Abstract
Evidence of the effect of lifestyle behaviors on health-related quality of life (HRQoL) is scarce or ambiguous. The objective of the present study was to explore the associations between multiple lifestyle behaviors and HRQoL among the elderly individuals with prediabetes.
Four hundred thirty-four elderly individuals with prediabetes were included in this cross-sectional study. The Medical Outcomes Study 36-Item Short Form Health Survey Questionnaire (SF-36) was used to assess HRQoL. Multiple lifestyle behaviors, including smoking, alcohol consumption, physical activity, dietary habits, daily sedentary time and sleep duration were self-reported. The associations between HRQoL and multiple lifestyle behaviors were examined using a multivariate linear regression analysis.
Lower-risk physical activities (β =3 .19, 95% CI: 1.32, 5.64), dietary habits (β = 2.60, 95% CI: 1.50, 4.72), and sedentary time (β = 2.49, 95% CI: 1.21, 4.81) were positively associated with the physical health component score (PCS) for HRQoL. Meanwhile, a lower-risk behavior, such as sleep duration (β = 2.64, 95% CI: 1.77, 4.51), was associated with a higher mental health component score (MCS) for HRQoL after adjusting for socio-demographic factors, chronic diseases, anthropometric data and all other lifestyle behaviors. Engaging in a greater number of lower-risk lifestyle behaviors was positively correlated with a better HRQoL.
Multiple lifestyle behaviors were associated with HRQoL among the elderly individuals with prediabetes in rural areas. These results highlight the need for multiple lifestyle behavioral interventions to maintain and improve HRQoL in the elderly individuals with prediabetes.
Keywords: elderly, health-related quality of life, lifestyle behavior, prediabetes
1. Introduction
Prediabetes is defined as blood glucose concentrations that are higher than normal but do not meet the criterion for diabetes,[1] which is interpreted as either impaired fasting glucose (IFG) and/or impaired glucose tolerance (IGT). Approximately 148.2 million adults and more than 20% of the elderly population in both urban and rural areas in China have prediabetes.[2] Moreover, individuals with prediabetes have a higher risk of developing diabetes.[3] However, individuals with prediabetes may progress to normal plasma glucose levels after participating in lifestyle interventions.4,5
Health-related quality of life (HRQoL) is defined as the perception of overall satisfaction with life and involves measurements of status in the domains of physical and mental health. Moreover, HRQoL has become a fundamental assessment in understanding the health status that predicts mortality and chronic diseases.6,7 People who progress to prediabetes or diabetes experience a lower HRQoL than people with normal plasma glucose levels.8,9 Thus, more people have recognized the importance of identifying and understanding the factors contributing to a good HRQoL.[10]
Unhealthy lifestyle habits, such as smoking, physical inactivity, and extreme sleep durations, are associated with all-cause mortality, cardiovascular disease,11,12 psychological stress, depression, and anxiety.13,14 A large number of studies has already established associations between unhealthy lifestyle habits, such as insufficient physical activity,[15] a long sedentary time,[16] and short sleep duration,[17] with poorer HRQoL. However, these studies have only examined one or two unhealthy lifestyle behaviors. Although a few studies have explored the relationship between multiple lifestyle behaviors and HRQoL among school-aged children[18] and patients with type 1 diabetes,[19] no study in the present literature has investigated this association in a population with prediabetes. Considering that the evidence for the effect of lifestyle behaviors on HRQoL is scarce and relevant research is currently lacking, the objective of the present study was to explore the associations between six indicators of lifestyle behaviors and HRQoL among the elderly individuals with prediabetes in rural areas. It was hoped that the results of this study would provide a sound scientific basis for improving HRQoL in this population.
2. Materials and methods
2.1. Study design and participants
This was a cross-sectional study, whose participants were members of the baseline group of a cluster randomized controlled trial, which began in April 2015 and was conducted in the rural areas of Yiyang City, Hunan Province, China. A multi-stage random sampling method was used to select a sample of elderly individuals with prediabetes. According to the administrative regions of China, two counties of Yiyang City were first selected. Second, four townships were randomly selected within each chosen county. Third, a quarter of rural villages were randomly selected in each chosen township. Finally, all households with elderly individuals in each village were listed. Elderly people who had lived in the village for more than 3 years were allowed to participate in the prediabetes screening. People with diabetes or severe mental or physical diseases were excluded from the screening. The oral glucose tolerance test (OGTT) and the 1999 WHO criteria[1] for prediabetes were used to distinguish individuals with prediabetes from people with normal plasma glucose levels. Briefly, 2144 elderly individuals from 42 villages participated in the screening programme, and 461 had prediabetes. Twenty-seven elderly individuals with prediabetes were not investigated or refused to participate in the subsequent study for various reasons. Finally, 434 elderly individuals with prediabetes who completed the investigation were included in this study. The design and procedure have been described in detail in a previous study.[20]
2.2. Ethical statement
The present study was approved by the Medical Ethics Committee of Central South University (CTXY-1520002-7). Besides, all participants were provided written informed consent before participating in the study, and they consented to participate in the study.
