Abstract
Background:
Posterior lumbar interbody fusion (PLIF) surgery is associated with significant blood loss; however, few studies have focused on hidden blood loss (HBL) in PLIF or its regulatory factors. The purpose of this study was to explore the HBL in PLIF surgery as well as the influence of tranexamic acid (TXA) on blood loss in PLIF.
Methods:
We performed a randomized controlled trial (RCT) and recruited patients undergoing PLIF into the study from November 2013 to April 2017. All participants were assigned to one of 2 groups according to a simple equal probability randomization scheme. At the end of PLIF surgery, for patients in the TXA group, the surgical field was immersed in TXA (1 g in 100 mL of saline solution) for 5 min before stitching the wound. For the control group, the surgical field was immersed in the same volume of normal saline.
Results:
In our study, the drainage volume during the first 24 h and the total postoperative drainage volume were significantly lower in patients in the TXA group than in the control group (P = .001). The hematocrit (Hct) of the drainage and calculation of blood contained in the drainage showed similar results. The mean length of hospital stay and rate of blood transfusion in the TXA group were less than those in the control group (P < .05). HBL was responsible for 45.6% of the total blood loss in PLIF, and both of the indicators in the TXA group were much lower than those in the control group.
Conclusions:
PLIF is associated with massive perioperative HBL, but the application of topical TXA leads to less postoperative blood loss including less HBL, a lower blood product transfusion rate, and a shorter hospital stay for PLIF.
Keywords: hidden blood loss, PLIF, topical TXA
1. Introduction
Spinal fusion surgery is associated with substantial intraoperative and postoperative bleeding, which may result in anemia and require blood transfusion; therefore, it is imperative to obtain an accurate assessment of perioperative blood loss.[1] When evaluating a hemorrhage during orthopedic operations, true blood loss is always underestimated due to the neglect of hidden blood loss (HBL), which results from hemolysis and residual blood in the in dead space.[2] Excessive HBL may affect wound healing, increase the risk of infection, and prolong hospitalization, resulting in increased medical expenses.[3] Consequently, it is very important to control operative bleeding and to recognize the importance of HBL.
Posterior lumbar interbody fusion (PLIF) is a widely available spinal fusion technique for the treatment of unstable lumbar degenerative disorders. It provides three-column stabilization and satisfactory restoration of the disk space height because a bone graft is implanted in the intervertebral disc space.[4] However, the more invasive procedure and longer operative time may result in increased blood loss in PLIF.[5] Previous studies have shown that total hip arthroplasty (THA), total knee arthroplasty (TKA) and anterior spine interbody fusion all involve massive HBL. However, no research regarding HBL in PLIF has been performed.
Tranexamic acid (TXA) is a type of synthetic lysine analog that has been widely used in orthopedic surgery as an antifibrinolytic agent. It can be effective in reducing blood loss and transfusion requirements in THA and TKA by inhibiting plasminogen activation and blocking fibrinolysis.[6] Regarding spinal surgery, a previous study demonstrated that high-dose intravenous (IV) TXA can control perioperative blood loss and decrease the blood transfusion rate.[7] However, no study has reported the effect of topical TXA on HBL in PLIF. Therefore, in the present study, we measured the HBL in patients undergoing primary PLIF and explored the influence of TXA on blood loss in PLIF.
2. Methods
We performed a randomized controlled trial (RCT) at Peking Union Medical College Hospital from November 2013 to April 2017. Patients with lumbar degenerative disease were recruited into our study. The inclusion criteria were spondylolisthesis, spondylolysis, severe spinal instability or large disc herniation resulting in the loss of intervertebral space. The exclusion criteria were as follows:
-
1.
severe medical comorbidities, such as coagulation defects, anemia, and cardiovascular disease (malignant arrhythmia, the degree of angiostenosis was more than 75% or within 6 months after coronary stenting);
-
2.
involvement of more than 2 surgical levels;
-
3.
patients taking antiplatelet aggregates such as aspirin or other anticoagulants within the last month; or
-
4.
the Cell Saver blood salvage system was used during surgery.
Use of the Cell Saver has been reported to affect HBL, and the mechanism may be associated with erythrocyte hemolysis; therefore, we excluded cases in which the Cell Saver was used to avoid bias.
