Abstract
Quick diagnosis units (QDU) have become an alternative hospital-based ambulatory medicine strategy to inpatient hospitalization for potentially serious illnesses in Spain. Whether diagnosis of pancreatic cancer is better accomplished by an ambulatory or inpatient approach is unknown. The main objective of this retrospective study was to examine and compare the diagnostic effectiveness of a QDU or inpatient setting in patients with pancreatic cancer.
Patients with a diagnosis of pancreatic adenocarcinoma who had been referred to a university, tertiary hospital-based QDU or hospitalized between 2005 and 2018 were eligible. Presenting symptoms and signs, risk and prognostic factors, and time to diagnosis were compared. The costs incurred during the diagnostic assessment were analyzed with a microcosting method.
A total of 1004 patients (508 QDU patients and 496 inpatients) were eligible. Admitted patients were more likely than QDU patients to have weight loss, asthenia, anorexia, abdominal pain, jaundice, and palpable hepatomegaly. Time to diagnosis of inpatients was similar to that of QDU patients (4.1 [0.8 vs 4.3 [0.6] days; P = .163). Inpatients were more likely than QDU patients to have a tumor on the head of the pancreas, a tumor size >2 cm, a more advanced nodal stage, and a poorer histological differentiation. No differences were observed in the proportion of metastatic and locally advanced disease and surgical resections. Microcosting revealed a cost of €347.76 (48.69) per QDU patient and €634.36 (80.56) per inpatient (P < .001).
Diagnosis of pancreatic cancer is similarly achieved by an inpatient or QDU clinical approach, but the latter seems to be cost-effective. Because the high costs of hospitalization, an ambulatory diagnostic assessment may be preferable in these patients.
Keywords: diagnosis, emergency department, inpatient, length of stay, outpatient, pancreatic adenocarcinoma, pancreatic cancer, primary care, time to diagnosis
1. Introduction
With a 5-year-life expectancy of 5%, the prognosis of pancreatic cancer has remained practically unchanged over the last 20 years.[1] The low survival rates can be attributed to several factors but mainly the late stage at which the condition manifests and a diagnosis is made. The majority of patients will have metastasis or a locally advanced disease in the asymptomatic phase and, even after surgery for cure, most will have recurrence.[1,2] Because there are no effective screening methods for early detection, a high degree of suspicion remains decisive to make a diagnosis, especially by primary care (PC) physicians who are usually the first to see the patient. Yet diagnosis can be challenging.[1,3,4] Since early symptoms may be nonspecific, vague, and intermittent (eg, weight loss, nonspecific abdominal pain) and often attributed to coexisting disorders or ageing, diagnosis is commonly delayed.[1,5–8] In fact, the total diagnostic interval (ie, from onset of symptoms to diagnosis) of pancreatic cancer is longer than the interval of other cancers including other hard-to-suspect cancers. Due to the poor specificity of symptoms and delayed diagnosis, some experts have recommended to improve the diagnostic skills of PC physicians when caring for patients with an eventual diagnosis of pancreatic cancer.[9–11]
Delays in the diagnosis of suspected serious diseases in patients attending PC centers or hospital outpatient clinics are not uncommon in countries with public healthcare systems. Indeed, mostly owing to delayed investigations ordered by PC physicians, patients with suspected cancer, even with an appropriate performance status, have been traditionally hospitalized to speed-up diagnosis.[12,16,17] However, admission for diagnostic workup of such patients may only increase the costs of hospitalization, contributing to increased expenditures in healthcare. To avoid delays and potentially avoidable admissions, the Spanish healthcare system created in the 2000s hospital based-ambulatory facilities called quick diagnosis units (QDUs).[12] Compared to conventional hospitalization for workup, these clinics have various advantages. In addition to a time to diagnosis similar to the length-of-stay for similar conditions, QDUs are useful to decrease referrals from PC to emergency departments (EDs), are cost-saving, and are associated with higher patients’ satisfaction scores than hospitalization.[12–15]
Since an adequate performance status argues in theory against admission of patients who are eventually diagnosed with pancreatic cancer, QDUs may be a suitable setting for their evaluation. However, no study has evaluated how a QDU compares to an inpatient setting for its diagnosis. The main purpose of this study was to investigate the effectiveness and associated costs of a hospital-based ambulatory QDU versus inpatient setting for the diagnosis of pancreatic adenocarcinoma.
2. Methods
2.1. Settings
The QDU is based on the Adult Day Care Center of the Hospital Clínic, a public tertiary university hospital in Barcelona with a reference population of 550,000. Patients are referred to this unit from 15 PC centers and the hospital ED. Evaluable disorders and general characteristics of QDU have been reported elsewhere.[12–14] In addition, the General Internal Medicine Department of the hospital has 3 inpatients wards, each with 25 beds, and most patients are admitted to them from the ED.
2.2. Study population
To analyze a homogeneous population, patients aged ≥18 years with a diagnosis of pancreatic adenocarcinoma who had been referred to QDU or hospitalized between October 2005 and November 2018 were eligible. The study was approved by the Research Ethics Committee of the Hospital Clínic and need for written consent was waived due to the retrospective nature of clinical data.
Pathologists selected and reviewed cytologic and histopathological (herein referred to as “cyto/pathological”) specimens of consecutive cases with a diagnosis of pancreatic adenocarcinoma according to the World Health Organization classification criteria.[18] Attending and resident physicians from QDU and inpatient wards reviewed the medical records of all patients and entered the following data into an electronic database:
-
(1)
referral sources (ED or PC);
-
(2)
demographic and epidemiological data including age, sex, ethnic race (white or other), and socioeconomic status (measured by education and income);
-
(3)
domestic situation (living alone or living with partner or other);
-
(4)
presenting clinical manifestations including presence or absence of weight loss, asthenia, anorexia, nausea or vomiting, change in bowel habit, abdominal pain, back pain, pruritus, lethargy or depression, thrombophlebitis, jaundice, new-onset diabetes, abdominal mass, hepatomegaly, and peripheral lymphadenopathy;
-
(5)
relevant laboratory data (see prognostic factors below);
-
(6)
overall comorbidity according to the age-adjusted Charlson index (0–4 or 4.1–6 or >6)[19,20];
-
(7)
results from endoscopic and imaging reports including procedures used to obtain a cyto/pathological diagnosis (upper gastrointestinal endoscopic ultrasound [EUS]-, ultrasound-, and computed tomography [CT]-guided biopsy); and
-
(8)
onward referrals after diagnosis (pancreatic multidisciplinary unit or hospitalization from QDU or PC or palliative care).
Patients with pancreatic neuroendocrine, intraductal papillary mucinous, and mucinous cystic neoplasms were excluded as were patients with ampullary and duodenal tumors through reevaluation of cyto/pathological specimens. Patients with incomplete clinical or cyto/pathological information, lost to follow-up, or dead before staging were also excluded.
