Abstract
Obese individuals are apt to develop Stanford A acute aortic dissection (AAD) complicated with acute lung injury (ALI), but the mechanism is still not well defined. We aim to investigate whether oxidative stress and inflammatory are involved in the aortic dissection lung injury caused by obesity.
Seventy-nine patients were categorized into AAD with obesity group (n = 17) and AAD without obesity group (n = 62) according to body mass index (BMI). Inflammatory reactions including interleukin 1β (IL-1β), tumor necrosis factor-α (TNF-α), IL-6, C-reactive protein (CRP) and white blood cell (WBC) count, and oxidative stress including malondialdehyde (MDA), superoxide dismutase were determined using enzyme-linked immunosorbent assays and chemiluminescence. All the patients received ascending aorta replacement combined with total arch replacement and stented elephant trunk. The postoperative complications were recorded.
The incidence of preoperative hypoxemia (94.1% vs 35.5%, P < .01) and postoperative ALI (88.2% vs 40.3%, P < .01) in obese patients was significantly higher than that in non-obese patients. Besides, the ICU stay (119.2 ± 59.2 vs 87.8 ± 31.2 h, P < .01) and hospitalization duration (18.8 ± 8.5 vs 14.3 ± 8.1d, P = .048) were increased in the obese patients with AAD. The expression of IL-1β, TNF-α, IL-6, CRP, and WBC was remarkably increased (P < .01) in obese group compared with non-obese group.
Oxidative stress and inflammatory response may be involved in the process of ALI of aortic dissection caused by obesity, which provides new ideas for the treatment of ALI of the aortic dissection.
Keywords: acute aortic dissection, acute lung injury, inflammation, oxidative stress
1. Introduction
Obese individuals are more predisposed to developing acute aortic dissection (AAD) compared to the healthy counterparts.[1] For the obese patients with AAD, a higher incidence of acute lung injury (ALI) is observed, together with hypoxemia prior to surgery.[2] To date, the roles of obesity in the pathogenesis of AAD complicated with ALI remains to be fully elucidated.
Chronic inflammation is frequently reported in the obese individuals or animals,[3,4] and this may induce pulmonary complications in the presence of trauma.[5,6] In the pathogenesis of AAD, obese patients are likely to suffer from systemic inflammation, which results in subsequent injury to the blood gas exchange in the lung tissues. However, the exact mechanism remains unclear.
In the present study, we aimed to determine the expression of factors associated with oxidative stress and inflammation in patients with Stanford A AAD, as well as examine the correlation between obesity and preoperative hypoxemia in these patients.
2. Materials and methods
2.1. Patients
Seventy-nine Stanford A AAD patients who were admitted to the intensive care unit of Wuhan University Renmin Hospital, Wuhan, Hubei, China between May 2014 and October 2015 were included in this retrospective study. Multi-slice computed tomography and color Doppler ultrasonic examination were performed for the diagnoses. Patients with the following conditions were excluded from the study: those with chronic inflammation in lung, lung cancer, pulmonary emphysema, pulmonary tuberculosis, or connective tissue diseases. According to body mass index (BMI), patients were categorized into: AAD with obesity group (n = 17; patients with BMI ≥25) and AAD without obesity group (n = 62; patients with BMI of <25). Written informed consent was obtained from each patient. The study protocols were approved by the Ethical Committee of Wuhan University Renmin Hospital.
2.2. Data collection
Following admission, the case history, symptoms and vital signs of each patient was collected by a trained physician. For the Stanford A AAD patients, routine blood tests were performed within 1 h following diagnosis. Serum triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol (LDL-c), white blood cell count, and C-reactive protein (CRP) were determined using chemiluminescence. Body weight was measured using a calibrated scale and height was measured with a wall-mounted stadiometer.
