Abstract
Bronchoscopy has a lower diagnostic yield for peripheral lung lesions (PLL). Endobronchial ultrasound guide sheath transbronchial lung biopsy (EBUS GS TBLB) has been used to overcome such limitation. Recent studies revealed that combined methods (e.g., EBUS GS TBLB plus electromagnetic navigation [EMN] or virtual bronchoscopic navigation [VBN]) further improve the diagnostic yield. However, those systems are associated with a high cost burden. Accordingly, we attempted to use VBN by computed tomography (CT) workstation (Aquarius iNtuition, TeraRecon) not dedicated only for VBN as an adjunctive tool for EBUS GS TBLB. We performed a prospective registry study to investigate whether VBN by CT workstation could improve the diagnostic yield of PLL.
Between February 2017 and February 2018, 128 patients with PLL were divided into 2 groups (VBN and non-VBN [NVBN]). In NVBN group (n = 64), EBUS GS TBLB was performed using a hand-drawn bronchial map based on CT images. VBN group (n = 64) underwent EBUS GS TBLB using VBN images.
VBN using CT workstation did not improve the diagnostic yield of EBUS GS TBLB for PLL (VBN vs NVBN, 72% vs 80%, P = .284). VBN slightly reduced procedure time (minute [mean ± SD], 25.31 ± 10.33 vs 25.81 ± 9.22), navigation time (time to find the lesion) (9.10 ± 7.88 vs 9.50 ± 7.14), and fluoroscopy time (2.23 ± 2.39 vs 2.86 ± 4.61), while these differences were not statistically significant.
The diagnostic yield of EBUS GS TBLB was not improved with VBN (compared with using a hand-drawn bronchial map). Although VBN slightly shortened the procedure-related times, which were not significantly different.
Keywords: bronchoscopy, computed tomography, image-guided biopsy, solitary pulmonary nodule, ultrasonography
1. Introduction
Peripheral lung lesions (PLL) could be caused by a variety of diseases.[1,2] For tissue diagnosis, multiple approaches can be utilized. If probability of malignancy is high, surgical resection may be a preferred option. However, if the probability of malignancy is indeterminate, transthoracic needle biopsy (TTNB) or bronchoscopy considered first.[3] Of the 2, TTNB is currently used more often because of its high diagnostic yield (about 90%).[4,5] However, TTNB-associated complications are relatively common (pneumothorax 15–20% and hemoptysis 1–5%).[3,6]
Although diagnostic yield of bronchoscopy is low (∼30%) for PLL,[3] it may be much improved (50–70%) by using recently developed bronchoscopic techniques (e.g., radial endobronchial ultrasound [EBUS], virtual bronchoscopic navigation [VBN], electromagnetic navigation [EMN]). According to recent studies,[7,8] combined approaches such as “EBUS” plus “EMN” or “EBUS” plus “VBN” further improved the diagnostic yield of PLL (70–90%) without compromising safety and also helped to shorten procedure time.
A challenge with these bronchoscopic approaches is medical expenses. While EBUS is available at a relatively low cost, VBN and EMN are expensive. For EBUS, the mini probe and driving unit for EBUS cost 7 million won (6,000 USD) and 25 million won (21,000 USD) in Korean currency, respectively. In contrast, 2 commercially available EMN systems (Veran and superDimension) cost over 200 million won (170,000 USD), and commercially available VBN systems (LungPoint and DirectPath) cost over 100 million won (85,000 USD).[9,10]
As an alternative and adaptive approach, we used a computed tomography (CT) workstation that was widely used in coronary artery reconstruction[11] and virtual colonoscopy.[12–14] The CT workstation is capable of reconstructing 3D images and could be used as a VBN producer. Two previous retrospective studies revealed that “VBN by CT workstation” improved diagnostic yield for PLL and decreased procedure time as well.[15,16] In addition, the CT workstation was offered as a default program, so no additional cost would be charged.[17] We used a high performance CT workstation running a software program called “Aquarius iNtuition Viewer” to generate VBN (Fig. 1). In the present study, endobronchial ultrasound guide sheath transbronchial lung biopsy (EBUS GS TBLB) was done to sample PLL with or without the assistance of VBN created using a CT workstation. We performed a prospective registry study to investigate whether VBN by CT workstation could improve the diagnostic yield of PLL (as a primary outcome). As secondary outcomes, we investigated EBUS GS TBLB procedure-related results such as procedure durations, PLL-identified bronchial generation, and EBUS detection rate. Finally, factors affecting diagnostic yields were also analyzed.
Figure 1.
