Abstract
Bats have populated earth for approximately 52 million years, serving as natural reservoirs for a variety of viruses through the course of evolution. Transmission of highly pathogenic viruses from bats has been suspected or linked to a spectrum of potential emerging infectious diseases in humans and animals worldwide. Examples of such viruses include Marburg, Ebolavirus, Nipah, Hendra, Influenza A, Dengue, Equine Encephalitis viruses, Lyssaviruses, Madariaga and Coronaviruses, involving the now pandemic Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Herein, we provide a narrative review focused in selected emerging viral infectious diseases that have been reported from bats.
Keywords: Bats, Viruses, Evolution, Anthropocene, Transmission, Cross-Species
Introduction
Over the last decades of the Anthropocene, the role of wild animals and their ecosystems in the emergence and expansion of infectious diseases has public health's attention. Bats are especially important as they have been potentially related to the current evere cute espiratory yndrome oronavirus 2 (SARS-CoV-2) pandemic, the previous emergence of Middle East espiratory yndrome oronavirus (MERS-CoV) in 2012, and the SARS-CoV epidemic in 2002 (Ahmad et al., 2020, Biscayart et al., 2020, Bonilla-Aldana et al., 2020a, Gutiérrez et al., 2020, Tiwari et al., 2020, Bonilla-Aldana et al., 2020b).
Bats have populated earth for approximately 52 million years, serving as natural reservoirs for multiple viruses through the course of their existence (Simmons et al., 2008, Han et al., 2015). The evolution of their physical, physiological and behavioral characteristics has allowed them to expand to all continents except Antarctica, with ecological niches located in urban or rural areas, and especially in caves, mines and some types of foliage (Cascio et al., 2011). Evolutionary changes have also determined their current eating patterns and their role within the ecosystem (Zubaid et al., 2005). Herein, we provided a narrative review focused in selected emerging viral infectious diseases that have been reported from bats.
General characteristics of bats
Bats are the only mammals capable of flight; they have nocturnal habits during which they either feed or mate and a layer of short fur that protects them from humidity and cold temperatures (Kunz et al., 2011). Bats belong to the order Chiroptera (wings on the upper extremities), with over 1400 species subclassified according two main used systems (Simmons and Cirranello, 2020), one of this, into two suborders: the Megachiroptera and Microchiroptera, e.g. used by the NCBI-NIH Taxonomy Browser (https://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi); and the other classification subdivided into Yinpterochiroptera and Yangochiroptera (Teeling et al., 2018). Such classifications allow a better understanding of some of their behavioral patterns and their association with viruses (Han et al., 2015, Hayman et al., 2013, Hu et al., 2015). Additionally, some other researchers use the terms Pteropodiformes and Vespertilioniformes, basing the names on the oldest valid genus description in each group, Pteropus and Vespertilio (Hutcheon and Kirsch, 2006). Nevertheless, these changing discussions on bats taxonomy and phylogenetics are the focus of some reviews and recent studies on that (Jebb et al., 2020).
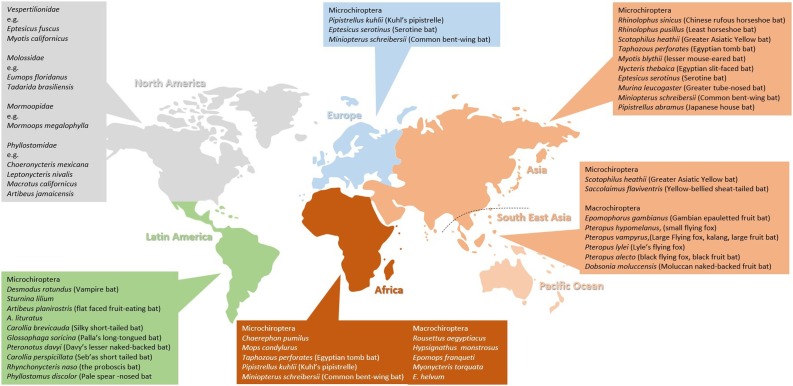
Bats from the Megachiroptera suborder, commonly known as megabats, account for approximately 170 species mainly located in Asia, Africa and Oceania or Pacific region (Figure 1 ); their size can vary between 40 and 150 cm with their wings spread, and they weigh 1 kg on average. Megabats feed exclusively on fruit, seeds and pollen and their main habitats include caves, mines, trees and some buildings. Megachiroptera bats cannot echo localize (Han et al., 2015). Some species of Pteropodidae, formerly representing “Megachiroptera”, may use lingual echolocation (Yovel et al., 2011). “Microbats”, formerly representing “Microchiroptera”, do not eat mainly flowers and fruit but insects. Furthermore, many bats of both suborders undergo considerable migrations (Fleming, 2019).
Figure 1.
Geographic distribution of bats associated with human disease.
The Microchiroptera or microbats include over 930 species distributed throughout the entire planet with the exception of some islands and the poles. Their size ranges from 4 to 16 cm and feed mostly on flowers and fruit. They possess an echolocation system that allows hematophagous bats to search and capture birds and mammals. Their primary habitats include forests and tropical areas although they are also capable of coexisting with humans in some urban settings (Han et al., 2015, Hayman et al., 2013, Hu et al., 2015).
Another notable feature is that microbats can travel long distances of up to 2000 km during migratory season to fulfill their nutritional needs, which is relevant to understand local and intercontinental spread of colonies and coexistence with other animals of the same species (Neves et al., 2020, Han et al., 2015, Muscarella and Fleming, 2007).
Characteristics of bats that allow transmission of pathogens
Bats that coexist within the same geographical area often host common microorganisms. The importance of cross‐species transmission events in seasonally changing communities may vary with respect to pathogen or variants of a pathogen, but is not really clear for any system. In some cases, infection cycles may be maintained in co‐ roosting species without cross species transmission (Kunz et al., 2011, Hayman et al., 2013). Transmission rates depend on contact rate and susceptibility to infections of a specific population (Hayman et al., 2013).
Some authors indicated that evolutionary processes granted bats with skills to facilitate air maneuvering. They have an efficient immune response, making bats asymptomatic carriers for a long list of viruses (Han et al., 2015). Bats are mammals, bone marrow (Zhou et al., 2016) and B cells (Banerjee et al., 2020). Additionally, several species are facultative heterotherms capable of entering profound lethargy during periods of physiological stress to compensate for energy and water deficits, favoring viral persistence (Hayman et al., 2013).
Continuous physical contact within bats of the same colony facilitates viral circulation, especially during breeding and migration seasons (Drexler et al., 2012). The proposed mechanisms of viral transmission between microbats is aerosol release produced by larynx vibrations that occur during echolocation in addition to close contact with other types of secretions such as fecal matter and urine (Calisher et al., 2006). Then, bats carry multiple emerging and reemerging pathogens, especially viral threats (Table 1 ).
Table 1.
Bat species carrying different emerging and reemerging viruses.
| Virus | Bats |
|---|---|
| Marburg | Rousettus aegyptiacus |
| Ebolavirus | Hypsignathus monstrosus, Epomops franqueti, Myonycteris torquata.; Chaerephon pumilus, Mops condylurus; E. helvum; Epomophorus gambianus, Nanonycteris veldkampii, Epomops buettikoferi |
| Nipah | Pteropus hypomelanus, Pteropus vampyrus, Pteropus lylei |
| Influenza A | Sturnina lilium, Artibeus planirostris, A. lituratus, Artibeus jamaicensis,Rousettus aegyptiacus |
| SARS | Rhinolophus sinicus, Hipposideros, Chaerophon, Rhinolophus pusillus, R. pearsoni, R. macrotis, R. ferrumequinum, Pteropus alecto, Dobsonia moluccensis, Scotophilus heathii |
| MERS | Taphozous perforatus, Rhinopoma hardwickii, Pipistrellus kuhlii |
| Hendra | Pteropus alecto, P. conspicillatus |
| Lyssavirus | Desmodus rotundus, Myotis blythii, Pteropus spp, Saccolaimus flaviventris, Myotis nattereri, Nycteris thebaica, Eptesicus serotinus, Myotis daubentonii, Myotis dasycneme, Pteropus medius, Murina leucogaster, Myotis mystacinus, Myotis brandtii, Epomophorus wahlbergi, Rousettus aegyptiacus, Eidolon helvum, Miniopterus schreibersii, Hipposideros vittatus, Miniopterus schreibersii, Pipistrellus abramus |
| Rabies | Desmodus rotundus, Diaemus youngi, Diphylla ecaudata |
| Dengue | Desmodus rotundus, Artibeus jamaicensis, Carollia brevicauda, Myotis nigricans, Glossophaga soricina, Artibeus literatus, Artibeus planirostris, Carollia perspicillata, Myotis lucifugus, Artibeus intermedius, Molossus sinaloae, Molossus pretiosus, Rhogeessa bickhami, Molossus rufus, Eumops glaucinus, Myotis nigricans, Pteronotus parnellii, Natalus stramineus, Artibejus jamaicensis, Artibeus spp., Uroderma spp., Molossus spp., Chaerephon pumilus, Mops condylurus, Anoura geoffroyi, Artibeus cinereus, Artibeus literatus, Carollia perspicillata, Molossus ater, Molossus molossus, Phyllostomus hastatus, Pteronotus davyi, Pteronotus parnellii, Sturnira spp., Pteropus gouldii, Pteropus giganteus, Glossophaga soricina, Artibeus intermedius, Molossus sinaloae, Rhogeessa io, Molossus pretiosus, Balantiopteryx plicata, Molossus rufus, Rhogeessa bickhami, Epomophorus labiatus. |
| Equine Encephalitis | Artibeus intermedius, Artibeus jamaicensis, Artibeus literatus, Artibeus turpis, Glossophaga soricina, Rhynchonycteris naso, Sturnira lilium, Carollia perspicillata, Phyllostomus hastatus, Vampyrops helleri, Eptesicus fuscus, Myotis lucifugus, Myotis keenii |
| Madariaga | Carollia castanea, Phyllostomus discolor, Carollia perspicillata, Phyllostomus hastatus |
Role of bats in the origin of emerging and re-emerging infectious diseases
Studies from the EcoHealth Alliance suggest that bats are possible carriers of emerging infectious agents that can potentially affect other mammals, including humans (Anderson et al., 2019, Morens et al., 2020, Olival et al., 2020). There are unique features of bats in relation to infectious diseases. Bats have several characteristics that combine to make them uniquely qualified to serve as viral hosts (Beltz, 2018).
