Supplemental Digital Content is available in the text
Keywords: antifungal, candida, mortality, susceptibility
Abstract
As detection rates of non-albicans Candida species are increasing, determining their pathogen profiles and antifungal susceptibilities is important for antifungal treatment selection. We identified the antifungal susceptibility patterns and predictive factors for mortality in candidemia.
A multicenter retrospective analysis of patients with at least 1 blood culture positive for Candida species was conducted. Candida species were classified into 3 groups (group A, Candia albicans; group B, Candida tropicalis, and Candida parasilosis; group C, Candida glabrata and Candida krusei ) to analyze the susceptibility patterns, first-line antifungal administered, and mortality. Univariate and multivariate comparisons between outcomes were performed to identify mortality risk factors.
In total, 317 patients were identified, and 136 (42.9%) had recorded mortality. Echinocandin susceptibility was higher for group A than group B (111/111 [100%] vs 77/94 [81.9%], P < .001). Moreover, group A demonstrated higher fluconazole susceptibility (144/149 [96.6%] vs 39/55 [70.9%], P < .001) and lower mortality (68 [45.3%] vs 34 [61.8%], P = .036) than those of group C. In the multivariate analysis, the sequential organ failure assessment score (odds ratio OR 1.351, 95% confidence interval 1.067–1.711, p = 0.013) and positive blood culture on day 7 of hospitalization (odds ratio 5.506, 95% confidence interval, 1.697–17.860, P = .004) were associated with a higher risk of mortality.
Patients with higher sequential organ failure assessment scores and sustained positive blood cultures have an increased risk of mortality.
1. Introduction
Candida species are normal flora of the gastrointestinal and genitourinary tracts. However, in hosts with a decreased immune response, widespread dissemination can result in multi-organ failure.[1] The term candidemia refers to the presence of Candida species in the blood.[2]Candida species identified from blood should never be considered contaminants, because that is the most common manifestation of invasive candidiasis.[2] Risk factors for candidemia include patients who have been treated in an intensive care unit (ICU) and those who are immunocompromised.[3] Identified risk factors for developing candidemia are patients in the ICU with indwelling central venous catheters (CVC), those receiving total parental nutrition, and those who have undergone gastrointestinal procedures.[4]
Although infection with Candida albicans (C albicans) is most common, the identification of the casual species is important, because some species are more resistant to azole antifungal agents.[2] The isolation of non-albicans Candida species has been increasing, and they have been frequently identified in the following order: Candida glabrata (C glabrata) and Candida parapsilosis, followed by Candida tropicalis (C tropicalis) and Candida krusei (C krusei).[5,6] Some C glabrata isolates are resistant to fluconazole, and all C krusei isolates are considered to be intrinsically resistant to fluconazole.[7] The minimal inhibitory concentrations (MICs) for echinocandins for Candida parasilosis (C parapsilosis) are higher than those of other Candida species. Moreover, resistance to fluconazole is highly predictive of resistance to voriconazole.[7] Therefore, the identification of changes in pathogen profiles and antifungal susceptibilities is important for antifungal treatment selection. Our study specifically compared the clinical, epidemiological, and antifungal susceptibility patterns in candidemia and identified risk factors for mortality.
2. Materials and methods
2.1. Study population and definitions
A multicenter retrospective analysis of episodes of candidemia in adults, collected from the electronic databases of 2 tertiary care hospitals in South Korea, was performed over a 4-year (2012–2015) period. Patients with at least 1 positive blood culture for a Candida species were included in the analysis. Patients with isolated yeasts other than Candida species were excluded from the study.