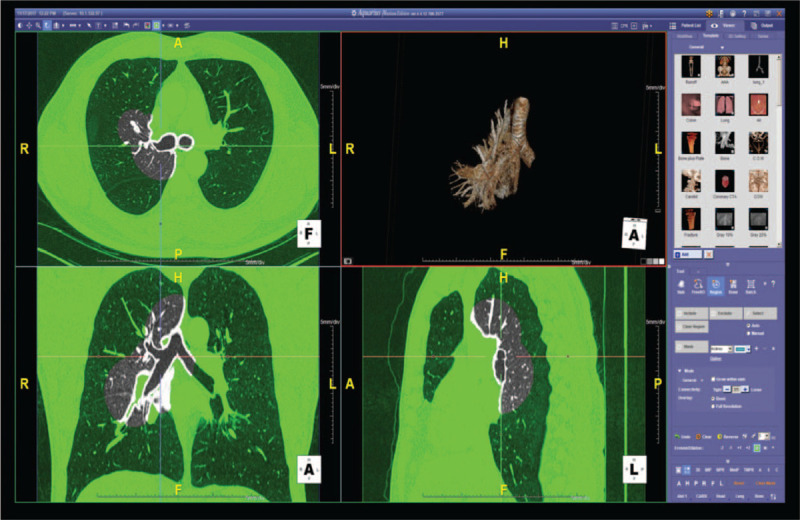
VBN image-creation process. Radiologist processes CT acquisition data to VBN images (the right upper area of the figure) with a high-performance workstation (Aquaris iNtuition, TeraRecon, Foster City, CA). CT = computed tomography, VBN = virtual bronchoscopic navigation.
2. Materials and methods
2.1. Patients: VBN group and non-VBN (NVBN) group
Between February 2017 and February 2018, adult (≥20 years) patients with PLL visible on chest CT scans who visited Ulsan University Hospital were enrolled in this study (Fig. 2). PLL were defined as lesions in outer half of the lungs[18] that are surrounded by normal lung parenchyma and connected by small bronchus (<5 mm in diameter) which is unlikely to be accessed by conventional bronchoscopy (outer diameter, 5.0–6.0 mm).[7,19] EBUS GS TBLB was conducted to those who agreed with the study, with 2 sessions per week, one with VBN (VBN group) and the other with hand-drawn bronchial maps (NVBN group). The exclusion criteria were as follows: evidence of endobrochial disease revealed by chest CT, percutaneous oxygen saturation <90%, severe comorbid conditions (i.e., unstable angina, acute myocardial infarction with the past 3 months, severe asthma, or uncontrolled pulmonary infection), pregnancy, and need for continuous anticoagulant or antiplatelet medication.[7,19] Eligible patients were prospectively registered, and all data were meticulously collected. Written informed consent was obtained from all patients and the study protocol was approved by the institutional review board (IRB) of Ulsan University Hospital (IRB number UUH-2017–01-013).
Figure 2.

Diagrammatic representation of the present study. VBN (n = 64) and NVBN groups (n = 64) were equally randomized. EBUS GS TBLB was conducted in all patients. Ten patients were excluded from diagnostic yield analysis because of the lack of final diagnosis: 4 (2 each from the VBN and NVBN groups) refused further work-up (TTNA or surgical biopsy) after failing EBUS GS TBLB (non-visualization on EBUS or failed TBLB), and 6 (VBN group [n = 5], NVBN group [n = 1]), who were diagnosed with non-malignant pathology using EBUS GS TBLB, were not followed-up. Of the remaining 118 patients, 90 (76%) had a positive diagnostic result via EBUS GS TBLB and the remaining 28 required a subsequent TTNA or surgical biopsy to establish final histological diagnosis. EBUS GS TBLB = endobronchial ultrasound guide-sheath transbronchial lung biopsy, NVBN = non-VBN, VBN = virtual bronchoscopic navigation.
2.2. Hand-drawn bronchial map and VBN images
All patients received CT scans prior to bronchoscopy to ensure high-quality CT raw images, enabling hand drawing of a bronchial map or converting into VBN images (256-MDCT scanner: Somatom Definition AS+ and Somatom Definition Flash, Siemens Healthcare). Images were reconstructed twice with slice thicknesses of 1.0 and 2.0 mm with a high spatial-frequency-reconstruction kernel (B40) without slice interval.
In NVBN group, EBUS GS TBLB was performed with a hand-drawn bronchial map based on the collected CT images (Fig. 3).[20,21] To create hand-drawn bronchial maps, we used Kurimoto's method.[20,21] Two experienced bronchoscopists (with 5 and 10 years’ experience of bronchoscopy [≥500 cases/year]) (SB and TL) drew their own maps, then the most improved one was created by discussion. The present study was initiated when bronchosopists were confident in the procedure (i.e., after >50 cases of EBUS GS TBLB).
Figure 3.

A representative example of the NVBN group: (A) PLL was located at right upper lobe apical segment on chest CT; (B) hand-drawn bronchial map generated following a detailed examination of chest CT; (C) EBUS visualization of probe within PLL. CT = computed tomography, NVBN = non-VBN; PLL = peripheral lung lesion, VBN = virtual bronchoscopic navigation.