2.3. Data collection and measures
2.3.1. General information
Socio-demographic characteristics were collected through a self-administered questionnaire and analyzed by trained investigators. The questionnaire included age, gender (1 = female and 2 = male), marital status (1 = non-married and 2 = married), education (1 = <1 year, 2 = 1–6 years, and 3 = 6 years or more), a history of hyperglycaemia (0 = no and 1 = yes), a family history of diabetes (0 = no and 1 = yes), and the presence of chronic diseases (0 = absent and 1 = present). Chronic diseases referred to non-communicable diseases, including coronary heart disease, hyperlipidemia, chronic obstructive pulmonary disease, stroke, and other diseases.
The anthropometric measurements included height, weight, blood pressure, waist circumference, and hip circumference. These were assessed using standard tools as described in detail in a previous study.[20] The body mass index (BMI) was calculated using the formula for weight in kilograms divided by height in square meters, m2 (kg/m2), and this was then divided into three categories (1: <24 kg/m2, 2: 24–28 kg/m2, 3: ≥28 kg/m2).[21] People with a systolic blood pressure >140 mm Hg and/or diastolic blood pressure >90 mm Hg were identified as hypertensive (0 = no and 1 = yes).[22] The waist-to-hip ratio (WHR) was calculated by dividing the waist circumference by the hip circumference. A WHR >0.9 in men and >0.8 in women was defined as a high WHR.
2.3.2. Lifestyle behavior measurements
Smoking status was classified into three categories, non-smoker, ex-smoker, and current smoker. These were self-reported by the participants. In addition, the following classifications were made: a lower-risk group (1 = non-smoker or ex-smoker) or a higher-risk group (0 = current smoker). Alcohol consumption was evaluated using two questions:
-
1.
do you consume alcohol currently; and
-
2.
the number of alcoholic drinks consumed per day when alcohol was consumed.
These items classified participants into the lower-risk group, if they never consumed alcohol, or drink ≤25 g/day for males and ≤15 g/day for females; and the higher-risk group if they reported that they drink more than 25 g/day for males and 15 g/day for females when alcohol was consumed (1 = lower-risk and 0 = higher-risk).23,24 Physical activities within the past week were assessed using the International Physical Activity Questionnaire Long Version (IPAQ), a valid and reliable measurement for four main domains of physical activities,[25] with a minimum duration of 10 min per physical activity session. Activities were classified into three levels according to the criteria of the IPAQ: low, moderate, and high. Participants were classified as the higher-risk group if they reported a low level of physical activity (1 = high or moderate level and 0 = low level). Daily sedentary time in the last 7 days was self-reported and subsequently dichotomized into a lower-risk behavior group (1: <8 h) or higher-risk group (0: ≥8 h).[26] Sleep duration in the last week was self-reported and participants were classified into three categories (<7 h per night, 7–8 h per night, and <8 h per night). Based on recommendations for the optimal amount of sleep for the elderly,[27] participants were dichotomized into a lower-risk group (1 = 7–8 h) or higher-risk group (0 = <7 h or ≥8 h). Dietary behavior in the past year were assessed using Questionnaire of the Chinese Nutrition and Health Surveillance,[28] which covered the frequency and quantity of fruits, vegetables, fish, meat, and other types of foods consumed. Additionally, the Diet Balance Index (DBI-07) was calculated to evaluate the dietary quality.[29] Participants with a total DBI-07 score ranging from 1 to 29 points were classified into the lower-risk group (1 = balanced diet); otherwise, individuals were classified into the higher-risk group (0 = unbalanced diet).
2.3.3. HRQoL measurement
The HRQoL was assessed using the Medical Outcomes Study 36-item Short Form Health Survey (SF-36),[30] which was translated into Chinese and exhibits high reliability and validity in the Chinese elderly population.[31] The SF-36 contains 36 items grouped into eight main domains that constitute two components. Thus, physical functioning (PF), role-physical (RP), bodily pain (BP), and general health (GH) constitute the physical health component, while vitality (VT), social functioning (SF), role-emotional (RE), and mental health (MH) constitute the mental health component. The eight domains were scored on a scale from 0 to 100 points, indicating the worst to best possible health, respectively. Scores for all domains were further summarized and standardized into the physical health component score (PCS) and the MH component score (MCS) according to a user manual, with higher scores representing a better HRQoL.[32]
2.4. Statistical analyses
Categorical variables are reported as n (%) and continuous variables are presented as the means ± SD. Comparison of socio-demographics, anthropometric data, HRQoL, and multiple lifestyle behaviors between male and female were conducted using chi-square test for categorical variables, and student's t test for continuous variables. Multivariate linear regression models were used to explore the associations between multiple lifestyle behavior and physical and mental components of HRQoL. In this regard, the strength of the association between high-risk and low-risk groups for each of lifestyle behavior was presented as β coefficient and 95% confidence interval (CI). Also, the relationship between each lifestyle behavior and HRQoL, when adjusted for age, gender, education, marital status, a history of hyperglycemia, a family history of diabetes, presence of chronic disease, BMI, hypertension, and WHR, was examined in model 1. Then, the relationship between each lifestyle behavior and HRQoL, when adjusted for all covariates included in model 1 as well as all other lifestyle behaviors, was examined in model 2. Finally, a multiple linear regression was used to examine the combined effects of the lower-risk lifestyle behavior index on both physical and mental health components of HRQoL, using the lower-risk lifestyle behavior index as a categorical variable, and adjusting for the above stated covariates. The number of lower-risk lifestyle behaviors was summarized as the lower-risk lifestyle behavior index, which was the sum of the number of lower-risk lifestyle behaviors a subject was engaged in. The possible values of this index ranged between 0 and 6 points. These values were classified into three categories, 0–2, 3–4, and 5–6, depending on the distribution of the data. The statistical analyses were conducted in SPSS version 20.0 (SPSS/IBM, Armonk, New York). All statistical tests were two-tailed and P < .05 was considered statistically significant.
