Abstract
Objective:
The aim of this systematic review was to evaluate the effect of therapies for cognitive impairment on patients’ perceived cognitive function in breast cancer survivors with chemotherapy-related cognitive impairment.
Method:
A literature search of PubMed, Embase, and the Cochrane Library was conducted up to April 2019. Search terms included breast cancer, chemotherapy, and cognitive impairment.
Result:
Six randomized controlled trials with a total of 305 patients were included in this review. A total of 6 randomized controlled trials using various treatments (Tibetan sound meditation, donepezil, memory and attention adaptation training, aerobic exercise, acupuncture, Qigong) for chemotherapy-related cognitive impairment met the eligibility criteria and were included. This review showed that meditative interventions (Tibetan sound meditation, Qigong) and cognitive therapy (memory and attention adaptation training) may partially improve some aspects of patients’ perceived (self-reported) cognitive functioning, particularly patients’ perceived cognitive impairment and ability.
Conclusion:
In this systematic review, the results showed that meditative interventions (Tibetan sound meditation, Qigong) and cognitive therapy (memory and attention adaptation training) may be optional therapies. We hope to have more randomized controlled trials to support this result in the future.
Keywords: breast cancer, chemotherapy, cognitive impairment, systematic review, therapy
1. Introduction
Globally, breast cancer (BC) is the most commonly occurring cancer and the most common cause of death among women.[1] With advances in the diagnosis and treatment of breast neoplasms, the survival rate has improved significantly.[2,3] Chemotherapy, as one of the treatments, plays an important role in decreasing the incidence and risk of death from BC. However, it also has severe side effects. According to a number of studies, up to 75% of patients with BC experience cognitive deficits after the end of cancer treatment,[4–8] and patients often report problems with memory, attention, executive function, and information processing speed.[7,9–13] These symptoms associated with chemotherapy are referred to as chemotherapy-related cognitive impairment (CRCI), also termed “chemobrain”.[13,14] The special mechanisms of this phenomenon remain unclear, but we do know that CRCI can negatively affect patients’ occupational performance and interpersonal relationships and undermine their overall quality of life (QOL).[15–18] Therefore, much more attention has been paid to the long-term effects of cancer treatment by clinicians and researchers.[19] In 2015, Morean et al[20] carried out a systematic review of the outcomes of objective neuropsychological measures of cognitive function to evaluate the effects of therapies for breast cancer survivors (BCS) with CRCI. Researchers have described relevant outcomes of cognitive function to assess the effects of therapies; moreover, since 2015, new treatments for CRCI have been developed. Thus, the objective of this systematic review is to comprehensively summarize the evidence and evaluate the effects of therapies on patients’ perceived cognitive functioning for BCS treated with chemotherapy.
2. Methods
The guidelines for this systematic review were based on PRISMA recommendations, and a protocol for this review was published in PROSPERO with the registration number CRD42019133469.
2.1. Literature search strategy
An electronic search of 3 databases (PubMed, Embase, and the Cochrane Library) was conducted from their inception to April 2019 using the following keywords: (“breast cancer”) and (“cognitive impairment”) and (“randomized controlled trial”). In addition, the references of relevant articles were hand-searched for records that may have been missed.
2.2. Criteria for inclusion and exclusion
The inclusion criteria were as follows:
-
(1)
studies of women with BC who received (or had received) chemotherapy and reported cognitive dysfunction;
-
(2)
randomized controlled trials (RCTs); and
-
(3)
study results were provided by a subjective measurement, Functional Assessment of Cancer Therapy-Cognitive Function (FACT-COG) version 3, of cognition.[21]
Exclusion criteria were as follows:
-
(1)
studies of women with BC combined with other cancers or having brain metastasis;
-
(2)
studies of women having a disease that would impact brain function (such as brain injury, central nervous system (CNS) disease, psychiatric disorders);
-
(3)
studies focusing on side effects other than CRCI;
-
(4)
studies with a design other than an RCT (eg, case report, review);
-
(5)
studies with no full-text access; or
-
(6)
studies without detailed data.