2.3. Markers of oxidative stress, inflammation, and cytokines
Serum malondialdehyde (MDA) and superoxide dismutase (SOD) were determined by enzyme linked immunosorbent assay (ELISA) using commercial kits (cat nos. C10446, PA5-23245, Invitrogen; Thermo Fisher Scientific, Inc, Waltham, MA). Serum IL-1β, IL-6, and TNF-α were measured using commercial ELISA kits (cat nos. EK0392, EK0410, and EK0525, respectively, Boster Co., Ltd. Wuhan, China) according to the manufacture's instructions. All tests were carried out at least three times.
2.4. Treatment and postoperative complications
All patients received ascending aorta replacement (AAR), total arch replacement (TAR), and stented elephant trunk. The procedures were carried out by physicians as previously described.[7] ALI was defined as PaO2/FiO2 ≤ 300 mm Hg, and acute respiratory distress syndrome (ARDS) was defined as PaO2/FiO2 ≤200 mm Hg.[8] Sleep apnea syndrome (SAS) was defined as ≥30 episodes of apnea (cessation of airflow for ≥10 s) during 7 h of sleep in a single night or ≥5 episodes of apnea per hour of sleep (apnea index, ≥5).[9] Data were collected using a standardized data form, which included information on demographics, case history, symptoms, physical findings, imaging findings, medical and surgical treatment, and outcomes.
2.5. Statistical analysis
SPSS version 20.0 software (SPSS, Inc., Chicago, IL) was used for data analysis. Quantitative data are presented as mean ± standard deviation. The distribution of quantitative data was determined by a test of homogeneity variance. An independent t test was used for comparing quantitative sizes of two independent samples. The χ2 test was used for the evaluation of dependence of qualitative variables. Multiple linear regression analysis was used to measure the relevance of the information. P < .05 was considered to indicate a statistically significant difference.
3. Results
3.1. Patient characteristics
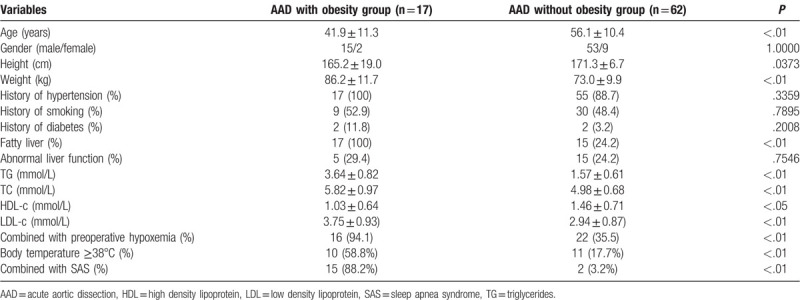
A total of 79 patients (68 males and 11 females) were included in the present study. The median age of the patients was 51.8 ± 12.3 years (38–77 years). Thirty-eight patients (48.1%) showed preoperative lung injury. The preoperative variables of patients were shown in Table 1. Significant differences were detected in the following variables, including age (P < .01), fatty liver (P < .01), TG (P < .01), TC (P = .0001), LDL-c (P < .01), combined with preoperative hypoxemia (P < .0001), body temperature ≥38°C (P < .01), and combined with SAS (P < .0001).
Table 1.
Patient characteristics.
