Abstract
Background:
Telerehabilitation in cardiology has the potential to become the alternative to regular outpatient cardiac rehabilitation. Our study focuses on the wrist heart rate monitor as a telerehabilitation device, defines detected limitations, and compares results between home-based and regular outpatient rehabilitation methods, related to physical fitness, quality of life, and training adherence. The study design was a randomized controlled trial.
Methods:
Eligible 56 cardiac rehabilitation patients were randomized into a 12-week regular outpatient training group (ROT) and interventional home-based telerehabilitation group (ITG). For both groups, the intensity of the training was prescribed to be performed at 70% to 80% of heart rate reserve for 60 minutes, 3 times a week. The ITG patients started their training with a wrist heart rate monitor in their home environment. These patients received feedback once a week, reflecting data uploaded on the internet application. The ROT patients performed their exercise under the direct supervision of a physical specialist in a regular outpatient clinic. Physical fitness and health-related quality of life were assessed at baseline and after 12 weeks. Training adherence in both groups was determined and compared.
Results:
Fifty-one patients comleted the intervention (91%); no serious adverse events were recorded. Physical fitness expressed as peak oxygen uptake showed significant improvement (P < .001) in ROT group from 23.4 ± 3.3 to 25.9 ± 4.1 mL/kg/min and (P < .01) in ITG group from 23.7 ± 4.1 to 26.5 ± 5.7 mL/kg/min without significant between-group differences after 12 weeks of intervention. The training adherence between groups was similar.
Conclusion:
Our study shows that telerehabilitation via wrist heart rate monitor could become an alternative kind of cardiac rehabilitation which deserves attention and further analyzing.
Keywords: cardiac rehabilitation, exercise training, health-related quality of life, mobile health, physical fitness, telerehabilitation
1. Introduction
Cardiovascular disease (CVD), including coronary heart disease and stroke, remains a leading cause of morbidity and mortality globally.[1] It has been well known for many decades that physical activity and exercise provide substantial benefits for the secondary prevention of CVD.[2,3] Hospitals and medical centers have provided patients with CVD with cardiac rehabilitation (CR) as an effective way to positively influence their physical fitness levels, which have a major role in reducing cardiovascular mortality.[4] Despite all the benefits that CR brings, patients’ training adherence remains low for many reasons.[5] These reasons are well known as a lack of structure, long waiting times of acceptance, transportation problems, early return to work, and a lack of knowledge of the beneficial effects of CR by both patients and healthcare providers.[6] Therefore, there is a need for innovative rehabilitation methods aiming at an increase in CR uptake.[7] Recommendations for secondary prevention of myocardial infarction support new strategies for providing CR and its development, such as the telerehabilitation (TR) model. They identified cardiac TR as a possible complementary form of center-based or outpatient CR programs.[8] TR is described as the use of information and telecommunication technologies to provide healthcare services between a patient and a healthcare professional over a long distance. It includes a multicomponent approach, such as remote monitoring, electronic learning, and tele-coaching.[9] One recent meta-analysis concluded nonsignificant differences in results, including functional capacity, quality of life (QOL), and adverse events among patients with CVD in cardiac TR or outpatient CR programs.[10] In contrast, the heart network council supports further research in cardiac TR to gain broader and more comprehensive findings on the effectiveness and efficiency of this method before implementation in existing healthcare systems.[11] Currently, physical activity in CR studies is often evaluated using questionnaires or accelerometers.[12] With the development of wearable technology, the ability to assess levels of physical activity is more reliable and accurate.[13,14]
Our study focuses on the use of the wrist heart rate monitor as a TR device, defines detected limitations, and compares the effect between home-based TR and regular outpatient CR methods related to physical fitness, QOL, and training adherence. The hypothesis of the study was determined that interventional home-based TR would lead to at least the same efficiency as regular outpatient CR. This manuscript reports the main results of the study.