VBN group underwent EBUS GS TBLB using VBN images (Fig. 4). The assistant changed the VBN images to the path leading to a PLL according to the actual bronchoscopy images. To make VBN images, 2 thoracic radiologists with 3 and 14 years’ experience (SL and W-JK) analyzed CT acquisition data and selected optimal endobronchial route(s) to reach the target PLL in consensus. Considering the distance from large airway and path complexity, they chose 1 or 2 (upto 3) routes for the target PLL that were short from large airway and not complex. After route(s) selection, post-processing to create VBN paths was performed by 1 of 2 radiologists by using a high-performance workstation running a software program called “Aquarius iNtuition” (TeraRecon, Foster City, CA).
Figure 4.

A representative example of the VBN group: (A) Left: PLL was located at the border between anterior and posterior segment of right upper lobe on chest CT, Right: VBN image of right upper lobe. (B) Practical implementation of VBN. The assistant (not visible in the picture) changes the VBN images of the path leading to a PLL according to the actual bronchoscopy images. CT = computed tomography, EBUS GS TBLB = endobronchial ultrasound guide-sheath transbronchial lung biopsy, PLL = peripheral lung lesion, VBN = virtual bronchoscopic navigation.
2.3. Endobronchial ultrasound guide sheath transbronchial lung biopsy (EBUS GS TBLB)
Two bronchoscopists (SB and TL) performed EBUS GS TBLB as previously described.[21–23] Firstly, a thin bronchoscope (outer diameter, 4.0–4.2 mm, BF-P260F or BF-P290, Olympus) was inserted as far into the bronchus nearest to the PLL as possible. Secondly, a radial EBUS probe (UM-S20–17S, Olympus) was inserted with GS (K-201, Olympus) through the working channel of bronchoscope; EBUS imaging confirmed that the probe reached the target lesion. Biopsy (TBLB) and brush were performed only when a lesion was confirmed by EBUS visualization (within or adjacent). Lastly, after the lesion was confirmed, the EBUS probe was removed leaving only the GS. Brush and biopsy forceps were introduced via the GS to obtain cytology and pathology samples. Following 1 brush, 2 biopsies were followed; this process was repeated ≥3 times until at least 4 biopsy specimens were obtained. After tissue acquisition, bronchoscopy was wedged for 2 to 5 minutes to confirm that there was no bleeding and the procedure was terminated. Most procedures were carried out with the help of fluoroscopy (69% [44/64] of VBN group and 73% [47/64] of NVBN group). All cases were performed with conscious sedation and under the guidance of anesthesiologists (SEP, IH, HK, and MA). A routine chest radiograph was done within 2 hours following the conclusion of the procedure.
2.4. Baseline data gathering and final diagnosis establishing
Baseline characteristics (i.e., age, sex, PLL size, PLL distance from the pleura, lobar location of PLL, bronchus sign [presence of an open bronchus connected from a proximal airway in the PLL], and nature of PLL on chest CT) of all enrolled patients were collected.[24] PLLs were classified into 3 types depending on the chest CT nature: a ground glass opacity, a mixed opacity, and a solid opacity. The ground glass opacity is defined as focal densities in which underlying lung morphology is preserved.[3] A mixed opacity was defined when a lesion contains both solid and ground glass opacities but ground glass opacity is >50%.[3]
Final diagnoses were established according to pathologic results. If not diagnosed by EBUS GS TBLB, further examination (TTNB or surgical resection) was performed. If the pathologic results revealed no malignancy, a lesion was classified as benign if the size stayed the same or decreased on a 12-month follow-up CT scan.[15,25]
2.5. Outcome variables
The primary outcome of the present study is diagnostic yield in VBN group and NVBN group, which is the fraction of people whose final diagnosis has been confirmed by EBUS GS TBLB.[7,8,26] Secondary outcomes related to the EBUS GS TBLB procedure were also investigated[7,26,27] (i.e., success rate of EBUS visualization [EBUS probe within or adjacent to PLL],[27] total procedure time [from the time when the bronchoscopy passes through the vocal cords until the end of the procedure], navigation time [until the lesion is identified by EBUS], endoscopically inserted PLL-identified bronchial generation [the carina was defined as first generation], fluoroscopy exposure time). Safety assessments, including the development of complication (bleeding/hemoptysis, pneumothorax) were also monitored and recorded.
2.6. Statistical analysis
Statistical analyses were performed using SPSS 21 (IBM). Continuous variables are expressed as mean ± standard deviation (SD) or median with range, and categorical variables are presented as percentages. The independent t test (for continuous variables) and chi-square test (for dichotomous variables) were used to identify any potential associations. Diagnostic yields were analyzed using the chi-square test. To identify factors that may affect diagnosis yield, univariate and multivariate analyses were performed. Multivariate logistic regression analyses were performed using variables found to be significant (i.e., P < .05) in the univariate analysis (diagnosis [benign vs malignancy]; EBUS visualization type [within vs adjacent to]; bronchus sign on CT [yes vs no]), basic demographic variables (age; sex), and those reported to be associated diagnostic yield in previous studies (lesion size [≥20 mm vs <20 mm][28]; chest CT nature [non-solid vs solid][17]; use of VBN[15,16]). A P-value <.05 was considered statistically significant in all analyses.
