Abstract
High levels of serum alanine aminotransferase (ALT) and gamma-glutamyltransferase (GGT) are associated with increased diabetes risk. In the present study, we investigated the combined effects of ALT and GGT on the development of diabetes in a Korean population. A total of 9405 individuals (4020 women and 5385 men) without diabetes were enrolled in this study. From the baseline health screening to the follow-up examination, the development of diabetes, based on changes in ALT and GGT quartile levels, was analyzed. In addition, we analyzed the quartiles of ALT and GGT together to determine any synergistic effect from the fourth quartile of ALT and GGT on the development of diabetes. The development of diabetes gradually increased with an increase in the circulating levels of ALT and GGT. For the fourth quartile ALT and GGT, the hazard ratios of diabetes compared with the first quartile were 1.892 (95% confidence interval [CI]: 1.26–2.83, P = .002) and 3.526 (95% CI: 2.12–5.85, P < .001) after adjusting for confounders, respectively. Hazard ratios of diabetes after combining both fourth quartiles of ALT and GGT were 3.663 (95% CI: 2.42–5.52, P < .001), as compared with the first and second quartiles. Serum ALT and GGT levels are well associated with diabetes in Koreans after adjusting for confounders, and a combination of ALT and GGT levels can have a synergy in predicting the development of diabetes.
Keywords: alanine aminotransferase; diabetes mellitus; gamma-glutamyltransferase,
1. Introduction
Despite advances in medical care for decades, the incidence of diabetes has increased not only in the Western world but also in Asia. In general, patients diagnosed with diabetes need to take medication over a lifetime and should be constantly monitored to prevent diabetic complications. In addition, diabetes and its complications are a significant cause of increased morbidity and mortality, and result in a heavy economic burden.[1] Therefore, early identification of risk factors for diabetes is important for preventing more serious complications and increased expenditure.
Recently, several studies have reported the elevation of serum alanine aminotransferase (ALT) and gamma-glutamyltransferase (GGT) as risk factors for the development of diabetes.[2,3] ALT and GGT are considered to be surrogate markers of liver function. A systematic review and meta-analysis of prospective studies indicated moderate associations between ALT levels and the risk of developing diabetes.[4] An elevated serum GGT level also relates to incidence of diabetes in adults, and is an important biochemical risk indicator for diabetes prediction.[5,6] In addition, a meta-analysis reported that both ALT and GGT elevation were associated with increased risk of diabetes.[7]
In spite of the clinical effect these increased ALT and GGT levels have in many studies, little is known about the combined effect of these biomarkers on the development of diabetes after controlling for multiple potential confounding factors in the general population. The present study was based on the hypothesis that the incidence of diabetes was higher when both indicators were elevated than when only 1 of 2 was increased. This study was aimed to investigate the combined association of increased ALT and GGT levels in development of diabetes.
2. Methods
2.1. Study population
We analyzed the data of the subjects who had undergone health recheckups from 2009 to 2014 from among the 37,582 people who had received checkups from 1999 to 2001 at the Health Promotion Center, Ajou University Hospital, Suwon, Korea. If a person received more than 2 health checkups from 1999 to 2001, the first health checkup result was used. In addition, if the health checkup was received more than once from 2009 to 2014, the result of the last checkup was analyzed. For the purposes of this study, those who received health examinations from 1999 to 2001 were excluded if any of the following conditions were met (n = 27,985): subjects diagnosed with diabetes; subjects with missing fasting blood glucose, serum ALT or serum GGT values; subjects with a history of chronic liver disease, such as hepatitis B or C, or liver cirrhosis, or who were taking drugs influencing the liver function; subjects diagnosed with cardiovascular disease or cancer; subjects who had taken enough alcohol to damage the liver (>20 g/day in females or >30 g/day in male).[8,9] We further excluded patients (n = 192) whose serum GGT was 3 times greater than normal (≥198 U/L) to reduce this confusion, because serum GGT may be elevated because of viruses or toxic substances. Finally, the participant sample included 9405 individuals (4020 women and 5385 men).