Coronaviruses
Coronaviruses (CoV) belong to the family Coronaviridae, within the order Nidovirales. CoVs are enveloped, positive-strained RNA viruses that can infect mammals. In humans, CoVs generally cause mild upper respiratory tract symptoms. However, the clinical course can escalate from mild influenza-like presentations to severe pneumonia and even death (Ahmad et al., 2020, Tiwari et al., 2020).
In the 21 st century, oronaviruses have caused multiple outbreaks throughout the world, sometimes reaching epidemic proportions: SARS-CoV originated from China in late 2002, affecting 8000 people and killing 800; MERS-CoV outbreak in 2012 affected less than 2500 people but its mortality was higher, with 850 diseased; and the world is currently facing the oronavirus disease 2019 (COVID-19) pandemic caused by SARS-CoV-2 (Rodriguez-Morales et al., 2020), probably from the wet markets of Wuhan, China, with a total death toll of 1,341,360 people as of November 18, 2020. In these markets the trading of wild animals for human consumption is common, and the continuous contact with animal body fluids favors zoonotic transmission of diseases to human beings (Bonilla-Aldana et al., 2020c).
Although the precise reservoir of SARS-CoV-2 has not been established, one of the hypothesis is that bats of the genus Rhinolophus would be linked. Chinese studies have reported that SARS-CoV-2 is very similar to other coronaviruses naturally found in bats (Latinne et al., 2020, Lau et al., 2020, Morrison et al., 2020); however, these viruses are constantly evolving and mutating, making it difficult to pinpoint an exact reservoir (Figure 1). A recent study showed that the SARS-CoV-2 is probably a novel recombinant virus. Its genome is closest to that of SARS–related coronaviruses from horseshoe bats, and its receptor-binding domain is closest to that of pangolin viruses. Its origin and direct ancestral viruses have not been identified (Lau et al., 2020). SARS-CoV-2 showed high genome sequence identities (87.6%–87.8%) to SARSr-Rp-BatCoV-ZXC21/ZC45, detected in Rhinolophus pusillus bats from Zhoushan, China, during 2015 (Hu et al., 2018). Spillover events are largely the result of human activities and anthropogenic changes in the environment, where wildlife species are certainly not responsible of this emergence. In this setting, is important to providing correct bat species identifications in virological studies (Salazar-Bravo et al., 2006). All discovered coronaviruses that are causing illness for humans were originating from animals. Generally, these animals were either rodents or bats (Swelum et al., 2020). Currently, for the great majority of coronaviruses found in bats we only have sequences of conserved genome regions, useful to construct phylogenies, but there is still much to learn about their reservoirs, viral transmission, and the process leading to spillover to humans.
Since there is no effective treatment or vaccine for COVID-19 to date, strong regulationsincluding isolation, quarantine and social distancinghave been established by many countries in an effort to reduce expansion of the disease given the high person-to-person transmissibility of SARS-CoV-2, either directly by respiratory droplets with infective particles or indirectly by fluid-contaminated objects.
A study in Indonesia identified CoV genes in bareback fruit bats, where partial RNA-dependent RNA polymerase (RdRp) sequences and regions between helicase and RdRp genes were detected and amplified in feces and tissue samples (Anindita et al., 2015). Another study conducted in Zhoushan City, Zhejiang Province, found that out of 334 bats sampled, approximately 26% were naturally infected with oronaviruses (Hu et al., 2018). Recently, some authors has suggested that a complex concern such as the emergence of infectious diseases, especially in low-income countries, needs to be addressed using this key concept, in which particular characteristics of human, environmental, and animal health are considered in a unified way to more effectively detect, understand, prevent and, if necessary, to intervene aiming to solve related public health issues (Cibulski et al., 2020).
Marburg virus
Marburg virus is an RNA virus belonging to the Filoviridae family (genus Marburgvirus) (Table 2 ). It is the causal agent of disease, also known as Marburg hemorrhagic fever, a condition with a mortality rate of approximately 80%. The first evidence of MARV infection was reported in 1967 among laboratory workers manipulated biologic material from African green monkeys. From 1980 to 1987, new cases of infection were reported in patients from who had previously visited caves and mines; further molecular testing conducted on liver, spleen and lung samples of Egyptian fruit bat Rousettus aegyptiacus reported the presence of MARV RNA (Figure 1). Possible routes of transmission include fruit contamination and its consumption by humans or direct contact with bat's infected organs. MARV can also spread from human to human through contact with bodily fluids or fomites from sick patients (Table 3 ).
Table 2.
Summary of characteristics of virus transmitted from bats.
| Virus | Affected Host | Intermediate Host | Potential Reservoir/ Ancestral Host | Modes of transmission | Related factors |
|---|---|---|---|---|---|
| Marburg | Human | Non-human primates | Bat (Rousettus aegyptiacus) | Direct contact with an infected organ. Its spread occurs from one human to another when it comes into contact with bodily fluids or fomites from a sick person. | Travel, contact with animals or sick people, Prepare corpses for burial |
| Ebolavirus | Human, non-human primates | Non-human primates, pigs | Several fruit bats | Direct person-to-person contact through mucous membranes or skin, as well as objects contaminated with body fluids from a sick or dead person or animal. | consumption of raw meat, contact with blood burial practices, bad health practice, contact with wild animals |
| Nipah | Human, pigs | Pigs | Fruit bats (Genus Pteropus) flying foxes (Pteropus hypomelanus and Pteropus vampyrus) | Remains of infected food, direct contact with animals or sick humans | Climate changes, changes in agricultural practices, transportation of pigs as merchandise, social and cultural practices, destruction of habitat, food bitten by Bat s, poor handling of infected animals |
| Influenza A | Humans, birds, pigs, horses | Chicken, pigs | Sturnina lilium (small yellow-shouldered bat) and Artibeus planirostris (Bat flat-fronted bat) | Through drops of saliva and mucus from sick people, by leaving the virus in the air or on their hands, then infecting everything they touch. | socioeconomic conditions, immune system, age |
| Coronavirus | Mammals | Pangolins | Bat (Dobsonia moluccensis) | Interaction of dead animals, different body fluids of different species, blood and excretions, | Economic growth, desire for game meat, wildlife trade in wet markets, international travel, habitat destruction, contact with fluids |
| Hendra | Horse | Horse | Flying foxes and fruit bats (Pteropus) | Transmission to humans through close contact with sick horses, body fluids, or aerosols | Climate changes, Urbanization, Social and cultural practices. nutritional stress such as pregnancy / lactation |
| Lyssavirus | Mammals | Bovine, equine | Bats (Desmodus rotundus) | direct bites or scratches from an infected bat | socioeconomic, psychosocial and environmental conditions |
| Dengue | Humans | Mosquitos, Aedes | Bats (Desmodus rotundus) | Infected female mosquito bite healthy person | Tropical climate, Standing water, |
| Equine Encephalitis | Equine | Humans | Bats (Desmodus rotundus) | Arthropod bite | Climatic conditions, distribution of vectors and reservoirs, |
| Madariaga | Equine, human | Short-tail, rat (Zygodontomys brevicauda) | Short-tailed brown bats (Carollia castanea), Pale Spear Bat (Phyllostomus discolor), and Short-tailed bat (Seba) (Carollia perspicillata) | Close contact with infected animals, body fluids such as blood, organs, urine, feces and aerosols generated during defecation, consuming food contaminated with these viruses, direct bite or scratches from these mammals, or even eating it with poor cooking. | Work in forests, agriculture and fishing |
Table 3.
