Abstract
Effects of mutations on AML (acute myeloid leukemia) patients have been an area of clinical interest. The aim of this study was to analyze pre-chemotherapy WBC (white blood cell), platelet, monocyte, hemoglobin, and mean platelet volume (MPV) levels in acute myeloid leukemia patients with Wilms tumor 1 (WT1), FMS-like tyrosine kinase 3 (FLT3), or nucleophosmin (NPM) gene mutations, attempting to detect and compare possible differences in these values.
The study included 71 patients with acute myeloid leukemia known to have WT1, FLT3, or NPM gene mutations. The patients were divided into 3 groups: FLT3-mutated AML patients without any accompanying known mutations other than WT1 at the time of diagnosis (Group 1), NPM-mutated AML patients without any accompanying known mutations other than WT1 at the time of diagnosis (Group 2), WT1-mutated AML patients with no other accompanying known mutations at the time of diagnosis (Group 3). We carried out intergroup comparisons of WBC, platelet (PLT), monocyte, hemoglobin, and MPV levels before chemotherapy.
There was a statistically significant difference between the groups in terms of WBC parameters (P = .001). There were no statistically significant differences between the groups with respect to hemoglobin, platelet, and monocyte levels.
Higher white blood cell counts could be observed in patients with FLT3-mutated AML.
Keywords: FLT3, hemogram, mutation, NPM, WT1
1. Introduction
Wilms tumor 1 (WT1), FMS-like tyrosine kinase 3 (FLT3), or nucleophosmin (NPM) gene mutations could be seen in acute myeloid leukemia patients. While NPM gene mutations, along with FLT3–ITD (FMS-like tyrosine kinase 3–internal tandem duplication) mutations, are known to exert a prognostic impact, they also play a role in determining the treatment approach to acute myeloid leukemia. Patients with FLT3–ITD mutations are considered to have a poor prognosis, so they are advised to receive allogeneic stem cell transplant following the induction therapy. WT1 mutations have yet to influence the clinical approach in patients with acute myeloid leukemia; they can only be used in the follow-up of minimal residual disease (MRD).
Braoudaki et al reported that there was no significant association between NPM1 mutation and high white blood cell (WBC) count in pediatric acute myeloid leukemia (AML) patients.[1] Besides, NPM1 (Nucleophosmin 1) gene is also named as NPM gene, Nucleophosmin (Nucleolar Phosphoprotein B23, Numatrin) gene, nucleophosmin gene and/or Nucleolar Protein NO38 gene.[2] On the other hand, Colovic et al showed that AML patients with FLT3/ITD mutations had higher WBC counts than patients without FLT3/ITD mutations.[3] Hereby, the aim of this study was to analyze pre-chemotherapy WBC, platelet, monocyte, hemoglobin, and mean platelet volume (MPV) levels in acute myeloid leukemia patients with WT1, FLT3, or NPM gene mutations, attempting to detect and compare possible differences in these values.
2. Materials and methods
The study included 71 patients with acute myeloid leukemia known to have WT1, FLT3, or NPM gene mutations from the patient files in the archives of the Hematology Polyclinic at the University of Health Sciences Dr. Abdurrahman Yurtaslan Ankara Oncology Education and Research Hospital. Patients under 18 years of age and foreign nationals (those who were not citizens of Turkish Republic) were excluded from the study. The study retrospectively analyzed pre-chemotherapy WBC, PLT (platelet), monocyte, hemoglobin, and MPV levels in acute myeloid leukemia patients with WT1, FLT3, or NPM gene mutations. The patients who had complete blood count (CBC) results before chemotherapy were divided into 3 groups: FLT3-mutated AML patients without any accompanying known mutations other than WT1 at the time of diagnosis (Group 1), NPM-mutated AML patients without any accompanying known mutations other than WT1 at the time of diagnosis (Group 2), WT1-mutated AML patients with no other accompanying known mutations at the time of diagnosis (Group 3).
We also carried out intergroup comparisons of WBC, PLT, monocyte, hemoglobin, and MPV levels before chemotherapy. The study was a retrospective review of medical records. The patients diagnosed with mixed phenotype acute leukemia and/or biphenotypic acute leukemia were also excluded from the study. ANOVA test (F-table value) was used for comparison of 3 or more independent groups with normal distribution, and Kruskal–Wallis H test (χ2-table value) statistics was used to compare the data with no normal distribution. Statistical analyses were performed with IBM SPSS Statistics 24.0 software package. P < .05 was considered statistically significant. Known mutation statuses in the patient files were retrospectively evaluated for the study. This study was approved by the local ethics committee in the hospital.
