Abstract
Background:
A growing number of epidemiological studies have suggested a possible association between long-chain omega-3 polyunsaturated fatty acid (PUFA) intake and the risk of cancers, but the results have been inconsistent. We aimed to conduct a meta-analysis to assess the association of omega-3 PUFA consumption with digestive system cancers.
Methods:
Relevant observational studies were identified through a comprehensive search of PubMed, Embase, and the Web of Science through December 2019 and by reviewing the references of the retrieved articles. The relative risks (RRs) of digestive system cancers associated with omega-3 PUFA intake were estimated using a random-effect model and were stratified by region, sex, study design, type of omega-3 PUFAs, smoking status, alcohol consumption, BMI, and physical activity.
Results:
Twenty-five studies (8 case–control studies and 17 cohort studies) involving 1,247,271 participants and 23,173 patients with digestive system cancers were included in this analysis. The risk of digestive system cancers decreased by 17% in individuals who consumed omega-3 PUFAs (RR = 0.83, 95% confidence interval (CI), 0.76–0.91). The risk estimates of digestive system cancers varied by cancer sites, study location, study design, type of omega-3 PUFAs, and other confounders (smoking, alcohol consumption, body mass index, and physical activity). Visual inspection of funnel plots and the Begg's and Egger's tests revealed no evidence of publication bias.
Conclusion:
The findings show that omega-3 PUFAs should be as a healthy dietary component for the prevention of digestive system cancers. Cancer incidence decreases with increasing omega-3 PUFAs intake for most digestive system cancer sites. The relation between omega-3 PUFAs and digestive system cancers RR is similar among different populations.
Keywords: digestive system cancers, docosahexaenoic acid, omega-3 PUFAs, protective effect
1. Introduction
It has been estimated that by 2020, the number of new cases of cancer will increase to more than 15 million, with the number of deaths increasing to 12 million worldwide.[1] Digestive system cancers are the most common malignant tumors in the world with three million new cases adding each year (accounting for nearly 30% of all cancers).[1] The incidence of digestive system cancers will constantly increase, mainly due to trends in gastric cancer and colorectal cancer.[2] In the majority of developing countries, increasing trends in mortality rates associated with digestive system cancers have also been observed.[3,4]
Although increasing evidence has implicated omega-3 polyunsaturated fatty acids (PUFAs) in delaying the progression of anxiety,[5] cardiovascular disease,[6] and polycystic ovary syndrome,[7] their role in cancer etiology has yet to be established. Some observational epidemiological studies[8–10] have investigated the relationship between long-chain omega-3 PUFAs and the risk of cancers; however, the findings remain controversial. One previous meta-analysis[11] indicated that there was a null association between n-3 PUFAs and the risk of colorectal cancer. However, two systematic reviews of omega-3 PUFAs and cancer risk qualitatively concluded that there is inadequate[12] or limited evidence[13] to suggest an association between long-chain omega-3 PUFA intake and the risk of digestive system cancers. However, it had only been restricted to some types of cancer in most of these studies, and some cancer types, such as esophageal and oral cavity/pharynx cancers, were not investigated. Therefore, we conducted this comprehensive systematic review and meta-analysis study, in order to explore the relationship between omega-3 PUFA usage and the risk of digestive system cancers.
2. Methods
2.1. Search strategy
We followed the reporting standards for systematic reviews and meta-analyses of observational studies described in the Observational Studies in Epidemiology (MOOSE) guidelines.[14] For this meta-analysis, three electronic databases including PubMed, Embase, and the Web of Science were used to search from their inception to December 31, 2019, without language restrictions. Reviewing peer-review published articles and computer-aided literature searches using the following search terms with different combinations: (“omega” or “ω-3” or “n-3” or “PUFA” or “fish oil” or “EPA” or “DHA” or “PUFAs” or “eicosapentaenoic acid” or “docosahexaenoic acid” or “linolenic acid” or “docosapentaenoic acid” or “DPA” or “ALA”) and (“cancer” or “carcinoma” or “oncology” or “neoplasm”) were used to identify the eligible studies. The human studies were restricted in our study. The reference list of any article selected for consideration was manually checked for additional studies.
2.2. Inclusion criteria
We include studies if they met the following criteria:
-
1.
the study was designed using a case-control or cohort;
-
2.
the exposure of interest was omega-3 PUFA intake;
-
3.
the outcome of interest was diagnosed as digestive system cancers or first as digestive system cancers and had died during follow-up in cohort studies;
-
4.
the study reported estimates of odds ratios (ORs), relative risks (RRs), or hazard ratios (HRs) with 95% confidence intervals (CIs) (or information to calculate those effect sizes).
Only the most recent and informative study was included if the same population were published in multiple reports.
2.3. Data extraction and quality assessment
Two independent authors independently reviewed all the included studies. The following information which was extracted from each study using a data collection form: year of publication, the first author's name, study design, location, number of cases and participants, sex, types of cancer, exposure definition, risk estimates (ORs, RRs, or HRs) and 95% CIs, and covariates adjusted in the analysis. When data were missing, we contacted the authors for detailed information.