2.2. Measurements
Blood was collected on the morning of the day of hospital visit after fasting for more than 10 hours. Blood tests included fasting blood glucose, ALT, GGT, Uric acid, total cholesterol, triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C). Blood pressure was measured by mercury sphygmomanometer after resting for more than 5 minutes, and the average value of 2 measurements was used. Hypertension was defined as having a systolic Blood pressure ≥140 mm Hg or a diastolic Blood pressure ≥90 mm Hg or being on antihypertensive medications. Dyslipidemia was defined as LDL ≥160 mg/dL or TG ≥200 mg/dL, or by the use of antihyperlipidemic medications. Development of diabetes was defined as having a fasting blood glucose ≥126 mg/dL, or using oral hypoglycemic agents or insulin at the follow-up examination. Body mass index (BMI) was calculated as weight (kilograms) divided by height squared (square meters). We also used a self-reporting questionnaire for a history of hypertension, diabetes mellitus, hyperlipidemia, smoking status, and alcohol consumption. Smoking status was classified as being either a current smoker or nonsmoker. Alcohol consumption in individuals was calculated and then converted to weekly alcohol consumption levels (grams of ethanol per week) using the graduated frequency method.[8] Medical history and use of medications were based on information obtained by interviewers trained in collecting data.
2.3. Statistical analyses
We used a simple descriptive analysis for general characteristics. We grouped the study individuals into 4 quartiles according to the levels of their serum ALT and GGT grading. Analysis of variance trend analysis using polynomial contrasts was adapted to perform tests for trends according to the levels of serum ALT and GGT grading. Analyses relating the levels of serum ALT and GGT to development of diabetes used the Cox regression analysis, which was also used to assess the combined effect of serum ALT and GGT on development of diabetes. The distribution of alcohol consumption was right-skewed; therefore, a natural-log transformation was applied. We included covariates such as the baseline examination values of age, gender, BMI, log-transformed alcohol consumption, smoking status, hypertension, hyperlipidemia, and uric acid in the Cox regression analysis. All statistical analyses were done using the SPSS v20.0 software (SPSS, Inc, Chicago, IL), with P < .05 considered statistically significant.
3. Results
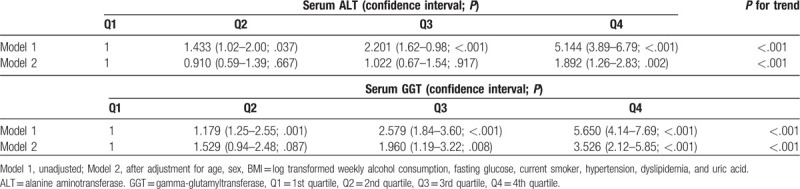
We divided the study subjects into 4 groups according to the ALT levels. The mean follow-up periods of the first to fourth quartiles of serum ALT values were 9.84, 9.39, 9.39, and 9.45 years, respectively. The range of the first to fourth quartiles of serum ALT values was 3 to 17, 18 to 23, 24 to 34, and 35 to 191 IU/L, respectively. We also divided the study subjects into 4 groups according to the GGT levels. The mean follow-up periods of the first to fourth quartiles of serum GGT values were 9.45, 9.51, 9.59, and 9.54 years, respectively. The range of the first to fourth quartiles of serum GGT values was 4 to 13, 14 to 20, 21 to 35, and 36 to 198 IU/L, respectively. Table 1 shows the baseline characteristics of the study subjects according to the quartiles of ALT and GGT. As the ALT quartile increased, traditional diabetes risk factors, such as hypertension, hyperlipidemia, alcohol consumption per week, and current smokers increased, as well as a new risk factor uric acid. Increased prevalence of males with increasing ALT quartiles was also associated with an increase in these factors. This trend was similarly observed with increasing GGT quartile. We did Cox proportional analysis for the odds ratio of diabetes in the second to fourth quartiles of serum ALT values compared to the lowest quartile of serum ALT values after allowing for confounding variables, such as age, gender, BMI, hypertension, hyperlipidemia, alcohol consumption per week, current smokers, and uric acid (Table 2). Compared with individuals with the first quartile of serum ALT, the adjusted odds ratio (95% confidence interval [CI]) of the second to fourth quartiles was 0.910 (0.59–1.39), 1.022 (0.67–1.54), and 1.892 (1.26–2.83), respectively. The odds ratio of the highest quartile of serum ALT values was attenuated after adjusting these confounding variables but remained statistically significant. Similarly for the GGT, the adjusted odds ratio (95% CI) of the second to highest quartiles was 1.529 (0.94–2.48), 1.960 (1.19–3.22), and 3.526 (2.12–5.85), respectively, compared with individuals with the lowest quartile of serum GGT. The odds ratio of the third to highest quartile of serum GGT values was also attenuated after adjusting for these confounders but remained statistically significant.
