Abstract
Objective:
The objective of this study was to illustrate the prognostic value of diversified galectins in patients with hepatic cancer via meta-analysis.
Methods:
We conducted a systematic search on PubMed, Embase, The Cochrane Library, Web of Science, the Chinese National Knowledge Infrastructure (CNKI) database, and Wanfang Data for studies that reported associations between galectin expression and the prognosis for hepatic cancer patients, from the inception of each database to March 20, 2019. The combined hazard ratio (HR) and 95% confidence interval (CI) were estimated to investigate the prognosis.
Results:
We collected 11 studies of 1957 patients in our meta-analysis. The pooled results indicated that overall galectin expression was not correlated with OS (HR = 1.23, 95% CI = 0.84–1.79, P = .29) or DFS/RFS (HR = 0.808, 95% CI = 0.376–1.735, P = .42) in liver cancer patients. In stratified analyses, we observed that high galectin-1 and galectin-3 expression was significantly associated with poor OS. The pooled HR of galectin-4 and galectin-9 was correlated with improved OS.
Conclusion:
Our results indicate that the high expression of galectin-1 and -3 and the low expression of galectin-4 and -9 may be predictive prognostic factors for poor OS in liver cancer patients.
Keywords: galectin, hepatic cancer, meta-analysis, prognostic biomarker
1. Introduction
The prevalence of hepatocellular carcinoma (HCC) is becoming a critical global health issue, as its sixth leading cancer death for the estimated cancer types worldwide and even most common cancer mortality in some African and Asian countries.[1] Although many therapeutic approaches have entered clinical practice, including surgical resection, radiofrequency ablation, anhydrous alcohol tumor intravenous injection, hepatic artery interventional embolization chemotherapy and other local treatment means, the local recurrence and distant metastasis remain occurring ranging from 40% to 70% in patients.2,3,4 Patients with local or distant progression could still benefit from early treatment, so it is an urgent need to identify the high-risk patients with poor prognosis and start a new intensive program to improve their survival as soon as possible.
The galectins family, defined by their carbohydrate recognition domains (CRDs) with specific β-galactoside-binding affinity, is widely distributed in mammalian tissues.[5] They are involved in the control of cell apoptosis, cell cycle, cell division, pre-mRNA splicing and metastasis.[6] In humans, the galectins family contains galectin-1, -2, -3, -4, -7, -8, -9, -10, -12, -13, -14, and -16. Recent studies suggest that different kinds of galectins expressed in HCC, as potential prognostic roles, are associated with different outcomes of survival and clinical characteristics. According to their reports, their views on the prognostic role of various galectins remain controversial.
Meta-analysis is regarded as a useful tool that can combine the existing different studies on variety mixtures. So we conducted this meta-analysis to systematically and comprehensively evaluate the prognostic value of different types of galectins in HCC.
2. Methods
2.1. Literature search strategy
We performed a systematic search through the following databases: PubMed, Embase, The Cochrane Library, Web of Science, the Chinese National Knowledge Infrastructure (CNKI) database, and Wanfang Data (Chinese). The search included available data up to March 20, 2019. The main search terms included:(“galectin” or ”galectins” or “beta galactoside binding lectin” or ”D galactoside binding lectin” or ”beta D galactosyl specific lectin” or ”S type lectin” or “galactose binding lectin”) and (“liver neoplasm” or “hepatic neoplasm” or “hepatic cancer” or “hepatic carcinoma” or “liver Cancer”,” liver carcinoma”). The reference list was also checked for relevant articles. The study protocol was approved by the Ethics Committee of the Third Affiliated Hospital of Soochow University.
2.2. Inclusion and exclusion criteria
Inclusion criteria were as follows:
-
(1)
studied patients with hepatic cancer were confirmed by pathological examination;
-
(2)
galectin expression was measured by immunohistochemical methods in cancer tissues;
-
(3)
correlation of galectin expression with overall survival (OS) and/or progression-free survival (PFS) and/or recurrence-free survival (RFS) and/or diseasefree survival (DFS) were reported;
-
(4)
sufficient data were available for direct or indirect estimation of the hazard ratio (HR) and CI.