3. Results
A total of 128 patients with PLL were registered into 2 groups (VBN [n = 64] and NVBN group [n = 64]). Age, sex, lesion size, distance from pleura, location, nature of PLL, and bronchus sign were not different between the groups (Table 1). EBUS GS TBLB was endeavored in all patients. In 4 patients (2 in VBN group and 2 in NVBN group), EBUS GS TBLB failed (non-visualization on EBUS or failed TBLB) but they refused further work-up (TTNA or surgical biopsy). Six patients (5 in VBN group, 1 in NVBN group), identified as having non-malignant pathology by EBUS GS TBLB, were not followed-up. Thus these 10 patients were excluded from diagnostic yield analysis because of the lack of final diagnosis (Fig. 2). Of the remaining 118 patients, 90 (76%) received a positive diagnostic result via EBUS GS TBLB; the remaining 28 required a subsequent TTNA or surgical biopsy to establish final histological diagnosis.
Table 1.
Clinical characteristics and final diagnosis.

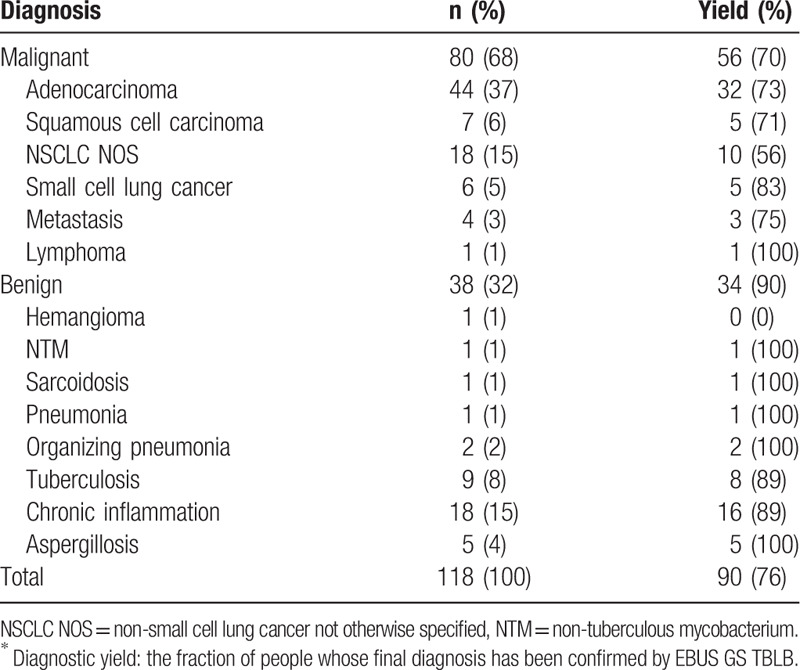
EBUS GS TBLB procedure-related results (secondary outcomes) are shown at Table 2. Although VBN slightly reduced total procedure time (minutes [mean ± SD], 25.31 ± 10.33 vs 25.81 ± 9.22), navigation time (9.10 ± 7.88 vs 9.50 ± 7.14), and fluoroscopy time (2.23 ± 2.39 vs 2.86 ± 4.61), which were not statistically significant. There was no difference in endoscopically inserted bronchial generation whether using VBN or not (median [range]: VBN group, 7 [4–10]; NVBN group 6 [4–12]; P = .250). Even when VBN was used, there was no improvement of EBUS detection rate (non-visualization/within/adjacent to, n [%]: VBN group, 1 [2]/44 [68]/19 [30]; NVBN group, 2 [3]/42 [66]/20 [31]; P = .559). No severe adverse events (e.g., massive bleeding, large pneumothorax) were identified during or after the procedure. A small amount of blood-tinged sputum was observed in 1 patient from the NVBN group, however, it disappeared without any treatment. Three cases (VBN [n = 2]; NVBN [n = 1]) of minimal pneumothorax were observed; all improved without chest tube insertion.
Table 2.
Procedure-related outcomes: VBN group versus NVBN group.
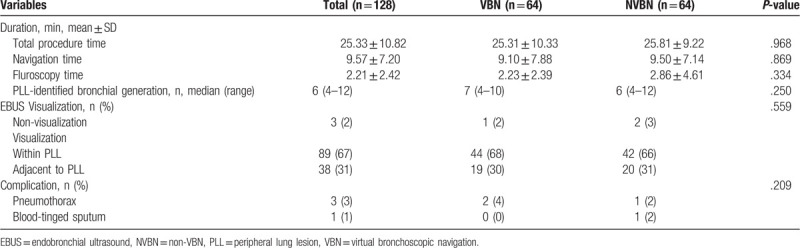
The final histologic diagnoses (n = 118) are shown in Table 3 along with the associated EBUS GS TBLB diagnostic yields: 80 (68%) were malignant (adenocarcinoma 37%, squamous cell carcinoma 6%, non-small cell lung cancer not otherwise specified [NSCLC NOS] 15%, small cell carcinoma 5%, and other malignancies 4%); 38 (32%) were benign (chronic inflammation 15%, tuberculosis 8%, aspergillosis 4%, organizing pneumonia 2%, and other benign lesions 4%). Of the initial 118 patients, 90 were diagnosed using EBUS GS TBLB (diagnostic yield, 76%). Benign lesions were associated with a higher diagnostic yield than malignant lesions (benign, 34/38 [90%]; malignant, 56/80 [70%]).