3.2. Operative variables and concomitant procedures
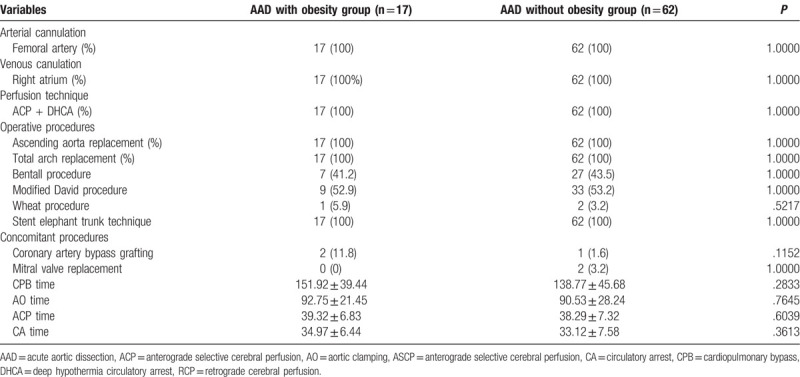
A total of 79 Stanford A AAD patients were treated by ascending aorta replacement (AAR), TAR and stented elephant trunk (Table 2). Concomitant procedures included coronary artery bypass grafting and mitral valve replacement, respectively. Compared with the AAD without obesity group, no statistical difference was noticed in the cardiopulmonary bypass (CPB) time, AO time, anterograde selective cerebral perfusion (ACP) time and circulatory arrest (CA) time.
Table 2.
Operative variables and concomitant procedures.
3.3. Postoperative complications
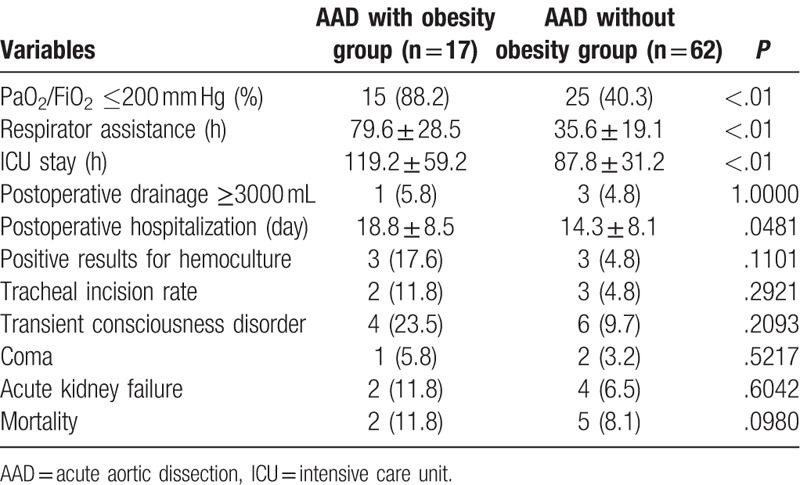
The most common complications were transient consciousness disorder, coma, acute renal failure, and even death (Table 3). In addition, the incidence of ARDS was elevated in obese patients with AAD. Moreover, the duration of respirator assistance was much longer in the obesity group compared with that of the AAD without obesity group. Furthermore, the ICU stay and hospitalization duration of the obesity group were higher than these of the AAD without obesity group.
Table 3.
Postoperative complications in Patients With AAD in surgery.
3.4. Comparison of serum oxidative stress and inflammatory factors in AAD complicated ALI patients and AAD patients
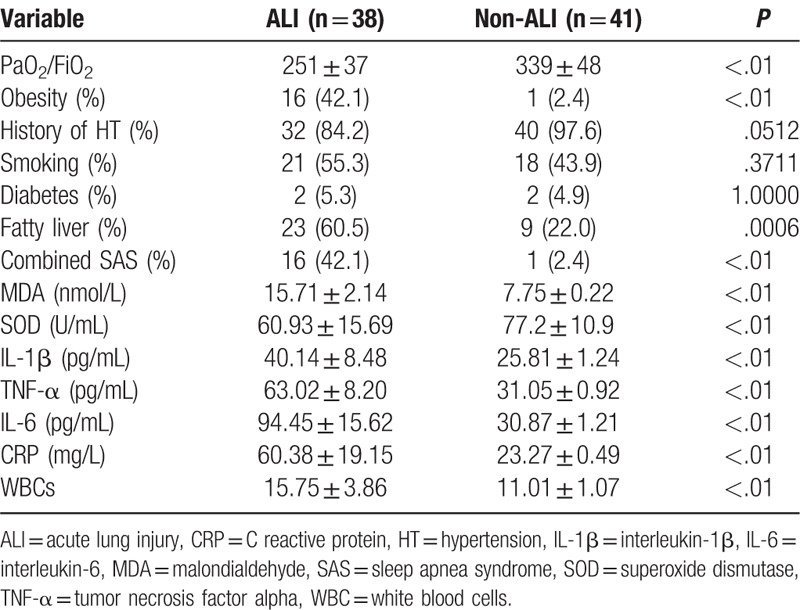