3. Results
There was a statistically significant difference between the groups in terms of WBC parameters (χ2 = 13.685; P = .001). Bonferroni-corrected values for paired comparisons conducted to determine from which group the significant difference stemmed revealed a statistically significant difference between the WBC value of the FLT3 and NPM group and that of WT1 group. WT1 group had significantly lower levels of WBC than those of FLT3 and NPM groups (Table 1). No difference was found between the groups in terms of hemoglobin, platelet, and monocyte levels (Table 1).
Table 1.
Comparison of the levels of WBC, PLT, monocyte, MPV, and hemoglobin according to the groups (patients with FLT3 mutation: Group 1, patients with NPM mutation: Group 2, patients with only WT1 mutation: Group 3).
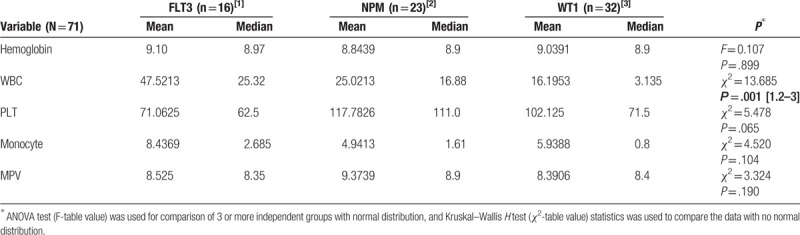
In the WT1 group, the MPV levels of 2 patients (6.25%) were higher than the normal range, and the MPV of 1 patient (3.125%) was lower than the normal range. In the FLT3 group, the MPV values of 2 patients (12.5%) were lower than the normal values, whereas the MPV of 1 patient (6.25%) was higher than the normal values. In the NPM group, MPV values of 4 patients (≈17.4%) were higher than the reference values, and the MPV values of 2 patients (≈8.7%) were lower than the normal values. On the other hand, no statistically significant difference was found between the groups in terms of MPV levels (Table 1).
4. Discussion
In their study, Santos et al reported a median WBC level of 37 × 109/L in AML patients with FLT3 mutation and a median WBC level of 14 × 109/L in those with no FLT3 mutation, showing that the median WBC level of patients with FLT3 mutation was significantly higher than that of patients with no FLT3 mutation.[4] They also found that there were no statistically significant differences between these groups in terms of platelet counts and hemoglobin levels.[4] Fröhling et al also reported that FLT3–ITD positivity was related to high white blood cell counts.[5] In addition, Hou et al demonstrated that there was no difference in the levels of WBC, hemoglobin, and PLT between AML patients with WT1 mutation and those with no WT1 mutation.[6] Chauhan et al also reported that patients with NPM1 mutation had higher platelet counts than those with no NPM1 and FLT3 mutations.[7] In our study, we found that AML patients with WT1 mutation only had lower WBC levels than those with FLT3 or NPM mutations. However, the platelet, monocyte, MPV, and hemoglobin levels showed no significant differences dependent on the existence of mutations. Furthermore, amino acid phosphorylation is an important process in signaling cascades. In their studies, Dwivedi et al performed phosphoproteomics analyses of granulocyte-colony stimulating factor receptors (G-CSFRs).[8,9] Phosphoproteomics analyses of the protein products of the aforementioned genes may give new information about the clinical findings of acute myeloid leukemia.