2.3. Data extraction and quality assessment
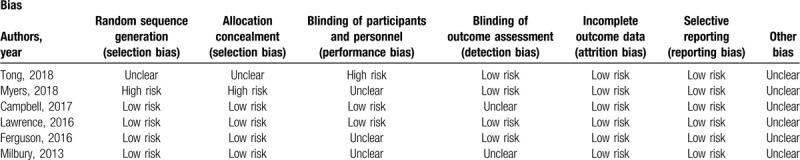
Two investigators independently extracted data on the characteristics of the included studies (eg, first author name, publication year, intervention types, sample size), and they assessed the risk of bias in individual studies by using the Cochrane Collaboration's Tool in the following aspects: The assessment includes sequence generation; allocation concealment; blinding of participants, personnel, and outcome assessors; incomplete outcome data; selective outcome reporting; and other sources of bias. Any differences between the authors on the data extraction and quality assessment were resolved by discussion.
3. Results
3.1. Study selection
A total of 700 records were identified from PubMed (346), Embase (202), and the Cochrane Library (152). Only 191 studies were left after the removal of obvious irrelevant studies and duplicates. Then, titles and abstracts of the 191 studies were reviewed, and 173 studies were excluded for various reasons. The remaining 18 records were further reviewed, 6 of which were eliminated because the full text was not accessible. Six RCTs did not provide outcomes of interest (the FACT-COG version 3, a subjective measure for patients’ self-perceived cognitive function) and were, therefore, excluded from this review.[18,22,25–28] Finally, 6 studies[12,13,16,17,23,24] were included in this review.
3.2. Quality assessment
The risk of bias in individual studies is shown in Table 1.
Table 1.
Risk of bias assessment of the included studies using the Cochrane risk of bias tool.
3.3. Description of the included studies
Due to differences in the reporting of outcome measures and interventions, a meta-analysis was not possible. Finally, the literature search revealed 6 RCTs in this review, and all these studies were published from 2013 to 2018 and were presented in English.
3.4. Types of participants
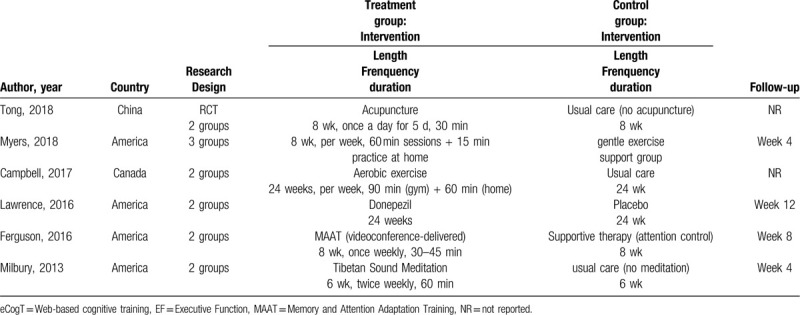
A total of 305 BC patients were included in the included studies. Characteristics of the patient populations included sample size of groups, age, education, menopausal status, BC stage and treatment. The total sample size of each included study was small and less than 100, and Campbell et al[23] only studied less than 20. Enrolled participants’ mean age ranged from 53 to 57 years old, and the average education level was college or above. Most of the patients included in the studies were post-menopausal; menopausal status was not reported in Ferguson et al.[12] BC stage and treatment were both well reported. All but one[17] of the included studies performed comparative statistics to demonstrate that there were no significant differences in the characteristics of participants in the intervention group and control group. Participant characteristics are outlined in Table 2.
Table 2.
Characteristics of participants.