Five primary parameters were compared between the 2 groups:
-
1.
volume of drainage on POD 1 (post-operative day 1) and POD 2 and the patient's total drainage output;
-
2.
Hct of drainage on POD 1, POD 2, and POD 3;
-
3.
pure blood contained in drainage at different time points;
-
4.
transfusion rate; and
-
5.
length of postoperative hospitalization.
Secondary parameters, including age, gender, body mass index (BMI), and operative time, were also compared between the 2 groups.
2.1. Calculation of sample size
Power calculations were based on the results of a previous study. We hypothesized that patients who received TXA application in the surgical field would exhibit an HBL of 350 mL compared to 500 mL in the control group. A sample size of 23 patients in each group was required to detect a difference with an alpha of 0.05 and a power of 0.80, assuming a 10% dropout rate.[8]
The operations were performed by the same surgeon. The surgeon did not know the intervention assigned. After posterior pedicle screw insertion and lumbar decompression, all patients underwent single-level PLIF. In addition, we allocated the patients into 2 groups according to a simple equal probability randomization scheme. For patients in the TXA group, the surgical field was immersed in TXA (1 g in 100 mL of saline solution) for 5 min at the end of the operation. For the control group, the same volume of normal saline was applied to the surgical field. Patient participation is presented in a flow diagram in Figure 1.
Figure 1.
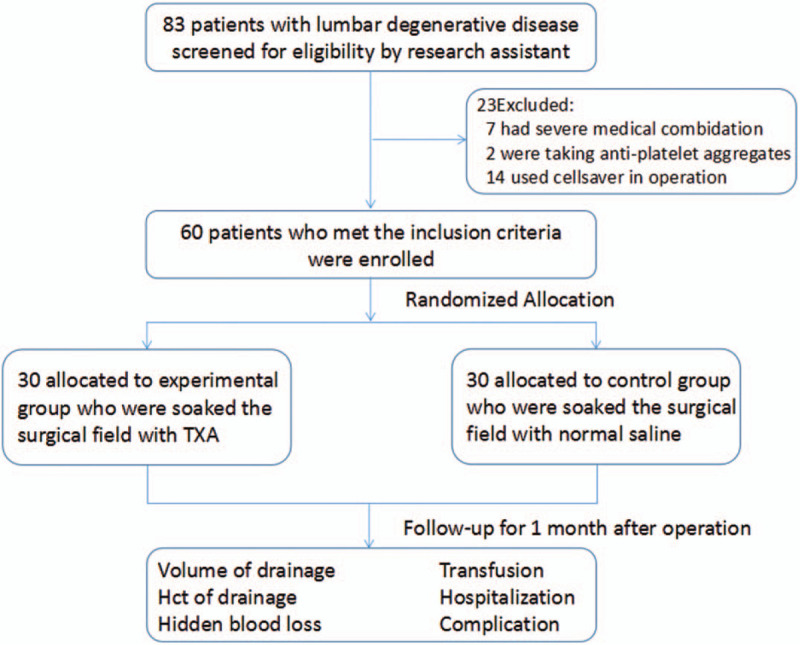
Flow chart.
Before wound closure, a deep drain was placed below the fascia. The amount of drainage on the first postoperative day (POD 1), second postoperative day (POD 2) and the total drainage volume were recorded. The drain was extracted when the output in 24 h was <50 mL. Clinical data including age, height, weight, BMI, operative duration, surgical level, intraoperative blood loss, related complications, and hospital duration were compiled for each case.
This study was approved by the Ethics Committee of Peking Union Medical College Hospital; all patients provided written informed consent for the study and surgery. The researchers who evaluated the results were blinded to the treatment received.
2.2. Calculation of HBL
To calculate HBL, we first need the patient's total blood volume (PBV), which can be calculated using the Nadler formula[9]:
PBV = k1 × height3 (m) + k2 × weight (kg) + k3, where k1 = 0.3669, k2 = 0.03219, and k3 = 0.6041 for men; and k1 = 0.3561, k2 = 0.03308, and k3 = 0.1833 for women.
Intraoperative blood loss was estimated according to the weight gain of soaked surgical sponges and the liquid volume in suction canisters that were used to remove the irrigation fluid added to the surgical field.