Intravenous catheter-related candidemia was defined in patients who had an intravascular device and ≥1 positive blood culture result, such that the same organism was isolated from the catheter and a peripheral blood culture, with clinical manifestations of infection (fever, chills, and/or hypotension) and no other apparent source for the bloodstream infection.[8] An intra-abdominal source of candidemia was considered for the patients who had a recent abdominal surgery or intra-abdominal events, such as peritonitis, abdominal abscess, and a purulent or necrotic infection at sites of gastrointestinal perforation or anastomotic leak, as confirmed by computed tomography scans.[1] Cardiovascular disease in patients was defined as the presence of hypertension, valvular heart disease, ischemic heart disease, or heart failure. Central nervous system disorder in patients was defined by a history of cerebrovascular accident or hemorrhage. Renal disease in patients was defined by chronic renal failure, stage 3 or 4, requiring hemodialysis or peritoneal dialysis. Lung disease in patients was defined as the presence of asthma, chronic obstructive lung disease, or idiopathic pulmonary fibrosis. Hematologic disease in patients was defined by aplastic anemia, lymphoma, or leukemia. The use of echinocandin was defined in patients who received first-line antifungal treatment with caspofungin, anidulafungin, or micafungin.
The demographic data, comorbidities, hospitalization and ICU stay, laboratory results, treatment outcomes, Candida species distribution, antifungal susceptibility results, first-line antifungal agents administered, and the complications (endocarditis, bone or joint infection, hepatosplenic candidiasis, and endophthalmitis) related to the candidemia were compared among the outcomes. The severity of illness was estimated by the sequential organ failure assessment (SOFA) score and the Charlson index. The laboratory data obtained on the first day of admission were analyzed. The presence of CVC insertion at the time of the positive blood culture and whether the patient was admitted to the ICU or the general ward were compared between outcomes. Blood culture results positive for a Candida species, obtained on the 7th, 14th, and 28th hospital day, were analyzed. The appropriate antifungal treatment was considered when the treatment was started within 48 hours, after the first blood culture was performed.[9] Univariate and multivariate comparisons between outcomes were performed.
Candida species were classified as C albicans (group A) and non-albicans Candida species groups (groups B and C). Among the non-albicans group, species with reduced susceptibility to fluconazole were classified as group C (C glabrata and C krusei), and species susceptible to fluconazole classified as group B (C tropicalis and C parapsilosis). The clinical and demographic factors, susceptibility patterns, first-line antifungal treatment, and mortality of each groups were analyzed.
2.2. Laboratory testing
Using the BACTEC 860 system (Becton Dickinson, Inc., Sparks, MD), isolated Candida were detected from blood cultures. Candida species were identified using the API-32C system (BioMerieux Vitek, Inc., St. Louis, MI). The commercial VITEK-2 yeast susceptibility test (BioMerieux, Hazelwood, MO) was used to derive the MICs for Candida species. Susceptibility to echinocandin and fluconazole was defined as follows according to the clinical breakpoints for interpreting the MICs from the Clinical and Laboratory Standards Institute (CLSI) guidelines. Susceptibility to echinocandins was defined in isolates of C albicans, C tropicalis, and C krusei with an MIC ≤0.25 mcg/mL, and C parasilosis with an MIC ≤2 mcg/mL for all 3 echinocandins (anidulafungin, caspofungin, and micafungin). C glabrata isolates with an MIC ≤0.12 mcg/mL to caspofungin and anidulafungin, and an MIC ≤0.06 mcg/mL to micafungin were considered susceptible.[10] The CLSI does not currently provide a susceptible breakpoint of fluconazole for C glabrata, with the intermediate breakpoint defined as MIC ≤32 and resistance as MIC ≥64. Susceptible was an MIC ≤2 mcg/mL for fluconazole in all species of Candida, except for C glabrata and C krusei (Supplementary Table 1).[11]
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to and the appropriate ethical review committee approval has been received.
2.3. Statistical analysis
Categorical variables have been presented as numbers and percentages, and continuous variables have been expressed as the mean ± standard deviation, unless otherwise indicated. Categorical variables were compared using a χ2 analysis, and continuous variables with normal distributions were compared using the Student t test. Single linear univariate correlations (Pearson correlation coefficients) and stepwise multivariate regression analyses were performed to evaluate the relationship between mortality and other variables. The covariates inserted in this model included the variables that differed with a P < .05 in the univariate analysis, which ensured the absence of significant multicollinearity. Multiple differences among groups of C albicans and non-albicans species were evaluated using a 1-way Analysis of Variance (ANOVA) with Tukey multiple comparison test. All categories were calculated as a percentage with a 95% confidence interval (CI). All statistical tests were performed using IBM SPSS software for Windows, version 20. P-values < .05 were considered statistically significant.
