Supplemental Digital Content is available in the text
Keywords: Meta-analysis, subacute combined degeneration, vitamin B12
Abstract
Background:
To determine the prevalence of subacute combined degeneration (SCD) patients with normal or elevated serum vitamin B12 level and to identify clinical characteristics of these patients.
Methods:
We searched PubMed, EMBASE, and Cochrane library, without language restriction up to June 2019 and included studies with SCD patients who were diagnosed with normal or elevated serum vitamin B12 levels. Meta-analysis was performed to estimate the prevalence of SCD in patients with normal or elevated serum vitamin B12 levels and compare the differences of clinical data between patients with low and no-low serum vitamin B12 level.
Results:
Six studies were included in our analysis, with a total number of 181 patients involved. The pooled proportion in patients with no-low serum vitamin B12 level was 31.0% (95% confidence interval [CI]: 22.5–40.8). There was no significant difference in the level of hemoglobin (Mean difference (MD): –3.05, 95% CI: –12.42 to 6.33. P = 0.52) and erythrocyte mean corpuscular volume (MD: –2.37, 95% CI: –11.17 to 6.43, P = 0.60) between SCD patients with no-low and those with low serum vitamin B12 levels. The meta-analysis showed that the functional disability rating scale on admission in patients with low serum vitamin B12 level was no worse than that with normal or elevated serum vitamin B12 level (MD: 0.29, 95% CI: –0.58 to 1.16, P = 0.51).
Conclusion:
Decreased level of serum vitamin B12 may not be a necessity for the diagnosis of SCD. Approximately one third of the SCD patients have normal or elevated serum vitamin B12 level. No differences were found in clinical severity between patients with normal or elevated serum vitamin B12 level and those with low level of serum vitamin B12 on admission.
1. Introduction
Subacute combined degeneration (SCD) is a demyelinating disease which is attributed to metabolic disturbances. Various manifestations such as gait or sensory disturbances, mental impairment and pyramidal tract damage may appear to different extents in patients with SCD.[1] It is believed to be linked with vitamin B12 deficiency, and low vitamin B12 concentration has been attached great importance in the diagnosis of SCD. Serum vitamin B12 is most commonly detected, and a declined value of serum vitamin B12 is strongly indicative of having SCD in clinically suspected patients. Recently there are literatures reporting SCD patients with normal or elevated serum vitamin B12.[2,3] However, it is unclear what proportion they account for and what features they have. We therefore performed a systematic review to determine the prevalence of SCD patients with normal or elevated level of serum vitamin B12, and to assess characteristics associated with those patients.
2. Methods
This systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The study was approved by the Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China. We aimed at identifying all published studies in which patients with SCD were confirmed with normal or elevated serum vitamin B12 level.
3. Search strategy
We systematically searched PubMed, EMBASE, and Cochrane library without language restrictions up to June 2019, using the following search terms: “subacute combined degeneration” or “sub-acute combined degeneration.” Relevant or any additional published studies were obtained by manually searching related reviews and the reference lists of original articles. All studies were reviewed in accordance with the following criteria:
-
1.
patients diagnosed with SCD;
-
2.
series of more than 5 patients;
-
3.
studies included patients with diagnosis of SCD with normal or elevated serum vitamin B12 level.
By definition, SCD patients with normal or elevated serum vitamin B12 level must had evidence of vitamin B12 deficiency or clinical improvement after regular vitamin B12 treatment. Studies of less than 5 patients, without diagnosis of SCD with normal or elevated serum vitamin B12 level or without extractable data were excluded. Two reviewers independently scanned records to exclude irrelevant studies and identify studies that met the eligibility criteria. Any questions or discrepancies were resolved through iteration and consensus.
4. Data extraction
We abstracted demographic and clinical data from each study. Demographic characteristics included study design (retrospective or prospective), number of patients with SCD, mean age, and sex distribution. The clinical data collected included the history of gastrointestinal disease, vegetarian and anemia, criteria for low serum vitamin B12 level, the number of patients with normal or elevated serum vitamin B12 level, level of red blood cell, hemoglobin, erythrocyte mean corpuscular volume (MCV), folic acid, spinal MRI, and functional disability rating scale score on admission.
5. Statistical analysis
Data were analyzed using Review Manager (version 5.3, the Nordic Cochrane Centre, the Cochrane Collaboration, 2014). We used the random effects model to estimate a pooled prevalence with 95% confidence intervals (CI), and calculated the weights using the inverse variance method for random effects. Differences in clinical data on admission between SCD with low and no-low serum vitamin B12 level were expressed as mean difference (MD) and 95% CI. Meta-analysis was performed for the level of hemoglobin and MCV, as well as functional disability rating scales on admission. We assessed heterogeneity using the I2 statistic. When I2>50%, the random effects model was used. Otherwise, the fixed effects model was used. Only 2 studies were included in each meta-analysis, we therefore did not assess reporting bias.