The exclusion criteria were as follows:
-
(1)
abstracts, case reports, letters, reviews, or nonclinical studies;
-
(2)
studies were not written in English or Chinese;
-
(3)
when studies had duplicated data or repeated patient cohorts, we used the most informative or up-to-date publication;
-
(4)
sample sizes were less than 50.
2.3. Data collection and quality assessment
All the candidate articles were assessed and collected respectively by two authors (Qi Shao and Jing He). If disagreements were present, the 2 authors discussed and reached a consensus with a third author (Zhiming Chen). For each study, the data were extracted and listed as follows: first author, year of publication, country, sample size, gender, galectin type, TNM (tumor, node, metastasis) stage, cut-off value, expression ratio, treatment strategy, HRs with 95% CIs. If HRs were not provided directly, we extracted the survival data from the Kaplan-Meier curves using the software Engauge Digitizer 4.1. The Newcastle-Ottawa Scale (NOS) was used to evaluate each study. NOS scores of 6 were designated as high-quality studies.
2.4. Statistical analysis
All the statistical analyses were performed using the statistical software STATA version 15.1 (Stata Corporation, College Station, TX). The correlation between galectins and clinical outcomes was evaluated by the HR and 95% CI. The pooled HRs and 95%CIs were used to assess the relationship between the galectin type and OS or RFS/DFS. The heterogeneity of the included studies was assessed using Cochran's Q test and Higgins I 2 test. Heterogeneity <0.10 or I 2 > 50% suggested significant heterogeneity in the literature and a random-effects model was used. When heterogeneity was not significant, a fixed-effects model was used. Subgroup analysis and sensitivity analysis were conducted to explore the origin of heterogeneity. Publication bias was assessed by Begg and Egger tests. All P values were 2-sided. A P < .05 was considered statistically significant.
3. Results
3.1. Study selection
A total of 557 studies were obtained from the 6 databases by following the systematic search strategy. A total of 307 studies were excluded by comprehensively screening the titles, abstracts, and publication types, and 338 studies remained after removing duplicates. Eventually, 11 retrospective studies7,8,9,10,11,12,13,14,15,16,17 involving of 13 cohorts and consisting of 1957 patients that were published between 2008 and 2017 were included in our meta-analysis. The flow diagram summarizes the study selection process (Fig. 1).
Figure 1.

Flow chart of the included studies.
3.2. Study characteristics
From the 11 selected studies, 9 studies were conducted on participants from China, 1 study was conducted on participants from Japan, and 1 study was conducted on participants from the Netherlands. Four studies reported the prognostic role of galectin-1, while 1 study reported the same for galectin-4, 3 studies reported the same for galectin-3, and 5 studies reported the same for galectin-9. All the studies reported OS or Kaplan-Meier curves, while RFS or DFS was assessed in 3 studies. We selected OS as the major survival outcome for all the available studies. HRs and 95%CIs were reported directly in 7 studies. In another 4 studies, the data was extracted from graphical survival plots. All selected studies used immunohistochemistry staining as the test method. The cut-off values for staining intensity differed between studies, although most studies choose score values of 3+ (or more) to designate the positive or high expression group. The baseline characteristics of the selected studies are summarized in Table 1.
Table 1.
Main clinical characteristics of all the studies included in the meta-analysis.

3.3. Correlation between galectins expression and OS
All studies evaluated the prognostic value of different types of galectins. Since the studies had significant statistical heterogeneity (I 2 = 88.5%, P < .001), we used the random-effects model to pool HRs. This pooled meta-analysis revealed that different galectin types had no significant association with OS in patients with hepatic tumors (HR = 1.23, 95% CI = 0.84 to 1.79, P = .29), as shown in Figure 2.