Comprehensive Geographical Classification of Bats that Cause Human Disease.
| Species | Common name | Macro vs. Microchiroptera | Virus detected | Possible modes of transmission | Region |
|---|---|---|---|---|---|
| Old World Bats | |||||
| Pteropus hypomelanus, Pteropus vampyrus Pteropus lylei | Small flying fox Large Flying fox Lyle’s flying fox | Macrochiroptera | Nipah virus | Feces of bat (Contamination of Savia from Phoenix dactylifera) Person to person and contact with fluids from infected animals (pigs) | South East Asia |
| Pteropus alectoa | black flying fox | Macrochiroptera | Hendra virus | Contact with fluids from infected animals (horses) | Australia |
| Australian bat lyssavirus (ABLV) | Scratchs and bites from bats | Australia | |||
| Rhinolophus sinicus | Chinese rufous horseshoe bat | Microchiroptera | SARS | unknown | Asia |
| Taphozous perforates | Egyptian tomb bat | Microchiroptera | MERS | unknown | Middle East Asia, Central Asia |
| Rousettus aegyptiacus | Egyptian fruit bat | Macrochiroptera | Marburg | Contact with fluids and/or meat of the bat Person to person (blood, fluids, burial ceremonies) | Africa |
| Lyssavirus phylogroup 1related bats | |||||
| Eptesicus serotinus | Serotine bat | Microchiroptera | European bat lyssavirus EBLV1 | Bat bite>>contact with urine or feces | Poland, Russia, Slovakia, Spain Belgium, Denmark, France, Germany, Netherlands |
| Myotis daubentonii | Daubenton’s bats | Microchiroptera | European bat lyssavirus EBLV2 | Bat bite >>contact with urine or feces | Denmark, Finland, France, Germany, Norway, Switzerland and United Kingdom |
| Miniopterus schreibersiia | Common bent-wing bat, Schreiber’s bat | Microchiroptera | Duvenhage lyssavirus (DUVV) | Bat bite | Kenya and South Africa |
| Myotis blythi | Lesser mouse-eared bat | Microchiroptera | Aravan lyssavirus (ARAV) | Bat bite | Kyrgyzstan |
| Myotis nattereri | Natterer’s bat | Microchiroptera | Bokeloh bat lyssavirus (BBLV) | Bat bite | France and Germany |
| Pteropus medius | Indian flying fox | Macrochiroptera | Gannoruwa bat lyssavirus (GBLV) | Contact with urine or feces | Sri Lanka |
| Murina leucogaster | Greater tube-nosed bat | Microchiroptera | Irkut virus (IRKV) | China and Russia | |
| Myotis mystacinus | Whiskered bat | Microchiroptera | Khujand virus (KHUV) | Bat bite | Tajikistan |
| Lyssavirus phylogroup 2related batsb | |||||
| Hipposideros commersoni | Commerson’s leaf-nosed bat | Microchiroptera | Shimoni bat lyssavirus (SHIV)c | – | Kenya |
| Eidolon helvum | Straw coloured fruit bat | Macrochiroptera | Lagos bat lyssavirus (LBV)c | Bite, saliva | Ghana, South Africa, Ethiopa, Zimbawe, Senegal, Nigeria, Central Africa |
| Lyssavirus phylogroup 3related bats | |||||
| Miniopterus schreibersiia | Common bent-wing bat, Schreiber’s bat | Microchiroptera | West Caucasian bat lyssavirus (WBCV) | bite | Russia |
| Lleida bat lyssavirus (LLEBV) | bite | Spain | |||
| New World Bats | |||||
| Desmodus rotundus | Common Vampire bat | Microchiroptera | Lyssavirus RABV (rabies virus) – phylogroup 1 | Bite and scratchs from the bat | Central and South America |
| Dhiphylla ecaudata | Hairy legged vampire bat | Microchiroptera | South America, Central America and southern Mexico | ||
| Diaemus youngi | White winged vampire bat | Microchiroptera | Mexico to northern Argentina, Trinidad Island and Margarita Island | ||
| Artibeus planirostris | flat faced fruit-eating bat | Microchiroptera | Bite | Trinidad Island | |
Causes more than one disease.
Phylogroup Lyssavirus-Mokola virus (MOKV) and Ikoma virus (IKOV) is not associated with bats.
No human cases.
In humans, the incubation period ranges from 3 to 9 days, and clinical presentation usually involves flu-like symptoms, fever between 39 and 40 degrees Celsius, conjunctivitis, cramps, cervical lymphadenopathy, and hemorrhagic manifestations. Death occurs as a consequence of cardiocirculatory collapse and multiple hemorrhages in the digestive tract and lungs.
A study detected a high seroprevalence of antibodies against in fruit bats in South Africa, with a 19.1% seroconversion rate in recaptured bats (Paweska et al., 2018); Another study detected MARV genome in bats captured in Zambia (Kajihara et al., 2019); and a posterior serosurvey identified filovirus-specific immunoglobulin G antibodies in 71 out of 748 serum samples collected from migratory fruit bats (Ogawa et al., 2015). Indirect contact with bat fecal shedding has been proposed as the transmission route (Adjemian et al., 2011), as the most recent outbreaks in humans have been after visiting a cave or mine where Rousettus aegyptiacus roosts. The main transmission of the disease is between humans and their contact with fluids from other humans. The eventual contact with non-human primates or bat feces could potentially lead to an outbreak, but these events are rarer than a nosocomial transmission.
Currently, there is no effective treatment for MARV infection other than symptomatic support and rehydration, but some hematologic, immunologic, and pharmacologic actions are under development to improve survival rates.
Ebolavirus
Ebolavirus also belongs to the Filoviridae family and displays a negative-stranded single RNA genome. First described in 1976 in the Democratic Republic of the Congo, this notorious virus has decimated populations of gorillas, chimpanzees and humans in Africa with mortality rates ranging from 25% to 90% (Table 2).
Evidence of infection has been reported in three different species of frugivorous bats associated with large outbreaks of Ebola hemorrhagic fever in 20142015, which later escalated to pandemic proportions resulting in the death of 11,301 people (Han et al., 2015, Leroy et al., 2005). Multiple studies point at bats of the genus Myotis as the main reservoir for Ebola virus given that these bats carry a copy of viral gene VP35 (Table 3), which may hinder human and animal immune responses and thus explaining its high mortality rates. These genes have been found in bats from Africa, Asia, Europe, North America and South America (Shi, 2013) (Figure 1).
Incubation ranges from 2 to 21 days and the infectious period begins with symptom onset. The virus could have been introduced to humans by direct contact with bodily fluids from infected animals since it is usually present in blood, secretions and mucus. Bats can harbor the virus as asymptomatic carriers until death, where transmission to other animals and humans occurs due to ingestion of contaminated tissue. Person-to-person transmission occurs by direct contact with skin or mucous membranes, as well as objects contaminated with body fluids.
Symptoms of Ebola virus infection in humans are similar to those of MARV, but Ebola can lead to kidney and liver failure. Another important difference is that Ebola virus can be vertically transmitted through breast milk or placental circulation.
Studies in Africa analyzed 4022 blood samples from bats, detecting antibodies against Ebola virus in one genus of insectivorous bats and six species of fruit bats (De Nys et al., 2018). Another study conducted in Sierra Leone identified the complete genome of a new Ebola virus, the Bombali virus, in free-tailed bats resting inside human dwellings, suggesting potential human transmission (Goldstein et al., 2018). In Kenya, researchers also identified the Bombali virus in the organs and excreta of free-tailed bats (Mops condylurus) (Forbes et al., 2019). For Ebola and other emerging viruses, transmission chains remain undocumented, deserving more research and studies. Despite the lack of evidence, direct transmission has been speculated based on annual fruit bat migration (Leroy et al., 2009), and on ashes from Mops condylurus in a burnt tree where a human outbreak occurred (Marí Saéz et al., 2015). None of this has been corroborated.
Nipah virus
In Malaysia, an outbreak in pigs and humans took place between September 1998 and April 1999, affecting 283 people and causing 109 human deaths and the slaughter of more than one million pigs (Table 2). Initially, the outbreak was attributed to the Japanese encephalitis virus; but later, researchers demonstrated that the causal agent was a virus that belonged to the Henipavirus genus, family Paramyxoviridae, closely related to the Hendra virus. Fruit bats (genus Pteropus) are the main natural reservoir for Nipah virus (NiV), while pigs serve as intermediate hosts (Table 3). The infection is transmitted from bats to pigs and subsequently from pigs to humans (Figure 1).
On the Island of Malaysia, researchers found NiV in the urine and saliva of flying foxes (Pteropus hypomelanus and Pteropus vampyrus). Initial circulation of NiV likely occurred in late 1997 through contaminated food debris from migrating flying foxes on which pigs fed. Fruit bat migration to cultivated orchards and pig farms was a consequence of the lack of fruit during droughts related to El Niño phenomenon and wild fires in Indonesia. A study conducted in 324 bats (predominantly Pteropus P. vampyrus and P. hypomelanus) from Malaysia found that 6.48% had neutralizing antibodies against NiV and the virus was subsequently isolated from the urine and fruits consumed by P. hypomelanus.