3. Results
3.1. Characteristics of the study sample
Altogether, 2144 elderly individuals participated in the screening, of which 461 had prediabetes; thus, the prevalence of prediabetes in the total sample was 21.5% (461/2144). However, 27 subjects with prediabetes in this sample refused to participate in this survey for various reasons and, finally, 434 individuals with prediabetes were included in this study. The participants’ characteristics are summarized in Table 1. The average age was 69.4 ± 6.4 years. The majority of participants were female, and a large proportion was married and had completed <6 years of education. A minor proportion of the participants had a family history of diabetes and a history of hyperglycemia. The average BMI and WHR of all participants was 23.7 ± 3.6 kg/m2 and 0.93 ± 0.1, respectively.
Table 1.
Characteristics of the elderly individuals with prediabetes.
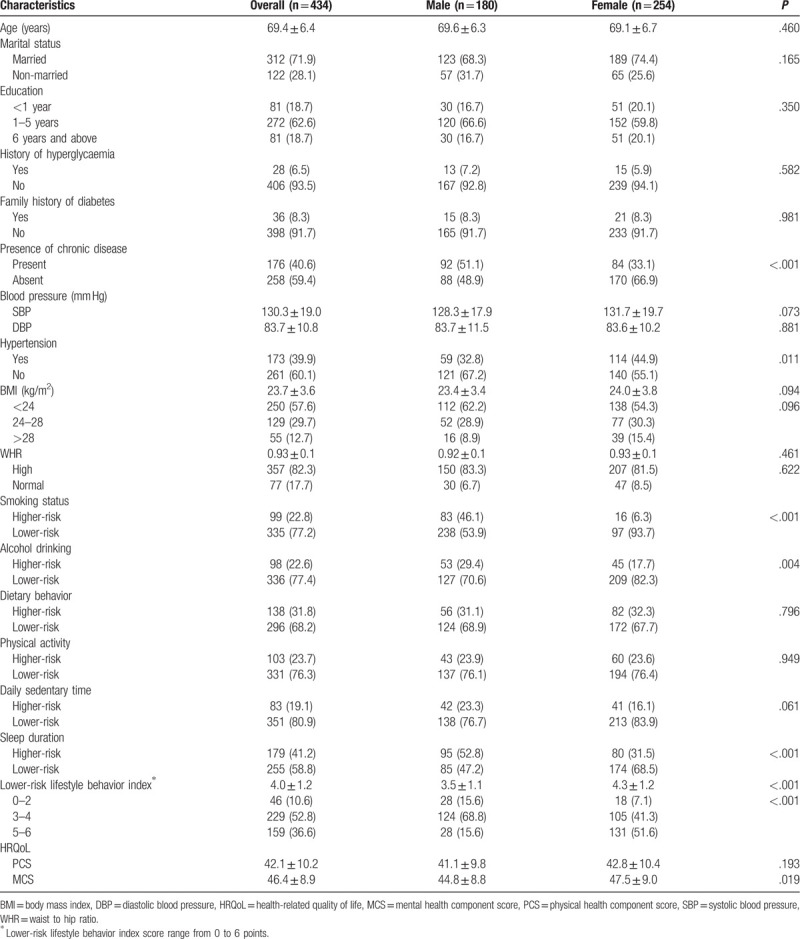
Approximately 77.2% of the participants were non-smokers or ex-smokers with lower-risk of alcohol drinking behaviors (77.4%), daily sedentary times (80.9%), sleep durations (58.8%), physical activity (76.3%), and dietary behavior (68.2%). The average lower-risk lifestyle behavior index score was 4.0 ± 1.2. Furthermore, 46 (10.6%) participants reported <2 lower-risk lifestyle behaviors and 159 (36.6%) participants reported 5 to 6 lower-risk lifestyle behaviors. Participants with prediabetes reported a mean ± SD PCS of 42.1 ± 10.2 and a mean ± SD MCS of 46.4 ± 8.9. The results were shown in Table 1.