Figure 2.

Forest plots for the correlation between galentins expression and overall survival among patients with hepatic cancer.
Subsequently, we employed a subgroup analysis according to galectin type to further explore the potential sources of heterogeneity. Galectins could be classified into 3 groups according to their molecular-structure characteristics: “proto-type” galectins(galectin-1, -2, -5, -7, -10, -11, -13, -14, -15), “chimera-type” galectins (galectin-3), and “chimera-type tandem repeat-type” galectins (galectin-4, -6, -8, -9, -12).[18] Subgroup analysis for galectin types indicated that increased galectin-1 expression was significantly correlated with poor OS for patients with HCC (HR = 1.87, 95% CI = 1.61 to 2.16, P < .001). Inter-study heterogeneity (I 2 = 0.0%, P = .82) was not found, and a fixed-effects model was used (Fig. 3A). Patients with higher expression of galectin-3 had poor survival outcomes compared to patient's lower expression levels (HR = 3.29, 95% CI = 1.10–9.83, P = .03). We used the random-effects model to pool HRs based on significant statistical heterogeneity (I 2 = 85.0%, P = .001; Fig. 3B). In contrast, galectin-4 and galectin-9 expression predicted beneficial outcomes in HCC patients (HR = 0.57, 95% CI = 0.46–0.71, P < .001), and a fixed-effects model was carried out as no heterogeneity was present (I 2 = 0.0%, P = .73; Fig. 3C, Table 2).
Figure 3.

A, Forest plots for the relationship between the high expression of galectin-1 and OS. B, Forest plots for the relationship between the high expression of galectin-3 and OS. C, Forest plots for the relationship between the high expression of galectin-4, -9 and OS. OS = overall survival.
Table 2.
Summary of HRs of galectins expressions.

3.4. Correlation between galectins expression and DFS/RFS
DFS and RFS, considered as another clinical outcome and given their similar meaning, were used as a single united parameter. Three studies reported a relationship between DFS/RFS and galectin expression. A random-effects model (I 2 = 93.0%, P < .001) was adopted. Combined data revealed that galectin expression was not associated with DFS/RFS given the resulting pooled HR of 0.808 (95% CI = 0.376 to 1.735, P = .42; Fig. 4).
Figure 4.
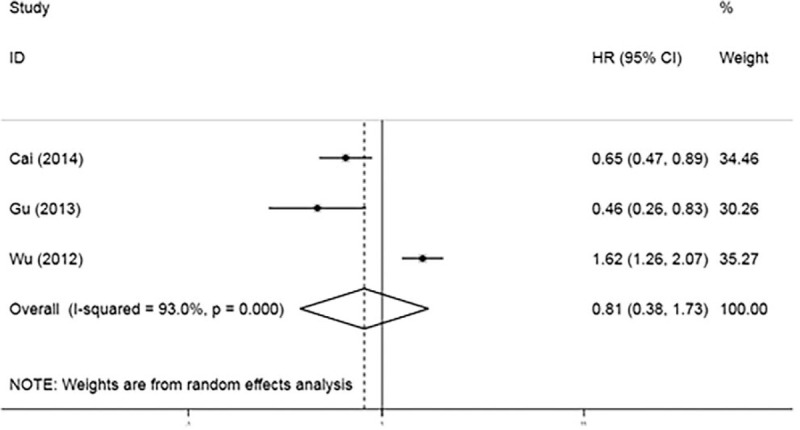
Forest plots for the correlation between galentins expression and disease-free survival/recurrence-free survival among hepatocellular carcinoma patients.
3.5. Sensitivity analysis and publication bias
We performed a sensitivity analysis to evaluate the stability of the combined HR and its 95%CI. As presented in Figure 5, credible results from the sensitivity analysis indicated that there was no significant heterogeneity among the included studies. Both Begg funnel plot and Egger test were used to assess the publication bias for OS. As presented in Figure 6, the results of Begg test (P = .76) and Egger test (P = .5) suggested that significant publication bias was not observed in this meta-analysis.