2.3. Staging
A contrast-enhanced thin-slice CT scan of the chest, abdomen, and pelvis was performed in all patients to determine the clinical stage.[21] Specifically, CT scans, and occasionally EUS, were used to establish the tumor site and size, presence or absence of metastatic disease, and local relationships of the tumor according to the American Joint Committee on Cancer (AJCC) (7th Edition [2010] staging system).[22,23]
2.4. Risk and prognostic factors
Risk factors of pancreatic cancer were compared between QDU patients and inpatients. These included age (<75 vs ≥75 years), smoking status (current vs ex-smoker vs never smoker), history of pancreatitis, family history of pancreatic cancer, body mass index (≥35 vs <35 kg/m2), long-standing type 2 diabetes mellitus, and heavy alcohol consumption (<6 vs ≥6 drinks/d).[1–3,21,24,25] When it was not possible to differentiate between acute and chronic pancreatitis, any history of pancreatitis was considered.
Reported prognostic factors of pancreatic cancer were recorded including the Eastern Cooperative Oncology Group (ECOG) performance score (0–<2 vs ≥2–4), primary tumor site (head vs body/tail), AJCC tumor stage (T1 [≤2 cm] vs T2 [>2 cm]), AJCC nodal stage (N0 vs N1 vs unknown [NX]), AJCC metastasis stage (M0 vs M1), and overall staging according to AJCC (resectable stages: I, II, and borderline resectable stage III subset; unresectable stages: locally advanced stage III subset and stage IV or metastatic subset).[21,25,26] The grade of differentiation of the specimens obtained by biopsy (well-differentiated, moderately differentiated, poorly differentiated, or unknown) and the results of several laboratory parameters formerly reported to have a potential prognostic role in pancreatic cancer including serum tumor markers carbohydrate antigen 19.9 (CA19.9) and carcinoembryonic antigen (CEA), lactate dehydrogenase (LDH), albumin, white blood cell count, platelet count, hemoglobin, aspartate aminotransferase (AST/SGOT), alanine aminotransferase (ALT/SGPT), alkaline phosphatase, total bilirubin, blood urea nitrogen, creatinine, and C-reactive protein (CRP) were also compared.[27–39] In patients with a total bilirubin level ≥2.0 mg/dL (ie, presumed altered biliary excretion), CA19.9 and CEA concentrations were adjusted by dividing their level by the total bilirubin level. In patients with normal biliary excretion (ie, total bilirubin level <2.0 mg/dL), the actual tumor marker concentration was used.[27,33]
Surgical characteristics of patients who underwent resection were analyzed. These characteristics included age and sex at time of surgery, presence or absence of preoperative jaundice, total bilirubin level >20 mg/dL, need for preoperative endoscopic biliary stenting, resectability according to the AJCC staging system,[21] primary tumor site on surgical resection, tumor size, nodal stage, histological grade, vascular, lymphatic, and perineural invasion by microscopically evaluated surgical specimens,[34] adjusted preoperative level of CA19.9, and resection margins on microscopically assessed specimens (negative or R0 resection: absence of tumor cells within 1 mm of the resection margin; positive or R1 resection: microscopically positive at margin or tumor within 1 mm of the margin).[2,31,34]
2.5. Waiting times
Waiting times between referral and appointment of QDU patients and admission of inpatients, QDU time to diagnosis in QDU patients and length-of-stay in inpatients were calculated. To allow for an equivalent measure of QDU time to diagnosis versus length-of-stay and associated costs between QDU and inpatient wards, QDU time to diagnosis and admission (instead of length-of-stay) time to diagnosis were defined as the time elapsed between the request of the decisive diagnostic procedure and the cyto/pathological diagnosis. This was done because patients with pancreatic cancer may require hospitalization not only to expedite diagnosis but also for management of symptoms. Thus, to control for the imbalance in patient- and pancreatic cancer-related characteristics that may bias the outcome comparisons, these intervals, instead of the full time until discharge, were considered a better reflection and comparable measure of the time to diagnosis between the 2 groups.
2.6. Patient factors associated with hospitalization
In a separate analysis, the potential independent predictors of emergency admission against ED referral to QDU were determined. A literature review was performed to identify candidate explanatory variables that could be associated with unplanned emergency admission in patients with pancreatic cancer or other types of cancer.[40] Associations between this outcome and the explanatory variables were tested using first univariate and then multivariate analysis (see Statistical analysis).
2.7. Resource use data collection and cost analysis
Costs of QDU patients and inpatients were analyzed and compared with the microcosting method, often considered a paradigm of hospital service costs since all relevant cost components are determined.[41–43] The microcosting methodology used by us for other disorders has been described elsewhere.[13,16,44,45] In brief, resource use for each patient evaluated was obtained. Resource use data included diagnostic examinations, pharmaceuticals and consumables, therapeutic procedures, adverse events, and consultations. Only treatments other than pancreatic cancer-specific treatments (ie, treatment of patients’ symptoms) were included in the analysis. Costs of all individual resource items were obtained from the institutional information system of the hospital. For QDU patients, the cost of an average ambulatory consultation corresponded to Catalan Health Service fees. The cost of examinations corresponded to hospital tariffs and were the same for QDU patients and inpatients. The analysis also integrated fractions of all staff wages. Staff at QDU includes a full-time consultant internist, a senior internal medicine resident, a full-time nurse, a part-time nurse coordinator, and 2 part-time administrative assistants. The unit is open 5 hours a day, 5 days a week. Staff in each of the 3 medical wards includes 2 full-time consultant internists, 2 residents, a full-time nurse coordinator, 3 teams of 3 full-time nurses and 3 teams of 2 full-time nursing assistants (8-hours daily shifts), and a full-time administrative assistant. Non-direct costs, which mainly corresponded to structural and general functioning costs such as costs related to maintenance, laundry, cleaning services, and administrative costs, as well as depreciation of fixed costs were included in the final analysis.
The mean number of visits during the QDU evaluation, cost per visit and cost per QDU patient, and the mean admission time to diagnosis, cost per day of stay and cost per inpatient were computed and compared. All costs were adjusted for the year of collection (2005–2018) to reflect 2018 Euros (€). Final costs and cost differences are presented in 2018 Euros.
2.8. Statistical analysis
The Chi-square or Fisher exact test were used to compare categorical variables, and results are reported as absolute frequencies (%). The t test was used to compare normally distributed continuous variables and results are expressed as means with standard deviations. When needed, continuous variables with skewed distributions were compared with the nonparametric Mann–Whitney U test. The nature and extent of missing data was included in the analysis.
For the study of factors associated with emergency admission, univariate tests were first done to compare patients admitted versus not admitted on each explanatory variable. Determinants with a P value <.20 in the univariate analysis were included in a multivariate logistic regression model and correlations between covariates were assessed. Collinearity was evaluated for the independent variables and those variables showing excessive collinearity were excluded. Because the existing literature emphasizes that age and sex are important determinants of admission, these variables were forced into the model irrespective of the univariate results. A backward selection analysis was done to construct the final model. Results are expressed as crude and adjusted odds ratios (ORs) with 95% confidence intervals (95% CI). The accuracy of the model was evaluated by the area under the receiver-operating-characteristic (ROC) curve. Reported P values are 2-sided and statistical significance was established at P < .05. Analyses were performed with SAS version 9.4 and Stata version 15.