3.2. Associations between lifestyle behaviors and HRQoL
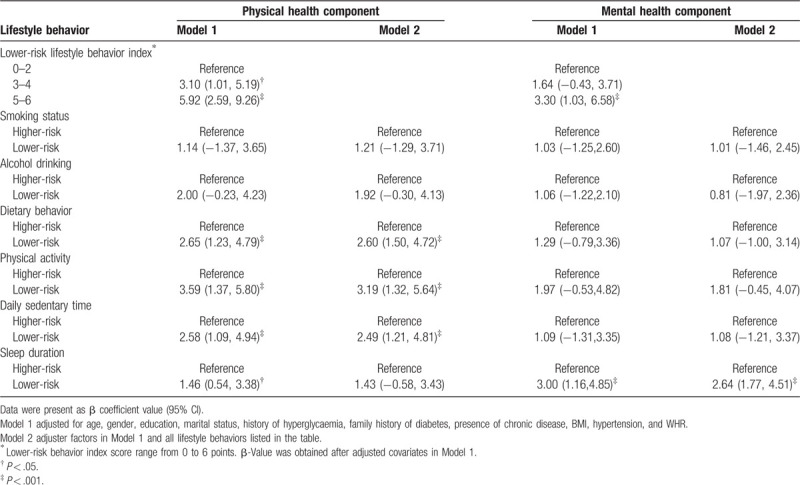
According to the multivariate linear regression analysis, adjusted for age, gender, education, marital status, a history of hyperglycaemia, a family history of diabetes, presence of chronic diseases, BMI, hypertension, WHR, and all lifestyle behaviors, lower-risk physical activity (β = 3.19, 95% CI: 1.32, 5.64), daily sedentary time (β = 2.49, 95% CI: 1.21, 4.81), and dietary habits (β = 2.60, 95% CI: 1.50, 4.72) were positively correlated with a higher PCS, while a lower-risk sleep duration (β = 2.64, 95% CI: 1.77, 4.51) was positively associated with a higher MCS.
Compared to participants engaging in 0 to 2 of lower-risk behaviors, a positive correlation with a better PCS was observed in the participants who engaged in 3 to 4 (β = 3.10, 95% CI: 1.01, 5.19) and 5 to 6 (β = 5.92, 95% CI: 2.59, 9.26) of lower-risk lifestyle behaviors. But a better MCS was observed only in participants who engaged in 5 to 6 (β = 3.30, 95% CI: 1.03, 6.58) of lower-risk lifestyle behaviors. The results are presented in Table 2.
Table 2.
Association between multiple lifestyle behaviors and physical and mental health components of HRQoL.
4. Discussion
The purpose of this study was to explore whether the lifestyle habits of smoking, alcohol consumption, daily sedentary time, physical activity, sleep duration, and dietary habits are associated with HRQoL. Lower-risk lifestyle behaviors, including physical activity, dietary habits, and daily sedentary time, were positively correlated with the physical health component of HRQoL, while lower-risk behavior in terms of sleep duration was positively correlated with the MCS component of HRQoL. Moreover, individuals who engaged in a greater number of healthy lifestyle behaviors had an increased likelihood of reporting better HRQoL.
Patients with prediabetes tend to report lower HRQoL than individuals with normal plasma glucose levels.33,34 From the public health perspective, older adults with prediabetes should be regarded as a target population for interventions and treatments, considering the high conversion rate of prediabetes to diabetes and the relationship between poor HRQoL and adverse health outcomes.[35] This study, therefore, provided significant evidence that individuals with a greater number of healthy lifestyle behaviors reported a better HRQoL, consistent with many previous studies. For instance, a study including 242 patients with type 1 diabetes reported an association between the clustering of unhealthy lifestyle habits and reduced HRQoL.[19] Another study conducted in a 10,000-step cohort indicated an association between engaging in a large number of poor lifestyle behaviors and a higher prevalence of poor HRQoL.[36] Furthermore, a longitudinal study revealed that each 5-point increase in the healthy lifestyle index score at baseline was associated with 1.7- and 2.5-fold higher scores for the change in physical and mental HRQoL components after 2.5 years of follow-up.[37] Many randomized controlled trials have reported the utility of comprehensive lifestyle interventions in delaying the progression from prediabetes to diabetes.[38] These phenomena may result from some type of cluster effect between healthy lifestyle behaviors and HRQoL, and provide new insights into lifestyle interventions to improve quality of life and health outcomes at the population level.[39]
The findings of an association between physical activity and HRQoL are consistent with other studies, which showed that a sufficient level of physical activity was associated with better physical and mental HRQoL.40,41,42 Specifically, a study conducted among 232 individuals residing in Northern Alberta indicated that participants with prediabetes, who reported adequate physical activity, scored 2.7 points higher on the PCS and 3.0 points higher on the MCS for HRQoL than those who had insufficient physical activity.[40] Moreover, a systematic review of 31 studies revealed a positive correlation between higher levels of physical activity and better HRQoL, and a dose–response relationship in this regard was also observed in several studies.[43] However, a bidirectional association between physical activity and HRQoL might exist, as subjects who perceive themselves as having better physical and MH are more likely to participate in a physical activity. In addition, the finding of an association between lower daily sedentary time and good physical health is also consistent with the results of previous research reports.36,44 For example, Duncan et al[36] indicated that people who spent more than 11 h per day sitting were 1.33 times more likely to report poor self-rated health than individuals who spent <8 h sitting per day. Similarly, prolonged sedentary time was associated with significantly lower PF and higher disability levels.[44] A potential explanation for these results is that people who report a longer sedentary time appear to be more likely to have lower daily living abilities and chronic conditions,[45] which exacerbates the poor PF and GH components of HRQoL.[10] Together with the evidence that physical activity interventions have been successful in increasing HRQoL,[46] the results of this study reinforce the need for increasing physical activity and reducing sedentary time, which is in line with many national physical activity guidelines of many countries.47,48