Previous human studies showed that most cases had a history of direct contact with live pigs. In humans, the disease can be fatal and is characterized by respiratory and particularly severe neurological manifestations, such as encephalitis and coma. Clinical signs in animals may vary, including agitation, spasms, seizures, rapid breathing, and harsh cough. Evidence of infection (virus isolation, immunohistochemistry, serology) and neurological involvement has been reported in dogs and horses. Transmission studies in Australia established that NiV could rapidly spread through pigs via oral and parenteral inoculation. Neutralizing antibodies were detectable 1014 days after infection (Kalodimou et al., 2019, Kenmoe et al., 2019, Ochani et al., 2019, Shuai et al., 2020, Singh et al., 2019).
Influenza a virus
Influenza A viruses (IAV) are one of the leading causes of disease in humans, with important animal reservoirs including birds, pigs, and horses that can potentially produce new zoonotic variants (Table 2). Studies in South America identified the first, named HL17NL10 and HL18NL11; affected bat species Sturnira lilium (small yellow-shouldered bat) and Artibeus planirostris (flat-faced frugivorous bat). A Brazilian study analyzed 533 fruit bats, detecting high concentrations of Influenza A HL18N11 subtype in the intestines and feces of two bats from the species A. lituratus. Another study in Guatemala evaluated 316 bats from 21 different species, identifying IAV in three S. lilium bats through PCR and sequencing (Table 3). Notably, small yellow-shouldered bats in Central America have been proposed as potential mammalian reservoirs of influenza (Figure 1).
Research has shown that bats are susceptible to IAV infection. A seroprevalence study identified IAV H9 in 30% of fruit bats sampled in Africa. Bats are believed to have the ability to harbor more genetic diversity of the Influenza virus than any other mammal and bird species. Nevertheless, more studies are necessary to confirm such findings. Transmission of IAV from bats have been not confirmed to other species.
Hendra virus
Hendra virus, formerly known as equine morbillivirus, is a henipavirus of the Paramyxoviridae family for which bats of the genus Pteropus serve as main vectors (Table 2). This virus is endemic in Australian flying foxes which are currently in danger of extinction and it can be detected in blood, urine, feces, and fetal and uterine tissue. Different studies affirm that Hendra virus is horizontally transmitted from bat to bat, but in rare instances vertical transmission has been reported (Figure 1).
Although horses are usually accidental hosts that can contract the virus from these megabats, it has been suggested that the virus could be found in the environment, entering equines through the upper airways and oropharynx. Transmission to humans occurs with close contact with infected horses, either through bodily fluids or aerosols; studies have ruled out person-to-person transmission.
Hendra virus was discovered after an outbreak that killed 20 equines and their trainer in Queensland, Australia, in 1994 (Murray et al., 1995). The virus remains viable for approximately four days in bat urine and fruit juice, but fails to survive in temperatures above 37 degrees Celsius. The disease has an incubation period of 512 days, and its main symptoms are similar to influenza but with a mild, progressive encephalitis. Since this infection is uncommon in humans, an effective antiviral has not been developed (Table 3).
Bat lyssavirus
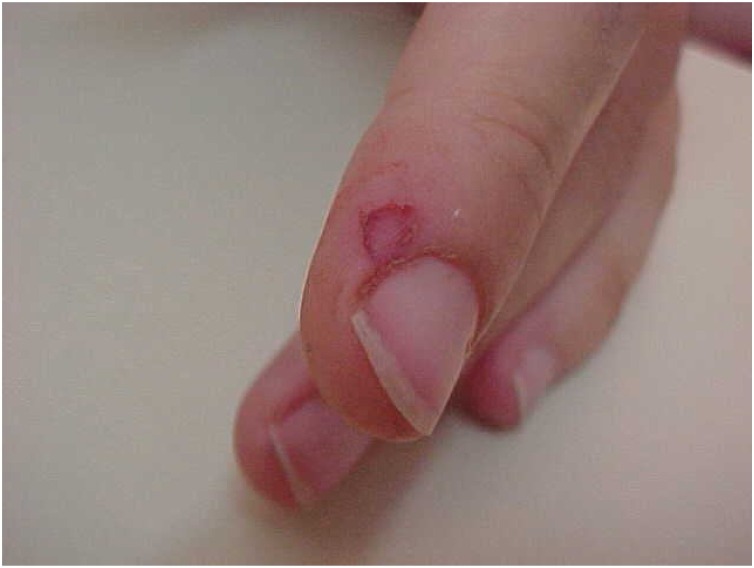
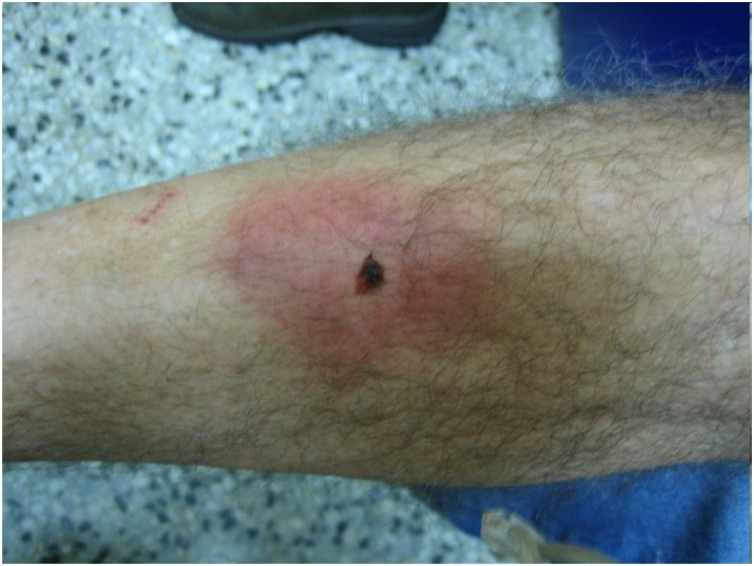
Lyssaviruses are a wide range of pathogens that cause rabies (Table 2). The first case of human rabies was reported in Ukraine in 1977. Hematophagous bat Desmodus rotundus, mainly located in Latin America, is considered the primary vector of this disease (Moran et al., 2015), which historically has resulted in large economic losses by causing the death of cattle and horses that get infected through bat bites. Occasionally, those bites are also seen in human beings (Figure 2, Figure 3 ). People needs to having the proper protection and vaccines especially when working with wildlife to avoid disease risks. Lyssaviruses have also been identified in other hematophagous bats such as Diphylla ecaudata and Diaemus youngi and frugivorous species such as Artibeus planirostris, Diclidurus albus, Hemiderma sp. and Artibeus lituratus (former Phyllostoma superciliatum) (Table 3).
Figure 2.
Biology researcher who suffered a Desmodus rotundus bite during a field work trapping bats in Venezuela. The bat resulted positive for RABV; however, the patient received prophylaxis post exposure and did not develop clinical disease. Courtesy of Jose Antonio Suarez.
Figure 3.
Male patient that suffered an intra-domiciliary bite, while he was trying to capture a bat. He sought medical attention; the bat species was identified as Desmodus rotundus. The bat was negative for RABV; however, the patient received post exposure prophylaxis. Courtesy of Jose Antonio Suarez.
A Colombian study performed on 286 brains from bats of six families and 23 species showed that two species, Artibeus lituratus and Artibeus planirostris, were positive for the rabies virus (Calderon et al., 2019a). Another study conducted in Chile reported that 9.5% of 15,000 bats captured were naturally infected (Escobar et al., 2015). In 2005, the Brazilian Ministry of Health reported a new focus of bat-transmitted human rabies infection (Desmodus rotundus) in northeast Brazil (Ministério da Saúde do Brasil, 2005). During December 2006, 23 cases of jungle rabies were recorded in human patients from Peru where a variant of the bat rabies virus was identified in deceased patients (Gomez-Benavides et al., 2007); a study later reported a seroprevalence rate of 10.3% in bats from Peru (Salmon-Mulanovich et al., 2009). A similar investigation was conducted on hematophagous bats in Mexico, finding antibody prevalence rates of 37% (Salas-Rojas et al., 2004) (Figure 1).
As a of the destruction of natural habitats, closer interaction between bats and humans has grown significantly, especially in mining areas (Bonilla-Aldana et al., 2019). Rabies transmission primarily occurs via direct bites or scratches from infected bats (Calderon et al., 2019a); secondary transmissions in humans take place through contact with infected pets. These viruses cause acute progressive encephalitis that is inevitably fatal from the onset of clinical signs. Initial symptoms are similar to influenza and evolve in a few days to severe neurological involvement that ultimately leads to death (Nguyen et al., 2019, Koury and Warrington, 2020). Currently, only preventive vaccines are available (Benitez et al., 2008, Astray et al., 2017, Rabies Vaccine, 2006, Rifakis et al., 2006, Mahl et al., 2014).