Table 3.
Diagnostic yields∗: all patients according to histology.
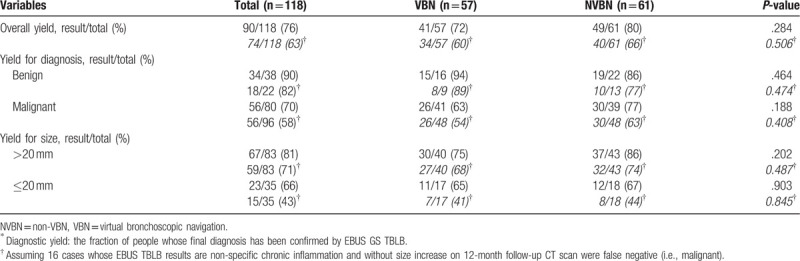
3.1. Diagnostic yield of EBUS GS TBLB in VBN group and NVBN group (primary outcome)
The use of VBN had no impact on diagnostic yield as the overall diagnostic yield was not statistically different between the VBN and NVBN groups (results/total [%]: VBN group, 41/57 [72]; NVBN group 49/61 [80]; P = .284). There was also no between-group difference in diagnostic yield according to final diagnosis (benign/malignant) or size (>20 mm vs ≤20 mm). In addition, further diagnostic yield analysis, assuming 16 cases whose EBUS TBLB results are non-specific chronic inflammation and without size increase on 12-month follow-up CT scan were false negative (i.e., malignant), also showed no between group differences (Table 4).
Table 4.
Diagnostic yields∗: VBN group versus NVBN group.
3.2. Factors affecting diagnostic yields
Multivariate analysis revealed that within-type EBUS visualization (adjusted OR [aOR] 6.981, 95% confidence interval [CI] 2.119–22.995, P = .001), and benign lesion (aOR 9.574, 95% CI 2.260–40.559, P = .002) were independent factors affecting high diagnostic yield. The use of VBN was not associated with diagnostic yield in either univariate or multivariate analyses (Table 5).
Table 5.
Factors affecting diagnostic yields (n = 118).

4. Discussion
The present study reveals that VBN (using CT workstation) did not improve the diagnostic yield of EBUS GS TBLB for PLL compared with EBUS GS TBLB without VBN. A hand-drawn bronchial map generated by an experienced practitioner following a CT preview prior to the EBUS GS TBLB yielded diagnostic results similar to EBUS GS TBLB plus VBN. In terms of the procedure time, VBN slightly shortened the time, but did not show a statistically significant difference. In addition, we confirmed that EBUS visualization of probe within a PLL is the most important factor in predicting the diagnostic success of EBUS GS TBLB. Furthermore, this study revealed that the diagnostic yield of EBUS GS TBLB for benign lesions was considerably high. If a benign pathology is present in a properly conducted EBUS GS TBLB (e.g., EBUS visualization of probe within PLL), the result is believed to be reliable.
Reports have suggested that with the help of VBN or EMN, the diagnostic yield of EBUS GS TBLB may increase (from 50–70% to 70–90%).[3,7,8] In the present study, we adopted VBN as an adjunctive tool of EBUS GS TBLB. The difference from previous studies is the process of obtaining VBN. We reconstructed the VBN using a CT workstation running a software program called “Aquarius iNtuition” (TeraRecon). The Aquarius iNtuition CT workstation requires manipulation by the radiologist, while the existing commercial VBNs (LungPoint and DirectPath) are software that reconstruct the CT image into VBN without the help of a radiologist. Through a retrospective analysis, Matsumoto et al[15] recently reported that VBN using a CT workstation helps improve the diagnostic yield of EBUS GS TBLB. We performed a prospective study and divided enrolled patients into 2 groups (VBN and NVBN) to determine whether the VBN using the CT workstation could improve the diagnostic yield of the EBUS GS TBLB.
Our results reveal that VBN did not improve diagnostic yield. Additionally, VBN was not helpful in EBUS visualization or endoscopically inserted bronchial generation. We note that these results are in conflict with previously reported studies.[7,15] In fact, small (<2 mm) bronchi near the PLL are often unclear in chest CT, and in this case they are not distinguishable in VBN as well.