Table 1.
The baseline characteristics of the study subjects by serum alanine aminotransferase and gamma-glutamyltransferase grading.
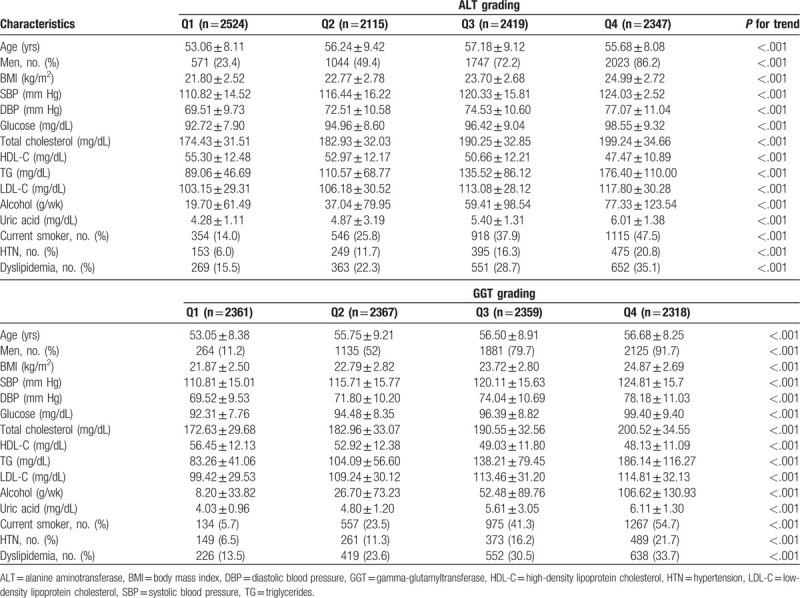
Table 2.
Hazard ratios of diabetes mellitus by serum alanine aminotransferase and gamma-glutamyltransferase.
We also divided the study subjects into 9 groups according to the ALT and GGT levels (1st and 2nd quartile, 3rd quartile, 4th quartile) (Fig. 1). Taking ALT and GGT into consideration together strengthened their association for the odds ratio of new-onset diabetes. Adjusted OR of development of diabetes in the fourth quartile of both ALT and GGT was 3.663 (95% CI: 2.42–5.52) compared with the reference group (1st and 2nd quartile of ALT and GGT). This finding was supported by the fact that the interaction term for ALT and GGT was statistically significant (P < .001).
Figure 1.