We found that higher WBC levels could be seen in patients with FLT3-mutated AML. This might be a potential reason for the worse prognosis of FLT3-mutations in AML. Rezaei et al showed that there were no significant differences in mean white blood cell counts between cytogenetically normal AML patients with wild-type and mutant FLT3–ITD and NPM1 genes.[10] But, we did not determine cytogenetics as an exclusion or inclusion criteria in our study. Furthermore, our study was a retrospective study. The patients were grouped according to their known mutation status, and the data in the patient files were used to gather the mutation status of the patients. Furthermore, only the known mutation statuses in the patient files could be used for analyses. On the other hand, mutations such as ASXL1 (Additional Sex Combs Like-1), CEBPA, RUNX1 (Runt-related transcription factor 1 gene) had not been routinely tested for the patients. Hilgendorf et al showed that ASXL1 could have a role in erythroid development and differentiation.[11] Walker et al reported an autosomaly dominant inherited point mutation of the RUNX1 gene that might cause thrombocytopenia.[12] One of the family members evaluated in the work of Walker et al developed AML.[12] Cai et al showed that early LOF (loss-of-function) RUNX1 mutations could reduce Ribi (ribosome biogenesis) in mice HSPCs (hematopoietic stem and progenitor cells).[13] In addition, we included all the patients whose records/files could be found during the period of study. Leukemia is an infrequent disease. Therefore, multiple center prospective studies may also help to include larger number of patients.
Further prospective studies which specify the mutations more detailly and more homogenous will give accurate results about the effect of mutations on hemogram parameters. Future studies in this area may also reveal new genetic mutations predicting worse prognosis in non-FLT3-mutated AML patients with high WBC levels.
Acknowledgments
The abstract of this study was presented at the 13th Balkan Congress of Human Genetics, 17–20 April 2019, in Edirne, Turkey. It was presented as oral presentation. The abstract number was S-35. And Alparslan Merdin is the main author of this study. The corresponding author Alparslan Merdin also wants to thank his father Hayri Merdin and his mother Zeynep Merdin for sharing their time during the study.
Author contributions
Conceptualization & Study Design: Alparslan Merdin.
Data curation: Alparslan Merdin, Mehmet Sinan Dal, Merih Kizil Çakar, Jale Yildiz, Bahar Uncu Ulu, Hikmetullah Batgi, Ayşegül Tetik, Nuran Ahu Baysal, Dicle İskender, Fevzi Altuntaş.
Formal analysis: Alparslan Merdin, Mehmet Sinan Dal, Merih Kizil Çakar, Jale Yildiz, Bahar Uncu Ulu, Hikmetullah Batgi, Ayşegül Tetik, Sema Sema Seçilmiş, Tahir Darçin, Derya Şahin, Mehmet Bakirtaş, Semih Başçi, Tuğçe Nur Yiğenoğlu, Nuran Ahu Baysal, Dicle İskender, Fevzi Altuntaş.
Investigation: Alparslan Merdin, Mehmet Sinan Dal, Merih Kizil Çakar, Jale Yildiz, Bahar Uncu Ulu, Fevzi Altuntaş.
Methodology: Alparslan Merdin, Mehmet Sinan Dal, Merih Kizil Çakar, Fevzi Altuntaş.
Project administration: Alparslan Merdin.
Software: Alparslan Merdin, Mehmet Sinan Dal, Merih Kizil Çakar, Jale Yildiz, Bahar Uncu Ulu, Hikmetullah Batgi, Ayşegül Tetik, Sema Seçilmiş, Tahir Darçin, Derya Şahin, Mehmet Bakirtaş, Semih Başçi, Tuğçe Nur Yiğenoğlu, Nuran Ahu Baysal, Dicle İskender, Fevzi Altuntaş.
Supervision: Alparslan Merdin, Mehmet Sinan Dal, Merih Kizil Çakar, Fevzi Altuntaş.
Writing – original draft: Alparslan Merdin, Mehmet Sinan Dal, Merih Kizil Çakar, Jale Yildiz, Bahar Uncu Ulu, Hikmetullah Batgi, Ayşegül Tetik, Sema Seçilmiş, Tahir Darçin, Derya Şahin, Mehmet Bakirtaş, Semih Başçi, Tuğçe Nur Yiğenoğlu, Nuran Ahu Baysal, Dicle İskender, Fevzi Altuntaş.
Footnotes
Abbreviations: AML = acute myeloid leukemia, ASXL1 = Additional Sex Combs Like-1, CBC = complete blood count, FLT3 = FMS-like tyrosine kinase 3, FLT3–ITD = FMS-like tyrosine kinase 3–internal tandem duplication, G-CSFRs = granulocyte-colony stimulating factor receptors, HSPCs = hematopoietic stem and progenitor cells, LOF = loss-of-function, MPV = mean platelet volume, MRD = minimal residual disease, NPM = nucleophosmin, PLT = platelet, Ribi = ribosome biogenesis, RUNX1 = Runt-related transcription factor 1 gene, WBC = white blood cell, WT1 = Wilms tumor 1.
