Supplemental Digital Content is available in the text
Keywords: depression, meta-analysis, motor, Parkinson disease, repetitive transcranial magnetic stimulation
Abstract
Background:
Clinical symptoms of Parkinson disease (PD) included both motor and nonmotor symptoms. Previous studies indicated inconsistent results for the therapeutic effects of repetitive transcranial magnetic stimulation (rTMS) on motor and depression in PD. The study aimed to assess the therapeutic effect of rTMS with different mode on motor and depression in PD using a meta-analysis.
Methods:
Articles published before July 2019 were searched based on the following databases (PubMed, Web of Science, Medline, Embase, and Google Scholar). The therapeutic effects were assessed by computing the standard mean difference (SMD) and a 95% confidence interval (CI).
Results:
The present study indicated that rTMS showed significant therapeutic effects on motor in PD (SMD 2.05, 95% CI 1.57–2.53, I2 = 93.0%, P < .001). Both high-frequency (HF)-rTMS and low-frequency rTMS showed therapeutic effects on motor; stimulation over primary motor cortex (M1), supplementary motor area, dorsal lateral prefrontal cortex (DLPFC) or M1+DLPFC showed therapeutic effects; stimulation during “on” and “off” states showed therapeutic effects; the study showed long-term effect of rTMS on motor in PD. In addition, the study indicated that rTMS showed significant therapeutic effects on depression in PD (SMD 0.80, 95% CI 0.31–1.29, I2 = 89.1%, P < .001). Stimulation over left DLPFC showed significant therapeutic effects on depression in PD; only HF-rTMS showed therapeutic effects; ages, disease durations, numbers of pulses, and session durations displayed influence on the therapeutic effects of rTMS on depression in PD; the therapeutic effects on depression was long term. However, no significant difference in therapeutic effects on depression were showed between rTMS and oral Fluoxetine (SMD 0.74, 95% CI −0.83 to 2.31, I2 = 92.5%, P < .001).
Conclusion:
The rTMS showed significant therapeutic effects on motor in PD. HF-rTMS showed a significant positive antidepressive effect in PD only over DLPFC.
1. Introduction
Epidemiologic data indicated that Parkinson disease (PD) is the 2nd most common neurodegenerative disease worldwide, after Alzheimer disease.[1] Clinical symptoms of PD included both motor and nonmotor symptoms. The loss of dopaminergic neurons in the substantia nigra pars compacta results in the characteristic motor symptoms of PD: resting tremors, bradykinesia, rigidity, and postural instability.[2] An amount of therapeutic methods have been tried for the treatment of PD. Motor symptoms could be controlled by drug therapy and deep brain stimulation.[3] However, these treatments are usually ineffective for nonmotor symptoms. Epidemiologic results showed that depression is the most common nonmotor symptom, with a frequency between 15% and 50%.[4] The frequent resistant to medication of depression in PD severely affects patients’ quality of life.[5]
Repetitive transcranial magnetic stimulation (rTMS) is a painless and noninvasive brain stimulation method over stimulating selected regions of the brain to treat neurologic and psychiatric disorders including stroke, Alzheimer disease, depression, and PD rehabilitation.[6,7] rTMS works by modulating the excitability of the cerebral cortex.[8] According to previous studies, the low-frequency rTMS (LF-rTMS, ≤1 Hz) works via decreasing cortical neural excitability, whereas high-frequency rTMS (HF-rTMS, >1 Hz) works via enhancing cortical neural activity.[9] In the recent years, some studies indicated incompatible results regarding the therapeutic effects of rTMS on motor and mood symptoms in PD. Maruo et al[10] reported that HF-rTMS over the primary motor cortex (M1) significantly improved motor scores in PD, compared to sham stimulation. However, Filipovic et al[11] found no significant difference in therapeutic effects on motor in PD between sham and real rTMS over M1. In addition, Pal et al[12] demonstrated the beneficial effect of the left dorsal lateral prefrontal cortex (DLPFC) rTMS on depression in PD lasting at least 30 days after treatment, whereas Yokoe et al[13] found that no significant improvements were demonstrated in the mood disturbances after the stimulations over any of the targets (M1, supplementary motor area [SMA], DLPFC]). Thus, a meta-analysis is essential to explore the therapeutic effect of rTMS on motor and depression in PD. Moreover, according to previous studies, these inconsistent results may come from different designs of rTMS protocols such as the frequency and stimulation target.[14] Previous meta-analyses on the topic of rTMS for motor and depression in patients with PD have showed that only rTMS was superior to sham-rTMS.[15,16] However, these studies did not clearly indicated which mode of rTMS represented the optimal parameters. The study aimed to assess the therapeutic effect of rTMS with different modes on motor and depression in PD using a meta-analysis.
2. Methods
The study was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.[17] The study is a meta-analysis, an analysis with secondary processing. Thus, ethical approval was not necessary in the study.