3. Results
3.1. General characteristics
Of 1147 eligible patients, 143 were excluded. Figure 1 shows the number of initially eligible patients from QDU and inpatient wards and the causes for their exclusion. The main reason for exclusion was incomplete clinical information. After exclusion, 1004 patients comprising 508 QDU patients and 496 inpatients were available for the analysis (Fig. 1). The general characteristics of the whole population are shown in Table 1. There were significant differences in the referral sources. While both QDU patients and inpatients were more commonly referred from the ED, the QDU was more often used by PC physicians (31.7% of QDU patients versus 21.4% of inpatients were referred from PC; P < .001). The mean age of QDU patients and inpatients was 71.2 (12.8) and 72.5 (13.2) years, respectively, and there was a slight predominance of males in both groups. No significant differences were observed in the socioeconomic status. Compared with inpatients, QDU patients were less likely to live alone and more likely to live with a partner (21.3 vs 26.6%; P = .024 and 69.7 vs 65.0%; P = .042, respectively). Regarding symptoms and signs on presentation, admitted patients were significantly more likely than QDU patients to have weight loss, asthenia, anorexia, abdominal pain, jaundice, and palpable hepatomegaly of stony consistency. Whereas some symptoms (nausea/vomiting, change in bowel habit, pruritus, and new-onset diabetes) never presented solitarily, the most frequent symptoms presenting solitarily were abdominal pain, jaundice, back pain, and the triad weight loss, asthenia, and anorexia. It is of note that 9 (1.8%) QDU patients and 6 (1.2%) inpatients (all of them with early-stage pancreatic adenocarcinoma) had no symptoms but their tumor was detected as an incidental imaging finding on medical check-up or during evaluation for other diseases.
Figure 1.
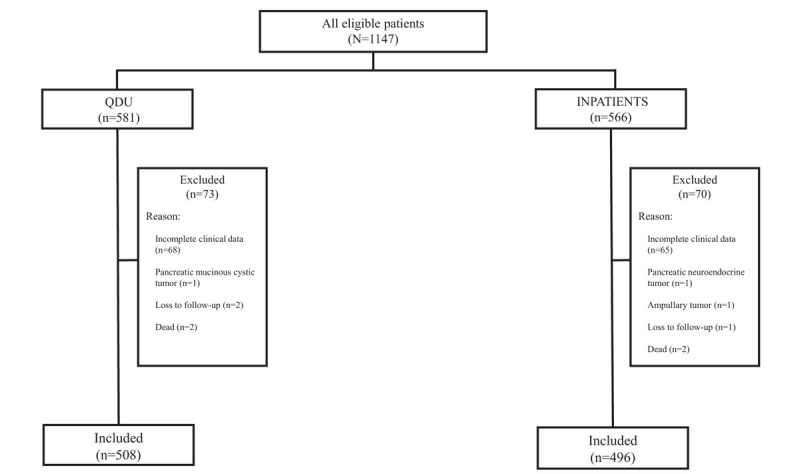
Flowchart of patients included in the study. QDU = quick diagnosis unit.
Table 1.
General characteristics of study patients.
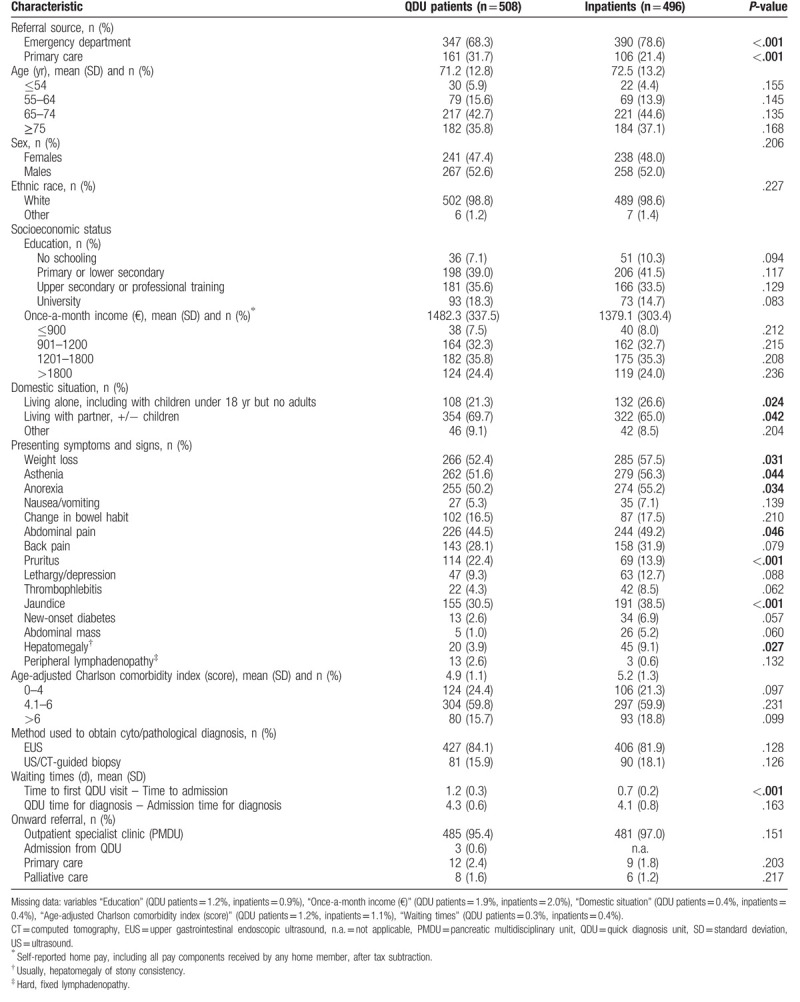
There were no significant differences in the age-adjusted Charlson comorbidity score between QDU patients and inpatients. As to waiting times, the time to admission was significantly shorter than the time to the first QDU visit (0.7 [0.2] vs 1.2 [0.3)] days; P < .001) and there were no differences between the admission time to diagnosis and the QDU time to diagnosis (4.1 [0.8] vs 4.3 [0.6] days; P = .163). Three patients had to be admitted to inpatient wards during the QDU assessment: 2 of them had a quick deterioration of their performance status as well as increased jaundice and the other a pulmonary thromboembolism. Table 1 shows the nature and extent of missing data in both groups of patients. There was a slight degree of missing data in the variables “Socioeconomic status,” “Domestic situation,” “Age-adjusted Charlson comorbidity index,” and “Waiting times,” which ranged from 0.3% to 1.9% in QDU patients and 0.4% to 2% in inpatients (see footnote of Table 1).