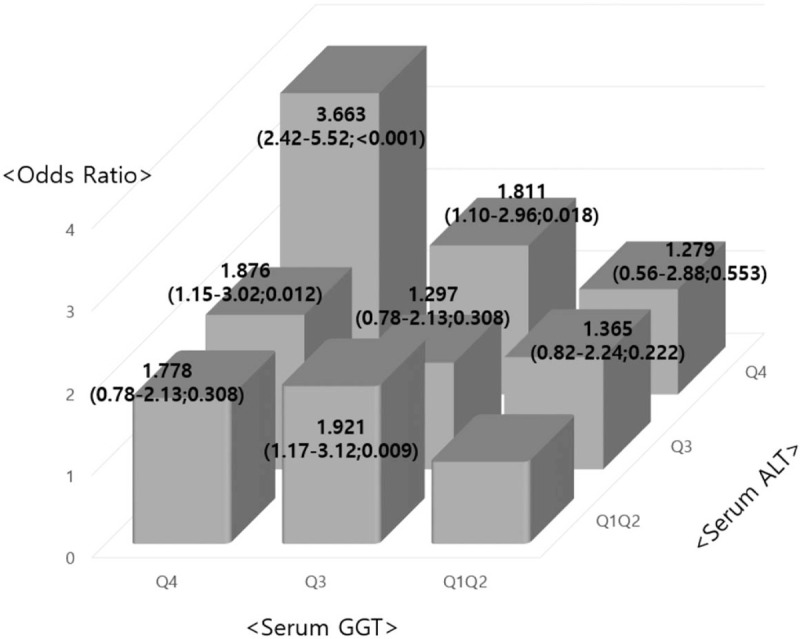
Adjusted hazard ratios∗ (95% confidence interval; P) of the development of diabetes mellitus according to the combined categories of ALT and GGT levels (1st and 2nd quartile, 3rd quartile, and 4th quartile). The reference category consisted of the 1st and 2nd quartile of ALT and GGT levels. Interaction terms for ALT and GGT combinations were statistically significant (P < .001). ALT = alanine aminotransferase, BMI = body mass index, GGT = gamma-glutamyltransferase. ∗The model was adjusted for age, sex, BMI = log-transformed weekly alcohol consumption, fasting glucose, current smoker, hypertension, dyslipidemia, and uric acid.
4. Discussion
In our longitudinal study, we found that the development of diabetes significantly increased according to serum GGT and ALT quartiles after adjusting for established diabetes risk factors (age, gender, BMI, log-transformed alcohol consumption, smoking status, hypertension, hyperlipidemia, and uric acid). In addition, the cut-off of fourth-quartile ALT and GGT levels was defined as > 35 IU/L and >36 IU/L, respectively. These values are considered to be abnormal ALT and GGT levels in most medical laboratories. The observations that the odds ratio of new-onset diabetes by ALT and GGT quartile was significantly increased within the normal reference range of ALT and GGT levels suggest that ALT and GGT may be independent factors of diabetes development, even in the normal range. Furthermore, we showed that the hazard ratios for development of diabetes were significantly increased in relation to the combination of serum ALT and GGT.
Some prospective studies for association between serum ALT and diabetes have arrived at results similar to ours. Song et al,[10] with a prospective cohort of 6484 individuals, found that, compared with the low ALT group, the odds ratios for incident diabetes of the high ALT groups were 1.40 (1.04–1.89) after adjusting for confounding factors. Zhang et al[11] reported that in the fatty liver group, which was adjusted for multiple confounding factors, ALT remained significantly associated with the risk for incident type 2 diabetes. In addition, the relationship between elevated serum GGT and diabetes mellitus was consistent with our results in many cross-sectional and prospective studies.[12,13] The Bogalusa Heart study reported that elevations in levels of GGT were related to incidence of prediabetes and diabetes in apparently healthy adults.[14] The Coronary Artery Risk Development in Young Adults Study demonstrated that serum GGT, even in the range regarded as physiologically normal, is associated with incident diabetes.[15]
Although the biological mechanisms are not completely understood, mounting evidence suggests that ALT and GGT may be involved in the development of diabetes. With regards to ALT levels, ALT, as a specific marker of accumulation of liver fat, was associated with hepatic insulin sensitivity.[16] ALT is commonly considered to be an epidemiologic biomarker of nonalcoholic fatty liver disease, which was associated with increased risk of developing diabetes.[17] In addition, ALT levels have been demonstrated to be connected with hepatic insulin resistance, which may contribute to the development of diabetes.[18] For GGT levels, serum GGT may be an oxidative stress marker for diabetes associated with glutathione.[19] The elevation of GGT is a response to oxidative stress, which means that glutathione is transported into the cell. In this respect, the elevation of GGT may indicate that oxidative stress is increasing at the cellular level. The beta cells of the pancreas that regulate insulin secretion are particularly sensitive to oxidative stress because there are relatively few antioxidant enzymes, such as superoxide dismutase, catalase, and glutathione peroxidase.[20] Indeed, oxidative stress is known to be a factor that reduces insulin secretion by damaging the beta cells of the pancreas.[21] These mechanisms may be responsible for the association of ALT and GGT levels with the onset of diabetes.