Figure 5.

Sensitivity analysis for the stability of the pooled hazard ratio and its 95% confidence interval.
Figure 6.

Begg and Egger test for the publication bias.
4. Discussion
A great number of studies have researched the effect of tissue galectin expression on the prognosis of HCC patients; however, conclusions regarding the prognostic role of galectin expression remained controversial. So we reviewed published studies and performed a meta-analysis to generate a more accurate estimate of the prognostic value of various galectin types. Our meta-analysis combined the outcomes of 1957 HCC patients and 4 types of galectins (galectin-1, -3, -4, -9) from 11 studies, and indicated that the 4 galectin types as a whole did not suggest any significant associations between OS (HR = 1.23, 95%CI = 0.84–1.79, P = .29) and RFS/DFS (HR = 0.808, 95%CI = 0.376–1.735, P = .42). Consequently, we conducted a subgroup analysis to find potential sources of heterogeneity. The subgroup analysis revealed that increased galectin-1 and galectin-3 expression were significantly correlated with poor OS, while the combined effects of galectin-4 and galectin-9 expression produced the opposite result. However, given that only 3 studies reported the relevant RFS/DFS data, further subgroup analyses could not be performed. Many enrolled studies analyzed the relationship between galectin expression and pathological parameters. Some results showed that galectins were related to cell differentiation, TNM stage, distant metastasis and recurrence, but the results were not consistent. As the parameters they selected were not exactly the same, we did not conduct statistical analysis. There are many similar and recent studies that support our findings. Wu[19] observed that high galectin-1 expression was associated with poor OS in digestive cancers. Wang[20] suggested that galectin-3 plays an oncogenic role, and is expressed in colorectal cancer, non-small cell lung cancer and ovarian cancer. In addition, Wang[21] reported that high galectin-9 expression in cancer tissue was correlated with improved CSS (cancer-specific survival) and weakly improved OS or DFS/RFS in cancer patients.
The family of galectins plays an important role in the control of apoptotic signaling pathways by regulating the immune response, inflammation, and angiogenesis.[22] Strikingly, very little attention has been given to understanding the molecular details behind this key regulatory network. Galectins families were found at both intracellular and extracellular sites. Galectin-1 and galectin-4 were expressed in both intracellular and extracellular compartments,[11] while galectin-3 and galectin-9 were mostly expressed in cytoplasm and a few expressed in the membrane and cytoplasm. Galectins family could serve as a tumor suppressor intracellularly and promote tumor metastases extracellularly during cancer development, which might confer opposing roles for galectins in cancer progression.[11] Galectin-1 is a prototype galectin with one carbohydrate recognition domain.[6] Recent studies showed that forced galectin-1 expression could trigger continuous activation of the MEK-ERK pathway and promote cell transformation.[23] This pathway works synergistically with other pathways to promote the expression of EMT-related genes.[24] Moreover, galectin-1 activates NF-κB in kidney cancer, inducing CXCR4 expression,[25] which may be a potential cause of galectin-1-induced HCC progression. Galectin-3 is the only chimera galectin.[6] It can be both antiapoptotic and proapoptotic. Galectin-3 can, like galectin-1, induces T-cell apoptosis by activating caspase-9 through the N-terminal end[26] and the coordination of CRDs.[27] Its antiapoptotic effect is dependent on caspase-3 activation[28] and the prevention of cytochrome c release.[29] Galectin-4 and -9 are tandem-repeat galectins with 2 CRDs joined by a linker sequence. Galectin-4 is mainly expressed in the gastrointestinal tract of healthy individuals,[30] and it reduces the production of proinflammatory cytokines in the intestine mucosa in a colitis model.[31] However, it can also promote intestinal inflammation by stimulating CD4+ T-cells to produce IL-6.32,33 Galectin-9 plays an important role in cancer immunotherapy.[34] The galectin-9/Tim-3 pathway is a key resistance mechanism to anti-PD-1.[35] Moreover, it mediates the close correlation of IgM and CD22, and Cao[36] suggested that the loss of this association provided an enhanced mechanism for the activation of galectin-9-deficient B cells. This meta-analysis was conducted to explore the relationship between expression level of galectins and survival of liver cancer patients. We think that different prognosis caused by different galectins was formed by their respective mechanisms. Additionally, owing to the limited number of studies, the prognostic accuracy and specificity of galectins remain controversial. In consequence, we look forward to more experimental studies and clinical trials on galectins.