Two reviewers independently assessed the study quality by using the Newcastle–Ottawa Scale (NOS) according to the procedures recommended in the Cochrane Handbook of Systematic Reviews.[15] The NOS was a 9-point scale that allocates points on the basis of the process of selection of the cohort study or case–control study (0–4 points), the comparability (0–2 points), and the identification of outcomes or exposures of study participants (0–3 points). We assigned scores of 0–3, 4–6, and 7–9 for low, moderate, and high quality of studies, respectively. Inter-observer agreement (κ) was 0.892. Any disagreement was resolved by review of the manuscript together to reach consensus.
2.4. Statistical analysis
RRs were used to measure the associations between omega-3 PUFAs and the risk of digestive system cancers, and HRs were considered equivalent to RRs. We combined the case–control and cohort studies in the primary meta-analysis as ORs and RRs provide similar estimates of risk when the outcome was rare.[16,17] Studies reported by different populations or by types of cancer were regarded as independent reports.
A random-effects model was used to estimate the pooled RRs for the relationship between omega-3 PUFAs and the risk of digestive system cancers,[18] if heterogeneity was detected, or the fixed-effects model was used otherwise. The heterogeneity across studies was assessed with I2 statistic, where value of 25% or less, near 50%, and near 75% or greater as having low, moderate, and high degrees of heterogeneity, respectively.[19] The Egger's and Begg's tests and inspecting the symmetry of funnel plots were adopted to evaluate publication bias, as recommended by the Cochrane handbook.[20,21] Subgroup analyses were conducted to explore the potential sources of heterogeneity, and were stratified by exposure measurement, study design, sex, study location, cancer site, smoking status, alcohol consumption, body mass index (BMI), and physical activity were conducted to assess the robustness of the results. Additionally, the differences among subgroups were tested using meta-regression analysis (using STATA “metareg” command). Sensitivity analyses were conducted to assess the influence of any single study on the pooled RRs. All statistical analyses were conducted with Stata version 13.0.
3. Results
3.1. Literature search
Figure 1 shows the process of the identification of eligible studies. Of the 5224 articles that were identified, 35 articles qualified for full-text evaluation. Among these articles that underwent full-text review, six articles were duplicate reports from the same study population, eight articles were unmatched with the study exposure, and four studies were added from reference lists ultimately, 25 studies[8–10,22–43] with 28 independent reports were included in the meta-analysis.
Figure 1.
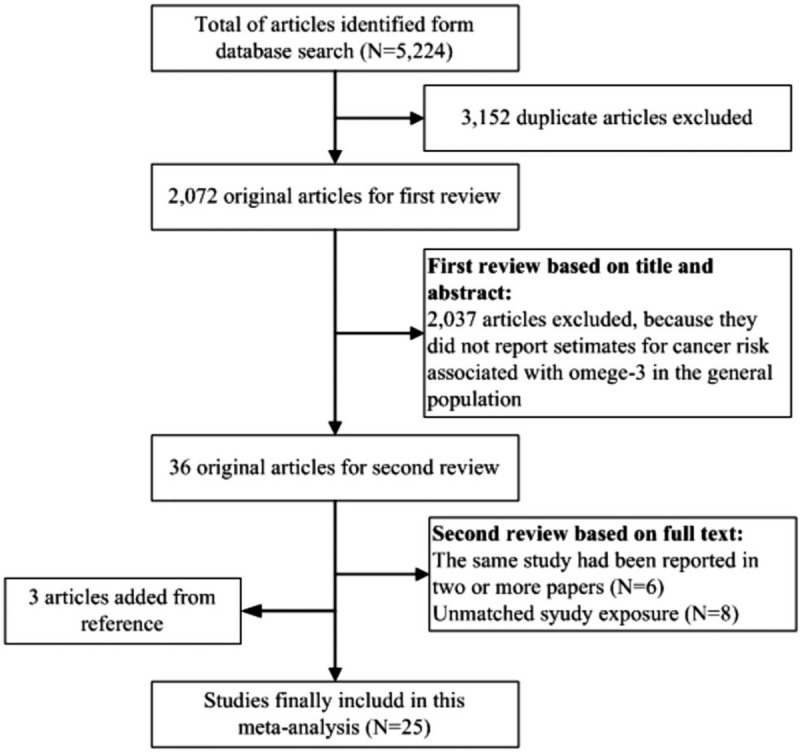
Flow chart of identification of relevant observational studies of omega-3 PUFA intake in relation to digestive system cancers.