Dengue virus
Dengue virus (DENV) belongs to the Flaviviridae family, which is transmitted by mosquitoes, most commonly Aedes aegypti (Table 2) (Rodriguez-Morales and Paniz-Mondolfi, 2015). There are four DENV serotypes (DENV-1, DENV-2, DENV-3 and DENV-4) that can cause febrile syndromes in humans. Dengue fever is recognized as an epidemic re-emerging disease that has affected many countries in recent decades (Zambrano et al., 2017, Wilder-Smith et al., 2019, Lorenz et al., 2020, Zambrano et al., 2019, Calderon et al., 2019b).
There is evidence that bats could naturally become infected with DENV (Platt et al., 2000). In Mexico, a study evaluated 162 bats and identified DENV nucleic acid and anti-DENV (Aguilar-Setien et al., 2008). Another study in Costa Rica evaluated 12 species of bats, reporting a cumulative seroprevalence of 21.2% (51/241) by PRNT and a prevalence of 8.8% (28/318) in organs tested by RT-PCR (Vicente-Santos et al., 2017). In French Guiana, DENV nucleic acid was detected in the liver and sera of wild-caught bats (de Thoisy et al., 2009).
Recently in Colombia, viral RNA obtained from bat tissues, and a nested-RT-PCR detected amplicons of 143 fragment of the NS5 gene were then sequenced by the Sanger method. In non-hematophagous bats such as Carollia perspicillata and Phyllostomus discolor captured in Ayapel and San Carlos (Córdoba) respectively, an amplicon corresponding to NS5 was detected; these amplicons showed high similarity DENV-2 (Calderon et al., 2019b). Yet, the clinical relevance of DENV isolation from bats is unclear, as well as the implications in transmission to humans. Similarly occurs with other arboviruses, such as Madariaga and the quine ncephalitis viruses. So far, the have been confirmed as infected hosts, probably reservoirs, but not a direct source for human infections (Figure 1). Although this, some authors have found that bats in urban environments do not sustain DENV amplification, and that they would not have a role as reservoirs there. These may be functioning as epidemiological dead-end hosts for this virus (Vicente-Santos et al., 2017). Dengue virus has been found in bats (RNA and antibodies); however, control infections in the laboratory show no replication of the virus (Cabrera-Romo et al., 2014, Perea-Martínez et al., 2013), the sequences found cluster with human strains (suggesting spillback instead of spillover) (de Thoisy et al., 2009).
Equine encephalitis viruses
The equine encephalitis group involves RNA viruses belonging to the Togaviridae family, of the Alphavirus genus. It includes the Venezuelan ncephalitis (EE), Eastern quine ncephalitis (EEE), Western quine ncephalitis (EE) and Highland J virus, which can be transmitted by blood-sucking arthropods and flies. However, it has been reported that animal reservoirs such as birds and bats play an important role in transmission. These arboviruses are very limited to climatic conditions and their dissemination depends entirely on the geographical distribution of their vectors, hosts and reservoirs. Some studies claim that bats of the genus Desmodus rotundus are the most susceptible to contracting EEV (Guzman et al., 2019, Guzman et al., 2020).
Commonly, equine encephalitis viruses are transmitted via mosquito bites. When these viruses infect equines, they will often present high fevers of 40.542.0 °C, restlessness, hyporexia, ataxia, bruxism, imbalance, and indifference toward the environment. These viruses have been detected in humans, which generally act as accidental hosts. Currently, only preventing vaccines are available.
Studies conducted in Mexico analyzed fractions of cardiac, pulmonary and hepatic tissue of 65 bats of the species Carollia perspicillata, Artibeus turpis and Glossophaga soricina, identifying EEV in a male bat of the Artibeus turpis species (Wong-Chia and Scherer, 1971). Another investigation carried out in the departments of Sucre and Córdoba managed to capture and classify 286 bats into 23 species, finding EEV RNA in bats from the species Artibeus planirostris and Sturnira lilium (Table 3). These findings suggest that fruit bats from the Caribbean region in Colombia could be involved in the enzootic cycle of EEV (Guzman et al., 2019) (Figure 1).
Madariaga virus
Madariaga virus is a strain of the eastern equine encephalitis virus. Rats and bats presumably serve as the main vectors given the reported seropositivy of brown short-tailed bats (Carollia castanea), Lanza bats (Phyllostomus discolor) and Seba's short-tailed bats (Carollia perspicillata) (Benvenuto et al., 2019).
Studies carried out in Panama detected neutralizing antibodies against MADV in blood samples of 16 different bats and the virus was isolated in Phyllostomus discolor and Phyllostomus hastatus. Additionally, 25% of pale spear bats (Phyllostomus discolor) and 11.1% of Seba's short-tailed bats (Carollia perspicillata) tested positive for MADV (Vittor et al., 2016). The virus was isolated by culture and the rest were diagnosed serologically (Figure 1).
Equines infected with MADV commonly develop blindness, depression, hyporexia, ataxia, walking in circles and fainting. At post mortem revision, corneal opacity and localized scarifications in head tissue can be observed, coupled by arachnoid and pia mater congestion (Benvenuto et al., 2019).
Factors that increase interaction between bat and human
Several factors increase the interaction between humans and bats. In most cases, this is due to the intrusion of humans into virgin territories inhabited by bats, fueled by the search of economic resources. This phenomenon forces bats to adapt to new settings occupied by men, such as buildings, tombs, mines and bridges. Additional illegal trading of bats for human consumption and traditional medicine in Asia, coupled with poor sanitation and hygiene practices, has facilitated the emergence of zoonotic diseases, some with pandemic potential. Financial openness and globalization involve close connections between countries and across continents and can serve as a mean of transportation that accelerates the spread of these emerging diseases.
Conclusions
Emerging diseases are a matter of concern, especially during the decades, and now even more with the 2020 pandemic of COVID-19. Bats are potential reservoirs of SARS-CoV-2 and other emerging pathogens. Recognizing bats as potential reservoirs or transmission sources for several pathogens that can be a threat to human beings is of upmost importance for health since many of these conditions may cause severe damage and even led to death in a significant proportion of individuals. However, we should not neglect the responsibility of the active role of humans in the invasion of natural habitats and illegal trafficking of bats. The One Health approach on this (Bonilla-Aldana et al., 2020d, Dhama et al., 2020a, Dhama et al., 2020b) became critical, the balance of human, animal and environmental health is of utmost importance in the assessment of emerging diseases. Although bats do not harbour more viruses than other groups once we control for species richness, their role in transmission of multiple emerging infectious diseases deserve more studies (Mollentze and Streicker, 2020).
Their complex and fascinating evolutionary history, great species diversity (representing 1/5 of mammals), ecological adaptations (even to human perturbation), ability of powered-flight, and many more unique characteristics, has made them excellent models for research in disease dynamics. Currently, more than 200 viruses of at least 29 families have been detected in bats (Moratelli and Calisher, 2015), and probably there are still many more to be discovered. However, in the vast majority of cases we do not know more than a simple taxonomic classification of these viruses. In recent years, bats have been proposed as the natural reservoirs and zoonotic source of spillover events. However, due to the complexity of viral transmission in natural systems, and the large variation in experimental designs and viral detection methods used, interpretation of the results is often difficult and rarely conclusive. In fact, only on rare occasions there has been evidence of direct bat-to-human transmission, indicating that there is still much to learn. Most viruses are associated with bats after they have detected the presence of viral RNA/DNA of antibodies against the virus. However, virus detection methods are challenging, and interpretation of results should be made with caution (Mansfield et al., 2011). Studies should be performed with higher sample sizes in order to have more confidence prevalence figures. In most of the reports this is overlooked and causes confusion, since many of the high prevalence numbers are not properly weighted for their small sample size. We consider that conducting new investigations centered the role of bats and their ecosystems in the transmission of emerging diseases should be a priority to global health.
Author contributions
DKBA and AJRM conceived the review, developed the preliminary search strategy, and drafted the manuscript. SDJD, JSAD, and MAF refined the search strategy by conducting iterative database queries and incorporating new search terms. SDJD, JSAD, MAF, DKBA and AJRM searched and collected the articles. AJRM and DKBA conducted the quality assessment. All authors critically reviewed the manuscript for relevant intellectual content. All authors have read and approved the final version of the paper.
Conflicts of interest
All authors report no potential conflicts.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Funding source
Universidad Tecnológica de Pereira. From the Dirección de Investigación Científica, Humanística y Tecnológica (2-05-01-01), National Autonomous University of Honduras, Tegucigalpa, MDC, Honduras, Central America, this article. L.I.Z. was the recipient of the UNAH (CU-0−041-05−2014/03−2014. Study sponsors had no role in the study design collection, analysis and interpretation of data; writing of the manuscript; and the decision to submit the manuscript for publication.