2. Methods
2.1. Study design
Our study team realized a single prospective randomized controlled trial among cardiac patients at the University Hospital (UH) in Brno, the Czech Republic, from August 2018 to May 2019. The study itself complied with the Declaration of Helsinki, and the protocol was approved by the Ethical Committee of the UH Brno, the Czech Republic; the study participants had to sign an informed consent before the trial randomization. The protocol is also registered at Australian New Zealand Clinical Trial Registry with registration number: ACTRN12618001170213. The study inclines to CONSORT guidelines of reporting trials.[15] For more detailed information, see the study protocol.[16]
2.2. Population and randomization
Study cardiologist recommended all eligible patients from the UH Brno (Fig. 1). Their clinical status allowed them to undergo cardiopulmonary exercise test (CPET), they understood and wrote in Czech language. They also had to meet participation conditions – age above 18 years, diagnosed with CVD (angina pectoris, myocardial infarction in the last 6 months, with left ventricular ejection fraction >45%). All the patients had a heart revascularization (percutaneous angioplasty and aortocoronary bypass). As the separation into groups was random, the technical equipment (mobile phone and free internet access) was required in all participants.
Figure 1.
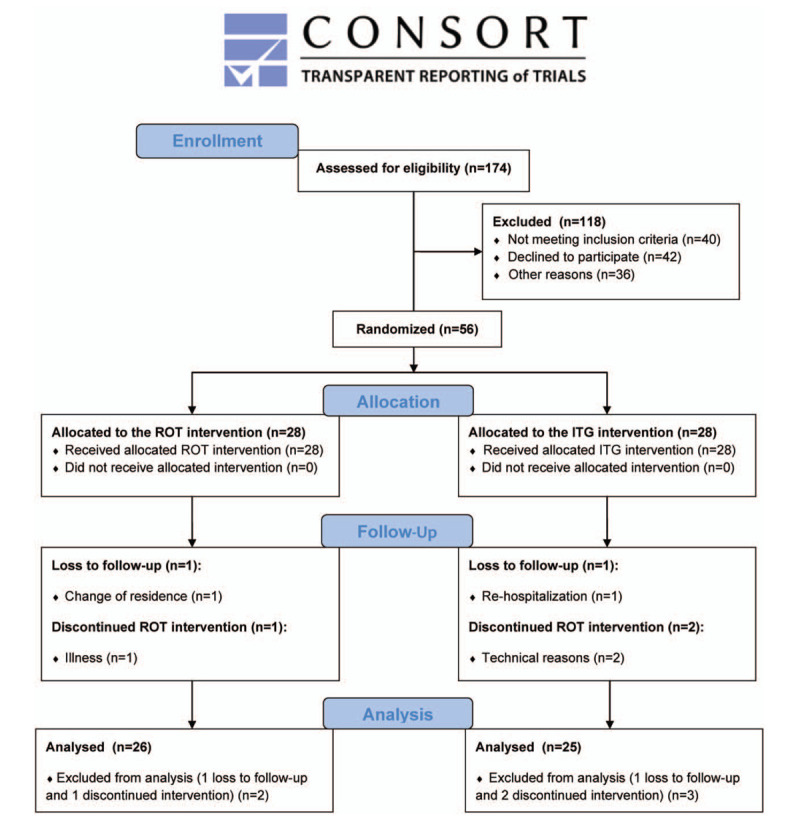
Flow chart diagram. ITG = interventional home-based telerehabilitation group, ROT = regular outpatient training group.
Exclusion participating criteria were significant cardiovascular risk, implanted cardioverter-defibrillator or pacemaker, residual coronary artery stenosis requiring revascularization, orthopedic or neurological disability to exercise, mental disadvantage making cooperation impossible, serious oncological disease, or treatment.
Fifty-six eligible patients had to undergo baseline assessment on cyclo ergometer Ergoselect 100 (Ergoline GmbH, Bitz, Germany), filled in the health-related QOL questionnaire SF-36 and study cardiologist tested their physical fitness through CPET. All the basement treatments were performed in the outpatient clinic. After that, they were given a study package containing personal data questionnaire (sex, age, diagnosis, and pharmacological treatment), a list of trial information, educational booklet (healthy diet advice, cardiovascular risk factors management, and smoking cessation) and an informed consent form. These patients were further separated into two groups via a computerized allocation system applying an algorithm in proportion 1:1, 28 participants using a telemedicine device in interventional home-based TR group (ITG), and 28 participants with regular outpatient training group (ROT).