Compared with the non-ALI AAD group, the ratio of PaO2/FiO2 in AAD with ALI patients was remarkably decreased (P < .01, Table 4). The incidence of obesity and SAS was increased. Interestingly, smoking and diabetes were not the cause for the AD. The serum MDA in the AAD patients was significantly elevated compared to that in the normal individuals (P < .01). Oxidative stress was identified in AAD with ALI patients as revealed by decrease in SOD concentration. Furthermore, several factors associated with inflammatory reactions were remarkably increased in the AAD with ALI patients compared to the non-ALI AAD individuals, including IL-1β, TNF-α, IL-6, CRP, and WBC. This indicated that oxidative stress and elevation of inflammatory factors were associated with the pathogenesis of AAD complicated ALI.
Table 4.
Clinical and chemical analysis comparison in ALI patients and non-ALI patients.
3.5. Comparison of serum oxidative stress and inflammatory factors in obese patients with AAD and non-obese patients with AAD
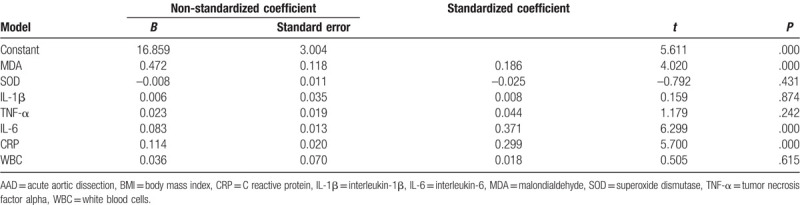
The ratio of PaO2/FiO2 was decreased in the obesity group compared with that of the AAD without obesity group. The serum MDA levels were elevated in the obese patients with AAD, however, no significant difference was observed in the expression of SOD in these patients. Moreover, the expression of IL-1β, TNF-α, IL-6, CRP, and WBC were significantly increased, as revealed in Table 5. Multiple linear regression analysis indicated MDA, IL-6, and CRP were closely associated with BMI (P < .01, Table 6). This indicated the oxidative stress and inflammatory reactions were elevated in obese patients with AAD.
Table 5.
Comparison of oxidative stress and inflammatory factors in AAD with obese patients and AAD without obese patients.
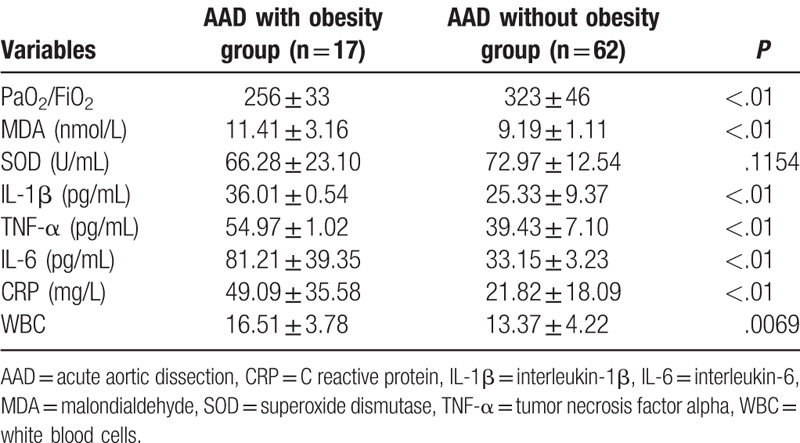
Table 6.