3. Results
During the study period, a total of 317 patients (88 cases originating from indwelling intravenous catheter, 89 cases from an intra-abdominal source, and 140 cases with an unidentifiable source of candidemia) were analyzed. Among these patients, mortality was observed for 136 (42.9%) patients.
The baseline characteristics and demographic data of the study population are presented in Table 1. Among the study patients, 198 (62.5%) were male with a median age of 68.3 ± 13.5 years. Patients with renal failure (36 [19.9%] vs 60 [44.1%], P < .001), chronic lung diseases (71 [39.2%] vs 93 [68.4%], P < .001), and hematologic disorders (48 [26.5%] vs 62 [45.6%], P < .001) presented with higher rates of mortality. Moreover, higher mortality was observed in the patients with higher SOFA scores (2.9 ± 2.9 vs 6.2 ± 4.3, P < .001), higher Charlson index (3.1 ± 1.8 vs 3.8 ± 1.9, P = .003), a CVC in place at the time of candidemia (74 [40.9%] vs 75 [55.1%], P = .012), and those admitted to the ICU (49 [27.1%] vs 67 [49.3%], P < .001). Patients with positive culture results on the 7th day of hospitalization demonstrated higher mortality rates than those with negative culture results (21 [35.6%] vs 21 [77.8%], P < .001).
Table 1.
Baseline characteristics of the study population.
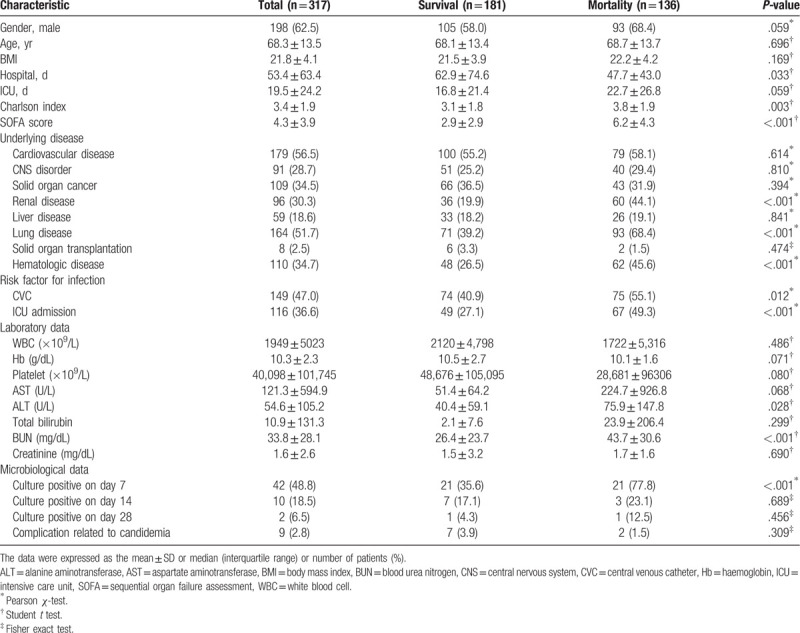
Table 2 presents the predictive factors associated with an increased risk of mortality. Higher SOFA scores (odds ratio [OR] 1.351, 95% CI 1.067–1.711, P = .013) and positive culture results on the 7th day of hospitalization (OR 5.506, 95% CI, 1.697–17.860, P = .004) were associated with an increased risk of mortality. The multivariate analysis demonstrated that there was no statistically significant difference in mortality based on the Candida species. Although the univariate analysis showed that echinocandin use resulted in a lower risk of mortality compared to no antifungal treatment (OR 0.148, 95% CI 0.041–0.541, P = .004), there was no statistically significant difference in the multivariate analysis.