2.3. Training description
The ITG patients had received 2 supervised training sessions in the outpatient clinic before they started a training program in their home environment. Patients were equipped with a wrist heart rate monitor M430 (Polar, Kempele, Finland) monitoring heart rate, time, training mode, duration, and distance of training physical activity.
The training period was set 3 times a week for 12 weeks altogether. One session consisted of 10 minutes warm-up, 60 minutes aerobic phase (walking or cycling, according to predefined training heart rate set at 70–80% heart rate reserve), and 10 minutes cool-down phase.
All training data were recorded by the wrist heart rate monitor and uploaded by each patient to a Polar Flow web application secured by individual login and password. Research assistant controlled these data further backed up on a separate secured external hard drive and later processed and evaluated.
During the trial, the physiotherapists checked the patients via telephone once a week and gave them feedback in the form of recommendations, advice, and training motivation.
The training period of the ROT patients was the same, set to 60 minutes per 1 session 3 times a week for 12 weeks. Unlike the ITG patients, they had to undergo a physical exercise workout under the direct supervision of a physiotherapist specializing in CR in the outpatient clinic as usual. Their training session consisted of 10 minutes warm-up, 60 minutes aerobic phase (cycling on ergometers and walking on treadmill, according to predefined training heart rate set at 70–80% heart rate reserve), and 10 minutes cool-down phase. Patients were also wearing a wrist heart rate monitor. All exercises were performed on cyclo ergometers E-Bike Basic (GE, Boston, MA, USA) and trademills Mill (Forcelink BV, Culemborg, Holland). The physiotherapist controlled each patient's heart rate data; they were uploaded to a secure external hard drive and later processed and evaluated. After 12 weeks of training, all patients, including ROT and ITG, were assessed one more time in physical fitness and filled in the SF-36 questionnaire.
2.4. Outcomes measures
2.4.1. Physical fitness
Primary outcomes were physical fitness values measured by individual maximal CPET test with respiratory gas analysis performed on a bicycle ergometer in the outpatient clinic using a ramp protocol. CPET was conducted according to the guidelines of the European Society of Cardiology and the American Cardiology Association.[17] The exercise consisted of 8 to 12 minutes cycling at 60 to 70 rpm frequency while recording a 12-lead electrocardiogram and blood pressure. Peak oxygen uptake (pVO2) was determined as the mean value of the last 30 seconds of exercise. Patients were supposed to exercise until they reached respiratory exchange ratio ≥1.10. Oxygen consumption was assessed by Metalyzer 3b (Cortex Biophysics GmbH, Leipzig, Germany).
2.4.2. Health-related quality of life
An SF-36 questionnaire is an option used for subjective evaluation of health-related QOL by the patient. The version of the questionnaire was translated into the Czech language. The SF-36 questionnaire assessed the secondary outcome values defining QOL consisted of 8 scaled scores, which are the weighted sums of the questions in 8 sections – vitality, physical functioning, bodily pain, general health perceptions, physical role functioning, emotional role functioning, social role functioning, and mental health. Each scale was directly transformed into a 0 to 100 scale on the assumption that each question carries equal weight. All the participants filled this questionnaire at baseline and after 12 weeks of training.[18]
2.4.3. Training adherence
Patients’ training adherence was defined as a percentage counted from the total number of accomplished training sessions of and individual participant. Patients in the ITG group recorded the training sessions in the Polar Flow web application using the wrist heart rate monitor. The ROT group adherence was determined as the number of attended training sessions at the outpatient clinic.
2.5. Statistical analysis
The sample size calculation was based on the Vysoký et al study, reporting improvement of pVO2 3.2 mL/kg/min with a standard deviation of 4.2 mL/kg/min.[19] We needed 56 participants to reach 80% of statistical power with the significance level set at P = .05. The estimated participant loss was 10%.[20] From the total number of 56 patients at discharge, we had to exclude (n = 2) participants from the ROT group and (n = 3) participants from the ITG during the trial. The reasons were illness, change of residence, rehospitalization, poor compliance, and technical problems. As we counted with the 10% loss, the number of participants needed to reach the statistical power was fulfilled. Excluded patients were not considered in the statistical analyses.