Previous research has shown that the hematocrit (Hct) of postoperative drainage may fluctuate over a wide range; therefore, the use of only the amount of drainage as the reference for postoperative blood loss is inaccurate.[10] In our study, we also examined the complete blood count (CBC) for every drainage sample to obtain Hct and hemoglobin (Hb) data, which were used to calculate the pure blood contained in the drainage.
The patient's total red blood cell volume can be obtained by multiplying the PBV by the Hct. Any change in red blood cell volume can therefore be calculated from the change in Hct. Total blood loss = PBV × (Hctpre – Hctpost)/Hctave. The HBL was estimated by subtracting the visible loss from the calculated total red blood cell volume loss and then adding the volume of the reinfusion. Except for the intraoperative blood loss, we regarded the pure blood contained in the drainage as postoperative blood loss, and the formula used to estimate the HBL in our study is as follows:
HBL = total blood loss + infused blood – operative blood loss – volume of drainage × Hctdrainage/Hctave (the drainage was calculated separately from the first day to the third day).
2.3. Transfusion
The Hb, Hct and coagulation index were examined postoperatively at 8, 24, 48, and 72 h. Transfusion was performed for patients with an Hb level less than 8 g/dL or for symptomatic patients with an Hb level between 8 and 10 g/dL.
2.4. Statistical analysis
Fisher's exact test was used to analyze categorical variables. After checking the normality of variables and confirming that they had a normal distribution, differences in perioperative data between the 2 groups were analyzed using an independent samples t test. In all analyses, the level of statistical significance was set at P < .05. All data analyses were performed with the SPSS 19.0 software package.
3. Results
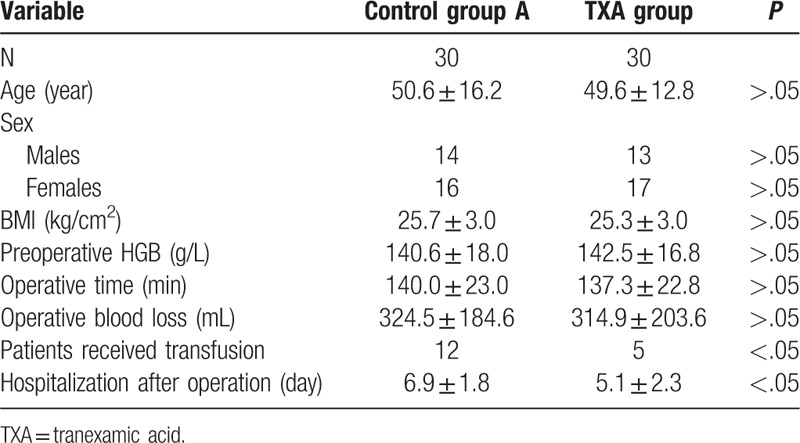
Sixty patients who met the inclusion criteria were enrolled in this study. Among these patients, 30 were assigned to the TXA group, and 30 were assigned to the control group. The mean age of the patients was 50.1 ± 14.3 years and the female/male ratio was 33:27. The average operative time was 138.8 ± 22.9 min and the operative blood loss was 317.5 ± 193.6 mL. No significant differences in age, height, weight, BMI, surgical level, preoperative Hb, coagulation indices, intraoperative blood loss, or operative duration were identified between the 2 groups, as presented in Table 1. However, the mean length of hospital stay (5.1 ± 2.3 vs 6.9 ± 1.8 days) and rate of blood transfusion (16.7% vs 40%) were significantly lower in the TXA group than in the control group.
Table 1.
Baseline data of patients in 2 groups.