3.2. Study characteristics
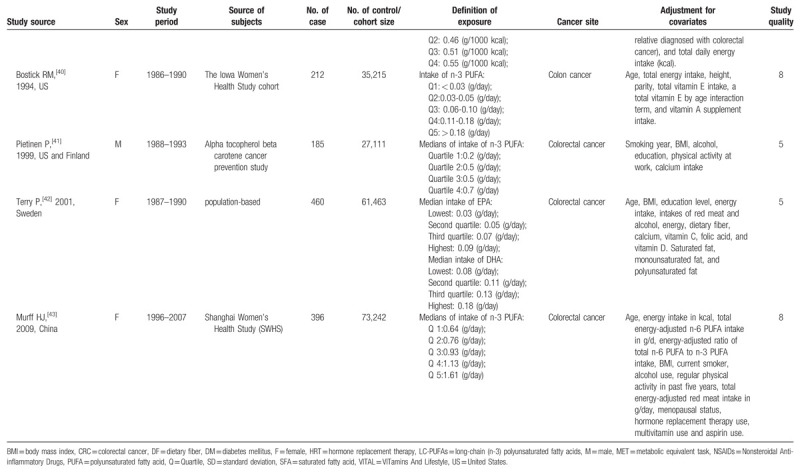
Table 1 presents the details of the 25 studies. Overall, data were available from 1,247,271 participants, including 23,173 cases with the following 6 cancer types: oral cavity/pharyngeal cancer (n = 736), esophageal cancer (n = 395), large bowel cancer (n = 2,280), pancreatic cancer (n = 1,132), hepatocellular carcinoma (n = 1,071), and colorectal cancer (n = 16,421). Most studies were performed in North America or Europe[9,10,22–27,29,30,33–35,37,38,40,42]; seven studies were conducted in Asia,[8,28,31,32,36,39,43] and one was an international study.[41] In total, four studies reported results for women only,[33,40,42,43] two studies reported results for men only,[9,41] six studies[26,27,35,36,38,39] reported results for men and women separately, and 13 studies reported results for both men and women combined.[8,10,22–25,28–32,34,37] The average NOS score of the included studies was 7.8 (ranging from 5 to 9).
Table 1.
Characteristics of included studies.
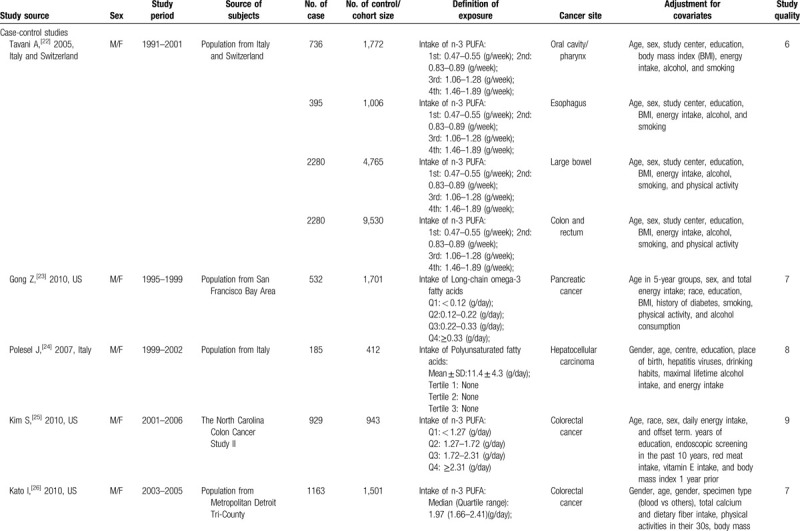
Table 1 (Continued).
Characteristics of included studies.
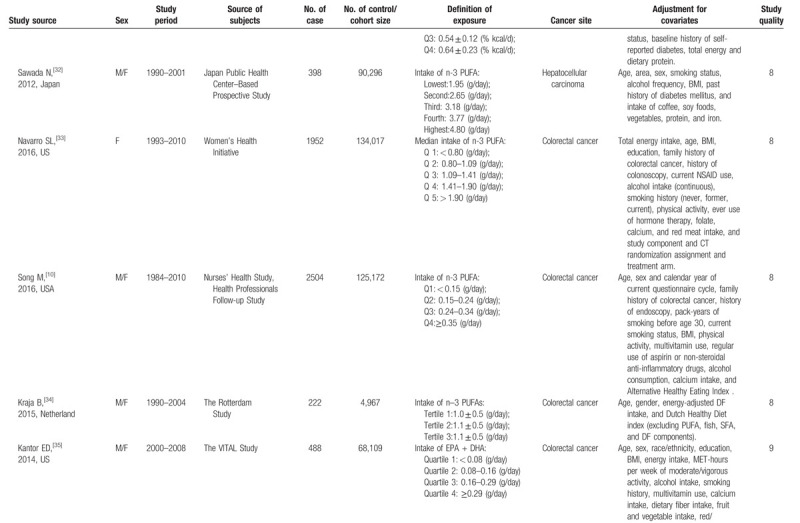
Table 1 (Continued).
Characteristics of included studies.
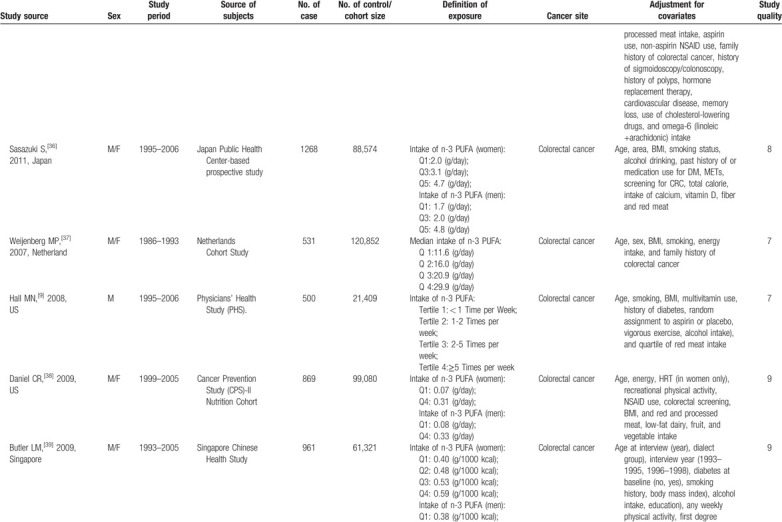
Table 1 (Continued).