6. Results
6.1. Literature search
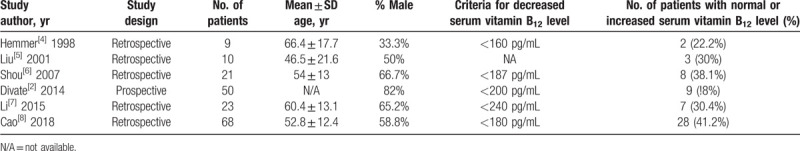
Our literature search yielded 1303 articles in total, of which 538 were identified via PubMed, 758 from EMBASE, and 7 from Cochrane searches, respectively. A total of 891 articles were excluded after screening of articles by abstract and title review. Finally, of the 37 full-text articles that were reviewed, 6 studies were included (Table 1). Five studies were retrospective and 1 study was prospective. In total, 181 patients were included in this systematic review and meta-analysis, and study size ranged from 9 to 68 patients. Characteristics of each study are given as below (Table 2).
Table 1.
PRISMA flow diagram of study search and selection for the systematic review and meta-analysis.
Table 2.
Characteristics of individual studies included in the systematic review and meta-analysis.
6.2. Prevalence of SCD with normal or elevated serum vitamin B12 level
These 6 studies included 57 SCD patients with normal or elevated serum vitamin B12 level. The meta-analytic synthesis of these 6 studies yielded a pooled prevalence of 31.0% (95% CI: 22.5–40.8) (supplementary Table).
6.3. Comparison in SCD patients with low and no-low serum vitamin B12 level
Two of 6 studies reported levels of hemoglobin on admission both in SCD patients with low and no-low serum vitamin B12 level. The meta-analysis of these 2 studies showed that there was no significant difference in the level of hemoglobin between these patients (MD: –3.05, 95% CI: –12.42 to 6.33, I2 = 0%, P = .52; Fig. 1A). Meanwhile, there was no statistically significant difference in the level of MCV between the 2 groups (MD: –2.37, 95% CI: –11.17 to 6.43, I2 = 0%, P = .60; Fig. 1B).
Figure 1.
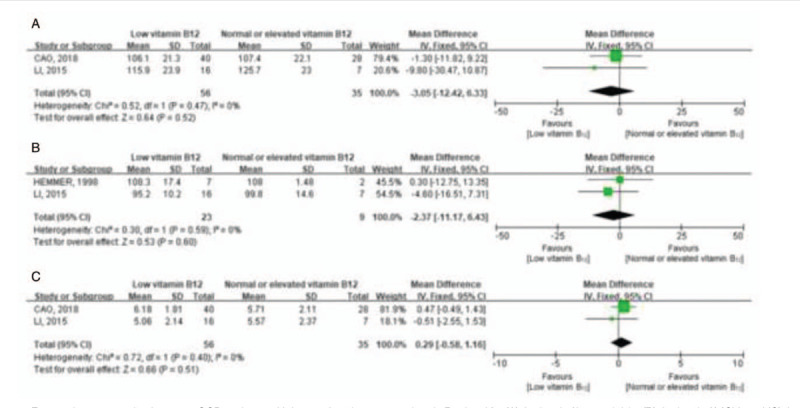
Forest plots comparing between SCD patients with low and no-low serum vitamin B12 level for (A) the level of hemoglobin, (B) the level of MCV, and (C) the functional disability rating scale on admission. No statistically significant difference in the level of hemoglobin, MCV, or the functional disability rating scale on admission was found between SCD patients with low and no-low serum vitamin B12 level. CI = confidence interval, MCV = mean corpuscular volume, SCD = subacute combined degeneration.
A meta-analysis for functional disability rating scale on admission was performed between 56 patients with low serum vitamin B12 level and 35 patients with normal or elevated serum vitamin B12 level in 2 studies. The MD value for normal or elevated vitamin B12 level relative to low vitamin B12 level was 0.29 (95% CI: –0.58 to 1.16, I2 = 0%, P = .51) (Fig. 1C). Thus, patients with low serum vitamin B12 level presented no more serious clinical manifestation than patients did with normal or elevated serum vitamin B12 level.
7. Discussion
As far as we know, this is the first systematic review and meta-analysis for the correlation between serum vitamin B12 and SCD to date. We found that as many as one third of SCD patients have normal or elevated serum vitamin B12. No differences were found in clinical severity between patients with normal or elevated levels of serum vitamin B12 and those with decreased levels of serum vitamin B12 on admission. These findings suggest the diagnosis of SCD should remain based on clinical manifestations and could not exclude patients only because of normal or elevated levels of serum vitamin B12.