2.1. Search strategy
Articles published before July 2019 were searched based on the following databases (PubMed, Web of Science, Medline, Embase, and Google Scholar). The following search terms were used: (“transcranial magnetic stimulation” OR “TMS”) AND (“Parkinson Disease” OR “Parkinson's Disease” OR “Parkinsonism” or “Parkinsonian”). After eliminating duplicates, 727 articles were included.
2.2. Inclusion and exclusion criteria
In the study, we included studies with randomized controlled or crossover design. These studies provided data regarding the comparison of pre- and posttreatment motor examination of the unified Parkinson's disease rating scale (UPDRS-III) or depression scale scores between patients with PD given rTMS and sham stimulation (or oral antidepressants). In addition, the outcomes were reported or could be calculated. We excluded articles as follows: Articles which did not provide sufficient data of pre- and posttreatment UPDRS-III or depression scale scores; Reviews, meta-analysis studies, and case reports.
2.3. Data collection
The data were extracted from these articles by 2 reviewers independently. These data covered author, publication year, study design, study location, information of included participants (sample size, gender, mean age, disease durations), target area of rTMS, rTMS frequency, intensity of rTMS, rTMS stimulation parameters, medication status during assessment (“on” or “off”), sham stimulation, adverse effects, and follow-up time.
2.4. Meta-analysis
The study used STATA 12.0 software to summarize the comparison of changes of motor and depression scales scores between patients with PD treated with rTMS and control therapy (sham stimulation or oral antidepressants). The treatment effect was assessed by computing the standard mean difference and a 95% confidence interval. We applied I2 to evaluate the heterogeneity among studies. We used fixed-effects models when I2 < 50%. Otherwise, random effects models were used. We conducted meta-regression analyses to explore source of the heterogeneity. Subgroup analyses (for short-term or long-term effects, medication status during assessment, target area of rTMS, different frequencies of rTMS) were conducted to explore the effect of these indicators on motor and mood symptoms in PD, respectively. Additionally, the stability of the meta-analysis was assessed by removing 1 individual study each time. Moreover, we used Begg test, Egger test and funnel plots to assess publication bias.
3. Results
3.1. Search and selection results
Figure 1 illustrates the procedure of search and subsequent selections. Supplementary Table 1 shows characteristics of 28 finally selected studies. Among these studies, 25 studies[10–14,18–37] investigated the therapeutic effect of rTMS on motor in PD (including 409 patients given rTMS and 378 patients given sham stimulation). In the 25 studies, 14 studies were with crossover design, 11 studies were with randomized controlled design. Additionally, 8 studies[12,13,26,29,30,33,35,36] explored the therapeutic effect of rTMS on depression in PD (including 177 patients given rTMS and 154 patients given sham stimulation). Among the 8 studies, 3 studies were with crossover design, 5 studies were with randomized controlled design. Moreover, 3 crossover studies[38–40] (including 45 patients given rTMS and 45 patients given oral Fluoxetine) compared the therapeutic effects of rTMS and oral antidepressants.
Figure 1.
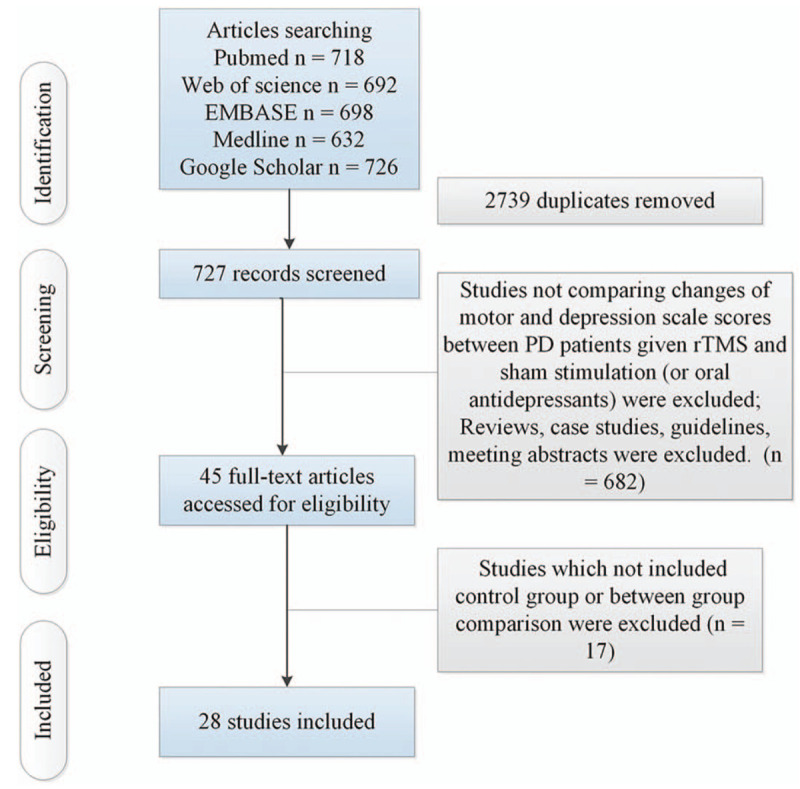
Flow of information through the different phases of a systematic review. PD = Parkinson disease; rTMS = repetitive transcranial magnetic stimulation.