Values are expressed as mean (standard deviation). The primary outcome of our study was to find out whether the patients significantly improved their physical fitness and QOL values in both groups during the intervention. One-way repeated measures analysis of variance was used to determine significant differences across time periods. Where a significant difference occurred, Bonferroni post-hoc analyses were performed. Response to patients’ results between study groups was analyzed with mixed-factorial 2-way (group × time) analysis of variance. Significant differences across time or between groups were reported at alpha level of P < .05.
Training adherence was defined as a total number of accomplished training sessions at the outpatient clinic in ROT group and the number of recorded training sessions in the web application using the wrist heart rate monitor. We used the data to determine training adherence, its frequency and intensity, and time spent in the prescribed training heart rate. All metric data were processed in the statistical software Statistica 12 (TIBCO Software Inc, Palo Alto, CA).
3. Results
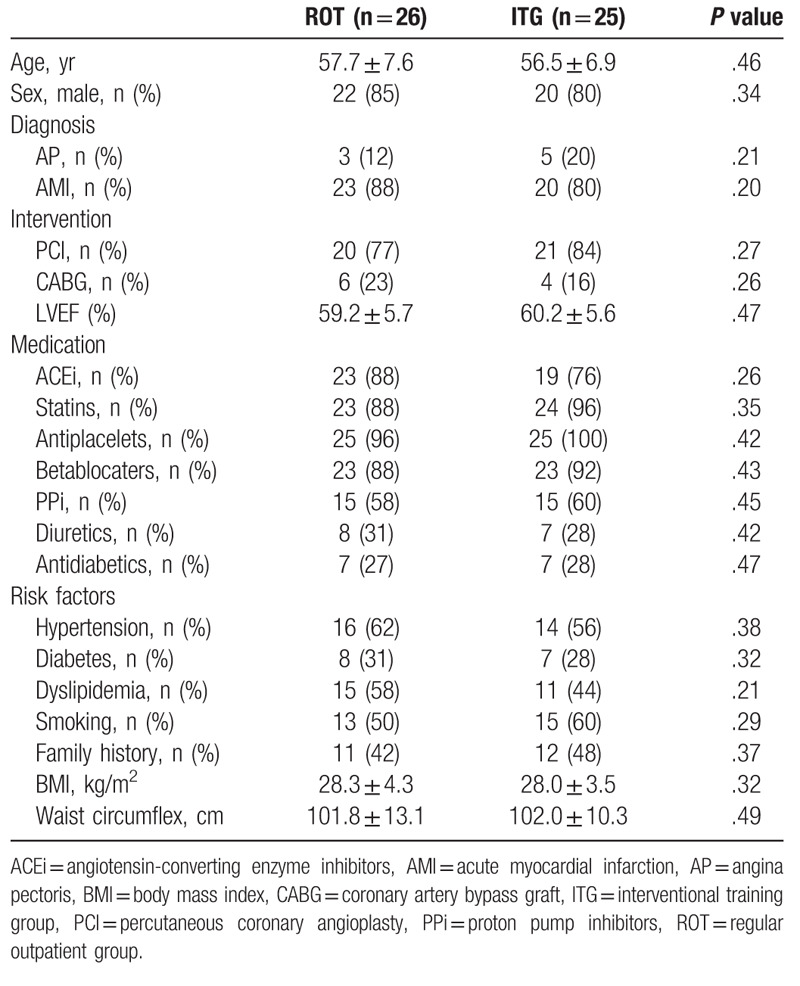
Out of the initial 174 patients with CVD of the UH Brno, 56 entered a controlled randomized 12-week trial. Remaining 118 of them did not meet inclusion criteria (n = 40), declined to participate (n = 42), or had other dropout reasons for not entering the trial (n = 36). Baseline characteristics of the ROT and ITG patients are described in Table 1.
Table 1.
Baseline characteristics.
Study participants were randomly separated into 2 groups – ITG group (n = 28) and ROT group (n = 28). During the trial another participants (n = 5) from ROT (n = 2) and from ITG (n = 3) left the study due to illness (n = 1), change of residence (n = 1), rehospitalization (n = 1), and technical reasons (n = 2).