3.2. Risk and prognostic factors
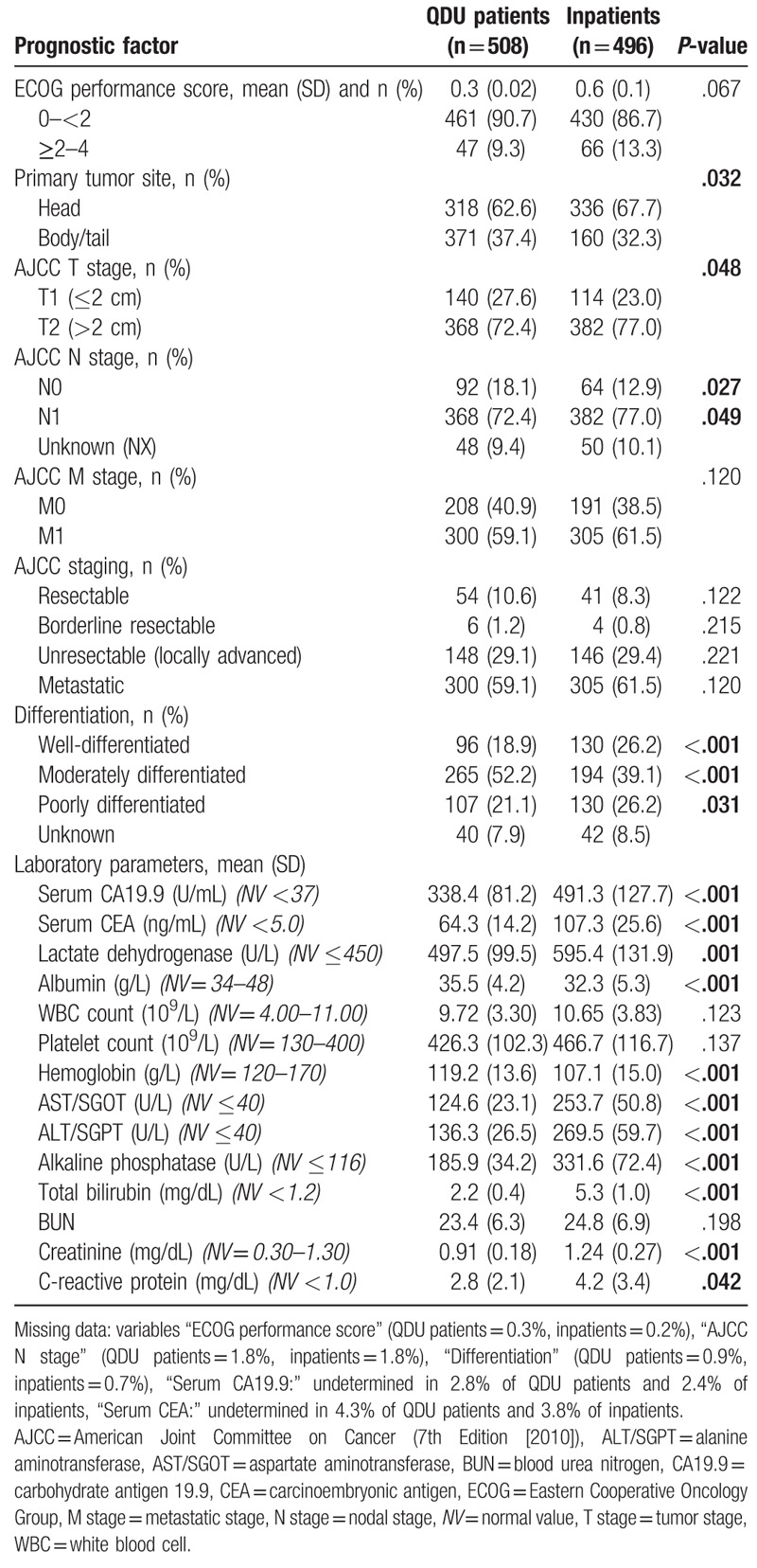
The frequency of risk factors of pancreatic cancer was slightly higher in inpatients than in QDU patients, but no significant differences were observed (Table 2). Table 3 shows the frequency of prognostic factors. Although the head of the pancreas was the most frequent primary tumor site in the 2 groups of patients, 67.7% of inpatients versus 62.6% of QDU patients had a tumor on this localization (P = .032). Inpatients were also more likely than QDU patients to have a mean tumor size >2 cm (77.0 vs 72.4%; P = .048), an N1 stage (75.6 vs 70.9%; P = .049), and a poorer grade of histological differentiation (26.2 vs 21.1%; P = .031). Moreover, 61.5 vs 59.1% of inpatients and QDU patients, respectively, had a metastatic stage on presentation (P = .120) and 29.4 versus 29.1% of inpatients and QDU patients, respectively, had an unresectable, locally advanced tumor (P = .221). Regional lymph nodes could not be identified in imaging studies in 8.5% of QDU patients and 9.5% of inpatients. The histological differentiation in 7.9% of QDU patients and 8.5% of inpatients was unknown for the pathologist. There were several statistically significant differences regarding laboratory results. Inpatients were more likely than QDU patients to have higher serum levels of CA19.9, CEA, LDH, AST/SGOT, ALT/SGPT, alkaline phosphatase, total bilirubin, creatinine, and CRP. The levels of albumin and hemoglobin were significantly lower in inpatients. There was some degree of missing data in the CA 19.9 and CEA determinations (Table 3).
Table 2.
Risk factors of study patients.
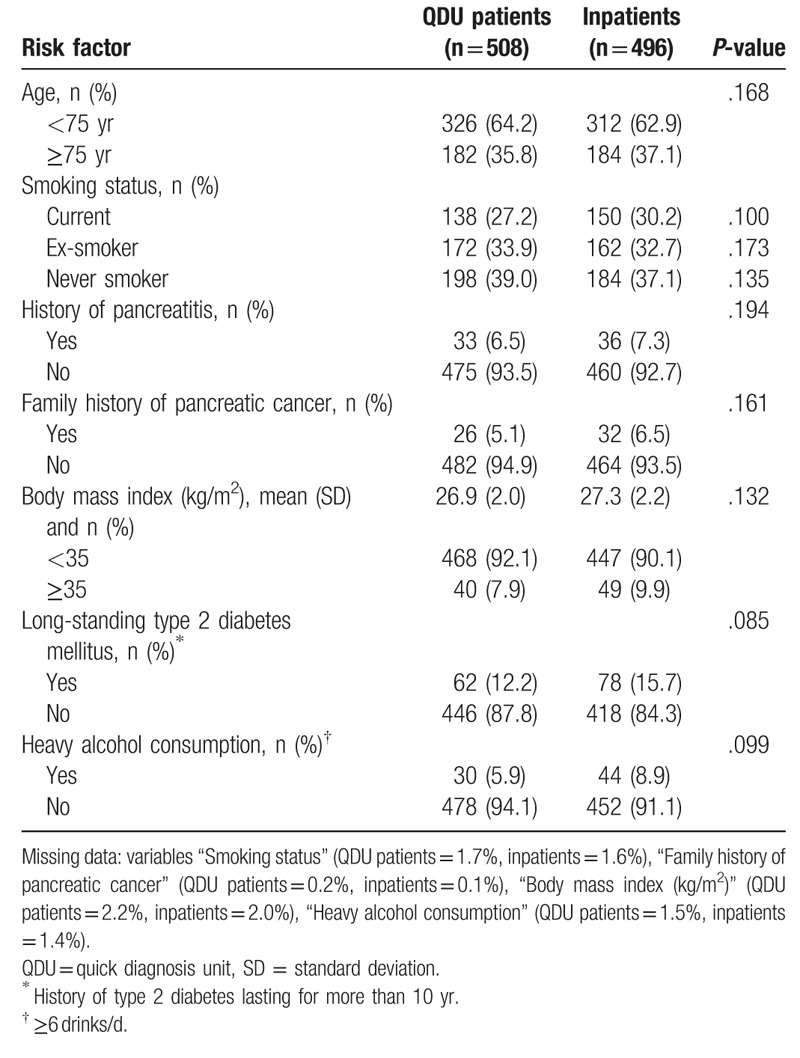
Table 3.