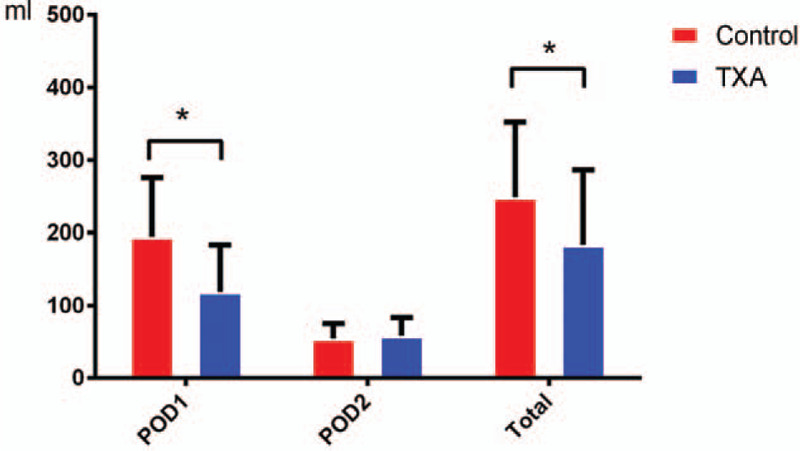
Detailed information about drainage in the 2 groups at various time points is presented in Table 2 and Figure 2. Compared to patients in the control group, patients in the TXA group had a significantly lower drainage volume on POD 1 and a lower total postoperative drainage volume (P < .05). The drainage volume on POD 2 was similar between the 2 groups and showed no significant difference.
Table 2.
Information of postoperative drainage.
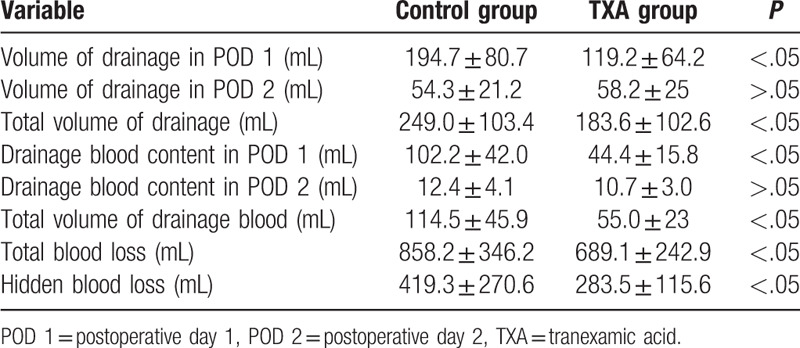
Figure 2.
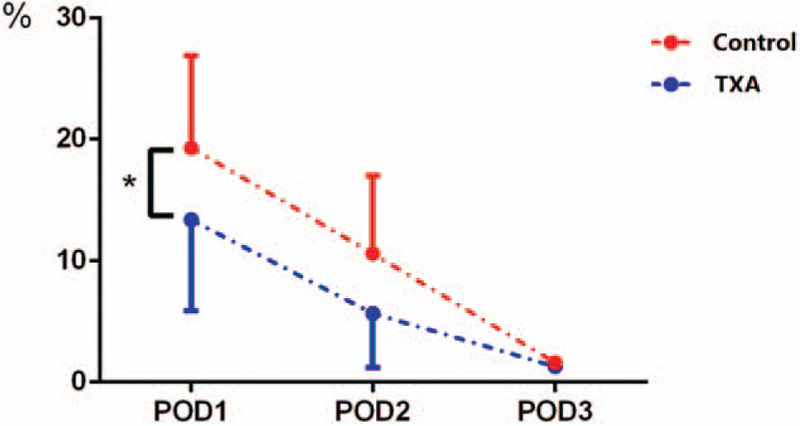
Postoperative drainage in the 2 groups: patients who received an application of topical tranexamic acid (TXA) were associated with a lower amount of drainage on postoperative day 1 (POD 1) and overall.
The Hct data of each drainage sample are presented in Figure 3; on POD1, the average Hct of the drainage was slightly lower than that in the whole blood CBC. However, the drainage Hct was reduced over time; on POD3, little blood content was present in the drainage. Using the daily volume and Hct of the drainage, the precise blood content in the drainage (volume of drainage × Hctdrainage/Hctave) was calculated as shown in Figure 4. The total amount of postoperative blood loss in the topical TXA group was much less than that in the control group, especially on POD1. Based on this calculation, we obtained the accurate total blood loss (TBL) (689.1 ± 242.9 vs 858.2 ± 346.2 mL) and HBL (283.5 ± 115.6 vs 419.3 ± 270.6 mL) values, as shown in Table 2. HBL was responsible for 45.6% of the TBL, and both of the indicators in the topical TXA group were much lower than those in the control group.