3.1. Exercise data
Table 2 shows the outcome values measured at discharge and after 12 weeks of the intervention. We can see significant improvement in pVO2 within both groups ROT (Δ2.5 ± 3.7 mL/kg/min, P < .001) and ITG (Δ2.8 ± 4.7 mL/kg/min, P < .01), while there is no significant difference between groups. For parameter pWL the difference was not statistically significant in ROT (Δ16.3 ± 20.1 W, P < .001) and ITG (Δ23.3 ± 31.0 W, P < .001), nor is there a significant difference between the groups. Total health-related QOL improved significantly in both groups (P < .01) with no significant difference between groups.
Table 2.
Exercise data.
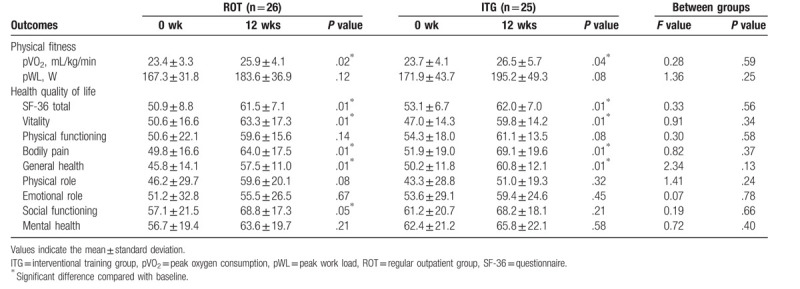
3.2. Training adherence
The ROT patients attended 30.1 ± 6.7 training units (83.6% of all sessions, ranging from 14 to 36). The total time duration of the aerobic training was 60.0 minutes. The total time duration of the training at the prescribed heart rate was 57.1 ± 3.9 minutes. The average intensity of the training was 75.5 ± 3.5% of heart rate reserve. No data was lost in the ROT group during the whole study.
The ITG group patients had 2 training sessions at the outpatient clinic, which were not included in the final sum of attended units. ITG performed 31.7 ± 8.9 training units (88.2% of all sessions, ranging from 10 to 48). The total time duration of the aerobic training phase was 58.0 ± 9.2 minutes. The total time duration of the training phase at the prescribed heart rate was 53.6 ± 9.6 minutes. The average intensity of the training was 74.8 ± 3.4% of heart rate reserve. Due to the low battery on the telemonitoring device, we have lost 5.4% of data (1.7 times per 1 participant on average). Total phone consultations with patients reached 68% of all calls (we have missed 42% of phone calls due to participants not being available).
3.3. Cardiac events and hospitalizations
Patients were advised to report any cardiac symptoms that occur during physical training in ITG and ROT group. None of the participants experienced an acute cardiac-related event, and there were no serious health complications associated with physical training during the 12-week intervention. There was one episode of chest pain symptom during the CPET; no further treatment was required, and the participant had recovered with the assistance under medical supervision. Another 2 patients (n = 1) from ITG and (n = 1) form the ROT group were admitted to the hospital due to cardiac symptoms during the 12-week intervention. After short observation in the UH, they were released without any required medical treatment.
4. Discussion
The most crucial reason to realize the study was to find out whether the cardiac TR could become a useful alternative model to regular outpatient CR without losing its clinical efficiency. Possibilities of home-based TR offer physical training for patients who cannot take part in regular outpatient CR in hospitals due to work, logistical reasons, or personal preferences.