3.2. Meta-analysis results
Table 1 shows the results about therapeutic effects of rTMS on motor in PD. The present study indicated that rTMS showed significant therapeutic effects on motor in PD (Fig. 2). Meta-regression analysis showed that ages, disease durations, numbers of pulses, and session durations were not responsible for heterogeneity across studies regarding effects of rTMS on motor in PD (Supplementary Table 2). Subgroup analyses showed significant short-term and long-term therapeutic effects of rTMS on motor in PD (Supplementary Fig. 1). Subgroup meta-analysis demonstrated that the site effect of rTMS therapy on motor in PD (Supplementary Fig. 2). Additionally, subgroup meta-analysis displayed that both HF-rTMS and LF-rTMS showed significant treatment effects on motor in PD (Supplementary Fig. 3). Moreover, subgroup analysis indicated significant therapeutic effects of rTMS on motor in PD during both “on” and “off” states (Supplementary Fig. 4).
Table 1.
Results about therapeutic effects of rTMS on motor in PD.
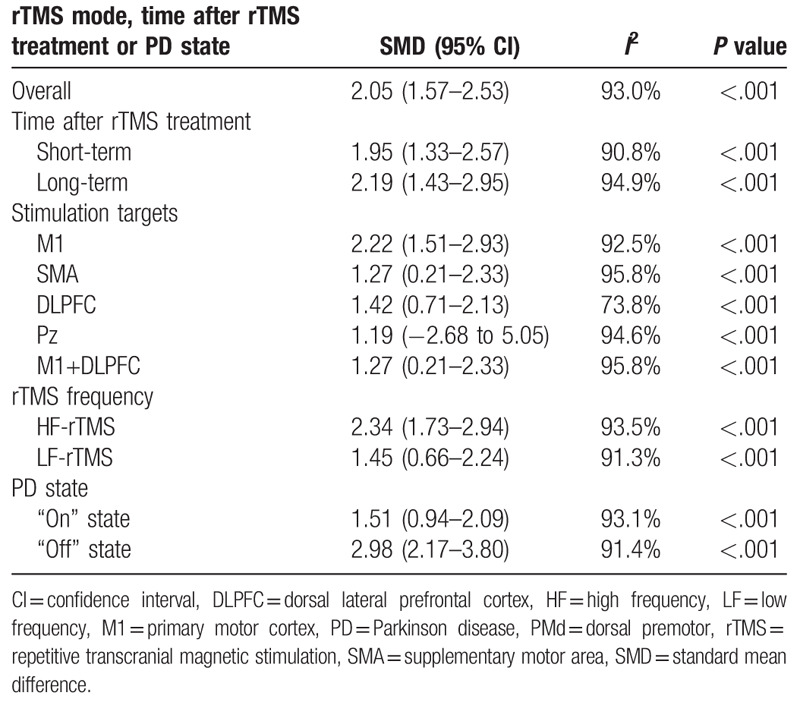
Figure 2.
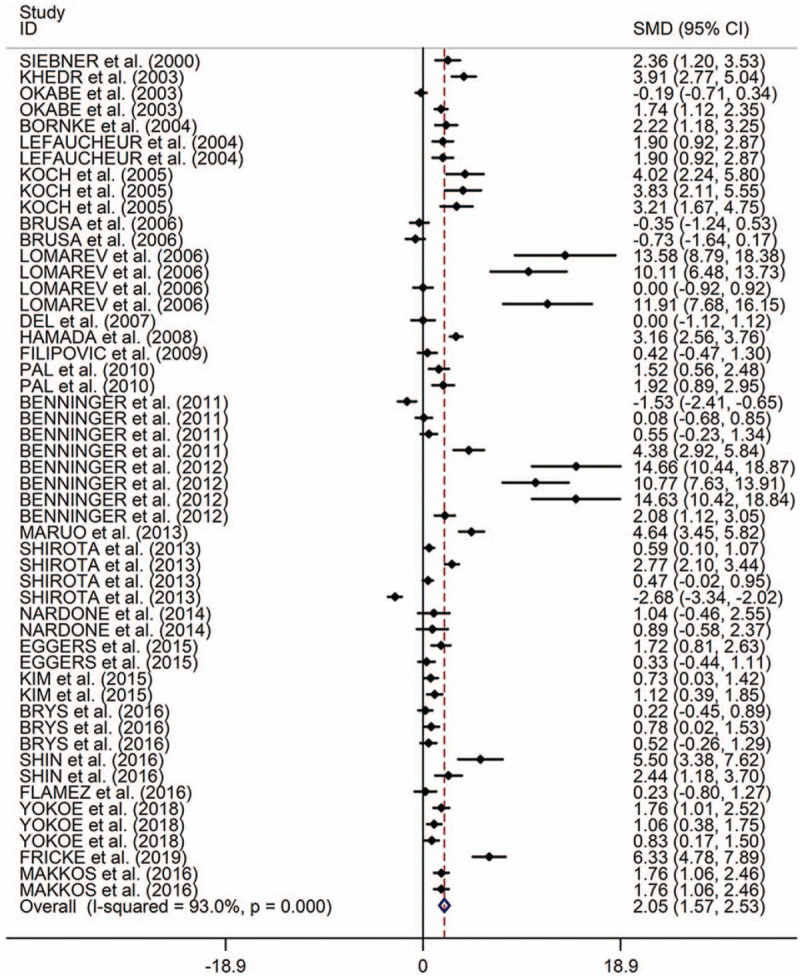
Forest plots of therapeutic effects of repetitive transcranial magnetic stimulation on motor in Parkinson disease. CI = confidence interval, SMD = standard mean difference.