Furthermore, there has been an increasing number of epidemiological studies examining the relationship between dietary habits and HRQoL.49,50 For example, a systematic review including 15 studies showed that older adults with balanced dietary habits were more likely to report better self-rated health.[51] Moreover, adherence to healthy dietary habits, such as the Mediterranean diet, was noticeably associated with improvements in at least one of the HRQoL domains.[51] However, based on the results of this study, lower-risk dietary behaviors were not significantly associated with the mental health component of HRQoL. Nonetheless, many studies have reported that higher fruit and vegetable consumption, and moderate fish consumption were associated with higher scores on mental health.52,53 Despite the connections between diet and MH, evidence on the association between dietary behaviors and MH components, such as depression, is mixed54,55,56 and requires further research.
Also, the result of this study regarding the association between sleep duration and MCS component of HRQoL was consistent with previous findings.57,58 For example, a cohort study of 3834 older adults was conducted in Spain and found that participants with extreme sleep durations had lower scores on the physical and mental components of the SF-36 scale.[59] Another population-based study including 1418 elderly individuals showed that subjects who slept for <6 h attained lower SF-36 scores for the vitality and MH components than those who slept for 7 to 8 h.[60] Additionally, both short- and long-sleep durations increased the risk for dissatisfaction with life and negative emotions.61,62 However, based on accumulating evidence, sleep quality, rather than sleep duration, is strongly associated with HRQoL.63,64 The mechanism by which sleep quality and sleep duration interact with HRQoL is currently being investigated and further research is needed to ascertain these interactions.
This study has several limitations. First, causal and effect relationships could not be established due to the cross-sectional design. It is possible that having good physical health and mental health may enhance engagement in more low-risk lifestyle behaviors, and this might have influenced the findings of this study. Secondly, lifestyle behaviors and HRQoL were self-reported, hence, these measurements may not be accurate, which may underestimate or overestimate the investigated outcomes. Also, self-administered questionnaires were used to assess some experiences of the participants 1 month or 1 year before the start of this investigation. Therefore, recall bias was unavoidable. Thirdly, information may be lost due to dichotomization of the measurements of lifestyle behavior, which limited the ability to examine dose–response relationships between lifestyle behavior and HRQoL. Moreover, every type of lifestyle behavior was equally weighted and contributed to the lower-risk lifestyle behavior index. This combined effect may not be accurate given the different β coefficients for each lifestyle behavior on HRQoL, and limited categories of the lower-risk lifestyle behavior index in this study. Finally, this study was conducted in the rural areas of one City in Hunan province in China. Therefore, the generalization of the results of this study to other populations should be carefully considered. Given these limitations, further cohort and longitudinal studies should be conducted to obtain a better understanding of the associations between multiple lifestyle behaviors and HRQoL.
5. Conclusions
In summary, lower-risk lifestyle behaviors were associated with both the physical and MH components of HRQoL among the elderly individuals with prediabetes in the rural areas of Yiyang City in China. Moreover, the participants with prediabetes, who engaged in a greater number of lower-risk lifestyle behaviors, were more likely to have a better HRQoL.
Acknowledgments
We thank all the older adults who participated in this study.
Author contributions
Conceptualization: Huilan Xu.
Investigation: Lulu Qin.
Writing – original draft: Zhao Hu.
Writing – review & editing: Lulu Qin, Atipatsa Chiwanda Kaminga, Huilan Xu.
Huilan Xu Orcid: 0000-0001-8948-3149
Footnotes
Abbreviations: BMI = body mass index, DBP = diastolic blood pressure, HRQoL = health-related quality of life, IFG = impaired fasting glucose, IGT = impaired glucose tolerance, MCS = mental health component score, PCS = physical health component score, SBP = systolic blood pressure, WHR = waist-to-hip ratio.