3.5. Types of interventions
These studies contained therapies for cognitive impairment associated with chemotherapy for BCS, including medical treatment and nonmedical treatment. In the eligible literature, only 1 study used medication to address CRCI.[17] The remaining 5 studies were nonmedical treatments, including Tibetan sound meditation, memory and attention adaptation training, aerobic exercise, acupuncture, and Qigong. All but 1 study took place in patients’ homes[17]; the other study took place in specific institutions and required participants to practice at home. Five studies had 2 intervention arms,[12,13,16,17,23] 1 had 3 arms,[24] and all the interventions aimed to improve patients’ cognitive function (self-reported cognitive function, neuropsychological test performance, or both). In addition, the intervention schedules of each included study were clearly reported, including length, frequency, duration and follow-up. The characteristics of the 6 eligible studies are outlined in Table 3.
Table 3.
Characteristics of the included studies.
3.6. Outcomes
In regard to the outcome measures, for the objective of this review, all studies that used FACT-COG (version 3) as a measurement for self-reported cognitive function were included. The FACT-COG (version 3) is a validated assessment for cancer patients’ perceived cognitive function and impact on QOL over the previous 7 days.[21] It yields 4 subscales (perceived cognitive impairment [PCI], perceived cognitive abilities, impact on QOL and comments from others); the higher the scale score, the better cognitive functioning.
In the eligible studies, the mean scale scores on the FACT-COG of the intervention group were all improved after treatment; however, compared to the control group, there was no statistically significant improvement in most of the included studies. Milbury et al[16] reported marginally significantly lower levels of PCI (P = 0.06) and better perceived cognitive ability (P = 0.08) after treatment in the Tibetan Sound Meditation (TSM) group. Ferguson et al[12] hypothesized that patients who received memory and attention adaptation training would have greater amelioration in self-reported cognitive impairment, and the results showed that the hypothesis for the FACT-Cog PCI scale was supported at follow-up time (P = 0.02). Myers et al[24] demonstrated significant improvement of the Qigong group in the FACT-Cog subscales for PCI and perceived cognitive ability (P = 0.01, P = 0.04).
4. Discussion
We carried out this systematic review to estimate the effect of therapies for chemotherapy-associated cognitive impairment on BCS’ perceived cognitive function. In this review, the results showed that meditative interventions (Tibetan sound meditation, Qigong) and cognitive therapy (memory and attention adaptation training) may partially improve some aspects of patients’ perceived (self-reported) cognitive functioning, particularly patients’ PCI and ability.
Cognitive dysfunction, as one of the side effects of chemotherapy, is not easier to detect than other side effects (such as nausea and vomiting), and only patients can perceive subtle cognitive changes and truly feel the effects of the treatments themselves. Therefore, patient-reported outcomes are an important indicator for evaluations of the effects of treatments. Previous reviews compared the objectively measured outcomes of various therapies for BCS with CRCI and found favorable effects of these therapies designed to improve patients’ verbal memory, attention, and processing speed hold the most promise.[20] To date, no review has focused on the subjectively measured outcomes of treatments for CRCI in women with BC. This review found 6 trials that matched our criteria. All these studies provided subjective measurements using FACT-COG (version 3), which was our observational indicator. However, the interventions used in the included studies were different, and the results were mixed. Patients’ perceived (self-reported) cognitive functioning was not significantly improved in studies except for those of Milbury et al,[16] Ferguson et al,[12] and Myers et al.[24]
Some limitations of this review should be noted. First, the small number of included studies and sample size. Second, we included only full-text published RCTs, unpublished or published as abstracts. Moreover, probably because we included only one type of study, we did not find the exact same cognitive interventions. Third, due to the diverse measurements, we excluded some studies that used different scales. We strongly suggest consistent measurements to be used in future research to facilitate data consolidation. Finally, there may exist potential publication bias in our results because of the small number of included studies.