Correlation between BMI and markers of oxidative stress or inflammation in AAD patients.
4. Discussion
A large number of AAD patients experience acute respiratory failure such as ALI and/or ARDS.[10,11] In the present study, a small majority of type A AAD patients (55.7%) presented ALI together with hypoxemia (PaO2/FiO2 ≤300 mm Hg) following diagnosis. Additionally, obese patients with type A AAD were more likely to suffer from hypoxemia prior to surgery compared to the non-obese AAD patients (94.1% vs 35.5%). Recent studies reveal the onset of the AAD complicated lung injury is closely related to systemic inflammatory reactions.[11–14] However, few studies have investigated the pathogenesis of AAD complicated with ALI in obese patients.
Adipose tissue can release numerous peptides and cytokines into blood circulation,[15,16] and obesity has been associated with subclinical chronic inflammation.[3] Furthermore, abundant inflammatory cytokines have been detected in adipose cells, and are closely involved in fat metabolism. These factors have been previously reported to be positively associated with all indices of obesity, particularly in patients with abdominal obesity.[17] Moreover, oxygen deficits, oxidative stress, and fatty acid levels are hypothesized to play a crucial, synergistic role in obesity-associated inflammation.[17]
The underdeveloped vascular system within the expanded adipose tissue of patients with obesity predisposes adipocytes to oxygen deficits, and continued over-nutrition rapidly leads to a state of chronic persistent hypoxia within the adipose tissue. Protracted high levels of hypoxia have been demonstrated within the white adipose tissue of obese individuals.[18–23] Hypoxemia has been shown to be the most potent stimulus for the release of these proinflammatory cytokines such as TNF-α, IL-6, IL-8, leptin, and IL-1β,[24,25] and activates pro-inflammatory signaling pathways, such as c-Jun N-terminal kinase (JNK), IκB kinase (IκK)-β, nuclear factor (NF)-κB, and redox-sensitive transcription factor.[3,26] Cross-sectional and prospective studies have indicated that obesity contributes to the altered levels of inflammatory cytokines, and this provides a likely explanation for how obesity contributes to a more severe respiratory inflammatory response in humans and animal models.[27] Thus, obese patients with AAD are more likely to develop severe systemic inflammation compared to AAD patients. Additionally, the elevation of IL-1β, TNF-α, and IL-6 in these patients may induce pulmonary dysfunction and the onset of hypoxemia, which in turn promote the release of inflammatory factors from adipose cells. Finally, severe lung injury may be induced after this process.
BMI and fat accumulation contributes to the production of reactive oxygen species (ROS) and oxidative stress.[4,28–30] In adipose tissues, oxidative stress plays a significant role in the regulation of inflammation. Fat accumulation stimulates the release of NADPH oxidase 4 (Nox4), a key factor in enzymatic cellular ROS production.[31–33] ROS stimulates macrophage infiltration of adipose tissue via ROS-induced monocyte chemoattractant protein (MCP) production, which results in further promotion of local Nox4 expression and ROS production.[34,35] Taken together, these indicate that both adipocytes and macrophages contribute to elevated oxidative stress in obesity. Previous studies have demonstrated that oxidative stress is elevated in obesity. Moreover, the addition of oxidants suppresses the adipose tissue expression of adiponectin and increases the expression of inflammatory factors.[4] In the present study, MDA was elevated in obese patients with AAD (P < .05), which indicated an imbalance in the oxidation and anti-oxidation in the obese patients with AAD.