In the present study, VBN reduced procedure time, navigation time (time to find the lesion), and fluoroscopy time. These are consistent findings with previous studies although ours were not statistically significant. Ishida et al[7] showed that the use of VBN (dedicated for EBUS GS TBLB) could help shorten the examination time in their prospective study. Matsumoto et al[15] showed that VBN (using CT workstation not designed exclusively for VBN) helped to shorten examination time in their retrospective study. Our conclusion is that VBN using CT workstation might be helpful in shortening examination time, but it does not help to improve the diagnostic yield.
To create hand-drawn bronchial maps, we used Kurimoto's method,[20,21] who first introduced the EBUS GS TBLB.[29] The present study was initiated when we were confident in the procedure (i.e., after >50 cases of EBUS GS TBLB). Casutt et al[30] reported that a stable diagnostic yield of EBUS TBLB was established before experience of 11 cases. And Eom et al[31] also advocated that the accuracy of EBUS GS TBLB does not greatly differ between beginners and experts. Therefore it is thought that supervised training of >10 cases is enough to generate an accurate hand-drawn map. Typically, we could draw a bronchial map for the procedure in 10 to 20 minutes. In the future, if CT image quality continues to improve and artificial intelligence can be used to make VBN (or EMN), VBN (or EMN) will be able to create a very small bronchus (e.g., <2 mm, which is unclear in current chest CT as well as current VBN) into a virtual image. If so, the diagnostic yield and procedure time will likely be improved further.
We further investigated—using multivariate analysis—factors that may affect the diagnostic yield of EBUS GS TBLB. As a result, we found that EBUS visualization of probe within PLL and benign pathology independently correlated with improved diagnostic yield. EBUS visualization of probe within PLL is the most important factor in predicting diagnostic potential of EBUS GS TBLB. We note that this is aligned with previous studies.[17,27]
In the present study, benign, but not malignant lesions, were independently associated with high diagnostic yield. It was the only factor outside of EBUS-mediated visualization of probe within PLL that was associated with high diagnostic yield in multivariate analysis. In previous studies, however, malignant lesions were associated with a higher diagnostic rate than benign lesions.[8,26,27] Therefore, we were cautious in interpreting our result as follows: if a benign pathology is present in properly conducted EBUS GS TBLB (such as EBUS visualization of probe within PLL), the result is believed to be reliable. Among the benign lesions of our study, tuberculosis, aspergillosis, and organizing pneumonia were associated with a high diagnostic rate. In previous studies, the diagnosis rate of tuberculosis was consistently high; other benign lesions were associated with inconsistent diagnostic rates.[8,27]
In our study, pneumothorax occurred in 3% and hemoptysis occurred in 1%. Both were improved without special treatment. We confirmed once again that EBUS GS TBLB is very safe.[3,6–8]
The present study has some limitations. First, the CT workstation we used to generate the VBN was not designed exclusively for VBN. Therefore, manipulation of the radiologist was required for chest CT image reconstruction as VBN, and unintended errors might occur in this process. Second, the definition of a benign lesion can be obscure. In the present study, if the initial pathologic results revealed no malignancy, lesions that were the same size or smaller at a follow-up CT scan at 12 months were classified as benign. Since 12 months is a relatively short period of time to exclude a slow-growing malignancy, there could be false negative malignant lesions and errors in diagnostic yields. Because of the concerns, we showed further analysis results assuming that 16 benign cases by CT follow-up as malignant. Third, we did not perform needle aspiration. Addition of transbronchial needle aspiration could increase the diagnostic yield of PLL, especially if the EBUS probe is adjacent to (but not within) the PLL.[32,33] However, there is not a currently available needle that pass through the GS (K-201, Olympus) we used. In order to use a needle, we need to remove the GS or use a larger GS (K-203), which requires a thicker bronchoscope, which makes difficult to access the PLL with the bronchoscope. Lastly, this is a single center and a non-randomized study. The results could be more robust with a multicenter, randomized controlled design, however in reality it is difficult to implement. We conducted a prospective registry study, which is a realistically feasible method, and we think the results are kind of reliable.
In conclusion, through a prospective registry study, we found that the use of VBN using CT workstation did not improve the diagnostic yield of EBUS GS TBLB for PLL. Even without VBN, diagnostic yield of EBUS GS TBLB could be equivalent to the use of VBN, assuming the hand-drawn bronchial map is well-established (by sufficient CT preview of an experienced bronchoscopist). In terms of the procedure time, VBN slightly shortened the time, but did not show a statistically significant difference.
Author contributions
Conceptualization: Woon-Jung Kwon, Taehoon Lee.
Data curation: Soohyun Bae, Jong Joon Ahn, Yangjin Jegal, Kwang Won Seo, Seung Won Ra, Byung Ju Kang, Jin Hyoung Kim, Soon Eun Park, Ilsang Han, Hojun Kang, Mingi An, Taehoon Lee.
Formal analysis: Soyeoun Lim, Minsu Ock, Eun Ji Park, Woon-Jung Kwon, Taehoon Lee.
Funding acquisition: Taehoon Lee.