This article was the first study to summarize the prognostic role of overall galectins for liver cancer patients. However, there were several limitations in this paper that need to be carefully considered. First, because China has a high incidence of liver cancer, most of the selected studies came from China and all selected studies were retrospective in design. The main limitation of our research is that the quality of the published data was relatively low. Second, significant heterogeneity existed in the selected studies. Given that the 11 studies we selected were mostly representative of Asian patients, many clinical features such as treatment plans and follow-up times were not reported; therefore, advanced subgroup analysis based on study type, ethnicity, cut-off values, and other principal features could not be performed. In particular, only 3 studies were included in the RFS/DFS analysis, resulting in insufficient data for the subgroup analysis. The heterogeneity could not be completely traced despite the utility of the sensitivity analysis. Third, 4 studies did not report HR values directly and we extracted survival data from the survival curves through the Engauge software, which might inevitably invite statistical bias in the pooled HR.
In summary, this meta-analysis demonstrated that galectin-1 and -3 might be negative prognostic factors and that galectin-4 and -9 might be positive prognostic factors for HCC patients. Future studies with well-designed, large-scale, prospective, randomized, controlled tests, and mechanism-based research are needed to confirm our conclusion.
Acknowledgment
The authors thank Bin Xu and Zhuojun Zheng for their supports and guidance throughout the project.
Author contributions
Conceptualization: Qi Shao, Zhiming Chen.
Data curation: Qi Shao, Jing He, Zhiming Chen.
Formal analysis: Qi Shao, Zhiming Chen.
Funding acquisition: Qi Shao, Zhiming Chen.
Investigation: Qi Shao, Zhiming Chen.
Methodology: Qi Shao, Zhiming Chen.
Project administration: Zhiming Chen.
Resources: Qi Shao, Zhiming Chen.
Software: Qi Shao.
Supervision: Changping Wu.
Validation: Changping Wu.
Visualization: Changping Wu.
Writing – original draft: Qi Shao.
Writing – review & editing: Qi Shao.
Footnotes
Abbreviations: CI = confidence interval, CRD = carbohydrate recognition domains, CSS = cancer-specific survival, DFS = disease-free survival, HCC = hepatocellular carcinoma, HR = hazard ratio, NOS = Newcastle-Ottawa Scale, OS = overall survival, PFS = progression-free survival, RFS = recurrence-free survival, TNM = tumor, node, metastasis.
How to cite this article: Shao Q, He J, Chen Z, Wu C. Prognostic role of galectins expression in patients with hepatic cancer: a meta-analysis. Medicine. 2020;99:14(e19622).
This work was supported by grants from the National Key R&D Program (2018YFC1313400), National Natural Science Foundation of Overseas Scholars Cooperation Research Project (31729001), National Natural Science Foundation of China (31570877, 31570908), the Key R&D Project of Science and Technology Department of Jiangsu Province (BE2018645).
The authors report no conflicts of interest.