Characteristics of included studies.
3.3. Omega-3 PUFAs and the risk of digestive system cancers
Comparing participants in the highest omega-3 PUFA group with those in the lowest omega-3 PUFA group, the multivariable-adjusted RR for total digestive system cancers was 0.83 (95% CI: 0.76–0.91). There was high heterogeneity across studies (I2 = 58.0%).
The decreased risks were statistically significant for 4 cancer types: colorectal cancer (RR = 0.89; 95% CI: 0.81–0.98; I2 = 53.6%), oral cavity/pharyngeal cancer (RR = 0.50; 95% CI: 0.33–0.76), esophageal cancer (RR = 0.50; 95% CI: 0.33–0.76) and large bowel cancer (RR = 0.70; 95% CI: 0.57–0.86). There were no statistically significant associations between omega-3 PUFAs and pancreatic cancer (RR = 0.71; 95% CI: 0.45–1.13; I2 = 70.2%) or hepatocellular carcinoma (RR = 0.83; 95% CI: 0.62–1.11; I2 = 9.5%).
3.4. Subgroup analyses
Primary subgroup investigations for digestive system cancers were stratified by region, sex, study design, and type of omega-3 PUFAs; smoking status, alcohol consumption, BMI, and physical activity were controlled or not in the models to examine the stability of the primary results and explore the sources of potential heterogeneity (Table 2). The differences between n-3 PUFA and in relation to digestive system cancers risk were observed in gender and study design subgroups (P values for meta-regression < .05). However, no significant interactions were found for other stratified variables (all P values for interaction > .05).
Table 1 (Continued).
Characteristics of included studies.
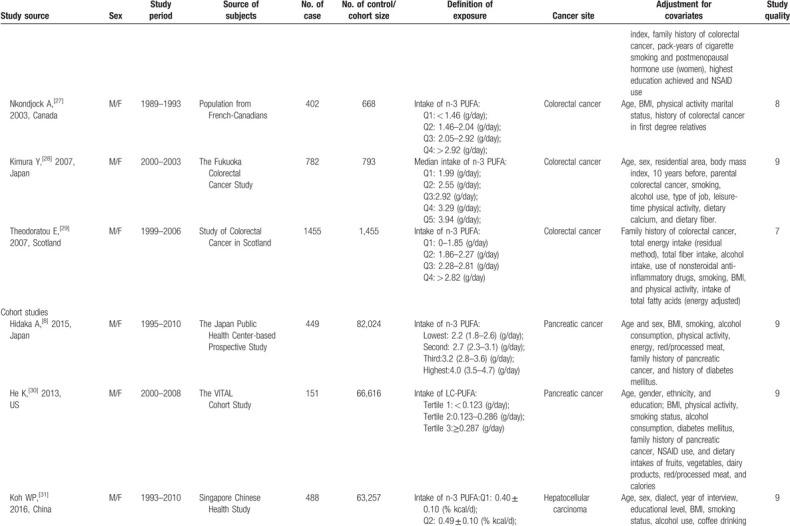
Table 2.
Subgroup analysis of relative risk of cancers.
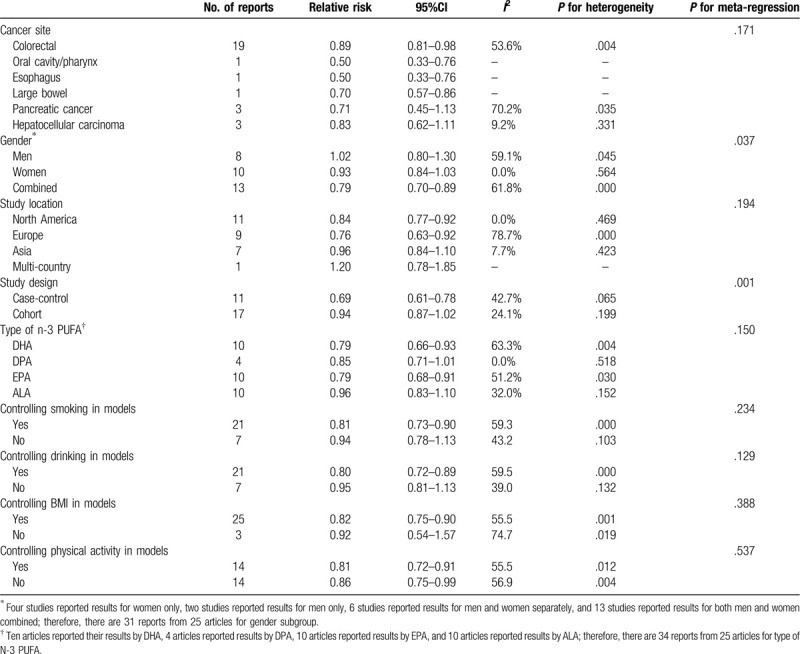
There was a lower risk of digestive system cancers in the case–control studies than in the cohort studies (the pooled RR was 0.69 [95% CI: 0.61–0.78] in the case–control studies and the pooled RR was 0.94 [95% CI: 0.87–1.02] in the cohort studies) (Table 2).