Table 2.
Predictive factors for mortality.
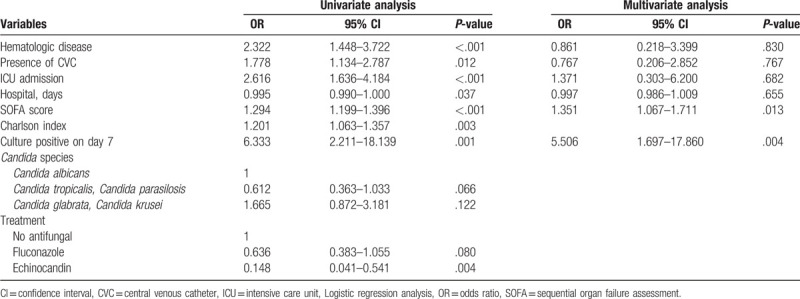
Table 3 presents the characteristics of the C albicans (group A) and non-albicans Candida species groups (groups B and C). The isolates were identified as C albicans, C parapsilosis or C tropicalis, and C glabrata or C krusei in 150, 112, and 55 cases (C albicans [n = 150], C parasilosis [n = 60], C tropicalis [n = 52], C glabrata [n = 47], and C krusei [n = 8]), respectively. No other Candida species were isolated. No multiple Candida isolates were identified from the same patient, and no mixed infections were identified. Susceptibility to echinocandin was higher for group A than group B (111/111 [100%] vs 77/94 [81.9%], P < .001). Group A presented higher susceptibility to fluconazole than that of group C (144/149 [96.6%] vs 39/55 [70.9%], P < .001). There was no difference in mortality between groups A and B. However, lower mortality was observed for group A than group C (68 [45.3%] vs 34 [61.8%], P = .036).
Table 3.
Characteristics of C albicans and non-albicans Candida species.
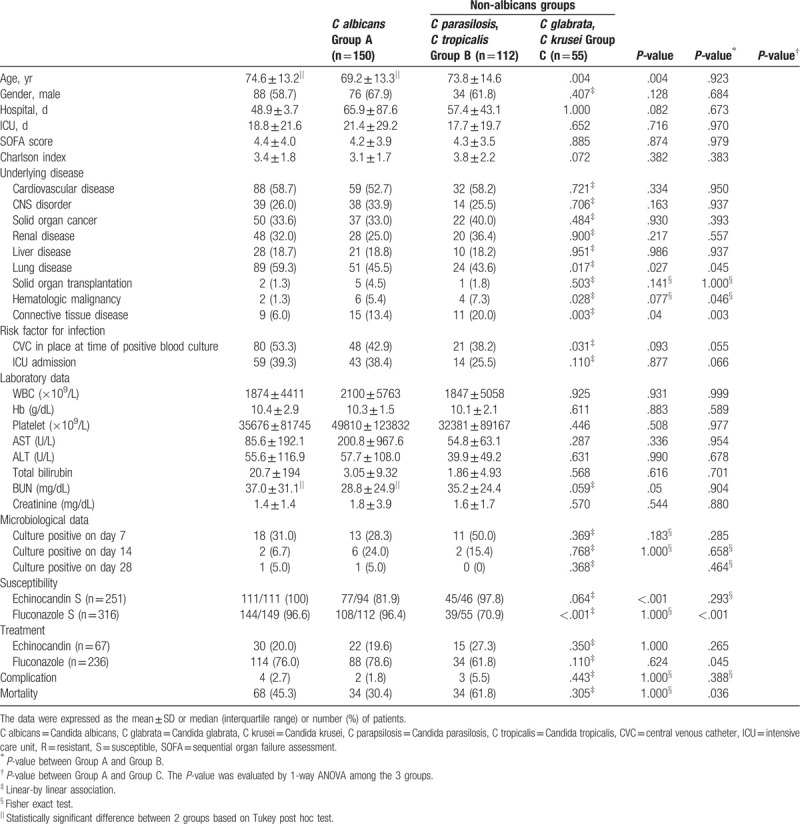
Figure 1 presents the percentages for echinocandin and fluconazole susceptibility for each group of Candida species.