Table 2 shows the results about therapeutic effects of rTMS on depression in PD. The present study indicated that rTMS showed significant therapeutic effects on depression in PD (Fig. 3). Meta-regression analysis showed that the four indicators (ages, disease durations, numbers of pulses, and session durations) were all responsible for heterogeneity across studies regarding effects of rTMS on depression in PD (Supplementary Table 2). Subgroup analyses showed significant long-term therapeutic effects of rTMS on depression in PD, whereas no significant short-term therapeutic effects of rTMS were found on depression in PD (Supplementary Fig. 5). Subgroup meta-analysis indicated that the site effect of rTMS therapy on depression in PD (Supplementary Fig. 6). Additionally, subgroup meta-analysis showed that HF-rTMS displayed significant treatment effects on depression in PD, whereas no significant therapeutic effect was found for LF-rTMS (Supplementary Fig. 7).
Table 2.
Results about therapeutic effects of rTMS on depression in PD and therapeutic effects compared to oral Fluoxetine.
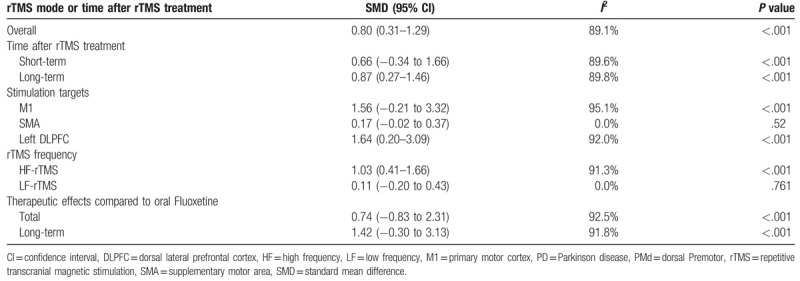
Figure 3.
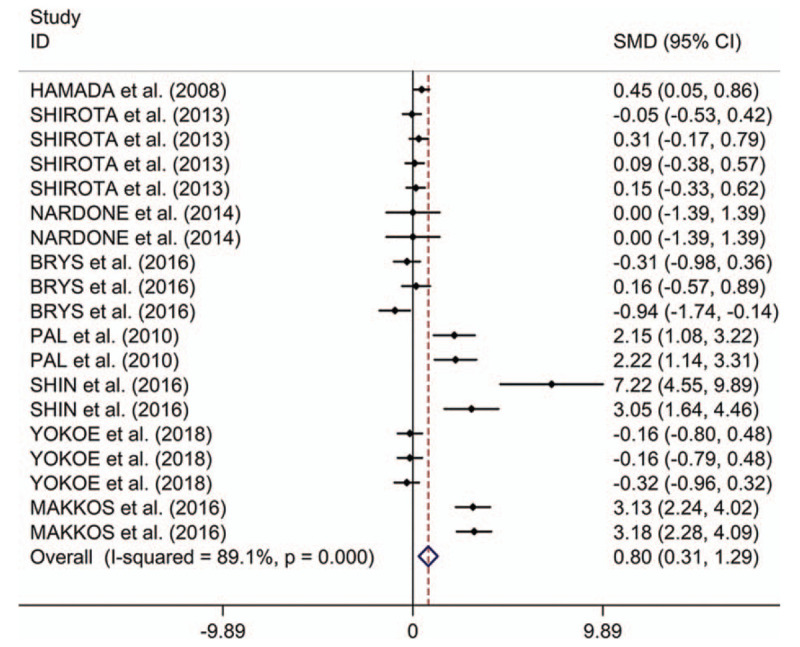
Forest plots of therapeutic effects of repetitive transcranial magnetic stimulation on depression in Parkinson disease. CI = confidence interval, SMD = standard mean difference.
Table 2 shows the results about therapeutic effects of rTMS on depression in PD compared to oral Fluoxetine. The present study showed no significant difference in therapeutic effects on depression between rTMS and oral Fluoxetine (Fig. 4). Subgroup analyses showed no significant difference in long-term therapeutic effects on depression between rTMS and oral Fluoxetine in PD (Supplementary Fig. 8).