Figure 3.
Time variation of the hematocrit (Hct) of the drainage: The Hct of postoperative drainage may fluctuate in a wide range and decline obviously over time; therefore, it is inaccurate to solely use the amount of drainage as the reference for postoperative blood loss.
Figure 4.
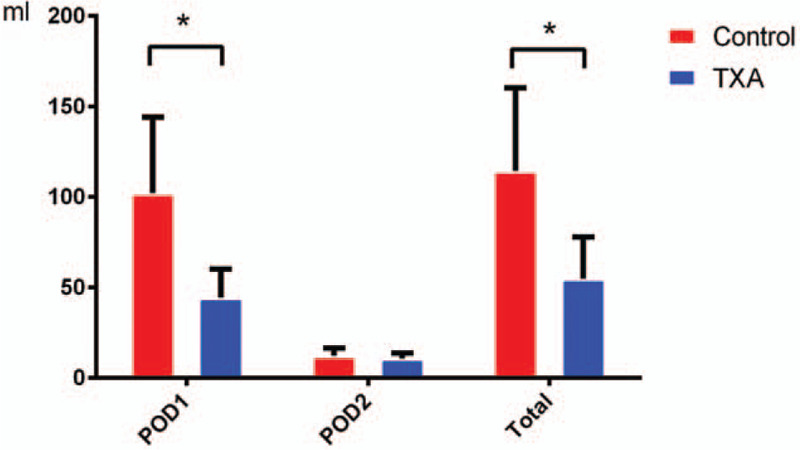
Blood content of the drainage in the 2 groups: accurate blood content in the drainage was calculated. The tranexamic acid (TXA) group showed less postoperative blood loss than the control group.
No perioperative complications, such as deep venous thrombosis/pulmonary embolism, postoperative hematoma or postoperative infection, occurred in either group.
4. Discussion
Few studies have focused on HBL in PLIF and its regulatory factors. In the present study, we analyzed the HBL of 60 patients who underwent PLIF to reveal the proportion of HBL and the influence of TXA on blood loss in PLIF. In previous studies, the postoperative drainage volume has been regarded as part of visible bleeding in the calculation of HBL. However, according to the Hct of the drainage measured at 24, 48, and 72 h in our study, the average Hct of the drainage declined obviously over consecutive days, and the drainage consisted mainly of the tissue fluid, while the blood volume decreased gradually. This result reminds us that we should not use the volume of drainage as the gold standard by which to judge postoperative blood loss and neglect the pure blood contained in the drainage. In our research, the mean TBL of 60 patients was 798.7 ± 302.5 mL, and the mean HBL was 351.4 ± 217.4 mL; therefore, the inferred HBL comprised 43.9% of the TBL. This result is consistent with previous findings from other spinal surgery research. In Yossi Smorgick's study, the calculated hidden loss was 600 mL or 42% of the total loss[11]; R.A. Hart also claimed that the HBL averaged 39.2% of the TBL in anterior lumbar interbody fusion surgery. HBL is caused by blood effusion into the tissues and residual cavity during surgery, and some research has suggested that hemolysis is also an important source of HBL. Excessive HBL may lead to a serious decrease in the Hb concentration and may influence postoperative rehabilitation; therefore, identifying techniques to effectively decrease HBL should be a priority.
TXA has been widely used in orthopedic surgery with positive hemostatic effects.[12] A meta-analysis performed by Xin Chen revealed that TXA significantly reduces HBL in TKA; therefore, they recommended using TXA as a standard drug to reduce HBL in arthroplasty surgery.[13] In Junichi Kushioka's study, the application of TXA reduced postoperative drainage by 52% after single-level PLIF.[13] In the present research, we planned to determine whether the utilization of topical TXA produced a similar outcome. The HBL of patients who received topical TXA was decreased by 32.5% compared to the control group; postoperative drainage was also decreased by 26.5%, and the TBL decreased by 19.7%. As a reversible lysine analog, TXA can compete with the lysine residues on fibrin to inhibit the conversion of plasminogen to plasmin, thus controlling fibrinolysis and stabilizing the clot. TXA is considered to be one of the most effective antifibrinolytic agents with minimal side effects.