The impact of omega-3 PUFAs on the risk of digestive system cancers appeared to be great in the combined sample of men and women (men: RR = 1.02; 95% CI: 0.80–1.30; women: RR = 0.93; 95% CI: 0.84–1.03; combined: RR = 0.79; 95% CI: 0.70–0.89). A decreased risk of digestive system cancers associated with omega-3 PUFA use was also identified in North America and Europe (North America: RR = 0.84; 95% CI: 0.77–0.92; Europe: RR = 0.76; 95% CI: 0.63–0.92). However, no statistically significant association was found in Asian countries (RR = 0.96; 95% CI: 0.84–1.10) (Fig. 2).
Figure 2.
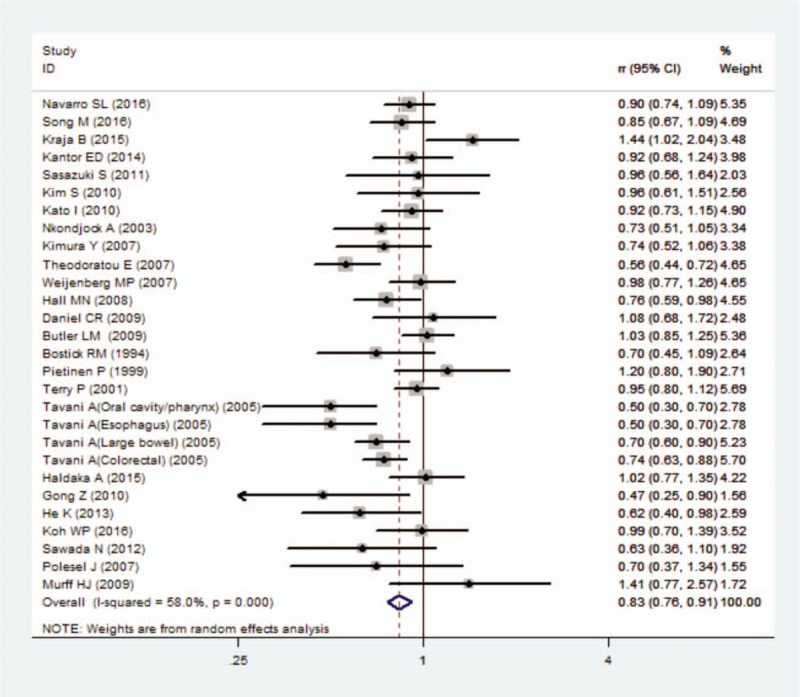
Forest plot for studies of omega-3 PUFA intake in relation to digestive system cancers.
The subgroup analysis indicated that an inverse association was observed in the docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) groups (DHA: RR = 0.79; 95% CI: 0.66–0.93; EPA: RR = 0.79; 95% CI: 0.68–0.91), but not in the docosapentaenoic acid’ (DPA) and alpha-linolenic acid (ALA) groups (DPA: RR = 0.85; 95% CI: 0.71–1.01; ALA: RR = 0.96; 95% CI: 0.83–1.10).
We conducted subgroup analysis by lifestyle factors. Additional subgroup analyses of lifestyle factors to assess the effect of smoking status, alcohol consumption, BMI, and physical activity on the digestive system cancers relationship showed no differences.
3.5. Sensitivity analyses
We excluded each study one by one and pooled the results of the remaining included studies. The pooled RR of cancer ranged from 0.82 (95% CI, 0.75–0.89) to 0.85 (95% CI, 0.78–0.93), which indicated that none of the studies substantially changed the combined RR.
3.6. Publication bias
Visual inspection of a funnel plot did not identify substantial asymmetry (Fig. 3). In addition, the Begg's test and Egger's test provided no evidence of publication bias across included studies (Begg's test P = .477; Egger's test P = .334).
Figure 3.