Prognostic factors of study patients.
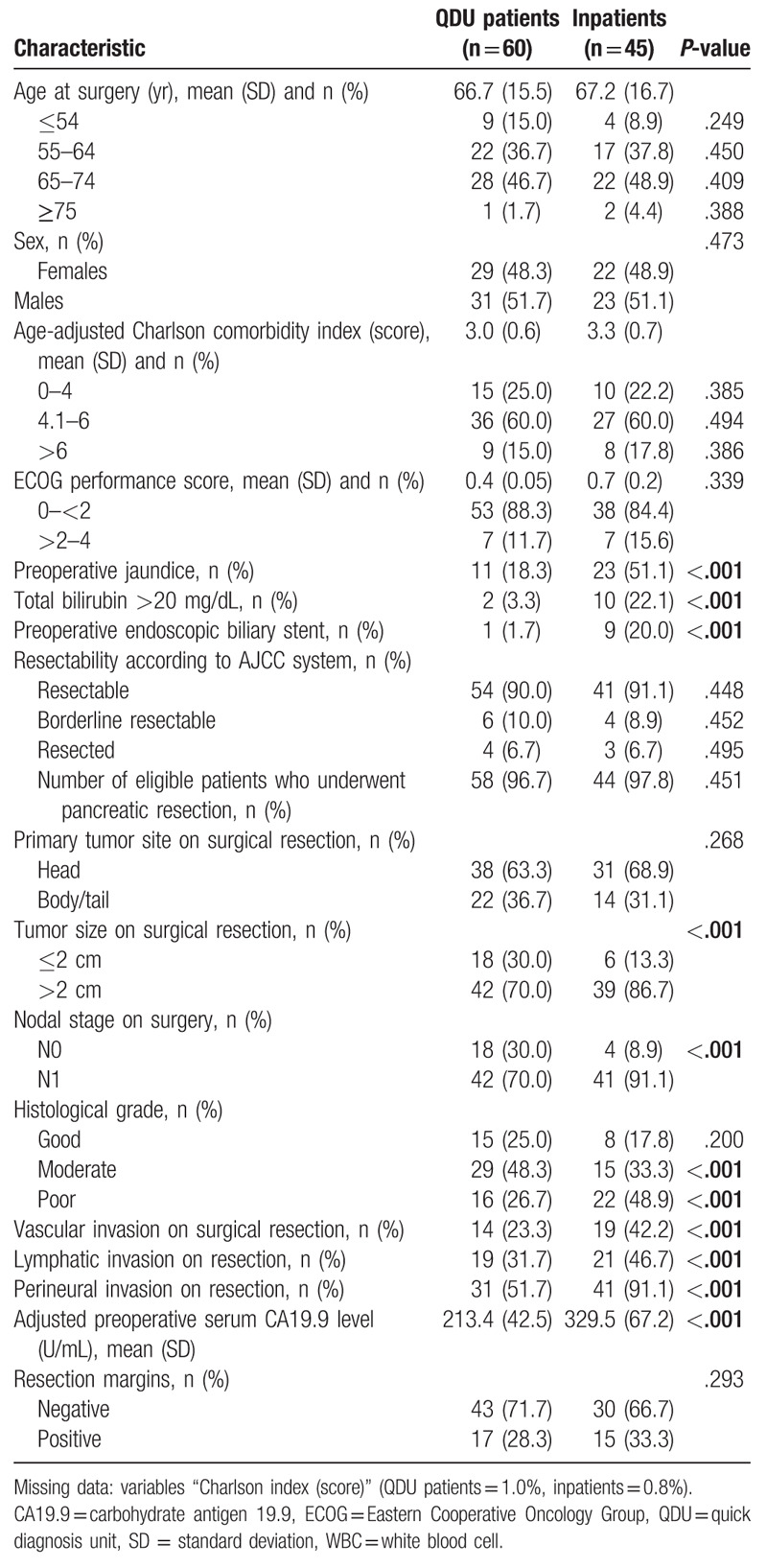
3.3. Surgical characteristics
A total of 105 (10.5% of the total population) patients underwent resection, corresponding to 60 QDU patients and 45 inpatients. Whereas 95 (54 QDU patients and 41 inpatients) patients with I and II AJCC stages had an immediate resection, 4 of 6 QDU patients and 3 of 4 inpatients with a borderline resectable tumor had a resection following neoadjuvant chemoradiotherapy (Table 4). The presence of preoperative jaundice was significantly more common among inpatients than QDU patients (P < .001), as it was having a total serum bilirubin level >20 mg/dL (P < .001) and an endoscopic biliary stent implanted (P < .001). Inpatients were also more likely to have a mean tumor size on surgical resection >2 cm (P < .001), an N1 nodal stage on resection (P < .001), a poorer histological grade on surgery (P < .001), and vascular, lymphatic and perineural invasion on surgical resection (P < .001 in all cases). The resection margins were not significantly different between QDU patients and inpatients (positive: 28.3% vs 33.3%, respectively). There was a minor degree of missing data in the variable Charlson index (Table 4).
Table 4.
Surgical characteristics of patients who underwent tumor resection.