The relationship between the use of TXA and thromboembolic events remains unclear, which is concerning. IV TXA has been reported to cause serious side effects in patients with hypercoagulability, severe ischemic heart disease and multiple organ failure.[14] In this study, we applied topical TXA at the end of the operation, and no thromboembolic events occurred. Compared to IV TXA, topical TXA provides a more convenient method to reach an effective concentration of TXA at the bleeding site. Joseph's research proposed that topical TXA is much more cost-effective and better at reducing perioperative blood loss than IV TXA for patients receiving THA and TKA.[15]
Decreased perioperative blood loss is associated with not only a reduced surgical risk but also a lower rate of transfusion and shorter hospitalization duration. Only 5 patients who received topical TXA received a transfusion after surgery, compared to 12 in the control group. Additionally, the average postoperative hospitalization duration of the topical TXA group was 5.1 days, which was significantly shorter than that in the control group (6.9 days). Many beneficial factors resulting from reduced perioperative blood loss, including a lower incidence of anemia, better physical condition and earlier functional exercise, may be reasons for this improvement.
Innovation is embodied in three aspects of our study. First, few studies have focused on exploring HBL in PLIF, which is rapidly becoming popular and is associated with massive blood loss. Second, although previous studies have examined the application of IV TXA in spinal fusion surgery, the validity of topical TXA needs to be further explored. Third, to evaluate HBL more accurately, the Hct of the drainage was measured in our study to precisely calculate postoperative blood loss. There are also limitations of our study that need to be addressed. First, only 60 cases were included in our RCT; thus, the sample size was relatively small. Moreover, patients with severe contraindications were excluded from our trial; therefore, the results are not sufficiently convincing to declare topical TXA as safe for all patients. Additional systematic and comprehensive research about the application of topical TXA is required in the future.
5. Conclusion
In the present study, we provide strong evidence that PLIF is associated with substantial perioperative HBL. Therefore, the Hb and drainage must be monitored in patients to prevent postoperative anemia. Additionally, we propose the application of topical TXA at the end of PLIF, which can significantly decrease HBL without causing any side effects. Reduced perioperative blood loss is associated with a safer surgery, faster recovery, and higher medical satisfaction. We hope that more effective measures to reduce perioperative blood loss can be evaluated in the future.
Acknowledgments
None.
Author contributions
Conceptualization: Xin Chen, Shugang Li.
Data curation: Derong Xu, Zhinan Ren.
Formal analysis: Zheng Li.
Methodology: Shugang Li.
Project administration: Shugang Li.
Supervision: Zheng Li, Shugang Li.
Writing – original draft: Derong Xu, Xin Chen.
Writing – review & editing: Derong Xu, Zheng Li, Qianyu Zhuang.
Footnotes
Abbreviations: BMI = body mass index, Hb = hemoglobin, HBL = hidden blood loss, Hct = hematocrit, IV TXA = intravenous TXA, PLIF = posterior lumbar interbody fusion, POD 1 = postoperative day 1, POD 2 = postoperative day 2, POD 3 = postoperative day 3, THA = total hip arthroplasty, TKA = total knee arthroplasty, TXA = tranexamic acid, VTEs = venous thrombosis events.
How to cite this article: Xu D, Chen X, Li Z, Ren Z, Zhuang Q, Li S. Tranexamic acid reduce hidden blood loss in posterior lumbar interbody fusion (PLIF) surgery. Medicine. 2020;99:11(e19552).
DX and XC are the co-first authors.
Registration number and name of trial registry: This RCT was approved by the Ethics Committee of Peking Union Medical College Hospital (reference number ZS-1000). All participants provided written informed consent for the study and surgery.
The trial registration number is ChiCTR-IIR-17010785: “A randomized controlled trial on effects of collagen sponge and topical tranexamic acid in posterior spinal fusion surgeries”. The URL is http://www.chictr.org.cn/listbycreater.aspx. The date of registration was 5/3/2017. The date of enrollment of the first participant into the trial was 01/11/2013.
Study Design: A randomized controlled trial study.
Consent for publication: We have obtained consent from the participants to report and publish individual patient data.