We also evaluated whether there was a synergism between serum ALT and GGT on the development of diabetes. We found that the adjusted hazard ratios for development of diabetes in the fourth quartile of serum ALT and GGT were significantly higher those for the reference group. This finding may be supported by the observation that both ALT and GGT were associated with systemic inflammation and oxidative stress.[22]
This study had several strengths. First, to the best of our knowledge, this was the first longitudinal study to examine the combined effects of serum ALT and GGT on the onset of diabetes in a general population. Second, this study included a relatively large number of subjects of both genders and had a long follow-up period. This study also had several limitations. First, from the self-reporting questionnaires, we accessed only parts of an individual's medical history; the smoking status or use of drugs or supplements might have been underreported. Second, our data were not necessarily representative of the whole population, because it was not derived from a random selection.
In conclusion, this study demonstrated that the combined effect of serum ALT and GGT is associated with a diabetes risk in Koreans and that using measurements of a combination of ALT and GGT appears to help predict the future development of diabetes. To prevent cardiovascular events, this greater relationship between both increased ALT and GGT levels and increased incidence of diabetes should be noted, even in subjects without diabetes mellitus at baseline.
Author contributions
Conceptualization: Kyu-Nam Kim.
Formal analysis: Kyu-Nam Kim, Sung-Hyun Choi, Bom-Taeck Kim, Junghwa Shin.
Methodology: Bom-Taeck Kim, Junghwa Shin.
Project administration: Kyu-Nam Kim, Sung-Hyun Choi.
Supervision: Kyu-Nam Kim, Bom-Taeck Kim.
Writing – original draft: Kyu-Nam Kim, Sung-Hyun Choi.
Writing – review & editing: Sung-Hyun Choi, Junghwa Shin.
Kyu-Nam Kim orcid: 0000-0002-1213-5004.
Sung-Hyun Choi orcid: 0000-0002-4151-8952.
Bom-Taeck Kim orcid: 0000-0002-0395-0410.
Junghwa Shin orcid: 0000-0002-4151-8952.
Footnotes
Abbreviations: ALT = alanine aminotransferase, BMI = body mass index, DBP = diastolic blood pressure, GGT = gamma-glutamyltransferase, HDL-C = high-density lipoprotein cholesterol, HTN = hypertension, LDL-C = low-density lipoprotein cholesterol, Q1 = 1st quartile, Q2 = 2nd quartile, Q3 = 3rd quartile, Q4 = 4th quartile, SBP = systolic blood pressure, TG = triglycerides.
How to cite this article: Choi SH, Kim BT, Shin J, Kim KN. Combined effect of serum alanine aminotransferase and gamma-glutamyltransferase on incidence of diabetes mellitus: A longitudinal study. Medicine. 2020;99:11(e18963).
Ethics committee approval was received for this study from The Institutional Review Board of Ajou University Hospital (No: AJIRB-MED-MDB-16–063). The requirement for informed consent was waived by the board because it was retrospective.
The authors have no conflicts of interest to disclose.