5. Conclusion
In conclusion, for BCS who reported cognitive deficits after receiving chemotherapy, effective intervention strategies to address this problem remain limited, and mixed results have been reported in many studies. In this systematic review, the results show that meditative interventions (Tibetan sound meditation, Qigong) and cognitive therapy (memory and attention adaptation training) may be optional therapies. We hope to have more RCTs to support this result in the future.
Author contributions
Conceptualization: Xiufei Gao, Shengliang Qiu.
Data curation: Shan Liu, Linghong Yu, Jiayan Zhu.
Formal analysis: Qiuhua Zhang, Shan Liu.
Software: Shan Liu, Linghong Yu, Jiayan Zhu.
Supervision: Xiufei Gao, Shengliang Qiu.
Validation: Shengliang Qiu.
Writing – original draft: Qiuhua Zhang.
Writing – review & editing: Xiufei Gao.
Footnotes
Abbreviations: CRCI = chemotherapy-related cognitive impairment, FACT-COG = Functional Assessment of Cancer Therapy-Cognitive Function, PCI = Perceived cognitive impairment.
How to cite this article: Zhang Q, Gao X, Liu S, Yu L, Zhu J, Qiu S. Therapies for cognitive impairment in breast cancer survivors treated with chemotherapy: a protocol for systematic review. Medicine. 2020;99:19(e20092).
QZ and XG are co-first authors.
This research was supported by the National Natural Science Foundation of China (No. 81503581), the Natural Science Foundation of Zhejiang Province (No. LY18H270006), and the Zhejiang Provincial Plan of Traditional Chinese Medicine (No. 2016ZQ015).
The authors have no conflicts of interests to disclose.
Our paper is a systematic review, and all the data in this review are from published clinical studies.
The datasets generated during and/or analyzed during the current study are publicly available.
References
- [1].Winters S, Martin C, Murphy D, et al. Breast cancer epidemiology, prevention, and screening. Prog Mol Biol Transl Sci 2017;151:1–32. [DOI] [PubMed] [Google Scholar]
- [2].Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med 2005;353:1784–92. [DOI] [PubMed] [Google Scholar]
- [3].Poppelreuter M, Weis J, Bartsch HH. Effects of specific neuropsychological training programs for breast cancer patients after adjuvant chemotherapy. J Psychosoc Oncol 2009;27:274–96. [DOI] [PubMed] [Google Scholar]
- [4].Vardy J. Cognitive function in breast cancer survivors. Cancer Treat Res 2009;151:387–419. [DOI] [PubMed] [Google Scholar]
- [5].Ahles TA, Root JC, Ryan EL. Cancer- and cancer treatment-associated cognitive change: an update on the state of the science. J Clin Oncol 2012;30:3675–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Janelsins MC, Kesler SR, Ahles TA, et al. Prevalence, mechanisms, and management of cancer-related cognitive impairment. Int Rev Psychiatry 2014;26:102–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wefel JS, Schagen SB. Chemotherapy-related cognitive dysfunction. Curr Neurol Neurosci Rep 2012;12:267–75. [DOI] [PubMed] [Google Scholar]
- [8].Jansen CE, Miaskowski C, Dodd M, et al. A metaanalysis of studies of the effects of cancer chemotherapy on various domains of cognitive function. Cancer 2005;104:2222–33. [DOI] [PubMed] [Google Scholar]
- [9].Kanaskie ML, Loeb SJ. The experience of cognitive change in women with breast cancer following chemotherapy. J Cancer Surviv 2015;9:375–87. [DOI] [PubMed] [Google Scholar]
- [10].Janelsins MC, Heckler CE, Peppone LJ, et al. Cognitive complaints in survivors of breast cancer after chemotherapy compared with age-matched controls: an analysis from a nationwide, multicenter, prospective longitudinal study. J Clin Oncol 2017;35:506–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Frank JS, Vance DE, Jukkala A, et al. Attention and memory deficits in breast cancer survivors: implications for nursing practice and research. J Neurosci Nurs 2014;46:274–84. [DOI] [PubMed] [Google Scholar]