3.4. Factors associated with hospitalization
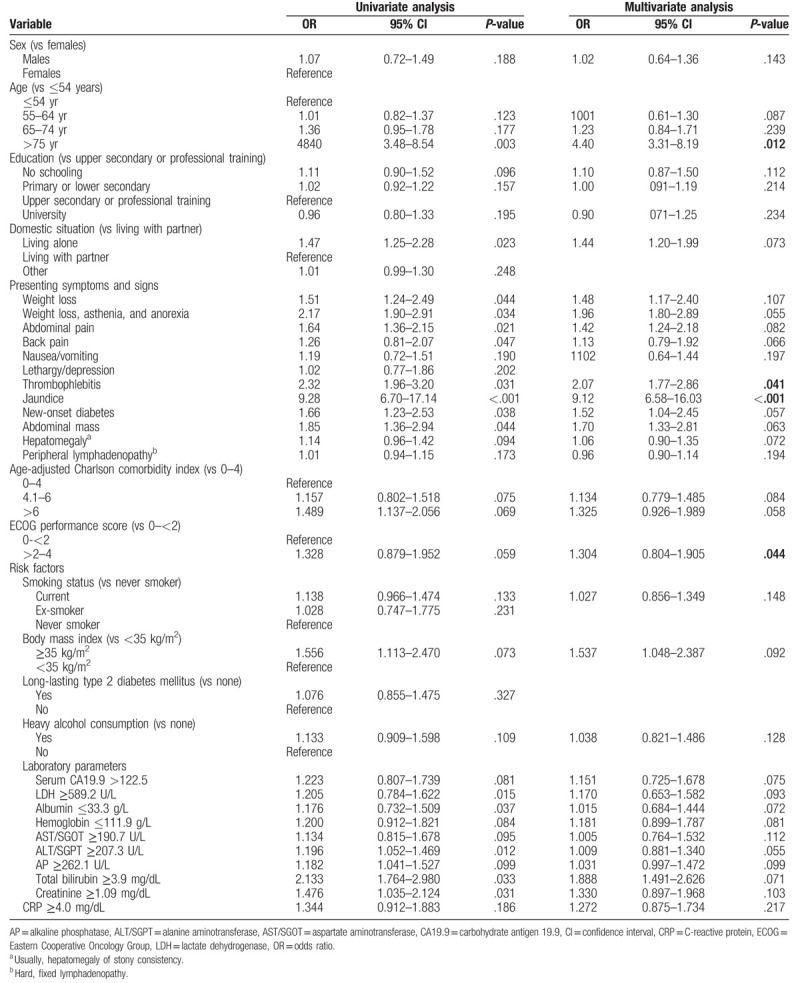
On multivariate logistic regression analysis with adjustment for other variables, 4 significant independent predictors of hospitalization were identified: age ≥75 years, thrombophlebitis, jaundice, and an ECOG performance score ≥2 to 4. The ORs and 95% CIs of each factor are listed in Table 5. Having jaundice was the strongest predictor of admission on multivariate analysis (OR 9.12, 95% CI: 6.58–16.03; P < .001). The quality of the model was assessed with the discrimination of the area under the ROC curve, which was 0.886.
Table 5.
Univariate and multivariate logistic regression analysis of factors associated with emergency admission against factors associated with referral from the emergency department to the quick diagnosis unit.
3.5. Results of cost analysis
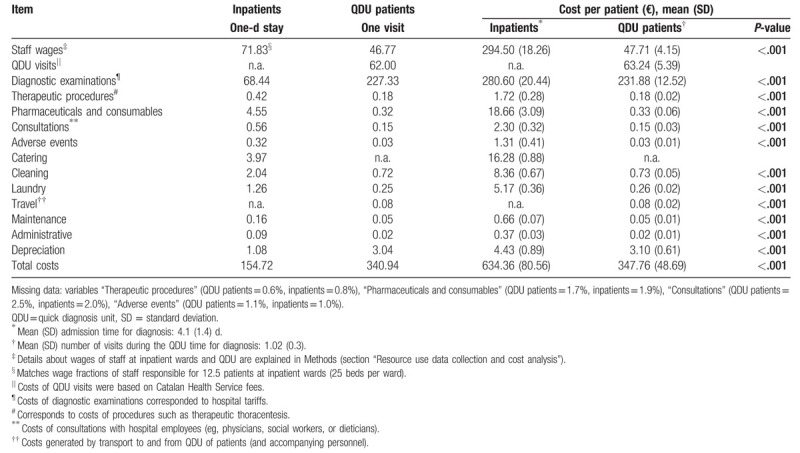
Table 6 shows the mean costs per day of hospitalization, per QDU visit, and per patient in inpatients and QDU patients. Considering that the mean admission time to diagnosis of inpatients was 4.1 (1.4) days and that the mean number of visits of QDU patients during the QDU time to diagnosis was 1.02 (0.3), the total cost per hospitalized patient was €634.36 (80.56), with 46.4% being attributable to personnel salaries and 44.2% to diagnostic tests, and the total cost per QDU patient was €347.76 (48.69), with 66.7% being attributable to diagnostic tests, 18.2% to ambulatory visits, and 13.7% to salaries. According to the analysis, the total saving with QDU was €286.6 per patient. There was some degree of missing data in the variables therapeutic procedures, drugs and consumables, consultations, and adverse events (see Table 6 footnote).
Table 6.
Mean costs (€) of QDU patients (n = 508) and inpatients (n = 496).
4. Discussion
This study revealed that diagnosis of pancreatic cancer is similarly achieved by conventional hospitalization and a hospital-based ambulatory quick diagnostic clinic, and that the latter approach appears to be cost-effective.
The general characteristics of pancreatic adenocarcinoma were largely consistent with known features. However, there were several salient differences between the inpatient and QDU cohorts. First, although the ED was the main referral source in all patients, QDU patients were more commonly referred from PC than inpatients, which may be compatible with the more indolent nature of pancreatic cancer in the QDU cohort. Second, in line with this observation, the differences in the presenting symptoms and signs between the 2 groups were consistent with a more advanced stage of disease in inpatients than QDU patients. Third, the differences became more evident when analyzing the prognostic factors reported to influence survival in patients with pancreatic cancer. The staging process revealed that inpatients were significantly more likely than QDU patients to have a greater tumor dimension, nodal disease, poor differentiation, and higher serum levels of laboratory parameters implicated in the prognosis of pancreatic cancer, more specifically CA19.9. However, the frequency of metastatic and locally advanced disease did not differ significantly between groups. Also, even though one might expect to find a higher occurrence of resectable tumors among QDU patients, no differences were observed in the proportion of resectable cases between QDU patients and inpatients. These observations may be explained by the “intrinsic” aggressiveness of pancreatic adenocarcinoma.
In our study, inpatients were more likely than QDU patients to be referred directly to the ED. Due to diagnostic difficulties by PC physicians in the presence of atypical symptoms and because inpatients have preferential access to examinations, patients with suspected cancer are frequently referred to the ED for admission.[12,14,46,47] It has been reported that a substantial proportion of cancer patients are diagnosed through an emergency presentation [48–50] and that patients with “harder-to-suspect” cancers with atypical symptoms such as pancreatic and stomach cancer and multiple myeloma have a higher proportion of emergency presentations.[9,46,51]
No previous study has reported the associated costs of an ambulatory versus inpatient setting for the diagnosis of pancreatic adenocarcinoma. The cost analysis was central to our study aims. The cost of the diagnostic evaluation in inpatients was almost the double that of QDU patients and savings were achieved at a similar time to diagnosis. It should be noted that nearly 50% of the costs incurred by the inpatient setting versus only 14% of those of the QDU unit owed to personnel wages. Although this finding may suggest an excess of staff in inpatient wards, several factors were likely involved during the stay of these patients which were not accounted for in the cost analysis. Patients with pancreatic cancer may require admission for diagnosis due to severe symptoms that may not be effectively managed in a hospital-based ambulatory unit that is considerably less staffed. The cost of an inpatient diagnosis may thus be worth if there are other complementary or competing diagnoses that are treated or managed at the same time.