References
- [1].Viswanathan V, Ranjan S. Diabetes: an ancient disease, epidemic & an economic burden for the present era. Indian J Med Res 2016;143:389–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Li Y, Wang J, Han X, et al. Serum alanine transaminase levels predict type 2 diabetes risk among a middle-aged and elderly Chinese population. Ann Hepatol 2019;18:298–303. [DOI] [PubMed] [Google Scholar]
- [3].Lee DH, Ha MH, Kim JH, et al. Gamma-glutamyltransferase and diabetes: a 4 year follow-up study. Diabetologia 2003;46:359–64. [DOI] [PubMed] [Google Scholar]
- [4].Kunutsor SK, Apekey TA, Walley J. Liver aminotransferases and risk of incident type 2 diabetes: a systematic review and meta-analysis. Am J Epidemiol 2013;178:159–71. [DOI] [PubMed] [Google Scholar]
- [5].Cho NH, Jang HC, Choi SH, et al. Abnormal liver function test predicts type 2 diabetes: a community-based prospective study. Diabetes Care 2007;30:2566–8. [DOI] [PubMed] [Google Scholar]
- [6].Mason JE, Starke RD, Van Kirk JE. Gamma-glutamyl transferase: a novel cardiovascular risk biomarker. Prev Cardiol 2010;13:36–41. [DOI] [PubMed] [Google Scholar]
- [7].Fraser A, Harris R, Sattar N, et al. Alanine aminotransferase, gamma-glutamyltransferase, and incident diabetes: the British Women's Heart and Health Study and meta-analysis. Diabetes Care 2009;32:741–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Greenfield TK. Ways of measuring drinking patterns and the difference they make: experience with graduated frequencies. J Subst Abuse 2000;12:33–49. [DOI] [PubMed] [Google Scholar]
- [9].Bellentani S, Saccoccio G, Costa G, et al. Drinking habits as cofactors of risk for alcohol induced liver damage. The Dionysos Study Group. Gut 1997;41:845–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Song BM, Kim HC, Kim DJ, et al. Aminotransferase levels, body mass index, and the risk of diabetes: a prospective cohort study. Ann Epidemiol 2018;28:675–80. [DOI] [PubMed] [Google Scholar]
- [11].Zhang J, Cheng N, Ma Y, et al. Liver enzymes, fatty liver and type 2 diabetes mellitus in a Jinchang cohort: a prospective study in adults. Can J Diabetes 2018;42:652–8. [DOI] [PubMed] [Google Scholar]
- [12].Ko SH, Baeg MK, Han KD, et al. Increased liver markers are associated with higher risk of type 2 diabetes. World J Gastroenterol 2015;21:7478–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lee YS, Cho Y, Burgess S, et al. Serum gamma-glutamyl transferase and risk of type 2 diabetes in the general Korean population: a Mendelian randomization study. Hum Mol Genet 2016;25:3877–86. [DOI] [PubMed] [Google Scholar]
- [14].Nguyen QM, Srinivasan SR, Xu JH, et al. Elevated liver function enzymes are related to the development of prediabetes and type 2 diabetes in younger adults: the Bogalusa Heart Study. Diabetes Care 2011;34:2603–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lee DH, Jacobs DR, Jr, Gross M, et al. Gamma-glutamyltransferase is a predictor of incident diabetes and hypertension: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Clin Chem 2003;49:1358–66. [DOI] [PubMed] [Google Scholar]
- [16].Vozarova B, Stefan N, Lindsay RS, et al. High alanine aminotransferase is associated with decreased hepatic insulin sensitivity and predicts the development of type 2 diabetes. Diabetes 2002;51:1889–95. [DOI] [PubMed] [Google Scholar]
- [17].Sung KC, Jeong WS, Wild SH, Byrne CD. Combined influence of insulin resistance, overweight/obesity, and fatty liver as risk factors for type 2 diabetes. Diabetes Care 2012;35:717–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Venkatesan C, Younossi ZM. Potential mechanisms underlying the associations between liver enzymes and risk for type 2 diabetes. Hepatology 2012;55:968–70. [DOI] [PubMed] [Google Scholar]
- [19].Rösen P, Nawroth PP, King G, et al. The role of oxidative stress in the onset and progression of diabetes and its complications: a summary of a Congress Series sponsored by UNESCO-MCBN, the American Diabetes Association and the German Diabetes Society. Diabetes Metab Res Rev 2001;17:189–212. [DOI] [PubMed] [Google Scholar]
- [20].Matsuoka T, Kajimoto Y, Watada H, et al. Glycation-dependent, reactive oxygen species-mediated suppression of the insulin gene promoter activity in HIT cells. J Clin Invest 1997;99:144–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Nakagawa T, Tuttle KR, Short RA, et al. Hypothesis: fructose-induced hyperuricemia as a causal mechanism for the epidemic of the metabolic syndrome. Nat Clin Pract Nephrol 2005;1:80–6. [DOI] [PubMed] [Google Scholar]
- [22].Yamada J, Tomiyama H, Yambe M, et al. Elevated serum levels of alanine aminotransferase and gamma glutamyltransferase are markers of inflammation and oxidative stress independent of the metabolic syndrome. Atherosclerosis 2006;189:198–205. [DOI] [PubMed] [Google Scholar]