- [12].Ferguson RJ, Sigmon ST, Pritchard AJ, et al. A randomized trial of videoconference-delivered cognitive behavioral therapy for survivors of breast cancer with self-reported cognitive dysfunction. Cancer 2016;122:1782–91. [DOI] [PubMed] [Google Scholar]
- [13].Tong T, Pei C, Chen J, et al. Efficacy of acupuncture therapy for chemotherapy-related cognitive impairment in breast cancer patients. Med Sci Monit 2018;24:2919–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Selamat MH, Loh SY, Mackenzie L, et al. Chemobrain experienced by breast cancer survivors: a meta-ethnography study investigating research and care implications. PLoS One 2014;9:e108002.doi: 10.1371/journal.pone.0117740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Piacentine LB, Miller JF, Haberlein S, et al. Perceived cognitive changes with chemotherapy for breast cancer: a pilot study. Appl Nurs Res 2016;29:9–11. [DOI] [PubMed] [Google Scholar]
- [16].Milbury K, Chaoul A, Biegler K, et al. Tibetan sound meditation for cognitive dysfunction: results of a randomized controlled pilot trial. Psychooncology 2013;22:2354–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lawrence JA, Griffin L, Balcueva EP, et al. A study of donepezil in female breast cancer survivors with self-reported cognitive dysfunction 1 to 5 years following adjuvant chemotherapy. J Cancer Surviv 2016;10:176–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kesler S, Hadi Hosseini SM, Heckler C, et al. Cognitive training for improving executive function in chemotherapy-treated breast cancer survivors. Clin Breast Cancer 2013;13:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Harrington CB, Hansen JA, Moskowitz M, et al. It's not over when it's over: long-term symptoms in cancer survivors--a systematic review. Int J Psychiatry Med 2010;40:163–81. [DOI] [PubMed] [Google Scholar]
- [20].Morean DF, O’Dwyer L, Cherney LR. Therapies for cognitive deficits associated with chemotherapy for breast cancer: a systematic review of objective outcomes. Arch Phys Med Rehabil 2015;96:1880–97. [DOI] [PubMed] [Google Scholar]
- [21].Costa DSJ, Loh V, Birney DP, et al. The structure of the FACT-Cog v3 in cancer patients, students, and older adults. J Pain Symptom Manage 2018;55:1173–8. [DOI] [PubMed] [Google Scholar]
- [22].Ferguson RJ, McDonald BC, Rocque MA, et al. Development of CBT for chemotherapy-related cognitive change: results of a waitlist control trial. Psychooncology 2012;21:176–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Campbell KL, Kam JWY, Neil-Sztramko SE, et al. Effect of aerobic exercise on cancer-associated cognitive impairment: a proof-of-concept RCT. Psychooncology 2018;27:53–60. [DOI] [PubMed] [Google Scholar]
- [24].Myers JS, Melissa M, Susan K, et al. Qigong intervention for breast cancer survivors with complaints of decreased cognitive function. Support Care Cancer 2019;27:1395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ah DV, Carpenter JS, Saykin A, et al. Advanced cognitive training for breast cancer survivors: a randomized controlled trial. Breast Cancer Res Treat 2012;135:799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gokal K, Munir F, Ahmed S, et al. Does walking protect against decline in cognitive functioning among breast cancer patients undergoing chemotherapy? Results from a small randomised controlled trial. PLoS One 2018;13:e0206874.doi: 10.1371/journal.pone.0206874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Damholdt MF, Mehlsen M, O’Toole MS, et al. Web-based cognitive training for breast cancer survivors with cognitive complaints-a randomized controlled trial. Psychooncology 2016;25:1293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Derry HM, Jaremka LM, Bennett JM, et al. Yoga and self-reported cognitive problems in breast cancer survivors: a randomized controlled trial. Psychooncology 2015;24:958–66. [DOI] [PMC free article] [PubMed] [Google Scholar]


