 alleles [23,25]. Several cases of collapsing glomerulopathy with COVID-19 have also been reported in the nontransplant setting in patients who possess a high-risk APOL1 genotype [5,19]. This suggests that SARS-CoV-2 infection could trigger a severe podocytopathy in susceptible individuals who carry the APOL1 risk alleles [5]. Clearly, our recipient who received a transplant with a kidney bearing 2 APOL1 risk alleles was doing well for 25 years after transplant, indicating that the high-risk APOL1 genotype alone is insufficient to cause a proteinuric kidney disease. A second hit, such as HIV or, in our case, SARS-CoV-2, may have been required for the development of disease.
Conclusions
This report describes MCD-like podocytopathy causing nephrotic syndrome in a patient with COVID-19. This case extends the spectrum of COVID-19–associated nephropathy that can be seen in transplant recipients. The podocytopathy occurred in a recipient who had received a kidney bearing 2 high-risk APOL1 alleles and who had stable kidney allograft function for years after transplant. We speculate that cytokine activation within the podocytes following SARS-CoV-2 infection, in the presence of a susceptible APOL1 genotype, resulted in a clinical manifestation that included MCD-like podocytopathy.
Acknowledgments
We thank Mr Paul J. Casella for editorial assistance.
Footnotes
Masaaki Yamada and Prerna Rastogi contributed equally to this article.
Disclosure Statement: DI receives research funding from Gilead Sciences Inc (Foster City, Calif, United States) for a clinical trial of remdesivir use in COVID-19. However, the presented case did not meet eligibility criteria at a time of admission, and she was not enrolled in the clinical trial. Other authors have no conflict of interest to declare.
References
- 1.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirsch J.S., Ng J.H., Ross D.W., Sharma P., Shah H.H., Barnett R.L. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98:209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larsen C.P., Bourne T.D., Wilson J.D., Saqqa O., Sharshir M.A. Collapsing glomerulopathy in a patient with COVID-19. Kidney Int Rep. 2020;5:935–939. doi: 10.1016/j.ekir.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kissling S., Rotman S., Gerber C., Halfon M., Lamoth F., Comte D. Collapsing glomerulopathy in a COVID-19 patient. Kidney Int. 2020;98:228–231. doi: 10.1016/j.kint.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu H., Larsen C.P., Hernandez-Arroyo C.F., Mohamed M.M.B., Caza T., Sharshir M. AKI and collapsing glomerulopathy associated with COVID-19 and APOL 1 High-Risk Genotype. J Am Soc Nephrol. 2020;31:1688–1695. doi: 10.1681/ASN.2020050558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nair V., Jandovitz N., Hirsch J.S., Nair G., Abate M., Bhaskaran M. COVID-19 in kidney transplant recipients. Am J Transplant. 2020;20:1819–1825. doi: 10.1111/ajt.15967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Columbia University Kidney Transplant Program Early description of coronavirus 2019 disease in kidney transplant recipients in New York. J Am Soc Nephrol. 2020;31:1150–1156. doi: 10.1681/ASN.2020030375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lazareth H, Pere H, Binois Y, Chabannes M, Schurder J, Bruneau T, et al: COVID-19-related collapsing glomerulopathy in a kidney transplant recipient [e-pub ahead of print]. Am J Kidney Dis https://doi.org/10.1053/j.ajkd.2020.06.009, accessed September 17, 2020. [DOI] [PMC free article] [PubMed]
- 9.Maas R.J., Deegens J.K., Smeets B., Moeller M.J., Wetzels J.F. Minimal change disease and idiopathic FSGS: manifestations of the same disease. Nat Rev Nephrol. 2016;12:768–776. doi: 10.1038/nrneph.2016.147. [DOI] [PubMed] [Google Scholar]
- 10.Waldman M., Crew R.J., Valeri A., Busch J., Stokes B., Markowitz G. Adult minimal-change disease: clinical characteristics, treatment, and outcomes. Clin J Am Soc Nephrol. 2007;2:445–453. doi: 10.2215/CJN.03531006. [DOI] [PubMed] [Google Scholar]
- 11.Kaneko K. Springer; Tokyo: 2016. Molecular mechanisms in the pathogenesis of idiopathic nephrotic syndrome. [Google Scholar]
- 12.Stangou M., Spartalis M., Daikidou D.V., Kouloukourgiotou T., Sampani E., Lambropoulou I.T. Impact of Tauh1 and Tauh2 cytokines in the progression of idiopathic nephrotic syndrome due to focal segmental glomerulosclerosis and minimal change disease. J Nephropathol. 2017;6:187–195. doi: 10.15171/jnp.2017.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacDonald N.E., Wolfish N., McLaine P., Phipps P., Rossier E. Role of respiratory viruses in exacerbations of primary nephrotic syndrome. J Pediatr. 1986;108:378–382. doi: 10.1016/s0022-3476(86)80876-9. [DOI] [PubMed] [Google Scholar]
- 14.Melgosa M., Madrid A., Alvarez O., Lumbreras J., Nieto F., Parada E. SARS-CoV-2 infection in Spanish children with chronic kidney pathologies. Pediatr Nephrol. 2020;35:1521–1524. doi: 10.1007/s00467-020-04597-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng Y., Luo R., Wang K., Zhang M., Wang Z., Dong L. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Su H., Yang M., Wan C., Yi L.X., Tang F., Zhu H.Y. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98:219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puelles V.G., Lutgehetmann M., Lindenmeyer M.T., Sperhake J.P., Wong M.N., Allweiss L. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020;383:590–592. doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez-Rojas M.A., Vega-Vega O., Bobadilla N.A. Is the kidney a target of SARS-CoV-2? Am J Physiol Renal Physiol. 2020;318:F1454–F1462. doi: 10.1152/ajprenal.00160.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Batal I., Santoriello D., Xu K., Barasch J., Peleg Y. Kidney Biopsy Findings in Patients with COVID-19. J Am Soc Nephrol. 2020;31:1959–1968. doi: 10.1681/ASN.2020060802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bromfield M., McQuillan R., John R., Avila-Casado C. The significance of tubuloreticular inclusions as a marker of systemic stimulation by interferons in a case of focal and segmental glomerulosclerosis associated with cytomegalovirus (CMV) infection. Clin Kidney J. 2014;7:174–178. doi: 10.1093/ckj/sft156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou Z., Ren L., Zhang L., Zhong J., Xiao Y., Jia Z. Heightened innate immune responses in the respiratory tract of COVID-19 patients. Cell Host Microbe. 2020;27:883–890. doi: 10.1016/j.chom.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bruggeman L.A., O’Toole J.F., Sedor J.R. APOL1 polymorphisms and kidney disease: loss-of-function or gain-of-function? Am J Physiol Renal Physiol. 2019;316:F1–F8. doi: 10.1152/ajprenal.00426.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robinson T.W., Freedman B.I. The impact of APOL1 on chronic kidney disease and hypertension. Adv Chronic Kidney Dis. 2019;26:131–136. doi: 10.1053/j.ackd.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenberg A.Z., Naicker S., Winkler C.A., Kopp J.B. HIV-associated nephropathies: epidemiology, pathology, mechanisms and treatment. Nat Rev Nephrol. 2015;11:150–160. doi: 10.1038/nrneph.2015.9. [DOI] [PubMed] [Google Scholar]