Methodology: Minsu Ock, Eun Ji Park, Woon-Jung Kwon, Taehoon Lee.
Supervision: Soon Eun Park, Jong Joon Ahn, Yangjin Jegal, Kwang Won Seo, Seung Won Ra, Byung Ju Kang.
Validation: Soohyun Bae, Soyeoun Lim, Soon Eun Park, Ilsang Han, Hojun Kang, Mingi An.
Writing – original draft: Soohyun Bae, Soyeoun Lim.
Writing – review & editing: Woon-Jung Kwon, Taehoon Lee.
Footnotes
Abbreviations: CT = computed tomography, EBUS = endobronchial ultrasound, EBUS GS TBLB = endobronchial ultrasound guide sheath transbronchial lung biopsy, EMN = electromagnetic navigation, GS = guide sheath, IRB = institutional review board, NVBN = non-VBN (virtual bronchoscopic navigation), PLL = peripheral lung lesions, TBLB = transbronchial lung biopsy, TTNB = transthoracic needle biopsy, UUH = Ulsan University Hospital, VBN = virtual bronchoscopic navigation.
How to cite this article: Bae S, Lim S, Ahn JJ, Jegal Y, Seo KW, Ra SW, Kang BJ, Kim JH, Park SE, Han I, Kang H, An M, Ock M, Park EJ, Kwon WJ, Lee T. Diagnosing peripheral lung lesions using endobronchial ultrasonography with guide sheath: a prospective registry study to assess the effect of virtual bronchoscopic navigation using a computed tomography workstation. Medicine. 2020;99:17(e19870).
SB and SL have equally contributed to the first authors.
This study has received funding by Ulsan University Hospital (Biomedical Research Center Promotion Fund, UUH-2016-01) and the National Research Foundation of Korea (NRF-2017R1C1B5076493).
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Swensen SJ, Silverstein MD, Edell ES, et al. Solitary pulmonary nodules: clinical prediction model versus physicians. Mayo Clin Proc 1999;74:319–29. [DOI] [PubMed] [Google Scholar]
- [2].Khouri NF, Meziane MA, Zerhouni EA, et al. The solitary pulmonary nodule. Assessment, diagnosis, and management. Chest 1987;91:128–33. [DOI] [PubMed] [Google Scholar]
- [3].Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e93S–120S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e142S–65S. [DOI] [PubMed] [Google Scholar]
- [5].Asano F, Eberhardt R, Herth FJ. Virtual bronchoscopic navigation for peripheral pulmonary lesions. Respiration 2014;88:430–40. [DOI] [PubMed] [Google Scholar]
- [6].Wiener RS, Schwartz LM, Woloshin S, et al. Population-based risk for complications after transthoracic needle lung biopsy of a pulmonary nodule: an analysis of discharge records. Ann Intern Med 2011;155:137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ishida T, Asano F, Yamazaki K, et al. Virtual bronchoscopic navigation combined with endobronchial ultrasound to diagnose small peripheral pulmonary lesions: a randomised trial. Thorax 2011;66:1072–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Eberhardt R, Anantham D, Ernst A, et al. Multimodality bronchoscopic diagnosis of peripheral lung lesions: a randomized controlled trial. Am J Respir Crit Care Med 2007;176:36–41. [DOI] [PubMed] [Google Scholar]
- [9].Shepherd RW. Bronchoscopic pursuit of the peripheral pulmonary lesion: navigational bronchoscopy, radial endobronchial ultrasound, and ultrathin bronchoscopy. Curr Opin Pulm Med 2016;22:257–64. [DOI] [PubMed] [Google Scholar]
- [10].Eberhardt R, Morgan RK, Ernst A, et al. Comparison of suction catheter versus forceps biopsy for sampling of solitary pulmonary nodules guided by electromagnetic navigational bronchoscopy. Respiration 2010;79:54–60. [DOI] [PubMed] [Google Scholar]
- [11].Becker A, Leber A, White CW, et al. Multislice computed tomography for determination of coronary artery disease in a symptomatic patient population. Int J Cardiovasc Imaging 2007;23:361–7. [DOI] [PubMed] [Google Scholar]
- [12].Pickhardt PJ, Hassan C, Halligan S, et al. Colorectal cancer: CT colonography and colonoscopy for detection--systematic review and meta-analysis. Radiology 2011;259:393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Johnson CD, Chen MH, Toledano AY, et al. Accuracy of CT colonography for detection of large adenomas and cancers. N Engl J Med 2008;359:1207–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Horton KM, Horton MR, Fishman EK. Advanced visualization of airways with 64-MDCT: 3D mapping and virtual bronchoscopy. AJR Am J Roentgenol 2007;189:1387–96. [DOI] [PubMed] [Google Scholar]