The primary outcome was to compare the effect of home-based TR to regular outpatient CR program on physical fitness in patients with CVD. Our 12-week interventional study shows that TR training results in similar improvement of physical fitness in comparison to regular outpatient CR. As expected, physical training resulted in an increase in pVO2 (approximately 10%) in both groups, and this finding was consistent with other CR studies.[19,21–23] The observed effects are likely to be clinically relevant. It has been shown in the earlier study, that an increase in physical fitness levels is associated with an almost 20% reduction in cardiovascular mortality.[4]
Secondary outcome of the study was QOL of participants, which significantly increased in both groups. Our short term results correspond with systematic reviews, which prove that home-based and outpatient CR lead to similar effects on improving exercise capacity and health-related QOL.[24,25]
The overall recorded rate of completion of the intervention was high (91%), comparable to previous observations.[26,27] Despite concerns about participants’ technical problems with the use of a telemonitoring device, our research has shown the willingness of older people to engage in the use of new technologies. We expected that operating more technically complex equipment and sharing training data would be an obstacle to older people who are likely to be less able to operate than younger populations. However, the findings show that this population is already sufficiently capable of using telemedicine technologies in CR. The results of training adherence were high in both groups, approximately 85%. In contrast, the study by Skobel et al, which included TR via ECG sensor and smartphone, reported poor results of training adherence.[28] Patients may be able to independently follow the established training program in their home environment by receiving adequate motivational guidance and appropriate feedback. The findings support the Fit@Home trial, which used a similar monitoring method during TR intervention.[27]
The outcomes indicate that the implementation of this program could represent progress in the quality of care provision in the CR and support proper adherence to the treatment. TR program could increase the number of potential participants for CR who could benefit from the better adaptation of the program according to their different needs or lifestyle.[29]
The next part of the discussion is about training modalities. As in other studies on home-based CR, the combined exercise modality of walking and cycling was included in our ROT group, and in the ITG it was mostly one preferred modality (walking or cycling)[23,27] A possible change in training modalities during the physical training could have caused a different effect compared to the ITG group, where the patients were trained without interruption. Marzolini et al concluded that it is essential to incorporate a combination of different training modalities during one session, which in the home-based settings can be challenging to handle.[30]
It is important to note that due to the low sample size and short follow-up time in the study, we cannot establish a significant conclusion for patients with CVD. Our study confirms that remotely monitored TR appears to be safe, and no adverse cardiac events occurred during the intervention, similarly concluding a systematic review of previous studies. Accent on safety in the home-based CR method remains still a challenge. This is particularly important nowadays when the population of patients with CVD is aging, and its comorbidity and cardiovascular risk increase.
4.1. Study limitations
To make deeper insight into TR possibilities and problems, further trial and studies are needed. First, it is necessary to mention problems related to various monitoring TR devices described in previous studies.[22,23,28] For example, Kraal et al used a chest strap sensor in his study, monitoring heart rate during training.[27] Despite positive results, patients were complaining about discomfort wearing a device on their chest while performing the exercise. Although the wrist heart rate monitor used in our study solved this problem, there remained other indisputable weaknesses related to technical issues, such as inability to use the software, poor internet connection, or low device battery (=data loss).
In our study, the intensity of physical training was determined using a wrist heart rate monitor, but several patients experienced insufficient device battery charging (approximately 5% of cases). As a result, some patients prematurely discontinued the prescribed physical activity, which could lead to less reliable data. We assume that the development of innovative charging options (e.g., wireless charging pad) could solve this problem in the future. These limitations have led to the loss of training data and may also prevent the use of the software platform, thereby limiting the effectiveness of telemonitoring management.
As part of our research method, we provided feedback to patients through telephone contact. This feedback method seems to limit us because it was successful only in 68% of cases. The problem was the unavailability of patients or inappropriate time of the phone call. The possibility of video communication could provide a more personalized approach and better adherence to a particular population, similar to the text form of feedback that has shown effectiveness in other studies.
Another limitation was the sample included in the study, which was a characteristic similar to those in previous CR reports. However, a large number of patients refused to participate, which may be a potentially confusing difference in measured characteristics between our sample and a typical CVD population.
The present research method has been suggested in the context of CR, but many patients with other diseases (such as metabolic syndrome, diabetes) would probably also benefit from a TR-based training program. This offers the possibility to adapt the program to their individual needs.
Wearable sensors and remote data acquisition provide unique opportunities to measure physiological responses during physical training in environments and contexts that have not been possible before. At the beginning of our research, several devices were unsuitable for remotely monitored training, but now wearable sensors have become more frequent, and this rapidly evolving field is likely to provide opportunities to refine and optimize remotely monitored training.
5. Conclusion
The CR is widely used all over the world as an efficient tool in decreasing the CVD mortality rate. Despite all the granted positives it brings, current technological development allows us to think about time-effective solutions in medical fields. Telemedicine offers a wide variety of possibilities to use telemonitoring systems as an alternative solution to those who are unable to attend regular outpatient CR, have different kinds of difficulties to do so, or do not want to perform their exercise with others. Our study shows that TR via a wrist heart rate monitor could become an alternative kind of CR, which deserves attention and further analyzing.