How to cite this article: Hu Z, Qin L, Kaminga AC, Xu H. Relationship between multiple lifestyle behaviours and health-related quality of life among elderly individuals with prediabetes in rural communities in China: A STROBE-compliant article. Medicine. 2020;99:15(e19560).
This study was funded by the Teachers Research Fund of Central South University (2013JSJJ034).
The authors have no conflicts of interest to disclose.
References
- [1]. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998;15:539–53. [DOI] [PubMed] [Google Scholar]
- [2]. Yang WY, Lu JM, Weng JP, et al. Prevalence of diabetes among men and women in China. N Engl J Med 2010;362:1090–101. [DOI] [PubMed] [Google Scholar]
- [3]. Forouhi NG, Luan J, Hennings S, et al. Incidence of type 2 diabetes in England and its association with baseline impaired fasting glucose: the Ely study 1990–2000. Diabet Med 2007;24:200–7. [DOI] [PubMed] [Google Scholar]
- [4]. Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–50. [DOI] [PubMed] [Google Scholar]
- [5]. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Ernstsen L, Nilsen SM, Espnes GA, et al. The predictive ability of self-rated health on ischaemic heart disease and all-cause mortality in elderly women and men: the Nord-Trondelag Health Study (HUNT). Age Ageing 2011;40:105–11. [DOI] [PubMed] [Google Scholar]
- [7]. Mishoe SC, Maclean JR. Assessment of health-related quality of life. Respir Care 2001;46:1236–57. [PubMed] [Google Scholar]
- [8]. Ghorbani A, Ziaee A, Esmailzadehha N, et al. Association between health-related quality of life and impaired glucose metabolism in Iran: the Qazvin Metabolic Diseases Study. Diabet Med 2014;31:754–8. [DOI] [PubMed] [Google Scholar]
- [9]. Hunger M, Holle R, Meisinger C, et al. Longitudinal changes in health-related quality of life in normal glucose tolerance, prediabetes and type 2 diabetes: results from the KORA S4/F4 cohort study. Qual Life Res 2014;23:2515–20. [DOI] [PubMed] [Google Scholar]
- [10]. Alonso J, Ferrer M, Gandek B, et al. Health-related quality of life associated with chronic conditions in eight countries: results from the International Quality of Life Assessment (IQOLA) Project. Qual Life Res 2004;13:283–98. [DOI] [PubMed] [Google Scholar]
- [11]. Ekelund U, Steene-Johannessen J, Brown WJ, et al. Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. Lancet 2016;388:1302–10. [DOI] [PubMed] [Google Scholar]
- [12]. Loef M, Walach H. The combined effects of healthy lifestyle behaviors on all cause mortality: a systematic review and meta-analysis. Prev Med 2012;55:163–70. [DOI] [PubMed] [Google Scholar]
- [13]. Teychenne M, Ball K, Salmon J. Physical activity and likelihood of depression in adults: a review. Prev Med 2008;46:397–411. [DOI] [PubMed] [Google Scholar]
- [14]. Hamer M, Stamatakis E, Steptoe A. Dose-response relationship between physical activity and mental health: the Scottish Health Survey. Br J Sports Med 2009;43:1111–4. [DOI] [PubMed] [Google Scholar]
- [15]. Jayasinghe UW, Harris MF, Parker SM, et al. The impact of health literacy and life style risk factors on health-related quality of life of Australian patients. Health Qual Life Outcomes 2016;14:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Raymond J, Johnson ST, Diehl-Jones W, et al. Walking, sedentary time and health-related quality life among kidney transplant recipients: an exploratory study. Transplant Proc 2016;48:59–64. [DOI] [PubMed] [Google Scholar]
- [17]. Magee CA, Caputi P, Iverson DC. Relationships between self-rated health, quality of life and sleep duration in middle aged and elderly Australians. Sleep Med 2011;12:346–50. [DOI] [PubMed] [Google Scholar]
- [18]. Dumuid D, Olds T, Lewis LK, et al. Health-related quality of life and lifestyle behavior clusters in school-aged children from 12 countries. J Pediatr 2017;183:178–83. [DOI] [PubMed] [Google Scholar]
- [19]. Mozzillo E, Zito E, Maffeis C, et al. Unhealthy lifestyle habits and diabetes-specific health-related quality of life in youths with type 1 diabetes. Acta Diabetol 2017;. 54:1073–80. [DOI] [PubMed] [Google Scholar]
- [20]. Qin L, Xu H. A cross-sectional study of the effect of health literacy on diabetes prevention and control among elderly individuals with prediabetes in rural China. BMJ Open 2016;6:e011077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Wu Y. Overweight and obesity in China. BMJ 2006;333:362–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Li Y, Yang L, Wang L, et al. Burden of hypertension in China: a nationally representative survey of 174,621 adults. Int J Cardiol 2017;227:516–23. [DOI] [PubMed] [Google Scholar]