Figure 4.
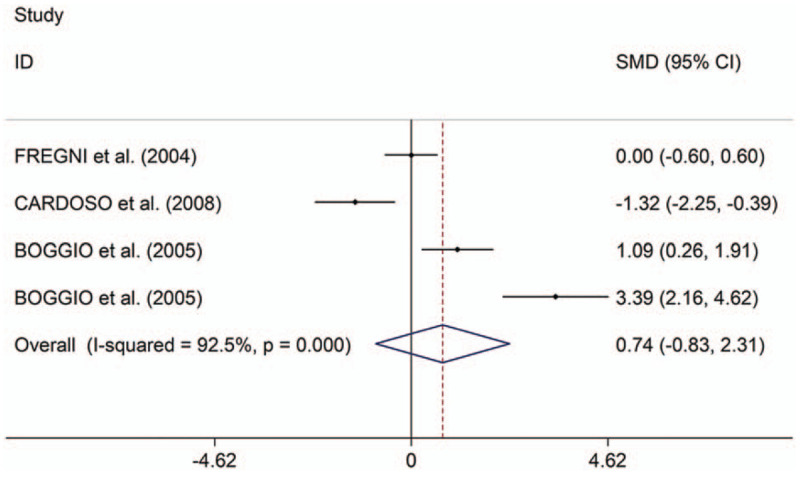
Forest plots of changes of depression scales scores between patients with Parkinson disease given repetitive transcranial magnetic stimulation and those given oral Fluoxetine. CI = confidence interval, SMD = standard mean difference.
In the present study, sensitivity analyses showed no changes in the direction of effect when any 1 study was excluded for all meta-analyses (Supplementary Fig. 9). Begg test, Egger tests, and funnel plots showed significant risks of publication bias for meta-analyses of therapeutic effects of rTMS on motor in PD (Supplementary Table 3 and Supplementary Fig. 10A). However, Begg test, Egger tests and funnel plots showed no significant risks of publication bias for meta-analyses of therapeutic effects of rTMS on depression in PD (Supplementary Table 3 and Supplementary Fig. 10B and C).
4. Discussion
The present meta-analysis indicated that rTMS showed significant therapeutic effects on motor and depression in PD. Both HF-rTMS and LF-rTMS showed therapeutic effects on motor; stimulation over M1, SMA, DLPFC, or M1+DLPFC showed therapeutic effects; stimulation during “on” and “off” states showed therapeutic effects. In addition, the study showed long-term effect of rTMS on motor in PD. Stimulation over left DLPFC showed significant therapeutic effects on depression in patients with PD; only HF-rTMS showed therapeutic effects; ages, disease durations, numbers of pulses, and session durations showed influences on the therapeutic effects of rTMS on depression in PD; the therapeutic effects on depression was long term. However, the study showed no significant difference in therapeutic effects on depression between rTMS and oral Fluoxetine.
The study showed that rTMS showed significant therapeutic effects on motor in PD. The results were corresponding to a recent meta-analysis,[41] which indicated that rTMS showed both short-term and long-term effects on motor function improvement of PD. However, the recent meta-analysis showed that HF-rTMS showed significant therapeutic effects, whereas LF-rTMS showed no significant therapeutic effects. The result was not inconsistent with our result. Compared to the previous published meta-analysis,[41] our study included more articles. Additionally, the present study explored sources of heterogeneity across studies with a meta-regression analysis. rTMS plays a role by modulating cortical excitability, with HF-rTMS (>1 Hz) being facilitatory and LF- rTMS (≤1 Hz) being inhibitory.[9] Additionally, the study showed that M1, SMA, DLPFC, or M1+DLPFC showed therapeutic effects were key targets of most therapeutic rTMS studies. rTMS on the motor cortex releases dopamine in the caudate and putamen corresponding to their cortico-striatal projections.[42] The motor cortex-basal ganglia-thalamo-cortical circuit might play a role in the therapeutic effects of rTMS on motor in PD.[43] SMA is another effective therapeutic target in the present study. SMA palys a role in self-initiated movements, which are impaired in PD.[44] DLPFC is active during self-initiated and externally cued motor activity.[30] DLPFC works as an effective therapeutic target in the present study. Additionally, stimulation over M1+DLPFC showed an effective therapeutic effect. Regarding the synergistic effect of stimulation over 2 brain regions, a recent study showed that there was no added benefit of M1+DLPFC stimulation on motor symptoms compared to M1 stimulation alone.[33] It is unknown about the neurophysiology of concomitant TMS stimulation over different sites. Another 2 previous studies have combined M1 and DLPFC rTMS in PD. All of the 2 reported motor benefit, but they differed from the recent study in important ways.[24,45] They lacked a sham control, used LF rather than HF rTMS to M1, and used a different type of TMS coil. Moreover, the study showed both short-term and long-term therapeutic effects of rTMS on motor in PD. Taken together, the present study indicated rTMS showed significant therapeutic effects on motor in PD.