Vitamin B12, also known as cobalamin, is a vital necessity for metabolism in human bodies. It promotes maturation of red blood cells, synthesis of DNA, and is also necessary for both fatty acid and amino acid metabolism.[9] The concentration of serum vitamin B12 has been regarded as a most straightforward indicator to vitamin B12 level.[10] Generally, a decreased serum vitamin B12 level has been regarded as vitamin B12 deficiency and help diagnose patients as having SCD. Nonetheless, it gradually seems that serum vitamin B12 cannot precisely reflect vitamin B12 deficiency.[11,12] An early study reported that there are 5% to 10% of patients with recognized clinically significant cobalamin deficiency in whom serum cobalamin was normal or elevated.[13] Another study also found even 45% of vitamin B12 deficient subjects being neglected when serum vitamin B12 is taken as the only screening test.[14] There are several reasons for falsely normal serum vitamin B12 levels, including myeloproliferative disorders, liver disease, congenital transcobalamin II deficiency, intestinal bacterial overgrowth, and nitrous oxide exposure.[10] Also, serum vitamin B12 is not good for detection of short-term deficiency. It is to note that the concentration of serum vitamin B12 decreases relatively late in depletion. It may even remain in the normal range for years in patients who stops administrating vitamin B12, although the decrease of tissue storage precedes alteration of concentration of serum cobalamin.[11] We assume that a normal or elevated level of serum vitamin B12 cannot rule out the “genuine” loss of vitamin B12 in tissues. On the other hand, a decreased serum vitamin B12 level seems not to be a guarantee for the lack of vitamin B12 in body. In fact, conditions including folate deficiency, pregnancy, use of oral contraceptives, congenital deficiency of serum haptocorrins, and multiple myeloma will cause a false low serum vitamin B12.[10] Of 238 SCD patients included in our study, 57 SCD patients are detected as having normal or elevated serum vitamin B12, taking up as many as an overall proportion of 31%. Hence, we believe that the declined serum vitamin B12 is not a compulsory element for a diagnosis of SCD. A SCD patient with normal serum vitamin B12 level may still lack vitamin B12 in the body, which is also known as “functional” vitamin B12 deficiency.[15]
Surrogate markers have been considered to evaluate vitamin B12 deficiency.[16] Although serum methylmalonic acid (MMA) and homocysteine are possible sensitive indicators to assess vitamin B12 deficiency,[3,11] the specificity of these tests are rather low.[17] In addition, both of them can be influenced by various factors such as age, renal dysfunction, and metabolic pathways. It is worth noting that MMA remains more expensive than serum vitamin B12 test.[16] Holotranscobalamin (Holo TC), known as transcobalamin-bound B12, transports active cobalamin. It is considered to be a convenient approach that measures the active portion of vitamin B12.[14] It becomes decreased before the development of the metabolic dysfunction, and applies to predict short-term as well as long-term status of cobalamin deficiency.[11] Studies manifested that the sensitivity of this assay is good but the specificity remains to be determined when evaluating functional vitamin B12 deficiency.[17] And it seems not to be a promising marker in predicting vitamin B12 status in renal patients.[14] In summary, to diagnose the minority whose serum cobalamin is falsely normal, serum MMA, homocysteine or Holo TC seems to play a complementary role, but changing the serum cobalamin to another one (e.g., MMA) as a fundamental marker of SCD is unlikely to occur in clinical practice. Whatever the situation, serum vitamin B12, which is the most widely available, should be regarded as a basic parameter for initial evaluation once vitamin B12 deficiency is in consideration.
Besides, in our study, no differences were found in clinical severity between patients with normal or elevated levels and those with decreased levels of serum vitamin B12 on admission according to meta-analysis. This is likely an illumination that the serum vitamin B12 level decrease and vitamin B12 deficiency are not the same concept. Thus, the declined serum vitamin B12 level cannot represent clinical severity.
Our study has limitations. First, due to lack of research in this rare disease, the quality of the included studies is limited. Secondly, because of the possibility that SCD with normal range of serum vitamin B12 would be possibly omitted by physicians especially when patients have only mild syndromes, the real proportion in patients with normal or elevated vitamin B12 tends to have been underestimated. Thirdly, studies included do not provide MMA, homocysteine or Holo TC assays, which could have certified “genuine” vitamin B12 deficiency if they are. Finally, we do not assess reporting bias as only 2 articles were included in each analysis.
In conclusion, our findings suggest the decreased level of serum vitamin B12 is not a necessity for the diagnosis of SCD. Clinicians should recognize the limitation of serum vitamin B12 for SCD diagnosis.