Ethical approval
Approval was not required.
References
- Adjemian J., Farnon E.C., Tschioko F., Wamala J.F., Byaruhanga E., Bwire G.S., et al. Outbreak of Marburg hemorrhagic fever among miners in Kamwenge and Ibanda Districts, Uganda, 2007. J. Infect. Dis. 2011;204(Suppl 3):S796–S799. doi: 10.1093/infdis/jir312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar-Setien A., Romero-Almaraz M.L., Sanchez-Hernandez C., Figueroa R., Juarez-Palma L.P., Garcia-Flores M.M., et al. Dengue virus in Mexican bats. Epidemiol Infect. 2008;136:1678–1683. doi: 10.1017/S0950268808000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad T., Khan M., Haroon Musa T.H., Nasir S., Hui J., Bonilla-Aldana D.K. Rodriguez-Morales AJ. COVID-19: Zoonotic aspects. Travel Med Infect Dis. 2020:101607. doi: 10.1016/j.tmaid.2020.101607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D.E., Islam A., Crameri G., Todd S., Islam A., Khan S.U., et al. Isolation and Full-Genome Characterization of Nipah Viruses from Bats, Bangladesh. Emerg Infect Dis. 2019;25:166–170. doi: 10.3201/eid2501.180267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anindita P.D., Sasaki M., Setiyono A., Handharyani E., Orba Y., Kobayashi S., et al. Detection of coronavirus genomes in Moluccan naked-backed fruit bats in Indonesia. Arch Virol. 2015;160:1113–1118. doi: 10.1007/s00705-015-2342-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astray R.M., Jorge S.A., Pereira C.A. Rabies vaccine development by expression of recombinant viral glycoprotein. Arch Virol. 2017;162:323–332. doi: 10.1007/s00705-016-3128-9. [DOI] [PubMed] [Google Scholar]
- Banerjee A., Baker M.L., Kulcsar K., Misra V., Plowright R., Mossman M. Novel insights into immune systems of bats. Front. Immunol. 2020;11:26. doi: 10.3389/fimmu.2020.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltz L.A. Wiley; 2018. Bats and human health, Ebola, SARS, Rabies and Beyond. [Google Scholar]
- Benitez J.A., Rodriguez-Morales A.J., Vivas P., Plaz J. Burden of zoonotic diseases in Venezuela during 2004 and 2005. Ann N Y Acad Sci. 2008;1149:315–317. doi: 10.1196/annals.1428.051. [DOI] [PubMed] [Google Scholar]
- Benvenuto D., Cella E., Fogolari M., De Florio L., Borsetti A., Donati D., et al. The transmission dynamic of Madariaga Virus by bayesian phylogenetic analysis: Molecular surveillance of an emergent pathogen. Microb Pathog. 2019;132:80–86. doi: 10.1016/j.micpath.2019.04.039. [DOI] [PubMed] [Google Scholar]
- Biscayart C., Angeleri P., Lloveras S., Chaves T., Schlagenhauf P., Rodriguez-Morales A.J. The next big threat to global health? 2019 novel coronavirus (2019-nCoV): What advice can we give to travellers? - Interim recommendations January 2020, from the Latin-American society for Travel Medicine (SLAMVI) Travel Med Infect Dis. 2020;33:101567. doi: 10.1016/j.tmaid.2020.101567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilla-Aldana D.K., Suarez J.A., Franco-Paredes C., Vilcarromero S., Mattar S., Gomez-Marin J.E., et al. Brazil burning! What is the potential impact of the Amazon wildfires on vector-borne and zoonotic emerging diseases? - A statement from an international experts meeting. Travel Med Infect Dis. 2019;31:101474. doi: 10.1016/j.tmaid.2019.101474. [DOI] [PubMed] [Google Scholar]
- Bonilla-Aldana D.K., Holguin-Rivera Y., Cortes-Bonilla I., Cardona-Trujillo M.C., Garcia-Barco A., Bedoya-Arias H.A., et al. Coronavirus infections reported by ProMED, February 2000-January 2020. Travel Med Infect Dis. 2020:101575. doi: 10.1016/j.tmaid.2020.101575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilla-Aldana D.K., Dhama K., Rodriguez-Morales A.J. Revisiting the One Health Approach in the Context of COVID-19: A Look into the Ecology of this Emerging Disease. Adv Anim Vet Sci. 2020;8:234–237. [Google Scholar]
- Bonilla-Aldana D.K., Jimenez-Diaz S.D., Patel S.K., Dhama K., Rabaan A.A., Sah R., et al. Importance of Bats in Wildlife: Not Just Carriers of Pandemic SARS-CoV-2 and Other Viruses. J Pure Appl Microbiol. 2020;14:709–712. [Google Scholar]
- Bonilla-Aldana D.K., Holguin-Rivera Y., Perez-Vargas S., Trejos-Mendoza A.E., Balbin-Ramon G.J., Dhama K., et al. Importance of the One Health approach to study the SARS-CoV-2 in Latin America. One Health. 2020;10:100147. doi: 10.1016/j.onehlt.2020.100147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera-Romo S., Recio-Tótoro B., Alcalá A.C., Lanz H., del Ángel R.M., et al. Experimental inoculation of Artibeus jamaicensis bats with dengue virus serotypes 1 or 4 showed no evidence of sustained replication. Am J Trop Med Hyg. 2014;91(6):1227–1234. doi: 10.4269/ajtmh.14-0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon A., Guzman C., Mattar S., Rodriguez V., Acosta A., Martinez C. Frugivorous bats in the Colombian Caribbean region are reservoirs of the rabies virus. Ann Clin Microbiol Antimicrob. 2019;18:11. doi: 10.1186/s12941-019-0308-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon A., Guzman C., Mattar S., Rodriguez V., Martinez C., Violet L., et al. Dengue Virus in Bats from Cordoba and Sucre, Colombia. Vector Borne Zoonotic Dis. 2019;19:747–751. doi: 10.1089/vbz.2018.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calisher C.H., Childs J.E., Field H.E., Holmes K.V., Schountz T. Bats: important reservoir hosts of emerging viruses. Clin Microbiol Rev. 2006;19:531–545. doi: 10.1128/CMR.00017-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascio A., Bosilkovski M., Rodriguez-Morales A.J., Pappas G. The socio-ecology of zoonotic infections. Clin Microbiol Infect. 2011;17:336–342. doi: 10.1111/j.1469-0691.2010.03451.x. [DOI] [PubMed] [Google Scholar]
- Cibulski S., de Lima F.E.S., Roehe P.M. Coronaviruses in Brazilian bats: a matter of concern? PLoS Negl Trop Dis. 2020;14(10):e0008820. doi: 10.1371/journal.pntd.0008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Nys H.M., Kingebeni P.M., Keita A.K., Butel C., Thaurignac G., Villabona-Arenas C.J., et al. Survey of Ebola Viruses in Frugivorous and Insectivorous Bats in Guinea, Cameroon, and the Democratic Republic of the Congo, 2015-2017. Emerg Infect Dis. 2018;24:2228–2240. doi: 10.3201/eid2412.180740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Thoisy B., Lacoste V., Germain A., Munoz-Jordan J., Colon C., Mauffrey J.F., et al. Dengue infection in neotropical forest mammals. Vector Borne Zoonotic Dis. 2009;9:157–170. doi: 10.1089/vbz.2007.0280. [DOI] [PubMed] [Google Scholar]
- Dhama K., Khan S., Tiwari R., Sircar S., Bhat S., Malik Y.S., et al. Coronavirus Disease 2019-COVID-19. Clin Microbiol Rev. 2020:33. doi: 10.1128/CMR.00028-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhama K., Patel S.K., Sharun K., Pathak M., Tiwari R., Yatoo M.I., et al. SARS-CoV-2 jumping the species barrier: zoonotic lessons from SARS, MERS and recent advances to combat this pandemic virus. Travel Med Infect Dis. 2020:101830. doi: 10.1016/j.tmaid.2020.101830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drexler J.F., Corman V.M., Muller M.A., Maganga G.D., Vallo P., Binger T., et al. Bats host major mammalian paramyxoviruses. Nat Commun. 2012;3:796. doi: 10.1038/ncomms1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar L.E., Restif O., Yung V., Favi M., Pons D.J., Medina-Vogel G. Spatial and temporal trends of bat-borne rabies in Chile. Epidemiol Infect. 2015;143:1486–1494. doi: 10.1017/S095026881400226X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming T.H. Encyclopedia of Animal Behavior. Elsevier; 2019. Bat migration; pp. 605–610. [Google Scholar]