The present meta-analysis indicated that rTMS showed significant therapeutic effects on depression in PD. The study showed that stimulation over left DLPFC showed significant therapeutic effects on depression in PD. The DLPFC is the only FDA-approved therapeutic target of rTMS in clinical practice.[46] In addition, the DLPFC is the prime target of rTMS for the treatment of depression.[46] Previous studies showed that the stimulation over the DLPFC improves mood compared to antidepressants.[38] However, the present meta-analysis showed no significant difference in changes of depression scale scores between patients with PD given rTMS or those given oral antidepressants. A group of European experts established guidelines for the therapeutic application of rTMS from articles published until March 2014.[47] They summarized a probable antidepressant effect of HF-rTMS of the left DLPFC in PD (level B).[47] Our study provided evidence for the expert consensus that only HF-rTMS showed therapeutic effects. In addition, our study demonstrated that ages, disease durations, numbers of pulses, and session durations might influence on the therapeutic effects of rTMS on depression in PD. Thus, the early diagnosis of depression in PD and treatment with rTMS, appropriate rTMS parameters were essential for the effective therapy of depression in PD. Compared to the previous published meta-analysis,[48] the present study explored sources of heterogeneity across studies with a meta-regression analysis. Additionally, the present study explored the therapeutic effects of rTMS, compared to oral antidepressants. However, there were only 3 studies exploring the therapeutic effects of rTMS on depression in PD, compared to oral antidepressants. More large-scale randomized controlled trials should be conducted to explore effects of rTMS on depression in PD, compared to oral antidepressants.
The present study showed some limitations. With respect to the therapeutic effect of rTMS on depression compared to oral antidepressants, there were a limited number of studies,[38–40] potentially limiting statistical power. Secondly, publication bias might exist due to the fact that the study only included articles published in English language journals. Thirdly, information about which mode of rTMS showed significant therapeutic effect could be obtained from the meta-analysis. However, information about which mode of rTMS showed optimal therapeutic effect could not be acquired from the meta-analysis.
5. Conclusion
In conclusion, rTMS showed significant therapeutic effects on motor in PD. HF-rTMS showed a significant positive antidepressive effect in PD only over DLPFC. However, information about which mode of rTMS showed optimal therapeutic effect could not be acquired from the meta-analysis. Additionally, more large-scale randomized controlled trials should be made to explore the effect of rTMS on depression in PD, compared to oral antidepressants Supplementary Reference
.
Author contributions
Data curation: Shuqian Li.
Investigation: Shuqian Li, Rui Jiao, Xiaomei Zhou.
Methodology: Shuqian Li, Rui Jiao, Xiaomei Zhou.
Writing – original draft: Shuqian Li.
Writing – review & editing: Shangjie Chen.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, DLPFC = dorsal lateral prefrontal cortex, HF = high frequency, LF = low frequency, M1 = primary motor cortex, PD = Parkinson disease, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses, rTMS = repetitive transcranial magnetic stimulation; SMA = supplementary motor area, SMD = standard mean difference.
How to cite this article: Li S, Jiao R, Zhou X, Chen S. Motor recovery and antidepressant effects of repetitive transcranial magnetic stimulation on Parkinson disease: a PRISMA-compliant meta-analysis. Medicine. 2020;99:18(e19642).
The authors have no funding and conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Pringsheim T, Jette N, Frolkis A, et al. The prevalence of Parkinson's disease: a systematic review and meta-analysis. Mov Disord 2014;29:1583–90. [DOI] [PubMed] [Google Scholar]
- [2].Meissner WG, Frasier M, Gasser T, et al. Priorities in Parkinson's disease research. Nat Rev Drug Discov 2011;10:377–93. [DOI] [PubMed] [Google Scholar]
- [3].Anderson D, Beecher G, Ba F. Deep brain stimulation in Parkinson's disease: new and emerging targets for refractory motor and nonmotor symptoms. Parkinsons Dis 2017;2017:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Weintraub D, Mamikonyan E. The neuropsychiatry of Parkinson disease: a perfect storm. Am J Geriatr Psychiatry 2019;27:998–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Johnston K, Powell LC, Anderson IM, et al. The burden of treatment-resistant depression: a systematic review of the economic and quality of life literature. J Affect Disord 2019;242:195–210. [DOI] [PubMed] [Google Scholar]