- [15].Matsumoto Y, Izumo T, Sasada S, et al. Diagnostic utility of endobronchial ultrasound with a guide sheath under the computed tomography workstation (ziostation) for small peripheral pulmonary lesions. Clin Respir J 2017;11:185–92. [DOI] [PubMed] [Google Scholar]
- [16].Iwano S, Imaizumi K, Okada T, et al. Virtual bronchoscopy-guided transbronchial biopsy for aiding the diagnosis of peripheral lung cancer. Eur J Radiol 2011;79:155–9. [DOI] [PubMed] [Google Scholar]
- [17].Okachi S, Imai N, Imaizumi K, et al. Factors affecting the diagnostic yield of transbronchial biopsy using endobronchial ultrasonography with a guide sheath in peripheral lung cancer. Intern Med 2016;55:1705–12. [DOI] [PubMed] [Google Scholar]
- [18].Santambrogio L, Nosotti M, Bellaviti N, et al. CT-guided fine-needle aspiration cytology of solitary pulmonary nodules: a prospective, randomized study of immediate cytologic evaluation. Chest 1997;112:423–5. [DOI] [PubMed] [Google Scholar]
- [19].Asano F, Shinagawa N, Ishida T, et al. Virtual bronchoscopic navigation combined with ultrathin bronchoscopy. A randomized clinical trial. Am J Respir Crit Care Med 2013;188:327–33. [DOI] [PubMed] [Google Scholar]
- [20].Kurimoto N, Isobe T, Miyazawa T, et al. Endobronchial ultrasonography for peripheral pulmonary lesions. Ultrasound Med Bio 2017;43:S30–1. [Google Scholar]
- [21].Kurimoto N. SC19.01 diagnosis of lung cancer: multimodal devices for peripheral pulmonary lesions. J Thorac Oncol 2017;12:S120–1. [Google Scholar]
- [22].Katsurada M, Izumo T, Nagai Y, et al. The dose and risk factors for radiation exposure to medical staff during endobronchial ultrasonography with a guide sheath for peripheral pulmonary lesions under X-ray fluoroscopy. Jpn J Clin Oncol 2014;44:257–62. [DOI] [PubMed] [Google Scholar]
- [23].Izumo T, Sasada S, Chavez C, et al. The diagnostic utility of endobronchial ultrasonography with a guide sheath and tomosynthesis images for ground glass opacity pulmonary lesions. J Thorac Dis 2013;5:745–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gaeta M, Pandolfo I, Volta S, et al. Bronchus sign on CT in peripheral carcinoma of the lung: value in predicting results of transbronchial biopsy. AJR Am J Roentgenol 1991;157:1181–5. [DOI] [PubMed] [Google Scholar]
- [25].Maekura T, Sugimoto C, Tamiya A, et al. Combination of virtual bronchoscopic navigation, endobronchial ultrasound, and rapid on-site evaluation for diagnosing small peripheral pulmonary lesions: a prospective phase II study. J Thorac Dis 2017;9:1930–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tamiya M, Okamoto N, Sasada S, et al. Diagnostic yield of combined bronchoscopy and endobronchial ultrasonography, under LungPoint guidance for small peripheral pulmonary lesions. Respirology 2013;18:834–9. [DOI] [PubMed] [Google Scholar]
- [27].Yamada N, Yamazaki K, Kurimoto N, et al. Factors related to diagnostic yield of transbronchial biopsy using endobronchial ultrasonography with a guide sheath in small peripheral pulmonary lesions. Chest 2007;132:603–8. [DOI] [PubMed] [Google Scholar]
- [28].Ali MS, Trick W, Mba BI, et al. Radial endobronchial ultrasound for the diagnosis of peripheral pulmonary lesions: a systematic review and meta-analysis. Respirology 2017;22:443–53. [DOI] [PubMed] [Google Scholar]
- [29].Kurimoto N, Miyazawa T, Okimasa S, et al. Endobronchial ultrasonography using a guide sheath increases the ability to diagnose peripheral pulmonary lesions endoscopically. Chest 2004;126:959–65. [DOI] [PubMed] [Google Scholar]
- [30].Casutt A, Prella M, Beigelman-Aubry C, et al. Fluoroscopic-guided radial endobronchial ultrasound without guide sheath for peripheral pulmonary lesions: a safe and efficient combination. Arch Bronconeumol 2015;51:338–43. [DOI] [PubMed] [Google Scholar]
- [31].Eom JS, Mok JH, Kim I, et al. Radial probe endobronchial ultrasound using a guide sheath for peripheral lung lesions in beginners. BMC Pulm Med 2018;18:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hayama M, Izumo T, Chavez C, et al. Additional transbronchial needle aspiration through a guide sheath for peripheral pulmonary lesions that cannot be detected by radial EBUS. Clin Respir J 2017;11:757–64. [DOI] [PubMed] [Google Scholar]
- [33].Chao TY, Chien MT, Lie CH, et al. Endobronchial ultrasonography-guided transbronchial needle aspiration increases the diagnostic yield of peripheral pulmonary lesions: a randomized trial. Chest 2009;136:229–36. [DOI] [PubMed] [Google Scholar]