- [23]. Rehm J, Mathers C, Popova S, et al. Alcohol and Global Health 1 Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet 2009;373:2223–33. [DOI] [PubMed] [Google Scholar]
- [24]. Yang YX, Wang XL, Leong PM, et al. New Chinese dietary guidelines: healthy eating patterns and food-based dietary recommendations. Asia Pac J Clin Nutr 2018;27:908–13. [DOI] [PubMed] [Google Scholar]
- [25]. Ren YJ, Su M, Liu QM, et al. Validation of the simplified Chinese-character version of the International Physical Activity Questionnaire-long form in urban community-dwelling Adults: a cross-sectional study in Hangzhou, China. Biomed Environ Sci 2017;30:255–63. [DOI] [PubMed] [Google Scholar]
- [26]. Chau JY, Grunseit AC, Chey T, et al. Daily sitting time and all-cause mortality: a meta-analysis. PLoS One 2013;8:e80000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Hirshkowitz M, Whiton K, Albert SM, et al. National Sleep Foundation's updated sleep duration recommendations: final report. Sleep Health 2015;1:233–43. [DOI] [PubMed] [Google Scholar]
- [28]. Zhang B, Zhai FY, Du SF, et al. The China Health and Nutrition Survey, 1989-2011. Obes Rev 2014;15: Suppl 1: 2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. Xu X, Hall J, Byles J, et al. Assessing dietary quality of older Chinese people using the Chinese Diet Balance Index (DBI). PLoS One 2015;10:e0121618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Ware JE, Jr, Gandek B. Overview of the SF-36 Health Survey and the International Quality of Life Assessment (IQOLA) Project. J Clin Epidemiol 1998;51:903–12. [DOI] [PubMed] [Google Scholar]
- [31]. Zhou B, Chen K, Wang JF, et al. Reliability and validity of a Short-Form Health Survey Scale (SF-36), Chinese version used in an elderly population of Zhejiang province in China. Zhonghua Liu Xing Bing Xue Za Zhi 2008;29:1193–8. [PubMed] [Google Scholar]
- [32]. Ware J, Kosinski M, Keller S. SF-36 physical and mental health summary scales: a user's manual. 5 Boston, MA: Health Assessment Lab, New England Medical Center; 1994. [Google Scholar]
- [33]. Tapp RJ, Dunstan DW, Phillips P, et al. Association between impaired glucose metabolism and quality of life: results from the Australian diabetes obesity and lifestyle study. Diabetes Res Clin Pract 2006;74:154–61. [DOI] [PubMed] [Google Scholar]
- [34]. Chittleborough CR, Baldock KL, Taylor AW, et al. Health status assessed by the SF-36 along the diabetes continuum in an Australian population. Qual Life Res 2006;15:687–94. [DOI] [PubMed] [Google Scholar]
- [35]. Ligthart S, van Herpt TTW, Leening MJG, et al. Lifetime risk of developing impaired glucose metabolism and eventual progression from prediabetes to type 2 diabetes: a prospective cohort study. Lancet Diabet Endocrinol 2016;4:44–51. [DOI] [PubMed] [Google Scholar]
- [36]. Duncan MJ, Kline CE, Vandelanotte C, et al. Cross-sectional associations between multiple lifestyle behaviors and health-related quality of life in the 10,000 Steps cohort. PLoS One 2014;9:e94184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37]. Leong TI, Weiland TJ, Jelinek GA, et al. Longitudinal associations of the healthy lifestyle index score with quality of life in people with multiple sclerosis: a prospective cohort study. Front Neurol 2018;9:874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38]. Yuen A, Sugeng Y, Weiland TJ, et al. Lifestyle and medication interventions for the prevention or delay of type 2 diabetes mellitus in prediabetes: a systematic review of randomised controlled trials. Austral N Z J Public Health 2010;34:172–8. [DOI] [PubMed] [Google Scholar]
- [39]. Owen N, Healy GN, Matthews CE, et al. Too much sitting: the population health science of sedentary behavior. Exerc Sport Sci Rev 2010;38:105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40]. Taylor LM, Spence JC, Raine K, et al. Physical activity and health-related quality of life in individuals with prediabetes. Diabetes Res Clin Pract 2010;90:15–21. [DOI] [PubMed] [Google Scholar]
- [41]. Thiel DM, Al Sayah F, Vallance JK, et al. Association between physical activity and health-related quality of life in adults with type 2 diabetes. Can J Diabetes 2017;41:58–63. [DOI] [PubMed] [Google Scholar]
- [42]. Paivarinne V, Kautiainen H, Heinonen A, et al. Relations between subdomains of physical activity, sedentary lifestyle, and quality of life in young adult men. Scand J Med Sci Sports 2018;28:1389–96. [DOI] [PubMed] [Google Scholar]