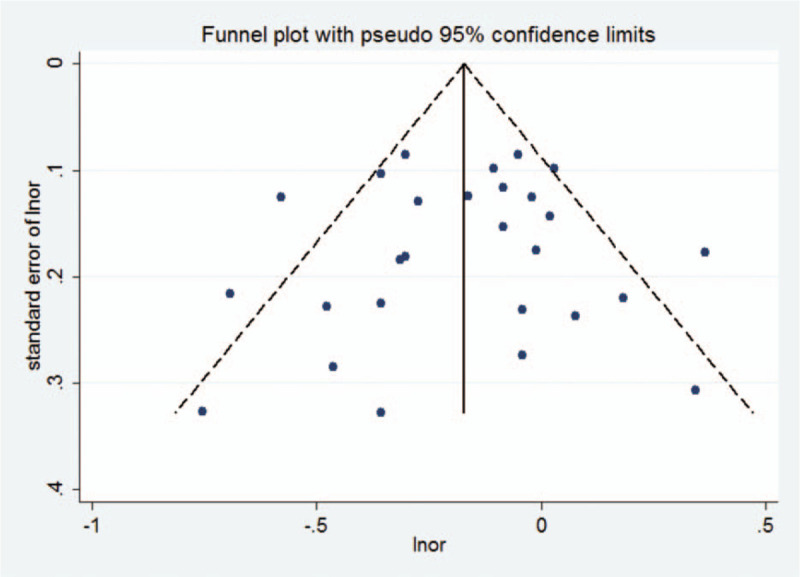
Funnel plot for studies of omega-3 PUFA intake in relation to digestive system cancers.
4. Discussion
In this meta-analysis of 25 studies, omega-3 PUFAs exerted a protective effect on the risk of digestive system cancers and was shown to possibly decrease the incident risk of cancers by as much as 17%. Inverse associations were also found between omega-3 PUFAs and the risk of colorectal, oral cavity/pharyngeal, esophageal and large bowel cancers. However, no significant association was observed between the intake of omega-3 PUFAs and the risk of pancreatic and hepatocellular cancers.
There are several potential mechanisms of the anticarcinogenic actions of omega-3 PUFAs. First, omega-3 PUFAs could inhibit the biosynthesis of arachidonic acid-derived eicosanoid, which resulted in an altered immune response to cancer cells and the modulation of inflammation, metastasis, apoptosis, cell proliferation, and angiogenesis.[44,45] Second, omega-3 PUFAs may affect gene expression, the activity of transcription factors, and signal transduction, which contributed to changes in metabolism and cell growth and differentiation.[46] Third, metabolites of omega-3 PUFAs increased the production of PGE3 and decreased the production of PGE2, which was expected to reduce estrogen-stimulated cell growth. Fourth, free radicals and reactive oxygen species could lead to the production of lipid hydroperoxides, which may further lead to genetic damage and cancer, and omega-3 PUFAs have been shown to decrease the levels of free radicals and reactive oxygen species, thus preventing cancer initiation. Final, PUFAs could reduce the risk of cancer through improving insulin sensitivity and membrane fluidity.[47]
Our subgroup analyses resulted in three valuable and significant findings. First, an interesting finding was that omega-3 PUFAs reduced the risk of digestive system cancers in the case–control studies but not in the cohort studies. One possible explanation for the difference might be that omega-3 PUFAs were assessed only at baseline in cohort studies, and the single baseline exposure assessment of omega-3 PUFA intake might reduce the ability to identify the relationship between omega-3 PUFAs and digestive system cancers. Moreover, cohort studies were more likely to have potential confounding bias, which might bias the statistical power.[48] Second, our results indicated that the strongest reduction in the risk of digestive system cancers associated with omega-3 PUFA intake was found in North American and European countries. However, no statistical significance was found in Asian countries. The reason may be that more than two-thirds of the included studies were performed in North American and European countries, and only a few studies were conducted in Asian countries, of which, 60% were from Japan. This might affect the accuracy of the results. Therefore, more researches are needed to investigate the differences among different countries and regions. Third, we found that the protective effects on digestive system cancers were observed in DHA and EPA but not in DPA or ALA. Muley et al[49] also indicated that marine n-3 PUFA had a better cardio-protective effects. However, the reasons for the discrepancies remain unclear, and additional studies on investigating the underlying mechanism by which these differences arise are clearly warranted.
This meta-analysis had some strength. This is the first evidence showing that omega-3 PUFAs can decrease the risk of colorectal cancer. Additionally, a large sample size provided reliable results with greater precision and statistical power. Moreover, there was no publication bias in the outcomes, showing that the statistical data obtained from the included studies may approximate actual results. Potential limitations did exist, however, which should be noted. Due to the limitation of the included data, we did not conduct a dose–response relationship analysis; thus, the relationship between omega-3 PUFAs and digestive system cancers could not be accurately assessed. In addition, although we established strict inclusion criteria, the different definitions of omega-3 PUFA intake might influence the results.
5. Conclusion
In summary, n-3 PUFA intake is inversely associated with the risk of digestive system cancers, especially colorectal, esophageal, large bowel, and oral cavity/pharyngeal cancers. The association between omega-3 PUFA intake and the risk of cancer might underlie part of the difference in the incidence of cancer across populations. Future studies, particularly studies with a universal definition of omega-3 PUFA intake and prospective studies with larger sample sizes, should be assessed to explore the association between omega-3 PUFAs and the development of digestive system cancers.
Acknowledgments
We would like to thank Dr. Yong Gan for his critical review and helpful discussions of this manuscript.
Author contributions
J.W, Y. Z and L. Z conceived and designed the study. J.W and Y. Z carried out the data collection. Y. Z performed the statistical analyses. J.W and Y. Z drafted the manuscript. All authors read and approved the final manuscript. L.Z is the guarantors of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Abbreviations: ALA = alpha-linolenic acid, BMI = body mass index, CI = confidence interval, DPA = docosehexaenoie acid, EPA = eicosapentaenoic acid, HR = hazard ratio, OR = odd ratio, PUFA = polyunsaturated fatty acid, RR = relative risk.