Acknowledgments
We thank Dr Da Feng for the support of statistical consultation.
Author contributions
Conceptualization: Jie Cao, Chenchen Liu.
Data curation: Jie Cao, Chenchen Liu.
Formal analysis: Jie Cao, Chenchen Liu.
Methodology: Jie Cao, Chenchen Liu.
Resources: Chenchen Liu.
Software: Jie Cao, Chenchen Liu.
Validation: Chenchen Liu.
Writing – original draft: Jie Cao, Shabei Xu.
Writing – review & editing: Chenchen Liu.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, Hb = hemoglobin, Holo TC = holotranscobalamin, MCV = mean corpuscular volume, MD = mean difference, MMA = methylmalonic acid, MRI = magnetic resonance imaging, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses, SCD = subacute combined degeneration.
How to cite this article: Cao J, Xu S, Liu C. Is serum vitamin B12 decrease a necessity for the diagnosis of subacute combined degeneration? A meta-analysis. Medicine. 2020;99:14(e19700).
The authors have no funding and conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Briani C, Dalla Torre C, Citton V, et al. Cobalamin deficiency: clinical picture and radiological findings. Nutrients 2013;5:4521–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Divate PG, Patanwala R. Neurological manifestations of B(12) deficiency with emphasis on its aetiology. J Assoc Physicians India 2014;62:400–5. [PubMed] [Google Scholar]
- [3].Ulrich A, Müller D, Linnebank M, et al. Pitfalls in the diagnostic evaluation of subacute combined degeneration. BMJ Case Rep 2015;2015: bcr2014208622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hemmer B, Glocker FX, Schumacher M, et al. Subacute combined degeneration: clinical, electrophysiological, and magnetic resonance imaging findings. J Neurol Neurosurg Psychiatry 1998;65:822–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Liu M, Jiang Y, Wang W, et al. The natural history of electrophysiological and magnetic resonance changes of subacute combined degeneration. Zhonghua Nei Ke Za Zhi 2001;40:180–2. [PubMed] [Google Scholar]
- [6].Shou YH, Li CF, Fan DS, et al. Diagnosis and clinical manifestations of subacute combined degeneration of the spinal cord: analysis of 21 cases. Neural Regen Res 2007;2:112–6. [Google Scholar]
- [7].Li J, Ren M, Dong A, et al. A retrospective study of 23 cases with subacute combined degeneration. Int J Neurosci 2016;126:872–7. [DOI] [PubMed] [Google Scholar]
- [8].Cao J, Su ZY, Xu SB, et al. Subacute combined degeneration: a retrospective study of 68 cases with short-term follow-up. Eur Neurol 2018;79:247–55. [DOI] [PubMed] [Google Scholar]
- [9].Naidich MJ, Ho SU. Case 87: subacute combined degeneration. Radiology 2005;237:101–5. [DOI] [PubMed] [Google Scholar]
- [10].Snow CF. Laboratory diagnosis of vitamin B12 and folate deficiency: a guide for the primary care physician. Arch Intern Med 1999;159:1289–98. [DOI] [PubMed] [Google Scholar]
- [11].Green R. Indicators for assessing folate and vitamin B-12 status and for monitoring the efficacy of intervention strategies. Am J Clin Nutr 2011;94:666S–72S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Herrmann W, Obeid R. Causes and early diagnosis of vitamin B12 deficiency. Dtsch Arztebl Int 2008;105:680–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lindenbaum J, Savage DG, Stabler SP, et al. Diagnosis of cobalamin deficiency: II. Relative sensitivities of serum cobalamin, methylmalonic acid, and total homocysteine concentrations. Am J Hematol 1990;34:99–107. [DOI] [PubMed] [Google Scholar]
- [14].Herrmann W, Obeid R, Schorr H, et al. The usefulness of holotranscobalamin in predicting vitamin B12 status in different clinical settings. Curr Drug Metab 2005;6:47–53. [DOI] [PubMed] [Google Scholar]
- [15].Turner MR, Talbot K. Functional vitamin B12 deficiency. Pract Neurol 2009;9:37–41. [DOI] [PubMed] [Google Scholar]
- [16].Sobczyńska-Malefora A, Gorska R, Pelisser M, et al. An audit of holotranscobalamin (“active” B12) and methylmalonic acid assays for the assessment of vitamin B12 status: application in a mixed patient population. Clin Biochem 2014;47:82–6. [DOI] [PubMed] [Google Scholar]
- [17].Oberley MJ, Yang DT. Laboratory testing for cobalamin deficiency in megaloblastic anemia. Am J Hematol 2013;88:522–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