Acknowledgment
The authors thank medical doctors, nurses, and physiotherapists of the Cardiovascular Rehabilitation Division, University Hospital Brno.
Author contributions
Conceptualization: Ladislav Batalik, Filip Dosbaba.
Data curation: Ladislav Batalik, Katerina Batalikova.
Funding acquisition: Filip Dosbaba, Martin Hartman.
Investigation: Ladislav Batalik, Martin Hartman.
Methodology: Martin Hartman.
Project administration: Filip Dosbaba.
Supervision: Jindrich Spinar.
Validation: Martin Hartman, Katerina Batalikova.
Writing – original draft: Ladislav Batalik, Katerina Batalikova.
Writing – review & editing: Filip Dosbaba, Katerina Batalikova, Jindrich Spinar.
Ladislav Batalik orcid: 0000-0003-2147-1541.
Footnotes
Abbreviations: CPET = cardiopulmonary exercise test, CR = cardiac rehabilitation, CVD = cardiovascular disease, ITG = interventional home-based telerehabilitation group, pVO2 = peak oxygen uptake, QOL = quality of life, ROT = regular outpatient training group, TR = telerehabilitation, UH = university hospital.
How to cite this article: Batalik L, Dosbaba F, Hartman M, Batalikova K, Spinar J. Benefits and effectiveness of using a wrist heart rate monitor as a telerehabilitation device in cardiac patients: a randomized controlled trial. Medicine. 2020;99:11(e19556).
This work was created with the sustained support of the Department of Cardiology and Internal Medicine, the UH Brno and Rehabilitation Department of the UH Brno.
This work was supported by Ministry of Health, Czech Republic, conceptual development of research organization (FNBr, 65269705, grant no NIG02/16).
The authors have no conflicts of interest to disclose.
References
- [1].Townsend N, Wilson L, Bhatnagar P, et al. Cardiovascular disease in Europe: epidemiological update 2016. Eur Heart J 2016;37:3232–45. [DOI] [PubMed] [Google Scholar]
- [2].Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: the evidence. CMAJ 2006;174:801–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lavie CJ, Arena R, Swift DL, et al. Exercise and the cardiovascular system clinical science and cardiovascular outcomes. Circ Res 2015;117:207–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lee DC, Sui XM, Artero EG, et al. Long-term effects of changes in cardiorespiratory fitness and body mass index on all-cause and cardiovascular disease mortality in men the aerobics center longitudinal study. Circulation 2011;124:2483–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Dunlay SM, Witt BJ, Allison TG, et al. Barriers to participation in cardiac rehabilitation. Am Heart J 2009;158:852–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cohen-Solal A. Ambulatory cardiac rehabilitation facilities should be present in every cardiology department. Eur J Prev Cardiol 2018;25:1704–6. [DOI] [PubMed] [Google Scholar]
- [7].Grace SL, Chessex C, Arthur H, et al. Systematizing inpatient referral to cardiac rehabilitation 2010 Canadian Association of Cardiac Rehabilitation and Canadian Cardiovascular Society Joint Position Paper. J Cardiopulm Rehabil Prev 2011;31:E1–8. [DOI] [PubMed] [Google Scholar]
- [8].PiepoliF MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice. Int J Behav Med 2017;24:321–419. [DOI] [PubMed] [Google Scholar]
- [9].Frederix I, Vanhees L, Dendale P, et al. A review of telerehabilitation for cardiac patients. J Telemed Telecare 2015;21:45–53. [DOI] [PubMed] [Google Scholar]
- [10].Chan C, Yamabayashi C, Syed N, et al. Exercise telemonitoring and telerehabilitation compared with traditional cardiac and pulmonary rehabilitation: a systematic review and meta-analysis. Physiother Can 2016;68:242–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. European Heart Network. Fighting Heart Disease and Stroke. EHN paper on eHealth; 2013. Available at: http://www.ehnheart.org/patients/papers/publication/698-ehn-paper-on-ehealth.html. Accessed 02 June 2019. [Google Scholar]
- [12].Kaminsky LA, Brubaker PH, Guazzi M, et al. Assessing physical activity as a core component in cardiac rehabilitation: a position statement of the american association of cardiovascular and pulmonary rehabilitation. J Cardiopulm Rehabil Prev 2016;36:217–29. [DOI] [PubMed] [Google Scholar]