How to cite this article: Wang J, Zhang Y, Zhao L. Omega-3 PUFA intake and the risk of digestive system cancers: A meta-analysis of observational studies. Medicine. 2020;99:19(e20119).
Ethical approval is not required for this systematic review.
The authors have no funding and support to disclose.
The authors have no conflicts of interest to disclose.
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
- [1].Kanavos P. The rising burden of cancer in the developing world. Ann Oncol 2006;17: Suppl 8: viii15–23. [DOI] [PubMed] [Google Scholar]
- [2].Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA-Cancer J Clin 2005;55:74–108. [DOI] [PubMed] [Google Scholar]
- [3].Jemal A, Center MM, DeSantis C, et al. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev 2010;19:1893–907. [DOI] [PubMed] [Google Scholar]
- [4].Hitt NP, Hendryx M. Ecological integrity of streams related to human cancer mortality rates. Ecohealth 2010;7:91–104. [DOI] [PubMed] [Google Scholar]
- [5].Su KP, Tseng PT, Lin PY, et al. Association of use of omega-3 polyunsaturated fatty acids with changes in severity of anxiety symptoms: a systematic review and meta-analysis. JAMA Netw Open 2018;1:e182327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Marklund M, Wu JHY, Imamura F, et al. Biomarkers of dietary omega-6 fatty acids and incident cardiovascular disease and mortality. Circulation 2019;139:2422–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yang K, Zeng L, Bao T, et al. Effectiveness of omega-3 fatty acid for polycystic ovary syndrome: a systematic review and meta-analysis. Reprod Biol Endocrinol 2018;16:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hidaka A, Shimazu T, Sawada N, et al. Fish, n-3 PUFA consumption, and pancreatic cancer risk in Japanese: a large, population-based, prospective cohort study. Am J Clin Nutr 2015;102:1490–7. [DOI] [PubMed] [Google Scholar]
- [9].Hall MN, Chavarro JE, Lee IM, et al. A 22-year prospective study of fish, n-3 fatty acid intake, and colorectal cancer risk in men. Cancer Epidemiol Biomarkers Prev 2008;17:1136–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Song M, Nishihara R, Cao Y, et al. Marine omega-3 polyunsaturated fatty acid intake and risk of colorectal cancer characterized by tumor-infiltrating T cells. JAMA Oncol 2016;2:1197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chen GC, Qin LQ, Lu DB, et al. N-3 polyunsaturated fatty acids intake and risk of colorectal cancer: meta-analysis of prospective studies. Cancer Causes Control 2015;26:133–41. [DOI] [PubMed] [Google Scholar]
- [12].Gerber M. Omega-3 fatty acids and cancers: a systematic update review of epidemiological studies. Br J Nutr 2012;107: Suppl 2: S228–39. [DOI] [PubMed] [Google Scholar]
- [13].MacLean CH, Newberry SJ, Mojica WA, et al. Effects of omega-3 fatty acids on cancer risk: a systematic review. JAMA 2006;295:403–15. [DOI] [PubMed] [Google Scholar]
- [14].Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- [15].Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa (ON): Ottawa Health Research Institute; 2014. [Google Scholar]
- [16].McNutt LA, Wu C, Xue X, et al. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol 2003;157:940–3. [DOI] [PubMed] [Google Scholar]
- [17].Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev 1987;9:1–30. [DOI] [PubMed] [Google Scholar]
- [18].Harris RJ, Bradburn MJ, Deeks JJ, et al. Metan: fixed- and random-effects meta-analysis. Stata Journal 2008;8:3–28. [Google Scholar]
- [19].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [21].Egger M, Davey SG, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Tavani A, Franceschi S, Levi F, et al. Fish, omega-3 polyunsaturated fat intake and cancer at selected sites. World Rev Nutr Diet 2005;94:166–75. [DOI] [PubMed] [Google Scholar]
- [23].Gong Z, Holly EA, Wang F, et al. Intake of fatty acids and antioxidants and pancreatic cancer in a large population-based case-control study in the San Francisco Bay Area. Int J Cancer 2010;127:1893–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Polesel J, Talamini R, Montella M, et al. Nutrients intake and the risk of hepatocellular carcinoma in Italy. Eur J Cancer 2007;43:2381–7. [DOI] [PubMed] [Google Scholar]
- [25].Kim S, Sandler DP, Galanko J, et al. Intake of polyunsaturated fatty acids and distal large bowel cancer risk in whites and African Americans. Am J Epidemiol 2010;171:969–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kato I, Majumdar AP, Land SJ, et al. Dietary fatty acids, luminal modifiers, and risk of colorectal cancer. Int J Cancer 2010;127:942–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Nkondjock A, Shatenstein B, Maisonneuve P, et al. Assessment of risk associated with specific fatty acids and colorectal cancer among French-Canadians in Montreal: a case-control study. Int J Epidemiol 2003;32:200–9. [DOI] [PubMed] [Google Scholar]