- [6].Rajji TK. Transcranial magnetic and electrical stimulation in Alzheimer's disease a mild cognitive impairment: a review of randomized controlled trials. Clin Pharmacol Ther 2019;106:776–80. [DOI] [PubMed] [Google Scholar]
- [7].He Y, Sun N, Wang Z, et al. Effect of repetitive transcranial magnetic stimulation (rTMS) for insomnia: a protocol for a systematic review. BMJ Open 2019;9:e029206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cirillo G, Di Pino G, Capone F, et al. Neurobiological after-effects of non-invasive brain stimulation. Brain Stimul 2017;10:1–8. [DOI] [PubMed] [Google Scholar]
- [9].Sasaki N, Kakuda W, Abo M. Bilateral high- and low-frequency rTMS in acute stroke patients with hemiparesis: a comparative study with unilateral high-frequency rTMS. Brain Inj 2014;28:1682–6. [DOI] [PubMed] [Google Scholar]
- [10].Maruo T, Hosomi K, Shimokawa T, et al. High-frequency repetitive transcranial magnetic stimulation over the primary foot motor area in Parkinson's disease. Brain Stimul 2013;6:884–91. [DOI] [PubMed] [Google Scholar]
- [11].Filipovic SR, Rothwell JC, van de Warrenburg BP, et al. Repetitive transcranial magnetic stimulation for levodopa-induced dyskinesias in Parkinson's disease. Mov Disord 2009;24:246–53. [DOI] [PubMed] [Google Scholar]
- [12].Pal E, Nagy F, Aschermann Z, et al. The impact of left prefrontal repetitive transcranial magnetic stimulation on depression in Parkinson's disease: a randomized, double-blind, placebo-controlled study. Mov Disord 2010;25:2311–7. [DOI] [PubMed] [Google Scholar]
- [13].Yokoe M, Mano T, Maruo T, et al. The optimal stimulation site for high-frequency repetitive transcranial magnetic stimulation in Parkinson's disease: a double-blind crossover pilot study. J Clin Neurosci 2018;47:72–8. [DOI] [PubMed] [Google Scholar]
- [14].Okabe S, Ugawa Y, Kanazawa I. 0.2-Hz repetitive transcranial magnetic stimulation has no add-on effects as compared to a realistic sham stimulation in Parkinson's disease. Mov Disord 2003;18:382–8. [DOI] [PubMed] [Google Scholar]
- [15].Xie CL, Chen J, Wang XD, et al. Repetitive transcranial magnetic stimulation (rTMS) for the treatment of depression in Parkinson disease: a meta-analysis of randomized controlled clinical trials. Neurol Sci 2015;36:1751–61. [DOI] [PubMed] [Google Scholar]
- [16].Hai-Jiao W, Ge T, Li-Na Z, et al. The efficacy of repetitive transcranial magnetic stimulation for Parkinson disease patients with depression. Int J Neurosci 2019;130:1–9. [DOI] [PubMed] [Google Scholar]
- [17].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Revista Española De Nutrición Humana Y Dietética 2009. [PMC free article] [PubMed] [Google Scholar]
- [18].Siebner HR, Rossmeier C, Mentschel C, et al. Short-term motor improvement after sub-threshold 5-Hz repetitive transcranial magnetic stimulation of the primary motor hand area in Parkinson's disease. J Neurol Sci 2000;178:91–4. [DOI] [PubMed] [Google Scholar]
- [19].Khedr EM, Farweez HM, Islam H. Therapeutic effect of repetitive transcranial magnetic stimulation on motor function in Parkinson's disease patients. Eur J Neurol 2003;10:567–72. [DOI] [PubMed] [Google Scholar]
- [20].Bornke C, Schulte T, Przuntek H, et al. Clinical effects of repetitive transcranial magnetic stimulation versus acute levodopa challenge in Parkinson's disease. J Neural Transm Suppl 2004;68:61–7. [DOI] [PubMed] [Google Scholar]
- [21].Lefaucheur JP, Drouot X, von Raison F, et al. Improvement of motor performance and modulation of cortical excitability by repetitive transcranial magnetic stimulation of the motor cortex in Parkinson's disease. Clin Neurophysiol 2004;115:2530–41. [DOI] [PubMed] [Google Scholar]
- [22].Koch G, Brusa L, Caltagirone C, et al. rTMS of supplementary motor area modulates therapy-induced dyskinesias in Parkinson disease. Neurology 2005;65:623–5. [DOI] [PubMed] [Google Scholar]
- [23].Brusa L, Versace V, Koch G, et al. Low frequency rTMS of the SMA transiently ameliorates peak-dose LID in Parkinson's disease. Clin Neurophysiol 2006;117:1917–21. [DOI] [PubMed] [Google Scholar]
- [24].Lomarev MP, Kanchana S, Bara-Jimenez W, et al. Placebo-controlled study of rTMS for the treatment of Parkinson's disease. Mov Disord 2006;21:325–31. [DOI] [PubMed] [Google Scholar]
- [25].del Olmo MF, Bello O, Cudeiro J. Transcranial magnetic stimulation over dorsolateral prefrontal cortex in Parkinson's disease. Clin Neurophysiol 2007;118:131–9. [DOI] [PubMed] [Google Scholar]
- [26].Hamada M, Ugawa Y, Tsuji S. High-frequency rTMS over the supplementary motor area for treatment of Parkinson's disease. Mov Disord 2008;23:1524–31. [DOI] [PubMed] [Google Scholar]