Figure 1.
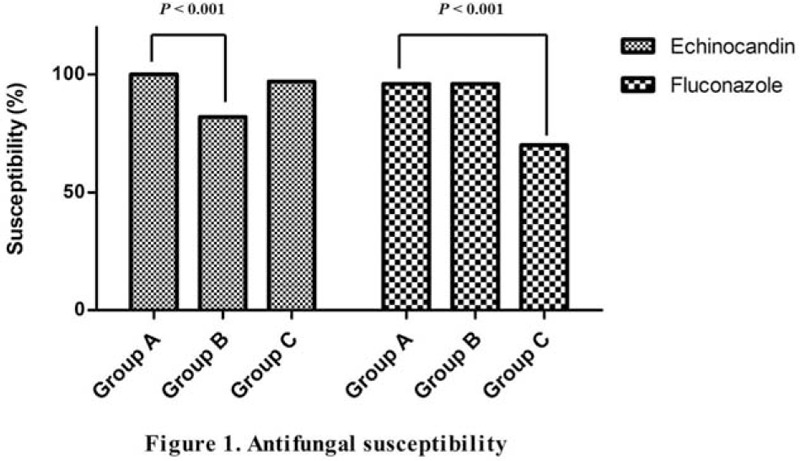
Antifungal susceptibility.
4. Discussion
Candidemia is 1 of the most common causes of health-care associated blood stream infections.[1] Among 1,314 Candida isolates tested from the Asia-Pacific region, C albicans (46.0%) was the most commonly isolated species followed by C glabrata (17.9%), C tropicalis (14.1%), C parapsilosis (12.9%), and C krusei (1.8%).[12] Although C albicans is the most commonly isolated species, an increase in non-albicans Candida species has been observed worldwide.[1]C glabrata was the most common non-C albicans species detected in all geographic regions except for Latin America, where C parapsilosis and C tropicalis were more common.[12] Both Europe (EUR) and North America (NA) demonstrated similar species distribution with C albicans (EUR 52.5%, NA 42.7%) the most commonly isolated species followed by C glabrata (EUR 16.0%, NA 24.3%).[12] In South Korea, although C albicans remains the leading Candida species that causes blood stream infection, an increase of C glabrata (from 21.3% to 28.5%) and a decrease of C parapsilosis (from 36.5% to 24.7%) over the past years were noticed.[13]C auris an emerging pathogen because it is intrinsically fluconazole resistant, and increasing numbers of infections have been identified in multiple countries.[14,15] This pathogen has caused invasive health care-associated infections, and it has been associated with high mortality rates.[15] The earliest known strain of C auris dates to 1996 in South Korea.[16] In this study, C albicans (n = 150) was the most commonly isolated pathogen, followed by C parapsilosis (n = 60), C tropicalis (n = 52), C glabrata (n = 47), and C krusei (n = 8).
A reduced dose-dependent susceptibility to fluconazole was observed for the C glabrata isolates compared to the other Candida species.[17] The highest rates of fluconazole resistant isolates were seen in C glabrata from North America (10.6%) and in C tropicalis from the Asia-Pacific region (9.2%).[12] Fluconazole resistance for C albicans was low in both North America (0.4%), and Asia-Pacific regions (0.2%).[12] In this study, 3.4% of C albicans were resistant to fluconazole, with C glabrata, and C krusei isolates exhibiting higher resistance to fluconazole than C. albicans. Due to an altered cytochrome P450 isoenzyme, C krusei is intrinsically resistant to fluconazole.[18] In a large international surveillance study that included 326 bloodstream isolates of C krusei, all of the isolates were susceptible to echinocandins.[19] Echinocandin resistance ranged from 3.5% for C glabrata to 0.1% for C albicans and C parapsilosis across the globe.[12] In the present study, C tropicalis and C parapsilosis isolates were less susceptible to echinocandins, as has been reported in prior studies.[17,20]
Prior studies have identified increasing age, higher Acute Physiology and Chronic Health Evaluation II scores, the Candida species, underlying renal dysfunction, and the primary antifungal agent selected as factors that are associated with an increased risk of mortality.[21–23] Although a higher SOFA score demonstrated an association with an increased risk of mortality, which has been shown in prior studies, there was no statistically significant difference in mortality among the Candida species or the primary antifungal agent administered.