- [43]. Wu XY, Han LH, Zhang JH, et al. The influence of physical activity, sedentary behavior on health-related quality of life among the general population of children and adolescents: A systematic review. PLoS One 2017;12:e0187668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44]. van Roekel EH, Winkler EA, Bours MJ, et al. Associations of sedentary time and patterns of sedentary time accumulation with health-related quality of life in colorectal cancer survivors. Prev Med Rep 2016;4:262–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45]. Elmesmari R, Reilly JJ, Martin A, et al. Accelerometer measured levels of moderate-to-vigorous intensity physical activity and sedentary time in children and adolescents with chronic disease: A systematic review and meta-analysis. PLoS One 2017;12:e0179429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46]. Eaglehouse YL, Schafer GL, Arena VC, et al. Impact of a community-based lifestyle intervention program on health-related quality of life. Qual Life Res 2016;25:1903–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47]. Nelson ME, Rejeski WJ, Blair SN, et al. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc 2007;39:1435–45. [DOI] [PubMed] [Google Scholar]
- [48]. Sparling PB, Howard BJ, Dunstan DW, et al. Recommendations for physical activity in older adults. BMJ 2015;350: doi:10.1136/bmj.h100. [DOI] [PubMed] [Google Scholar]
- [49]. Turner-McGrievy G, Davidson CR, Billings DL. Dietary intake, eating behaviors, and quality of life in women with polycystic ovary syndrome who are trying to conceive. Hum Fertil (Camb) 2015;18:16–21. [DOI] [PubMed] [Google Scholar]
- [50]. Mardas M, Jamka M, Madry R, et al. Dietary habits changes and quality of life in patients undergoing chemotherapy for epithelial ovarian cancer. Support Care Cancer 2015;23:1015–23. [DOI] [PubMed] [Google Scholar]
- [51]. Govindaraju T, Sahle BW, McCaffrey TA, et al. Dietary patterns and quality of life in older adults: a systematic review. Nutrients 2018. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52]. Ruiz-Cabello P, Soriano-Maldonado A, Delgado-Fernandez M, et al. Association of dietary habits with psychosocial outcomes in women with fibromyalgia: the Al-Andalus project. J Acad Nutr Diet 2017;117:422–32. [DOI] [PubMed] [Google Scholar]
- [53]. Haapasalo V, de Vries H, Vandelanotte C, et al. Cross-sectional associations between multiple lifestyle behaviours and excellent well-being in Australian adults. Prev Med 2018;116:119–25. [DOI] [PubMed] [Google Scholar]
- [54]. Meegan AP, Perry IJ, Phillips CM. The association between dietary quality and dietary guideline adherence with mental health outcomes in adults: a cross-sectional analysis. Nutrients 2017. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55]. Simsek S, Baysoy G, Gencoglan S, et al. Effects of gluten-free diet on quality of life and depression in children with celiac disease. J Pediatr Gastroenterol Nutr 2015;61:303–6. [DOI] [PubMed] [Google Scholar]
- [56]. Jacka FN, Mykletun A, Berk M, et al. The association between habitual diet quality and the common mental disorders in community-dwelling adults: the Hordaland Health study. Psychosom Med 2011;73:483–90. [DOI] [PubMed] [Google Scholar]
- [57]. Chiu HF, Xiang YT, Dai J, et al. Sleep duration and quality of life in young rural Chinese residents. Behav Sleep Med 2013;11:360–8. [DOI] [PubMed] [Google Scholar]
- [58]. Alfano CM, Lichstein KL, Vander Wal GS, et al. Sleep duration change across breast cancer survivorship: associations with symptoms and health-related quality of life. Breast Cancer Res Treat 2011;130:243–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59]. Faubel R, Lopez-Garcia E, Guallar-Castillon P, et al. Sleep duration and health-related quality of life among older adults: a population-based cohort in Spain. Sleep 2009;32:1059–68. [PMC free article] [PubMed] [Google Scholar]
- [60]. Lima MG, Barros MB, Alves MC. Sleep duration and health status self-assessment (SF-36) in the elderly: a population-based study (ISA-Camp 2008). Cad Saude Publica 2012;28:1674–84. [DOI] [PubMed] [Google Scholar]
- [61]. Zhi TF, Sun XM, Li SJ, et al. Associations of sleep duration and sleep quality with life satisfaction in elderly Chinese: the mediating role of depression. Arch Gerontol Geriatr 2016;65:211–7. [DOI] [PubMed] [Google Scholar]
- [62]. Zhai L, Zhang H, Zhang DF. Sleep duration and depression among adults: a meta-analysis of prospective studies. Depression Anxiety 2015;32:664–70. [DOI] [PubMed] [Google Scholar]
- [63]. Pan CW, Cong X, Zhou HJ, et al. Self-reported sleep quality, duration, and health-related quality of life in older Chinese: evidence from a rural town in Suzhou, China. J Clin Sleep Med 2017;13:967–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64]. Ertan P, Yilmaz O, Caglayan M, et al. Relationship of sleep quality and quality of life in children with monosymptomatic enuresis. Child Care Health Dev 2009;35:469–74. [DOI] [PubMed] [Google Scholar]