- [13].Warren JM, Ekelund U, Besson H, et al. Assessment of physical activity-a review of methodologies with reference to epidemiological research: a report of the exercise physiology section of the European Association of Cardiovascular Prevention and Rehabilitation. Eur J Cardiovasc Prev Rehabil 2010;17:127–39. [DOI] [PubMed] [Google Scholar]
- [14].Brage S, Ekelund U, Brage N, et al. Hierarchy of individual calibration levels for heart rate and accelerometry to measure physical activity. J Appl Physiol 2007;103:682–92. [DOI] [PubMed] [Google Scholar]
- [15].Schulz KF, Altman DG, Moher D, et al. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Batalik L, Dosbaba F, Hartman M, et al. Rationale and design of randomized controlled trial protocol of cardiovascular rehabilitation based on the use of telemedicine technology in the Czech Republic (CR-GPS). Medicine 2018;97:e12385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Guazzi M, Adams V, Conraads V, et al. EACPR/AHA Scientific Statement. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation 2012;126:2261–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dempster M, Donnelly M. Measuring the health related quality of life of people with ischaemic heart disease. Heart 2000;83:641–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Vysoký R, Fiala J, Dosbaba F, et al. Preventive training programme for patients after acute coronary event – correlation between selected parameters and age groups. Cent Eur J Public Health 2015;23:208–13. [DOI] [PubMed] [Google Scholar]
- [20].Suresh K, Chandrashekara S. Sample size estimation and power analysis for clinical research studies. J Hum Reprod Sci 2012;5:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [21].Korzeniowska-Kubacka I, Bilinska M, Dobraszkiewicz-Wasilewska B, et al. Hybrid model of cardiac rehabilitation in men and women after myocardial infarction. Cardiol J 2015;22:212–8. [DOI] [PubMed] [Google Scholar]
- [22].Bravo-Escobar R, Gonzalez-Represas A, Gomez-Gonzalez AM, et al. Effectiveness and safety of a home-based cardiac rehabilitation programme of mixed surveillance in patients with ischemic heart disease at moderate cardiovascular risk: a randomised, controlled clinical trial. BMC Cardiovasc Disord 2017;17:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Maddison R, Rawstorn JC, Stewart RA, et al. Effects and costs of real-time cardiac telerehabilitation: randomised controlled non-inferiority trial. Heart 2019;105:122–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Anderson L, Sharp GA, Norton RJ, et al. Home-based versus centre-based cardiac rehabilitation. Cochrane Database Syst Rev 2017;6:CD007130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Huang K, Liu W, He D, et al. Telehealth interventions versus center-based cardiac rehabilitation of coronary artery disease: a systematic review and meta-analysis. Eur J Prev Cardiol 2015;22:959–71. [DOI] [PubMed] [Google Scholar]
- [26].Pardaens S, Willems AM, Clays E, et al. The impact of drop-out in cardiac rehabilitation on outcome among coronary artery disease patients. Eur J Prev Cardiol 2017;24:1490–7. [DOI] [PubMed] [Google Scholar]
- [27].Kraal JJ, Van den Akker-Van Marle ME, Abu-Hanna A, et al. Clinical and cost-effectiveness of home-based cardiac rehabilitation compared to conventional, centre-based cardiac rehabilitation: Results of the FIT@Home study. Eur J Prev Cardiol 2017;24:1260–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Skobel E, Knackstedt C, Martinez-Romero A, et al. Internet-based training of coronary artery patients: the heart cycle trial. Heart Vessels 2017;32:408–18. [DOI] [PubMed] [Google Scholar]
- [29].Kachur S, Chongthammakun V, Lavie CJ, et al. Impact of cardiac rehabilitation and exercise training programs in coronary heart disease. Prog Cardiovasc Dis 2017;60:103–14. [DOI] [PubMed] [Google Scholar]
- [30].Marzolini S, Oh PI, Brooks D. Effect of combined aerobic and resistance training versus aerobic training alone in individuals with coronary artery disease: a meta-analysis. Eur J Prev Cardiol 2012;19:81–94. [DOI] [PubMed] [Google Scholar]