Lipids play important roles in the modulation of inflammatory signaling pathways. Free fatty acids bind innate immune receptors such as toll-like receptor 4 (TLR4). They are reported to be directly linked to the development of inflammation in states of hyperlipidemia, such as obesity. In addition, the binding of free fatty acids to TLR4 may result in subsequent induction of cascades of events, which finally resulted in the activation of the IκKβ/NFκB system and the induction of proinflammatory cytokines.[36]
In the present study, most of the obese patients with AAD showed concurrent SAS, which was significantly higher than that of the patients with AAD (88.2% vs 3.2%). For the obese patients with excessive growth of upper respiratory tract and adipose tissues, the incidence of the upper respiratory tract wall was increased. On this basis, the muscle group of the upper respiratory tract is relaxed during sleep, resulting in stenosis of the upper airway. Concurrently, obesity may trigger elevation of sympathetic activity. The aberrant changes of autonomic nerves may promote the obstructive apnea, which induces anaerobic conditions[37] and deterioration of inflammation and oxidative stress.[38,39]
There are some limitations in this study. First, the number of obese patients diagnosed with ALI of aortic dissection in this study was relatively small in size. Second, the retrospective, observational nature of the study is also a limitation of this study. Third, one of the biggest limitations of these types of studies is that they are cross-sectional in nature. However, to a certain degree, they can explain that oxidative stress and inflammation may be involved in ALI of aortic dissection caused by obesity through multi-factor comparison and the application of linear regression. In future, studies with long-term follow-up and randomization should be performed to compare obese and non-obese clinical outcomes, and the findings of these studies will be more convincing underlying pathogenesis of aortic dissection-induced ALI caused by obesity.
In conclusion, AAD complicated with ALI is a type of distal organ injury caused by systemic inflammation. Obese patients are more likely to develop AAD complicated with ALI. Adipose tissue has been recognized as an active participant in numerous physiological and pathophysiological processes. In adipose tissues, the production and secretion of a wide range of inflammatory molecules is increased, which may have systemic effects on other organs. Interestingly, obese patients aged 40 to 50 years are most likely to develop AAD complicated with ALI compared to the patients of all other ages.
Author contributions
Conceptualization: Zhiwei Wang.
Data curation: Zhiwei Wang.
Formal analysis: Zhiwei Wang.
Funding acquisition: Hongbing Wu, Rui Hu.
Investigation: Hongbing Wu, Rui Hu.
Methodology: Hongbing Wu, Rui Hu.
Project administration: Wei Ren, Zhipeng Hu.
Resources: Wei Ren, Zhipeng Hu.
Software: Wei Ren, Zhipeng Hu.
Supervision: Jinxing Chang.
Validation: Jinxing Chang.
Visualization: Jinxing Chang.
Writing – original draft: Zhiyong Wu.
Writing – review & editing: Zhiyong Wu.
Footnotes
Abbreviations: AAD = acute aortic dissection, AAR = ascending aorta replacement, ALI = acute lung injury, ARDS = acute respiratory distress syndrome, BMI = body mass index, CRP = C-reactive protein, ELISA = enzyme linked immunosorbent assay, HDL-c = high-density lipoprotein cholesterol, IL-1β = interleukin 1β, LDL-c = low-density lipoprotein cholesterol, MDA = malondialdehyde, SAS = sleep apnea syndrome, SOD = superoxide dismutase, TAR = total arch replacement, TC = total cholesterol, TG = triglyceride, TNF-α = tumor necrosis factor-α, WBC = white blood cell.
How to cite this article: Wu Z, Wang Z, Wu H, Hu R, Ren W, Hu Z, Chang J. Obesity is a risk factor for preoperative hypoxemia in Stanford A acute aortic dissection. Medicine. 2020;99:11(e19186).
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
The authors have no conflict of interest to disclose.