- Forbes K.M., Webala P.W., Jaaskelainen A.J., Abdurahman S., Ogola J., Masika M.M., et al. Bombali Virus in Mops condylurus Bat, Kenya. Emerg Infect Dis. 2019:25. doi: 10.3201/eid2505.181666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein T., Anthony S.J., Gbakima A., Bird B.H., Bangura J., Tremeau-Bravard A., et al. The discovery of Bombali virus adds further support for bats as hosts of ebolaviruses. Nat Microbiol. 2018;3:1084–1089. doi: 10.1038/s41564-018-0227-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Benavides J., Manrique C., Passara F., Huallpa C., Laguna V.A., Zamalloa H. Outbreak of human rabies in Madre de Dios and Puno, Peru, due to contact with the common vampire bat, Desmodus rotundus. Proceedings of 56th Annual Meeting of the American Society of Tropical Medicine and Hygiene; 2007 Nov 4–8; Philadelphia, PA, USA p 150–99; 2007. [Google Scholar]
- Gutiérrez A.B., Rodríguez-Morales A.J., Narváez Mejía ÁJ., García Peña ÁA., Giraldo Montoya ÁM., Cortes Muñoz A.J., et al. Colombian consensus recommendations for diagnosis, management and treatment of the infection by SARS-COV-2/ COVID-19 in health care facilities - Recommendations from expert's group based and informed on evidence. Infectio. 2020;24:1–102. [Google Scholar]
- Guzman C., Calderon A., Martinez C., Oviedo M., Mattar S. Eco-epidemiology of the Venezuelan equine encephalitis virus in bats of Cordoba and Sucre, Colombia. Acta Trop. 2019;191:178–184. doi: 10.1016/j.actatropica.2018.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman C., Calderon A., Oviedo T., Mattar S., Castaneda J., Rodriguez V., et al. Molecular and cellular evidence of natural Venezuelan equine encephalitis virus infection in frugivorous bats in Colombia. Vet World. 2020;13:495–501. doi: 10.14202/vetworld.2020.495-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H.J., Wen H.L., Zhou C.M., Chen F.F., Luo L.M., Liu J.W., et al. Bats as reservoirs of severe emerging infectious diseases. Virus Res. 2015;205:1–6. doi: 10.1016/j.virusres.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman D.T., Bowen R.A., Cryan P.M., McCracken G.F., O’Shea T.J., Peel A.J., et al. Ecology of zoonotic infectious diseases in bats: current knowledge and future directions. Zoonoses Public Health. 2013;60:2–21. doi: 10.1111/zph.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B., Ge X., Wang L.F., Shi Z. Bat origin of human coronaviruses. Virol J. 2015;12:221. doi: 10.1186/s12985-015-0422-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D., Zhu C., Ai L., He T., Wang Y., Ye F., et al. Genomic characterization and infectivity of a novel SARS-like coronavirus in Chinese bats. Emerg Microbes Infect. 2018;7:154. doi: 10.1038/s41426-018-0155-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheon J.M., Kirsch J.A.W. A moveable face: deconstructing the Microchiroptera and a new classification of extant bats. Acta Chiropterologica. 2006;8:1–10. [Google Scholar]
- Jebb D., Huang Z., Pippel M., Hughes G.M., Lavrichenko K., Devanna P., et al. Six reference-quality genomes reveal evolution of bat adaptations. Nature. 2020;583(7817):578–584. doi: 10.1038/s41586-020-2486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajihara M., Hang’ombe B.M., Changula K., Harima H., Isono M., Okuya K., et al. Marburgvirus in Egyptian Fruit Bats, Zambia. Emerg Infect Dis. 2019;25:1577–1580. doi: 10.3201/eid2508.190268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalodimou G., Veit S., Jany S., Kalinke U., Broder C.C., Sutter G., et al. A Soluble Version of Nipah Virus Glycoprotein G Delivered by Vaccinia Virus MVA Activates Specific CD8 and CD4 T Cells in Mice. Viruses. 2019:12. doi: 10.3390/v12010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenmoe S., Demanou M., Bigna J.J., Nde Kengne C., Fatawou Modiyinji A., Simo F.B.N., et al. Case fatality rate and risk factors for Nipah virus encephalitis: A systematic review and meta-analysis. J Clin Virol. 2019;117:19–26. doi: 10.1016/j.jcv.2019.05.009. [DOI] [PubMed] [Google Scholar]
- Koury R., Warrington S.J. 2020. Rabies. StatPearls, Treasure Island (FL) [Google Scholar]
- Kunz T.H., Braun de Torrez E., Bauer D., Lobova T., Fleming T.H. Ecosystem services provided by bats. Ann N Y Acad Sci. 2011;1223:1–38. doi: 10.1111/j.1749-6632.2011.06004.x. [DOI] [PubMed] [Google Scholar]
- Latinne A., Hu B., Olival K.J., Zhu G., Zhang L., Li H., et al. Origin and cross-species transmission of bat coronaviruses in China. Nat Commun. 2020;11(1):4235. doi: 10.1038/s41467-020-17687-3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lau S.K.P., Luk H.K.H., Wong A.C.P., Li K.S.M., Zhu L., He Z., et al. Possible bat origin of severe Acute Respiratory Syndrome Coronavirus 2. Emerg Infect Dis. 2020;26(7):1542–1547. doi: 10.3201/eid2607.200092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy E.M., Epelboin A., Mondonge V., et al. Human Ebola outbreak resulting from direct exposure to fruit bats in Luebo, Democratic Republic of Congo, 2007. Vector Borne Zoonotic Dis. 2009;9(6):723–728. doi: 10.1089/vbz.2008.0167. [DOI] [PubMed] [Google Scholar]
- Leroy E.M., Kumulungui B., Pourrut X., Rouquet P., Hassanin A., Yaba P., et al. Fruit bats as reservoirs of Ebola virus. Nature. 2005;438:575–576. doi: 10.1038/438575a. [DOI] [PubMed] [Google Scholar]
- Lorenz C., Azevedo T.S., Chiaravalloti-Neto F. COVID-19 and dengue fever: A dangerous combination for the health system in Brazil. Travel Med Infect Dis. 2020:101659. doi: 10.1016/j.tmaid.2020.101659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahl P., Cliquet F., Guiot A.L., Niin E., Fournials E., Saint-Jean N., et al. Twenty year experience of the oral rabies vaccine SAG2 in wildlife: a global review. Vet Res. 2014;45:77. doi: 10.1186/s13567-014-0077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield K.L., Horton D.L., Johnson N., Li L., Barrett A., Smith D.J., et al. Flavivirus-induced antibody cross-reactivity. J Gen Virol. 2011;92(Pt 131 12):2821–2829. doi: 10.1099/vir.0.031641-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marí Saéz A., Weiss S., Nowak K., Lapeyre V., Zimmermann F., Düx A., et al. Investigating the zoonotic origin of the West African Ebola epidemic. EMBO molecular medicine. 2015;7(1):17–23. doi: 10.15252/emmm.201404792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministério da Saúde do Brasil . 2005. Raiva Humana transmitida por morcegos en Estado do Pará e Maranhão.http://portal.saude.gov.br/portal/arquivos/pdf/nota_raiva.pdf [Google Scholar]
- Mollentze N., Streicker D.G. Viral zoonotic risk is homogenous among taxonomic orders of mammalian and avian reservoir hosts. Proc Natl Acad Sci U S A. 2020;117:201919176. doi: 10.1073/pnas.1919176117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran D., Juliao P., Alvarez D., Lindblade K.A., Ellison J.A., Gilbert A.T., et al. Knowledge, attitudes and practices regarding rabies and exposure to bats in two rural communities in Guatemala. BMC Res Notes. 2015;8:955. doi: 10.1186/s13104-014-0955-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moratelli R., Calisher C.h.H. Bats and zoonotic viruses: can we confidently link bats with emerging deadly viruses? Memórias do Instituto Oswaldo Cruz. 2015;110(1):1–22. doi: 10.1590/0074-02760150048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morens D.M., Daszak P., Taubenberger J.K. Escaping Pandora’s Box - Another Novel Coronavirus. N Engl J Med. 2020;382:1293–1295. doi: 10.1056/NEJMp2002106. [DOI] [PubMed] [Google Scholar]
- Morrison J.H., Miller C., Bankers L., Crameri G., Wang L.F., Poeschla E.M. A Potent Postentry Restriction to Primate Lentiviruses in a Yinpterochiropteran Bat. mBio. 2020;11(5):e01854–20. doi: 10.1128/mBio.01854-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray K., Selleck P., Hooper P., Hyatt A., Gould A., Gleeson L., et al. A morbillivirus that caused fatal disease in horses and humans. Science. 1995;268:94–97. doi: 10.1126/science.7701348. [DOI] [PubMed] [Google Scholar]
- Muscarella R., Fleming T.H. The role of frugivorous bats in tropical forest succession. Biol Rev Camb Philos Soc. 2007;82:573–590. doi: 10.1111/j.1469-185X.2007.00026.x. [DOI] [PubMed] [Google Scholar]