In this study, positive blood culture results obtained 1 week after hospitalization were associated with a nearly 5 times greater risk of mortality. For cases of deep-seated candidemia, blood culture positivity is estimated to be less than 50%.[24] Considering the low sensitivity of culture positivity in deep-seated candidemia, we believe that this finding raises awareness for clinicians, by indicating that Candida species isolated from blood should never be considered contaminants, and that patients with a positive blood culture after 1 week are at higher risk for a poor outcome.
There are several limitations to this study. First, this study is limited by its retrospective nature. Second, the clinical MIC breakpoints for both the echinocandins and fluconazole were defined according to the CLSI guidelines. Third, describing geometric means, MIC ranges, MIC 50 and MIC 90 values was not possible in this study.
The results of our study demonstrate the antifungal susceptibility patterns of Candida species at 2 hospitals in South Korea. Moreover, patients with higher SOFA scores at the time of admission and a positive culture on the 7th day of hospitalization are at an increased risk of mortality.
Author contributions
IJY designed the study and acquired data, analyzed and interpreted the data, drafted the initial manuscript, reviewed, and critically revised and approved the final manuscript as submitted. SJS conceptualized the study and is responsible for the content of the manuscript, including the data and analysis. YKK, HYK, YGS, JMK, and JYC provided statistical assistance and revised and approved the final manuscript. All authors read and approved the final manuscript.
Supplementary Material
Footnotes
Abbreviations: C albicans = Candida albicans, C glabrata = Candida glabrata, C krusei = Candida krusei, C parapsilosis = Candida parasilosis, C tropicalis = Candida tropicalis, CI = confidence interval, CLSI = Clinical and Laboratory Standards Institute, CVC = central venous catheters, EUR = Europe, ICU = intensive care unit, MICs = minimal inhibitory concentrations, NA = North America, OR = odds ratio, SOFA = sequential organ failure assessment.
How to cite this article: Jung IY, Jeong SJ, Kim YK, Kim HY, Song YG, Kim JM, Choi JY. A multicenter retrospective analysis of the antifungal susceptibility patterns of Candida species and the predictive factors of mortality in South Korean patients with candidemia. Medicine. 2020;99:11(e19494).
Ethics approval and consent to participate was not applicable.
Consent for publication was not applicable.
All data generated or analyzed during this study are included in this published article and its supplementary/additional information files. However raw dataset are available from the corresponding author on reasonable request.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of Candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016;62:e1–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fridkin SK. The changing face of fungal infections in health care settings. Clin Infect Dis 2005;41:1455–60. [DOI] [PubMed] [Google Scholar]
- [3].Kullberg BJ, Arendrup MC. Invasive candidiasis. N Engl J Med 2015;373:1445–56. [DOI] [PubMed] [Google Scholar]
- [4].Chow JK, Golan Y, Ruthazer R, et al. Risk factors for albicans and non-albicans candidemia in the intensive care unit. Crit Care Med 2008;36:1993–8. [DOI] [PubMed] [Google Scholar]
- [5].Horn DL, Neofytos D, Anaissie EJ, et al. Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clin Infect Dis 2009;48:1695–703. [DOI] [PubMed] [Google Scholar]