References
- [1]. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [2]. Ozakyol A. Global epidemiology of hepatocellular carcinoma (HCC epidemiology). J Gastrointest Cancer 2017;48:238–40. [DOI] [PubMed] [Google Scholar]
- [3]. Liu X, Wang Z, Chen Z, et al. Efficacy and safety of transcatheter arterial chemoembolization and transcatheter arterial chemotherapy infusion in hepatocellular carcinoma: a systematic review and meta-analysis. Oncol Res 2018;26:231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Raoul JL, Gilabert M, Adhoute X, et al. An in-depth review of chemical angiogenesis inhibitors for treating hepatocellular carcinoma. Expert Opin Pharmacother 2017;18:1467–76. [DOI] [PubMed] [Google Scholar]
- [5]. Long B, Yu Z, Zhou H, et al. Clinical characteristics and prognostic significance of galectins for patients with gastric cancer: a meta-analysis. Int J Surg 2018;56:242–9. [DOI] [PubMed] [Google Scholar]
- [6]. Brinchmann MF, Patel DM, Iversen MH. The role of galectins as modulators of metabolism and inflammation. Mediators Inflamm 2018;2018:1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Sideras K, Biermann K, Verheij J, et al. PD-L1, Galectin-9 and CD8+ tumor-infiltrating lymphocytes are associated with survival in hepatocellular carcinoma. OncoImmunology 2017;6:e1273309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. J L. Clinical significance of Galectin-1 in HCC and Galectin-1 modulates sensitivity of hepatocellular carcinoma cells to the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) (Chinese) 2016. [Google Scholar]
- [9]. You Y, Tan JX, Dai HS, et al. MiRNA-22 inhibits oncogene galectin-1 in hepatocellular carcinoma. Oncotarget 2016;7:57099–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Zhang PF, Li KS, Shen YH, et al. Galectin-1 induces hepatocellular carcinoma EMT and sorafenib resistance by activating FAK/PI3K/AKT signaling. Cell Death Dis 2016;7:e2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Cai Z, Zeng Y, Xu B, et al. Galectin-4 serves as a prognostic biomarker for the early recurrence/metastasis of hepatocellular carcinoma. Cancer Sci 2014;105:1510–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Jiang S, Weng D, Wang Q, et al. Galectin-3 is associated with a poor prognosis in primary hepatocellular carcinoma. J Transl Med 2014;12:273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Kong F, Ma HX, Jiang J, et al. Expressions of Galectin-3 and Galectin-9 in hepatocellular carcinoma patients and their relationship with prognosis. J Jilin Univ Med Ed 2014;40:351–7. [Google Scholar]
- [14]. Gu CJ, Wu H, Sheng CY, et al. Expression and prognostic value of galectin-9 in hepatocellular carcinoma patients (Chinese). Zhonghua Yi Xue Za Zhi 2013;93:2025–8. [PubMed] [Google Scholar]
- [15]. Wu H, Chen P, Liao R, et al. Overexpression of galectin-1 is associated with poor prognosis in human hepatocellular carcinoma following resection. J Gastroenterol Hepatol 2012;27:1312–9. [DOI] [PubMed] [Google Scholar]
- [16]. Zhang ZY, Dong JH, Chen YW, et al. Galectin-9 acts as a prognostic factor with antimetastatic potential in hepatocellular carcinoma. Asian Pac J Cancer Prev 2012;13:2503–9. [DOI] [PubMed] [Google Scholar]
- [17]. Matsuda Y, Yamagiwa Y, Fukushima K, et al. Expression of galectin-3 involved in prognosis of patients with hepatocellular carcinoma. Hepatol Res 2008;38:1098–111. [DOI] [PubMed] [Google Scholar]