- Neves B., Kessous I.M., Moura R.L., Couto D.R., Zanella C.M., Antonelli A., et al. Pollinators drive floral evolution in an Atlantic Forest genus. AoB Plants. 2020;12(5):plaa046. doi: 10.1093/aobpla/plaa046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen H.T.T., Afriyie D.O., Tran C.H., Dang A.D., Tran D.N., Dang T.Q., et al. Progress towards rabies control and elimination in Vietnam. Rev Sci Tech. 2019;38:199–212. doi: 10.20506/rst.38.1.2953. [DOI] [PubMed] [Google Scholar]
- Ochani R.K., Batra S., Shaikh A., Asad A. Nipah virus - the rising epidemic: a review. Infez Med. 2019;27:117–127. [PubMed] [Google Scholar]
- Ogawa H., Miyamoto H., Nakayama E., Yoshida R., Nakamura I., Sawa H., et al. Seroepidemiological Prevalence of Multiple Species of Filoviruses in Fruit Bats (Eidolon helvum) Migrating in Africa. J Infect Dis. 2015;(212 Suppl 2):S101–8. doi: 10.1093/infdis/jiv063. [DOI] [PubMed] [Google Scholar]
- Olival K.J., Latinne A., Islam A., Epstein J.H., Hersch R., Engstrand R.C., et al. Population genetics of fruit bat reservoir informs the dynamics, distribution and diversity of Nipah virus. Mol Ecol. 2020;29:970–985. doi: 10.1111/mec.15288. [DOI] [PubMed] [Google Scholar]
- Paweska J.T., Jansen van Vuren P., Kemp A., Storm N., Grobbelaar A.A., Wiley M.R., et al. Marburg Virus Infection in Egyptian Rousette Bats, South Africa, 2013-2014(1) Emerg Infect Dis. 2018;24:1134–1137. doi: 10.3201/eid2406.172165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea-Martínez L., Moreno-Sandoval H.N., Moreno-Altamirano M.M., Salas-Rojas M., García-Flores M.M. Experimental infection of Artibeus intermedius bats with serotype-2 dengue virus. Comp Immunol Microbiol Infect Dis. 2013;36(2):193–198. doi: 10.1016/j.cimid.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Platt K.B., Mangiafico J.A., Rocha O.J., Zaldivar M.E., Mora J., Trueba G., et al. Detection of dengue virus neutralizing antibodies in bats from Costa Rica and Ecuador. J Med Entomol. 2000;37:965–967. doi: 10.1603/0022-2585-37.6.965. [DOI] [PubMed] [Google Scholar]
- Rabies Vaccine N.I.H. Drugs and Lactation Database (LactMed) Bethesda (MD) 2006 [Google Scholar]
- Rifakis P.M., Benitez J.A., Rodriguez-Morales A.J., Dickson S.M., De-La-Paz-Pineda J. Ecoepidemiological and Social Factors Related to Rabies Incidence in Venezuela during 2002-2004. Int J Biomed Sci. 2006;2:1–6. [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Morales A.J., Paniz-Mondolfi A.E. Venezuela: far from the path to dengue and chikungunya control. J Clin Virol. 2015;66:60–61. doi: 10.1016/j.jcv.2015.02.020. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Morales A.J., Cardona-Ospina J.A., Gutierrez-Ocampo E., Villamizar-Pena R., Holguin-Rivera Y., Escalera-Antezana J.P., et al. Latin American Network of Coronavirus Disease C-REahwlo. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis. 2020;34:101623. doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas-Rojas M., Sanchez-Hernandez C., Romero-Almaraz M.de L., Schnell G.D., Schmid R.K., Aguilar-Setien A. Prevalence of rabies and LPM paramyxovirus antibody in non-hematophagous bats captured in the Central Pacific coast of Mexico. Trans R Soc Trop Med Hyg. 2004;98:577–584. doi: 10.1016/j.trstmh.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Salazar-Bravo J., Phillips C.J., Bradley R.D., Baker R.J., Yates T.L., Ruedas L.A. Voucher specimens for SARS-linked bats. Science. 2006;311:1099–1100. doi: 10.1126/science.311.5764.1099. [DOI] [PubMed] [Google Scholar]
- Salmon-Mulanovich G., Vasquez A., Albujar C., Guevara C., Laguna-Torres V.A., Salazar M., et al. Human rabies and rabies in vampire and nonvampire bat species, Southeastern Peru, 2007. Emerg Infect Dis. 2009;15:1308–1310. doi: 10.3201/eid1508.081522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z. Emerging infectious diseases associated with bat viruses. Sci China Life Sci. 2013;56:678–682. doi: 10.1007/s11427-013-4517-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuai L., Ge J., Wen Z., Wang J., Wang X., Bu Z. Immune responses in mice and pigs after oral vaccination with rabies virus vectored Nipah disease vaccines. Vet Microbiol. 2020;241:108549. doi: 10.1016/j.vetmic.2019.108549. [DOI] [PubMed] [Google Scholar]
- Simmons, N.B. and A.L. Cirranello. 2020. Bat Species of the World: A taxonomic and geographic database. Accessed on 11/18/2020. https://batnames.org/.
- Simmons N.B., Seymour K.L., Habersetzer J., Gunnell G.F. Primitive Early Eocene bat from Wyoming and the evolution of flight and echolocation. Nature. 2008;451:818–821. doi: 10.1038/nature06549. [DOI] [PubMed] [Google Scholar]
- Singh R.K., Dhama K., Chakraborty S., Tiwari R., Natesan S., Khandia R., et al. Nipah virus: epidemiology, pathology, immunobiology and advances in diagnosis, vaccine designing and control strategies - a comprehensive review. Vet Q. 2019;39:26–55. doi: 10.1080/01652176.2019.1580827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swelum A.A., Shafi M.E., Albaqami N.M., El-Saadony M.T., Elsify A., Abdo M., et al. COVID-19 in human, animal, and environment: a review. Front Vet Sci. 2020;7:578. doi: 10.3389/fvets.2020.00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teeling E.C., Vernes S.C., Dávalos L.M., Ray D.A., Gilbert M.T.P., Myers E., et al. Bat biology, genomes, and the Bat1K Project: to generate chromosome-level genomes for all living bat species. Annu Rev Anim Biosci. 2018;6:23–46. doi: 10.1146/annurev-animal-022516-022811. [DOI] [PubMed] [Google Scholar]
- Tiwari R., Dhama K., Sharun K., Iqbal Yatoo M., Malik Y.S., Singh R., et al. Rodriguez-Morales AJ. COVID-19: animals, veterinary and zoonotic links. Vet Q. 2020;40:169–182. doi: 10.1080/01652176.2020.1766725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente-Santos A., Moreira-Soto A., Soto-Garita C., Chaverri L.G., Chaves A., Drexler J.F., et al. Neotropical bats that co-habit with humans function as dead-end hosts for dengue virus. PLoS Negl Trop Dis. 2017;11 doi: 10.1371/journal.pntd.0005537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittor A.Y., Armien B., Gonzalez P., Carrera J.P., Dominguez C., Valderrama A., et al. Epidemiology of Emergent Madariaga Encephalitis in a Region with Endemic Venezuelan Equine Encephalitis: Initial Host Studies and Human Cross-Sectional Study in Darien, Panama. PLoS Negl Trop Dis. 2016;10 doi: 10.1371/journal.pntd.0004554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder-Smith A., Ooi E.E., Horstick O., Wills B. Dengue. Lancet. 2019;393:350–363. doi: 10.1016/S0140-6736(18)32560-1. [DOI] [PubMed] [Google Scholar]
- Wong-Chia C., Scherer W.F. [Isolation of the Venezuelan encephalitis virus from a frugivorous bat (Artibeus turpis) in Mexico] Bol Oficina Sanit Panam. 1971;70:339–343. [PubMed] [Google Scholar]
- Yovel Y., Falk B., Moss C.F., Ulanovsky N. Active control of acoustic field-of-view in a biosonar system. PLoS Biol. 2011;9:e1001150. doi: 10.1371/journal.pbio.1001150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambrano L.I., Rodriguez E., Espinoza-Salvado I.A., Rodriguez-Morales A.J. Dengue in Honduras and the Americas: The epidemics are back! Travel Med Infect Dis. 2019;31:101456. doi: 10.1016/j.tmaid.2019.07.012. [DOI] [PubMed] [Google Scholar]
- Zambrano L.I., Sierra M., Lara B., Rodriguez-Nunez I., Medina M.T., Lozada-Riascos C.O., et al. Estimating and mapping the incidence of dengue and chikungunya in Honduras during 2015 using Geographic Information Systems (GIS) J Infect Public Health. 2017;10:446–456. doi: 10.1016/j.jiph.2016.08.003. [DOI] [PubMed] [Google Scholar]
- Zhou P., Chionh Y., Irac S., et al. Unlocking bat immunology: establishment of Pteropus alecto bone marrow derived dendritic cells and macrophages. Scientific Report. 2016;6:8597. doi: 10.1038/srep38597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubaid A., McCracken G.F., Kunz T.H. 2005. Functional and evolutionary ecology of bats Oxford University Press, Inc. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.