- [6].Messer SA, Jones RN, Fritsche TR. International surveillance of Candida spp. and Aspergillus spp.: report from the SENTRY antimicrobial surveillance program (2003). J Clin Microbiol 2006;44:1782–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lyon GM, Karatela S, Sunay S, et al. Antifungal susceptibility testing of Candida isolates from the Candida surveillance study. J Clin Microbiol 2010;48:1270–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 2009;49:1–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Grim SA, Berger K, Teng C, et al. Timing of susceptibility-based antifungal drug administration in patients with Candida bloodstream infection: correlation with outcomes. J Antimicrob Chemother 2011;67:707–14. [DOI] [PubMed] [Google Scholar]
- [10].Pfaller MA, Diekema DJ, Andes D, et al. Clinical breakpoints for the echinocandins and Candida revisited: Integration of molecular, clinical, and microbiological data to arrive at species-specific interpretive criteria. Drug Resist Updat 2011;14:164–76. [DOI] [PubMed] [Google Scholar]
- [11]. Ghannoum MA, Clinical, Laboratory Standards I. Reference method for broth dilution antifungal susceptibility testing of yeasts ; fourth informational supplement M27-S3 Vol.28 No. 15, Volume 28, No. 15. [Google Scholar]
- [12]. Pfaller MA, Diekema DJ, Turnidge JD, Castanheira M, Jones RN. Twenty years of the SENTRY antifungal surveillance program: results for Candida species from 1997–2016. Open forum infectious diseases, Volume 6, Issue Supplement_1, March 2019, Pages S79–S94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ko J-H, Jung DS, Lee JY, et al. Changing epidemiology of non-albicans candidemia in Korea. J Infect Chemother 2019;25:388–91. [DOI] [PubMed] [Google Scholar]
- [14].Adams E, Quinn M, Tsay S, et al. Candida auris in healthcare facilities, New York, USA, 2013–2017. Emerg Infect Dis 2018;24:1816–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tsay S, Kallen A, Jackson BR, et al. Approach to the investigation and management of patients with Candida auris, an emerging multidrug-resistant yeast. Clin Infect Dis 2018;66:306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lee WG, Shin JH, Uh Y, et al. First three reported cases of nosocomial fungemia caused by Candida auris. J Clin Microbiol 2011;49:3139–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sanguinetti M, Posteraro B, Lass-Flörl C. Antifungal drug resistance among Candida species: mechanisms and clinical impact. Mycoses 2015;58:2–13. [DOI] [PubMed] [Google Scholar]
- [18].Pfaller M, Andes D, Diekema D, et al. Wild-type MIC distributions, epidemiological cutoff values and species-specific clinical breakpoints for fluconazole and Candida: time for harmonization of CLSI and EUCAST broth microdilution methods. Drug Resist Updat 2010;13:180–95. [DOI] [PubMed] [Google Scholar]
- [19].Pfaller M, Diekema D, Gibbs D, et al. Candida krusei, a multidrug-resistant opportunistic fungal pathogen: geographic and temporal trends from the ARTEMIS DISK antifungal surveillance program, 2001 to 2005. J Clin Microbiol 2008;46:515–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Pfaller M, Diekema D, Andes D, et al. Clinical breakpoints for the echinocandins and Candida revisited: integration of molecular, clinical, and microbiological data to arrive at species-specific interpretive criteria. Drug Resist Updat 2011;14:164–76. [DOI] [PubMed] [Google Scholar]
- [21].Pfaller M, Neofytos D, Diekema D, et al. Epidemiology and outcomes of candidemia in 3648 patients: data from the Prospective Antifungal Therapy (PATH Alliance®) registry, 2004–2008. Diagn Microbiol Infect Dis 2012;74:323–31. [DOI] [PubMed] [Google Scholar]
- [22].Andes DR, Safdar N, Baddley JW, et al. Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: a patient-level quantitative review of randomized trials. Clin Infect Dis 2012;54:1110–22. [DOI] [PubMed] [Google Scholar]
- [23].Schuster MG, Meibohm A, Lloyd L, et al. Risk factors and outcomes of Candida krusei bloodstream infection: a matched, case-control study. J Infect 2013;66:278–84. [DOI] [PubMed] [Google Scholar]
- [24].Clancy CJ, Nguyen MH. Finding the “missing 50%” of invasive candidiasis: how nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin Infect Dis 2013;56:1284–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