We received funding from Peking Union Medical College Hospital for the clinical collection, analysis, and interpretation of data.
The authors have no conflicts of interests to disclose.
Availability of data and materials: The raw data were collected and analyzed by the authors, who are not ready to share their data because the data have not been published.
Ethics approval and consent to participate: This RCT was approved by the Ethics Committee of Peking Union Medical College Hospital (reference number ZS-1000). All participants provided written informed consent for the study and surgery.
The trial registration number is ChiCTR-IIR-17010785: “A randomized controlled trial on effects of collagen sponge and topical tranexamic acid in posterior spinal fusion surgeries”. The URL is http://www.chictr.org.cn/listbycreater.aspx. The date of registration was 5/3/2017. The date of enrolment of the first participant into the trial was 01/11/2013.
References
- [1].Hossein Elgafy M. Blood loss in major spine surgery. Spine (Phila Pa 1976) 2010. [DOI] [PubMed] [Google Scholar]
- [2].Xu D, Ren Z, Chen X, et al. The further exploration of hidden blood loss in posterior lumbar fusion surgery. Orthop Traumatol Surg Res 2017;103:527–30. [DOI] [PubMed] [Google Scholar]
- [3].Liu X, Zhang X, Chen Y, et al. Hidden blood loss after total hip arthroplasty. J Arthroplasty 2011;26:1100–5. e1101. [DOI] [PubMed] [Google Scholar]
- [4].Fleege C, Rickert M, Rauschmann M. The PLIF and TLIF techniques. Indication, technique, advantages, and disadvantages. Orthopade 2015;44:114–23. [DOI] [PubMed] [Google Scholar]
- [5].Kang WS, Oh CS, Kwon WK, et al. Effect of mechanical ventilation mode type on intra- and postoperative blood loss in patients undergoing posterior lumbar interbody fusion surgery: a randomized controlled trial. Anesthesiology 2016;125:115–23. [DOI] [PubMed] [Google Scholar]
- [6].Xie J, Ma J, Yao H, et al. Multiple boluses of intravenous tranexamic acid to reduce hidden blood loss after primary total knee arthroplasty without tourniquet: a randomized clinical trial. J Arthroplasty 2016;31:2458–64. [DOI] [PubMed] [Google Scholar]
- [7].Luo W, Sun RX, Jiang H, et al. The efficacy and safety of topical administration of tranexamic acid in spine surgery: a meta-analysis. J Orthop Surg Res 2018;13:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sethna NF, Zurakowski D, Brustowicz RM, et al. Tranexamic acid reduces intraoperative blood loss in pediatric patients undergoing scoliosis surgery. Anesthesiology 2005;102:727–32. [DOI] [PubMed] [Google Scholar]
- [9].Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery 1962;51:224–32. [PubMed] [Google Scholar]
- [10].Xu D, Ren Z, Chen X, et al. A randomized controlled trial on effects of different hemostatic sponges in posterior spinal fusion surgeries. BMC Surg 2016;16:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Smorgick Y, Baker KC, Bachison CC, et al. Hidden blood loss during posterior spine fusion surgery. Spine J 2013;13:877–81. [DOI] [PubMed] [Google Scholar]
- [12].Ren Z, Li S, Sheng L, et al. Topical use of tranexamic acid can effectively decrease hidden blood loss during posterior lumbar spinal fusion surgery: a retrospective study. Medicine (Baltimore) 2017;96:e8233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chen X, Zhu X, Yang S, et al. Tranexamic acid treatment decreases hidden blood loss in total knee arthroplasty. Am J Ther 2016;23:e1397–405. [DOI] [PubMed] [Google Scholar]
- [14].Cho SK, Yi J-S, Park MS, et al. Hemostatic techniques reduce hospital stay following multilevel posterior cervical spine surgery. J Bone Joint Surg Am Vol 2012;94:1952–8. [DOI] [PubMed] [Google Scholar]
- [15].DiBlasi JF, Smith RP, Garavaglia J, et al. Comparing cost, efficacy, and safety of intravenous and topical tranexamic acid in total hip and knee arthroplasty. Am J Orthop (Belle Mead NJ) 2016;45:E439–e443. [PMC free article] [PubMed] [Google Scholar]