- [28].Kimura Y, Kono S, Toyomura K, et al. Meat, fish and fat intake in relation to subsite-specific risk of colorectal cancer: The Fukuoka Colorectal Cancer Study. Cancer Sci 2007;98:590–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Theodoratou E, McNeill G, Cetnarskyj R, et al. Dietary fatty acids and colorectal cancer: a case-control study. Am J Epidemiol 2007;166:181–95. [DOI] [PubMed] [Google Scholar]
- [30].He K, Xun P, Brasky TM, et al. Types of fish consumed and fish preparation methods in relation to pancreatic cancer incidence: the VITAL Cohort Study. Am J Epidemiol 2013;177:152–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Koh WP, Dan YY, Goh GB, et al. Dietary fatty acids and risk of hepatocellular carcinoma in the Singapore Chinese health study. Liver Int 2016;36:893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sawada N, Inoue M, Iwasaki M, et al. Consumption of n-3 fatty acids and fish reduces risk of hepatocellular carcinoma. Gastroenterology 2012;142:1468–75. [DOI] [PubMed] [Google Scholar]
- [33].Navarro SL, Neuhouser ML, Cheng TD, et al. The interaction between dietary fiber and fat and risk of colorectal cancer in the women's health initiative. Nutrients 2016;8:779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kraja B, Muka T, Ruiter R, et al. Dietary fiber intake modifies the positive association between n-3 PUFA intake and colorectal cancer risk in a Caucasian population. J Nutr 2015;145:1709–16. [DOI] [PubMed] [Google Scholar]
- [35].Kantor ED, Lampe JW, Peters U, et al. Long-chain omega-3 polyunsaturated fatty acid intake and risk of colorectal cancer. Nutr Cancer 2014;66:716–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sasazuki S, Inoue M, Iwasaki M, et al. Intake of n-3 and n-6 polyunsaturated fatty acids and development of colorectal cancer by subsite: Japan Public Health Center-based prospective study. Int J Cancer 2011;129:1718–29. [DOI] [PubMed] [Google Scholar]
- [37].Weijenberg MP, Luchtenborg M, de Goeij AF, et al. Dietary fat and risk of colon and rectal cancer with aberrant MLH1 expression, APC or KRAS genes. Cancer Causes Control 2007;18:865–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Daniel CR, McCullough ML, Patel RC, et al. Dietary intake of omega-6 and omega-3 fatty acids and risk of colorectal cancer in a prospective cohort of U.S. men and women. Cancer Epidemiol Biomarkers Prev 2009;18:516–25. [DOI] [PubMed] [Google Scholar]
- [39].Butler LM, Wang R, Koh WP, et al. Marine n-3 and saturated fatty acids in relation to risk of colorectal cancer in Singapore Chinese: a prospective study. Int J Cancer 2009;124:678–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bostick RM, Potter JD, Kushi LH, et al. Sugar, meat, and fat intake, and non-dietary risk factors for colon cancer incidence in Iowa women (United States). Cancer Causes Control 1994;5:38–52. [DOI] [PubMed] [Google Scholar]
- [41].Pietinen P, Malila N, Virtanen M, et al. Diet and risk of colorectal cancer in a cohort of Finnish men. Cancer Causes Control 1999;10:387–96. [DOI] [PubMed] [Google Scholar]
- [42].Terry P, Bergkvist L, Holmberg L, et al. No association between fat and fatty acids intake and risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev 2001;10:913–4. [PubMed] [Google Scholar]
- [43].Murff HJ, Shu XO, Li H, et al. A prospective study of dietary polyunsaturated fatty acids and colorectal cancer risk in Chinese women. Cancer Epidemiol Biomarkers Prev 2009;18:2283–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Calder PC. Omega-3 fatty acids and inflammatory processes: from molecules to man. Biochem Soc Trans 2017;45:1105–15. [DOI] [PubMed] [Google Scholar]
- [45].Zhao Y, Wang C. Effect of omega-3 polyunsaturated fatty acid-supplemented parenteral nutrition on inflammatory and immune function in postoperative patients with gastrointestinal malignancy: a meta-analysis of randomized control trials in China. Medicine (Baltimore) 2018;97:e0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Li J, Li K, Gao J, et al. Endogenously synthesized n-3 polyunsaturated fatty acids in pregnant fat-1 mice decreases mammary cancer risk of female offspring by regulating expression of long noncoding RNAs. Mol Nutr Food Res 2019;63:e1801150. [DOI] [PubMed] [Google Scholar]
- [47].Al-Jawadi A, Moussa H, Ramalingam L, et al. Protective properties of n-3 fatty acids and implications in obesity-associated breast cancer. J Nutr Biochem 2018;53:1–8. [DOI] [PubMed] [Google Scholar]
- [48].Song JW, Chung KC. Observational studies: cohort and case-control studies. Plast Reconstr Surg 2010;126:2234–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Muley A, Muley P, Shah M. ALA, fatty fish or marine n-3 fatty acids for preventing DM?: A systematic review and meta-analysis. Curr Diabetes Rev 2014;10:158–65. [DOI] [PubMed] [Google Scholar]