References
- [1].Aizawa K, Sakano Y, Ohki S, et al. Obesity is a risk factor of young onset of acute aortic dissection and postoperative hypoxemia. Kyobu Geka 2013;66:437–44. [PubMed] [Google Scholar]
- [2].Nakajima T, Kawazoe K, Izumoto H, et al. Risk factors for hypoxemia after surgery for acute type A aortic dissection. Surg Today 2006;36:680–5. [DOI] [PubMed] [Google Scholar]
- [3].Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol 2005;115:911. [DOI] [PubMed] [Google Scholar]
- [4].Furukawa S, Fujita T, Shimabukuro M, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 2004;114:1752–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hatakeyama N, Matsuda N. Alert cell strategy: mechanisms of inflammatory response and organ protection. Curr Pharma Des 2014;20:5766–78. [DOI] [PubMed] [Google Scholar]
- [6].Quante M, Dietrich A, Elkhal A, et al. Obesity related immune responses and their impact on surgical outcomes. Int J Obes 2015;39:877. [DOI] [PubMed] [Google Scholar]
- [7].Wu ZY, Wang ZW, Mao ZF, et al. The clinical study of modified total aortic arch replacement and stent elephant trunk technique treatment for patients with DeBakey I thoracic aortic dissection. Zhonghua wai ke za zhi 2011;49:236–9. [PubMed] [Google Scholar]
- [8].Artigas A, Bernard GR, Carlet J, et al. The American-European Consensus Conference on ARDS, part 2: ventilatory, pharmacologic, supportive therapy, study design strategies, and issues related to recovery and remodeling. Acute respiratory distress syndrome. Am J Respir Crit Care Med 1998;157(4 Pt 1):1332–47. [DOI] [PubMed] [Google Scholar]
- [9].Guilleminault C, A Tilkian A, Dement WC. The sleep apnea syndromes. Annu Rev Med 1976;27:465. [DOI] [PubMed] [Google Scholar]
- [10].Kurabayashi M, Okishige K, Azegami K, et al. Reduction of the PaO2/FiO2 ratio in acute aortic dissection—relationship between the extent of dissection and inflammation. Circ J 2010;74:2066–73. [DOI] [PubMed] [Google Scholar]
- [11].Hasegawa Y, Ishikawa S, Ohtaki A, et al. Impaired lung oxygenation in acute aortic dissection. J Cardiovasc Surg 1999;40:191–5. [PubMed] [Google Scholar]
- [12].Luo F, Zhou XL, Li JJ, et al. Inflammatory response is associated with aortic dissection. Ageing Res Rev 2009;8:31–5. [DOI] [PubMed] [Google Scholar]
- [13].Sugano Y, Anzai T, Yoshikawa T, et al. Serum C-reactive protein elevation predicts poor clinical outcome in patients with distal type acute aortic dissection: association with the occurrence of oxygenation impairment. Int J Cardiol 2005;102:39–45. [DOI] [PubMed] [Google Scholar]
- [14].Jo Y, Anzai T, Sugano Y, et al. Early use of beta-blockers attenuates systemic inflammatory response and lung oxygenation impairment after distal type acute aortic dissection. Heart Vessels 2008;23:334–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wisse BE. The inflammatory syndrome: the role of adipose tissue cytokines in metabolic disorders linked to obesity. J Am Soc Nephrol 2004;15:2792–800. [DOI] [PubMed] [Google Scholar]
- [16].Ikeoka D, Mader JK, Pieber TR. Adipose tissue, inflammation and cardiovascular disease. Revista Da Associação Médica Brasileira 2010;56:116–21. [DOI] [PubMed] [Google Scholar]
- [17].de Heredia FP, Gómezmartínez S, Marcos A. Obesity, inflammation and the immune system. Proc Nutr Soc 2012;71:332. [DOI] [PubMed] [Google Scholar]
- [18].Hosogai N, Fukuhara A, Oshima K, et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 2007;56:901–11. [DOI] [PubMed] [Google Scholar]