- [27].Benninger DH, Berman BD, Houdayer E, et al. Intermittent theta-burst transcranial magnetic stimulation for treatment of Parkinson disease. Neurology 2011;76:601–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Benninger DH, Iseki K, Kranick S, et al. Controlled study of 50-Hz repetitive transcranial magnetic stimulation for the treatment of Parkinson disease. Neurorehabil Neural Repair 2012;26:1096–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Shirota Y, Ohtsu H, Hamada M, et al. Supplementary motor area stimulation for Parkinson disease: a randomized controlled study. Neurology 2013;80:1400–5. [DOI] [PubMed] [Google Scholar]
- [30].Nardone R, de Blasi P, Holler Y, et al. Repetitive transcranial magnetic stimulation transiently reduces punding in Parkinson's disease: a preliminary study. J Neural Transm 2014;121:267–74. [DOI] [PubMed] [Google Scholar]
- [31].Eggers C, Gunther M, Rothwell J, et al. Theta burst stimulation over the supplementary motor area in Parkinson's disease. J Neurol 2015;262:357–64. [DOI] [PubMed] [Google Scholar]
- [32].Kim MS, Chang WH, Cho JW, et al. Efficacy of cumulative high-frequency rTMS on freezing of gait in Parkinson's disease. Restor Neurol Neurosci 2015;33:521–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Brys M, Fox MD, Agarwal S, et al. Multifocal repetitive TMS for motor and mood symptoms of Parkinson disease: a randomized trial. Neurology 2016;87:1907–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Flamez A, Cordenier A, de Raedt S, et al. Bilateral low frequency rTMS of the primary motor cortex may not be a suitable treatment for levodopa-induced dyskinesias in late stage Parkinson's disease. Parkinsonism Relat Disord 2016;22:54–61. [DOI] [PubMed] [Google Scholar]
- [35].Makkos A, Pal E, Aschermann Z, et al. High-frequency repetitive transcranial magnetic stimulation can improve depression in Parkinson's disease: a randomized, double-blind, placebo-controlled study. Neuropsychobiology 2016;73:169–77. [DOI] [PubMed] [Google Scholar]
- [36].Shin HW, Youn YC, Chung SJ, et al. Effect of high-frequency repetitive transcranial magnetic stimulation on major depressive disorder in patients with Parkinson's disease. J Neurol 2016;263:1442–8. [DOI] [PubMed] [Google Scholar]
- [37].Fricke C, Duesmann C, Woost TB, et al. Dual-site transcranial magnetic stimulation for the treatment of Parkinson's disease. Front Neurol 2019;10:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Fregni F, Santos CM, Myczkowski ML, et al. Repetitive transcranial magnetic stimulation is as effective as fluoxetine in the treatment of depression in patients with Parkinson's disease. J Neurol Neurosurg Psychiatry 2004;75:1171–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Boggio PS, Fregni F, Bermpohl F, et al. Effect of repetitive TMS and fluoxetine on cognitive function in patients with Parkinson's disease and concurrent depression. Mov Disord 2005;20:1178–84. [DOI] [PubMed] [Google Scholar]
- [40].Cardoso EF, Fregni F, Martins Maia F, et al. rTMS treatment for depression in Parkinson's disease increases BOLD responses in the left prefrontal cortex. Int J Neuropsychopharmacol 2008;11:173–83. [DOI] [PubMed] [Google Scholar]
- [41].Yang C, Guo Z, Peng H, et al. Repetitive transcranial magnetic stimulation therapy for motor recovery in Parkinson's disease: a meta-analysis 2018;8:e01132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Santos L, Opris I, Hampson R, et al. Functional dynamics of primate cortico-striatal networks during volitional movements. Front Syst Neurosci 2014;8:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Caligiore D, Mannella F, Arbib MA, et al. Dysfunctions of the basal ganglia-cerebellar-thalamo-cortical system produce motor tics in Tourette syndrome. PLoS Comput Biol 2017;13:e1005395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Hoorn AVD, Renken RJ, Leenders KL, et al. Parkinson-related changes of activation in visuomotor. Brain regions during perceived forward self-motion. PLoS One 2014;9:e95861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Cohen OS, Orlev Y, Yahalom G, et al. Repetitive deep transcranial magnetic stimulation for motor symptoms in Parkinson's disease: a feasibility study. Clin Neurol Neurosurg 2016;140:73–8. [DOI] [PubMed] [Google Scholar]
- [46].Randver R. Repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex to alleviate depression and cognitive impairment associated with Parkinson's disease: a review and clinical implications. J Neurol Sci 2018;393:88–99. [DOI] [PubMed] [Google Scholar]
- [47].Lefaucheur JP, Andre-Obadia N, Antal A, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol 2014;125:2150–206. [DOI] [PubMed] [Google Scholar]
- [48].Zhou L, Guo Z, Xing G, et al. Antidepressant effects of repetitive transcranial magnetic stimulation over prefrontal cortex of Parkinson's disease patients with depression: a meta-analysis. Front Psychiatry 2018;9:769. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