4.1. Strengths and limitations
The strengths of our study include the size of the sample (N = 1004), with a similar proportion of QDU patients and inpatients, and its duration (13 years). But it has limitations. As mentioned, it is likely that the cost analysis did not account for several hospitalization-related factors. The clinical and cytopathological information was carefully reviewed and registered, but some details may not have been captured and possible confounders were not determined, which is consistent with the retrospective design of the study. The exclusion of patients without complete information and the presence of missing data might have biased the results, but the low rate of 2 factors meant that study results were unlikely affected. The outcomes were not analyzed and the potential differences between the 2 cohorts could not be analyzed either. Finally, the management of patients referred to ambulatory clinics or admitted for investigation of clinical manifestations such as to those reported here and who have an eventual diagnosis of pancreatic cancer can be different in other settings, a circumstance that depends on various factors such as the type of hospital, the available resources, or the institution traditions.
5. Conclusions
In this study, the effectiveness of a hospital-based ambulatory clinic for the diagnosis of pancreatic adenocarcinoma was similar to that of hospitalization. Although there were no differences in the time to diagnosis between the 2 approaches, the costs of inpatients’ diagnosis were nearly the double than those of QDU patients. Because the high costs of hospitalization and the reported advantages for patients of a hospital-based ambulatory versus inpatient management,[15,17] an ambulatory instead of an inpatient diagnostic evaluation may be preferable in patients with suspected pancreatic cancer. However, it may be argued that admission has an added value for diagnosis since it is associated with issues which may not be properly treated or managed in an ambulatory setting.
Acknowledgments
The authors thank the pathologists and cytologists who reviewed cytologic and histopathological data. The authors are also indebted to the information system and the Department of Economics of the Hospital Clínic for providing the costs of individual resource items and for their assistance in the microcosting analysis.
Author contributions
Conceptualization: Xavier Bosch, Alfons López-Soto.
Formal analysis: Xavier Bosch, Alfons López-Soto.
Investigation: Xavier Bosch.
Methodology: Xavier Bosch, Pedro Moreno.
Project administration: Mar Guerra-García, Neus Guasch.
Resources: Pedro Moreno, Neus Guasch, Alfons López-Soto.
Supervision: Xavier Bosch.
Validation: Xavier Bosch, Mar Guerra-García, Alfons López-Soto.
Visualization: Pedro Moreno, Neus Guasch, Alfons López-Soto.
Writing – original draft: Xavier Bosch.
Footnotes
Abbreviations: 95% CI = 95% confidence intervals, AJCC= American Joint Committee on Cancer, ALT/SGPT = alanine aminotransferase, AST/SGOT = aspartate aminotransferase, CA19.9 = carbohydrate antigen 19.9, CEA = carcinoembryonic antigen, CRP = C-reactive protein, CT = computed tomography, ECOG = Eastern Cooperative Oncology Group, ED = emergency department, EUS = endoscopic ultrasound, LDH = lactate dehydrogenase, OR = odds ratio, PC = primary care, QDU = quick diagnosis unit, ROC = receiver-operating-characteristic.
How to cite this article: Bosch X, Moreno P, Guerra-García M, Guasch N, López-Soto A. What is the relevance of an ambulatory quick diagnosis unit or inpatient admission for the diagnosis of pancreatic cancer? A retrospective study of 1004 patients. Medicine. 2020;99:11(e19009).
The authors have no conflicts of interest to disclose.
References
- [1].Kamisawa T, Wood LD, Itoi T, et al. Pancreatic cancer. Lancet 2016;388:73–85. [DOI] [PubMed] [Google Scholar]
- [2].Ducreux M, Cuhna AS, Caramella C, et al. ESMO Guidelines Committee. Cancer of the pancreas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26: Suppl 5: v56–68. [DOI] [PubMed] [Google Scholar]
- [3].Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med 2014;371:1039–49. [DOI] [PubMed] [Google Scholar]
- [4].Maroni L, Ravaioli M, Pinna AD. Why is pancreatic adenocarcinoma not screened for earlier? Expert Rev Anticancer Ther 2016;16:1003–4. [DOI] [PubMed] [Google Scholar]
- [5].Hansen RP, Vedsted P, Sokolowski I, et al. Time intervals from first symptom to treatment of cancer: a cohort study of 2,212 newly diagnosed cancer patients. BMC Health Serv Res 2011;11:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. National Guideline Alliance (UK). Pancreatic cancer in adults: diagnosis and management. National Institute for Health and Care Excellence: Clinical Guidelines. London: National Institute for Health and Care Excellence (UK); 2018 Feb. [PubMed] [Google Scholar]
- [7].Bond-Smith G, Banga N, Hammond TM, et al. Pancreatic adenocarcinoma. BMJ 2012;344:e2476. [DOI] [PubMed] [Google Scholar]
- [8].Evans J, Chapple A, Salisbury H, et al. It can’t be very important because it comes and goes”--patients’ accounts of intermittent symptoms preceding a pancreatic cancer diagnosis: a qualitative study. BMJ Open 2014;4:e004215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lyratzopoulos G, Abel GA, McPhail S, et al. Measures of promptness of cancer diagnosis in primary care: secondary analysis of national audit data on patients with 18 common and rarer cancers. Br J Cancer 2013;108:686–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lyratzopoulos G, Saunders CL, Abel GA, et al. The relative length of the patient and the primary care interval in patients with 28 common and rarer cancers. Br J Cancer 2015;112: Suppl 1: S35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lyratzopoulos G, Wardle J, Rubin G. Rethinking diagnostic delay in cancer: how difficult is the diagnosis? BMJ 2014;349:g7400. [DOI] [PubMed] [Google Scholar]
- [12].Bosch X, Aibar J, Capell S, et al. Quick diagnosis units: a potentially useful alternative to conventional hospitalisation. Med J Aust 2009;191:496–8. [DOI] [PubMed] [Google Scholar]
- [13].Bosch X, Jordán A, Coca A, et al. Quick diagnosis units versus hospitalization for the diagnosis of potentially severe diseases in Spain. J Hosp Med 2012;7:41–7. [DOI] [PubMed] [Google Scholar]
- [14].Bosch X, Jordán A, López-Soto A. Quick diagnosis units: avoiding referrals from primary care to the ED and hospitalizations. Am J Emerg Med 2013;31:114–23. [DOI] [PubMed] [Google Scholar]
- [15].Sanclemente-Ansó C, Salazar A, Bosch X, et al. Perception of quality of care of patients with potentially severe diseases evaluated at a distinct quick diagnostic delivery model: a cross-sectional study. BMC Health Serv Res 2015;15:434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bosch X, Moreno P, Ríos M, et al. Comparison of quick diagnosis units and conventional hospitalization for the diagnosis of cancer in Spain: a descriptive cohort study. Oncology 2012;83:283–91. [DOI] [PubMed] [Google Scholar]