How to cite this article: Merdin A, Dal MS, Çakar MK, Yildiz J, Ulu BU, Batgi H, Tetik A, Seçilmiş S, Darçin T, Şahin D, Bakirtaş M, Başçi S, Yiğenoğlu TN, Baysal NA, İskender D, Altuntaş F. Analysis of pre-chemotherapy WBC, PLT, monocyte, hemoglobin and MPV levels in acute myeloid leukemia patients with WT1, FLT3 or NPM gene mutations . Medicine. 2020;99:14(e19627).
The authors have no conflicts of interest to disclose. The authors received no financial support for the research.
References
- [1].Braoudaki M, Papathanassiou C, Katsibardi K, et al. The frequency of NPM1 mutations in childhood acute myeloid leukemia. J Hematol Oncol 2010;3:41.doi: 10.1186/1756-8722-3-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. https://ghr.nlm.nih.gov/gene/NPM1#synonyms. [Google Scholar]
- [3].Colovic N, Tosic N, Aveic S, et al. Importance of early detection and follow-up of FLT3 mutations in patients with acute myeloid leukemia. Ann Hematol 2007;86:741–7. Epub 2007 Jun 20. [DOI] [PubMed] [Google Scholar]
- [4].Santos FP, Jones D, Qiao W, et al. Prognostic value of FLT3 mutations among different cytogenetic subgroups in acute myeloid leukemia. Cancer 2011;117:2145–55. doi: 10.1002/cncr.25670. Epub 2010 Nov 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fröhling S, Schlenk RF, Breitruck J, et al. Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: a study of the AML Study Group Ulm. Blood 2002;100:4372–80. doi: 10.1182/blood-2002-05-1440. [DOI] [PubMed] [Google Scholar]
- [6].Hou HA, Huang TC, Lin LI, et al. WT1 mutation in 470 adult patients with acute myeloid leukemia: stability during disease evolution and implication of its incorporation into a survival scoring system. Blood 2010;115:5222–31. doi: 10.1182/blood-2009-12-259390. Epub 2010 Apr 5. [DOI] [PubMed] [Google Scholar]
- [7].Chauhan PS, Ihsan R, Singh LC, et al. Mutation of NPM1 and FLT3 genes in acute myeloid leukemia and their association with clinical and immunophenotypic features. Dis Markers 2013;35:581–8. doi: 10.1155/2013/582569. Epub 2013 Oct 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Dwivedi P, Muench DE, Wagner M, et al. Phospho serine and threonine analysis of normal and mutated granulocyte colony stimulating factor receptors. Sci Data 2019;6:21.doi: 10.1038/s41597-019-0015-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dwivedi P, Muench DE, Wagner M, et al. Time resolved quantitative phospho-tyrosine analysis reveals Bruton's tyrosine kinase mediated signaling downstream of the mutated granulocyte-colony stimulating factor receptors. Leukemia 2019;33:75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rezaei N, Arandi N, Valibeigi B, et al. FMS-like tyrosine kinase 3 (FLT3) and Nucleophosmin 1 (NPM1) in Iranian adult acute myeloid leukemia patients with normal karyotypes: mutation status and clinical and laboratory characteristics. Turk J Haematol 2017;34:300–6. doi: 10.4274/tjh.2016.0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hilgendorf S, Folkerts H, Schuringa JJ, et al. Loss of ASXL1 triggers an apoptotic response in human hematopoietic stem and progenitor cells. Exp Hematol 2016;44:1188–96. doi: 10.1016/j.exphem.2016.08.011. Epub 2016 Sep 8. [DOI] [PubMed] [Google Scholar]
- [12].Walker LC, Stevens J, Campbell H, et al. A novel inherited mutation of the transcription factor RUNX1 causes thrombocytopenia and may predispose to acute myeloid leukaemia. Br J Haematol 2002;117:878–81. [DOI] [PubMed] [Google Scholar]
- [13].Cai X, Gao L, Teng L, et al. Runx1 deficiency decreases ribosome biogenesis and confers stress resistance to hematopoietic stem and progenitor cells. Cell Stem Cell 2015;17:165–77. doi: 10.1016/j.stem.2015.06.002. Epub 2015 Jul 9. [DOI] [PMC free article] [PubMed] [Google Scholar]