- [18]. Bacigalupo ML, Manzi M, Rabinovich GA, et al. Hierarchical and selective roles of galectins in hepatocarcinogenesis, liver fibrosis and inflammation of hepatocellular carcinoma. World J Gastroenterol 2013;19:8831–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Wu R, Wu T, Wang K, et al. Prognostic significance of galectin-1 expression in patients with cancer: a meta-analysis. Cancer Cell Int 2018;18:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Wang Y, Liu S, Tian Y, et al. Prognostic role of galectin-3 expression in patients with solid tumors: a meta-analysis of 36 eligible studies 11 Medical and Health Sciences 1112 Oncology and Carcinogenesis Aejaz Nasir. Cancer Cell Int 2018;18:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Wang K, Chen Z, Wu R, et al. Prognostic role of high gal-9 expression in solid tumours: a meta-analysis. Cell Physiol Biochem 2018;45:993–1002. [DOI] [PubMed] [Google Scholar]
- [22]. Chihara M, Kurita M, Yoshihara Y, et al. Clinical significance of serum galectin-9 and soluble CD155 levels in patients with systemic sclerosis. J Immunol Res 2018;2018:9473243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Astorgues-Xerri L, Riveiro ME, Tijeras-Raballand A, et al. Unraveling galectin-1 as a novel therapeutic target for cancer. Cancer Treat Rev 2014;40:307–19. [DOI] [PubMed] [Google Scholar]
- [24]. Jokinen E, Laurila N, Koivunen JP. Alternative dosing of dual PI3K and MEK inhibition in cancer therapy. BMC Cancer 2012;12:612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Huang CS, Tang SJ, Chung LY, et al. Galectin-1 upregulates CXCR4 to promote tumor progression and poor outcome in kidney cancer. J Am Soc Nephrol 2014;25:1486–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Fukumori T, Takenaka Y, Yoshii T, et al. CD29 and CD7 mediate galectin-3-induced type II T-cell apoptosis. Cancer Res 2003;63:8302–11. [PubMed] [Google Scholar]
- [27]. Xue H, Liu L, Zhao Z, et al. The N-terminal tail coordinates with carbohydrate recognition domain to mediate galectin-3 induced apoptosis in T cells. Oncotarget 2017;8:49824–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Takenaka Y, Fukumori T, Yoshii T, et al. Nuclear export of phosphorylated galectin-3 regulates its antiapoptotic activity in response to chemotherapeutic drugs. Mol Cell Biol 2004;24:4395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. Fukumori T, Oka N, Takenaka Y, et al. Galectin-3 regulates mitochondrial stability and antiapoptotic function in response to anticancer drug in prostate cancer. Cancer Res 2006;66:3114–9. [DOI] [PubMed] [Google Scholar]
- [30]. Paclik D, Danese S, Berndt U, et al. Galectin-4 controls intestinal inflammation by selective regulation of peripheral and mucosal T cell apoptosis and cell cycle. PLoS One 2008;3:e2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31]. Hokama A, Mizoguchi E, Sugimoto K, et al. Induced reactivity of intestinal CD4(+) T cells with an epithelial cell lectin, galectin-4, contributes to exacerbation of intestinal inflammation. Immunity 2004;20:681–93. [DOI] [PubMed] [Google Scholar]
- [32]. Kim SW, Park KC, Jeon SM, et al. Abrogation of galectin-4 expression promotes tumorigenesis in colorectal cancer. Cell Oncol (Dordr) 2013;36:169–78. [DOI] [PubMed] [Google Scholar]
- [33]. Satelli A, Rao PS, Thirumala S, et al. Galectin-4 functions as a tumor suppressor of human colorectal cancer. Int J Cancer 2011;129:799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34]. Sasidharan Nair V, Toor SM, Taha RZ, et al. DNA methylation and repressive histones in the promoters of PD-1, CTLA-4, TIM-3, LAG-3, TIGIT, PD-L1, and galectin-9 genes in human colorectal cancer. Clin Epigenetics 2018;10:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35]. Limagne E, Richard C, Thibaudin M, et al. Tim-3/galectin-9 pathway and mMDSC control primary and secondary resistances to PD-1 blockade in lung cancer patients. Oncoimmunology 2019;8:e1564505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36]. Cao A, Alluqmani N, Buhari FHM, et al. Galectin-9 binds IgM-BCR to regulate B cell signaling. Nat Commun 2018;9:3288. [DOI] [PMC free article] [PubMed] [Google Scholar]