- [17].Bosch X, Sanclemente-Ansó C, Escoda O, et al. Time to diagnosis and associated costs of an outpatient vs inpatient setting in the diagnosis of lymphoma: a retrospective study of a large cohort of major lymphoma subtypes in Spain. BMC Cancer 2018;18:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO Classification of Tumors of the Digestive System. 4th ed.Lyon (France): International Agency for Research on Cancer; 2010. 381–98. [Google Scholar]
- [19].Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- [20].Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol 1994;47:1245–51. [DOI] [PubMed] [Google Scholar]
- [21].Wolfgang CL, Herman JM, Laheru DA, et al. Recent progress in pancreatic cancer. CA Cancer J Clin 2013;63:318–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Pancreatric Section, British Society of Gastroenterology; Pancreatic Society of Great Britain and Ireland; Association of Upper Gastrointestinal Surgeons of Great Britain and Ireland; Royal College of Pathologists; Special Interest Group for Gastro-Intestinal Radiology. Guidelines for the management of patients with pancreatic cancer periampullary and ampullary carcinomas. Gut 2005;54: Suppl 5: v1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Shrikhande SV, Barreto SG, Goel M, et al. Multimodality imaging of pancreatic ductal adenocarcinoma: a review of the literature. HPB (Oxford) 2012;14:658–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Arslan AA, Helzlsouer KJ, Kooperberg C, et al. Pancreatic Cancer Cohort Consortium (PanScan). Anthropometric measures, body mass index, and pancreatic cancer: a pooled analysis from the Pancreatic Cancer Cohort Consortium (PanScan). Arch Intern Med 2010;170:791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Le N, Sund M, Vinci A. GEMS Collaborating group of Pancreas 2000. Prognostic and predictive markers in pancreatic adenocarcinoma. Dig Liver Dis 2016;48:223–30. [DOI] [PubMed] [Google Scholar]
- [26].Tas F, Sen F, Odabas H, et al. Performance status of patients is the major prognostic factor at all stages of pancreatic cancer. Int J Clin Oncol 2013;18:839–46. [DOI] [PubMed] [Google Scholar]
- [27].Kang CM, Kim JY, Choi GH, et al. The use of adjusted preoperative CA 19-9 to predict the recurrence of resectable pancreatic cancer. J Surg Res 2007;140:31–5. [DOI] [PubMed] [Google Scholar]
- [28].Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg 2000;4:567–79. [DOI] [PubMed] [Google Scholar]
- [29].Stocken DD, Hassan AB, Altman DG, et al. Modelling prognostic factors in advanced pancreatic cancer. Br J Cancer 2008;99:883–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Arnachellum RP, Cariou M, Nousbaum JB, et al. Pancreatic adenocarcinoma in the Finistère area, France, between 2002 and 2011 (1002 Cases): population characteristics, treatment and survival. Pancreas 2016;45:953–60. [DOI] [PubMed] [Google Scholar]
- [31].Butturini G, Stocken DD, Wente MN, et al. Pancreatic Cancer Meta-Analysis Group. Influence of resection margins and treatment on survival in patients with pancreatic cancer: meta-analysis of randomized controlled trials. Arch Surg 2008;143:75–83. [DOI] [PubMed] [Google Scholar]
- [32].Brown KM, Domin C, Aranha GV, et al. Increased preoperative platelet count is associated with decreased survival after resection for adenocarcinoma of the pancreas. Am J Surg 2005;189:278–82. [DOI] [PubMed] [Google Scholar]
- [33].Kim YC, Kim HJ, Park JH, et al. Can preoperative CA19-9 and CEA levels predict the resectability of patients with pancreatic adenocarcinoma? J Gastroenterol Hepatol 2009;24:1869–75. [DOI] [PubMed] [Google Scholar]
- [34].Strasberg SM, Gao F, Sanford D, et al. Jaundice: an important, poorly recognized risk factor for diminished survival in patients with adenocarcinoma of the head of the pancreas. HPB (Oxford) 2014;16:150–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Olson SH, Kurtz RC. Epidemiology of pancreatic cancer and the role of family history. J Surg Oncol 2013;107:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Winter JM, Yeo CJ, Brody JR. Diagnostic, prognostic, and predictive biomarkers in pancreatic cancer. J Surg Oncol 2013;107:15–22. [DOI] [PubMed] [Google Scholar]
- [37].Haas M, Heinemann V, Kullmann F, et al. Prognostic value of CA 199, CEA, CRP, LDH and bilirubin levels in locally advanced and metastatic pancreatic cancer: results from a multicenter, pooled analysis of patients receiving palliative chemotherapy. J Cancer Res Clin Oncol 2013;139:681–9. [DOI] [PubMed] [Google Scholar]
- [38].Ansari D, Bauden M, Bergström S, et al. Relationship between tumour size and outcome in pancreatic ductal adenocarcinoma. Br J Surg 2017;104:600–7. [DOI] [PubMed] [Google Scholar]
- [39].Ong SL, Garcea G, Thomasset SC, et al. Surrogate markers of resectability in patients undergoing exploration of potentially resectablepancreatic adenocarcinoma. J Gastrointest Surg 2008;12:1068–73. [DOI] [PubMed] [Google Scholar]
- [40]. Public Health England. National Cancer Intelligence Network Short Report. Routes to diagnosis 2015 update: pancreatic cancer. February, 2016. [Google Scholar]
- [41].Drummond MF, Sculpher MJ, Claxton K, et al. Methods for the Economic Evaluation of Health Care Programmes. 4th ed.Oxford: Oxford University Press; 2015. [Google Scholar]
- [42].Finkler SA, Ward DM, Baker JJ. Essentials of Cost Accounting for Health Care Organizations. 3rd ed.New York: Jones & Bartlett Learning; 2007. [Google Scholar]
- [43].Tan SS, Rutten FF, van Ineveld BM, et al. Comparing methodologies for the cost estimation of hospital services. Eur J Health Econ 2009;10:39–45. [DOI] [PubMed] [Google Scholar]
- [44].Bosch X, Palacios F, Inclán-Iríbar G, et al. Quick diagnosis units or conventional hospitalisation for the diagnostic evaluation of severe anaemia: a paradigm shift in public health systems? Eur J Intern Med 2012;23:159–64. [DOI] [PubMed] [Google Scholar]
- [45].Brito-Zerón P, Nicolás-Ocejo D, Jordán A, et al. Diagnosing unexplained fever: can quick diagnosis units replace inpatient hospitalization? Eur J Clin Invest 2014;44:707–18. [DOI] [PubMed] [Google Scholar]
- [46].Hamilton W. Emergency admissions of cancer as a marker of diagnostic delay. Br J Cancer 2012;107:1205–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Sheringham JR, Georghiou T, Chitnis XA, et al. Comparing primary and secondary health-care use between diagnostic routes before a colorectal cancer diagnosis: cohort study using linked data. Br J Cancer 2014;111:1490–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Abel GA, Shelton J, Johnson S, et al. Cancer-specific variation in emergency presentation by sex, age and deprivation across 27 common and rarer cancers. Br J Cancer 2015;112: Suppl 1: S129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Elliss-Brookes L, McPhail S, Ives A, et al. Routes to diagnosis for cancer - determining the patient journey using multiple routine data sets. Br J Cancer 2012;107:1220–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50]. Public Health England. National Cancer Intelligence Network Data Briefing. Routes to Diagnosis: Exploring Emergency Presentations. 2013. [Google Scholar]
- [51].Lyratzopoulos G, Neal RD, Barbiere JM, et al. Variation in number of general practitioner consultations before hospital referral for cancer: findings from the 2010 National Cancer Patient Experience Survey in England. Lancet Oncol 2012;13:353–65. [DOI] [PubMed] [Google Scholar]