- [19].Ye J. Emerging role of adipose tissue hypoxia in obesity and insulin resistance. Int J Obes 2009;33:54–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yin J, Gao Z, He Q, et al. Role of hypoxia in obesity-induced disorders of glucose and lipid metabolism in adipose tissue. American journal of physiology. Endocrinol Metab 2009;296:E333–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Pasarica M, Sereda OR, Redman LM, et al. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes 2009;58:718–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Rausch ME, Weisberg S, Vardhana P, et al. Obesity in C57BL|[sol]|6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int J Obes 2008;32:451. [DOI] [PubMed] [Google Scholar]
- [23].Ye J, Gao Z, Yin J, et al. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Phys Endocrinol Metab 2007;293:E1118–1128. [DOI] [PubMed] [Google Scholar]
- [24].Zemel MB, Sun X, Sobhani T, et al. Effects of dairy compared with soy on oxidative and inflammatory stress in overweight and obese subjects. Am J Clin Nutr 2010;91:16–22. [DOI] [PubMed] [Google Scholar]
- [25].Arvidsson E, Viguerie N, Andersson I, et al. Effects of different hypocaloric diets on protein secretion from adipose tissue of obese women. Diabetes 2004;53:1966–71. [DOI] [PubMed] [Google Scholar]
- [26].Weisberg SP, Leibel R, Tortoriello DV. Dietary curcumin significantly improves obesity-associated inflammation and diabetes in mouse models of diabesity. Endocrinology 2008;149:3549–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mancuso P. Obesity and respiratory infections: does excess adiposity weigh down host defense? Pulm Pharmacol Ther 2013;26:412–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Mohanty P, Hamouda W, Garg R, et al. Glucose challenge stimulates reactive oxygen species (ROS) generation by leucocytes. J Clin Endocrinol Metab 2000;85:2970–3. [DOI] [PubMed] [Google Scholar]
- [29].Dandona P, Mohanty P, Ghanim H, et al. The suppressive effect of dietary restriction and weight loss in the obese on the generation of reactive oxygen species by leukocytes, lipid peroxidation, and protein carbonylation. J Clin Endocrinol Metab 2001;86:355. [DOI] [PubMed] [Google Scholar]
- [30].Ramos LF, Shintani A, Ikizler TA, et al. Oxidative stress and inflammation are associated with adiposity in moderate to severe CKD. J Am Soc Nephrol 2008;19:593–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Inoguchi T, Li P, Umeda F, et al. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C—dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes 2000;49:1939–45. [DOI] [PubMed] [Google Scholar]
- [32].Sun X, Zemel MB. Dietary calcium regulates ROS production in aP2-agouti transgenic mice on high-fat/high-sucrose diets. Int J Obes 2006;30:1341–6. [DOI] [PubMed] [Google Scholar]
- [33].Sun X, Zemel MB. 1Alpha,25-dihydroxyvitamin D3 modulation of adipocyte reactive oxygen species production. Obesity (Silver Spring, Md) 2007;15:1944–53. [DOI] [PubMed] [Google Scholar]
- [34].Weisberg SP, McCann D, Desai M, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003;112:1796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sun X, Zemel MB. Calcium and 1,25-dihydroxyvitamin D3 regulation of adipokine expression. Obesity (Silver Spring, Md) 2007;15:340–8. [DOI] [PubMed] [Google Scholar]
- [36].Song MJ, Kim KH, Yoon JM, et al. Activation of Toll-like receptor 4 is associated with insulin resistance in adipocytes. Biochem Biophys Res Commun 2006;346:739–45. [DOI] [PubMed] [Google Scholar]
- [37].Shrivastava D. Impact of sleep-disordered breathing treatment on upper airway anatomy and physiology. Sleep Med 2014;15:733–41. [DOI] [PubMed] [Google Scholar]
- [38].De Lima FF, Mazzotti DR, Tufik S, et al. The role inflammatory response genes in obstructive sleep apnea syndrome: a review. Sleep Breath 2016;20:331–8. [DOI] [PubMed] [Google Scholar]
- [39].Adegunsoye A, Balachandran J. Inflammatory response mechanisms exacerbating hypoxemia in coexistent pulmonary fibrosis and sleep apnea. Mediators Inflamm 2015;2015:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]


