Abstract
The present study aimed to investigate the association between the expressions of serum progranulin (PGRN) and serum soluble Oxford 40 ligand (sOX40L) and determine their clinical significances in primary Sjögren's syndrome (pSS).
The present study included a total of 68 patients with pSS and 50 healthy controls. Demographic data and clinical basic information were collected. Enzyme-linked immunosorbent assay (ELISA) was performed to determine serum levels of PGRN, sOX40L and interleukins. Spearman's correlation coefficient and Mann–Whitney U test were used to determine the correlation between PGRN, and sOX40L and the association between PGRN and sOX40L and disease activity and disease severity.
Serum interleukin (IL)-4, IL-6, IL-10, PGRN, and sOX40L levels were significantly higher in pSS patients as compared to the healthy controls. A positive correlation was observed between PGRN and sOX40L. Patients with elevated levels of PGRN or sOX40L exhibited higher disease activity compared to those with lower levels. Patients with III to IV stages of pSS or multiple system damage showed higher serum levels of PGRN and sOX40L.
Elevated serum PGRN, and sOX40L levels were relevant with disease activity and severity in patients with pSS.
Keywords: oxford 40 ligand, primary Sjögren's syndrome, progranulin
1. Introduction
Primary Sjögren's syndrome (pSS) is a common rheumatic disease characterized by dryness of mouth and eye and multiple organ dysfunction.[1] The clinical symptoms of pSS at an early stage are relatively insidious and nonspecific, which is difficult to detect in time. It is widely accepted that the pathogenesis of pSS is closely related to the cytokines, especially for inflammatory cytokines involved in immune response and inflammatory reaction. Like other autoimmune diseases, pSS is mainly caused by the disorder of autoimmune tolerance mechanism, leading to abnormal T cell and B cell activation.[2] With the deepening of immunological research, the relationship among toll-like receptors, interleukin, T cells, lymphocytes, and the pathogenesis of pSS has been continuously revealed. Until now, interleukin is the most intensively studied cytokines in the field of rheumatic immunity. Recent studies suggested up-regulation of interleukin (IL)-4, IL-6, and IL-10 in pSS patients,[3,4] but their association with clinical manifestations has not been reported yet.
Oxford 40 (OX40) is a kind of tumor-necrosis-factor receptor (TNFR), which is activated by its cognate ligand OX40L that functions as a T cell co-stimulator to amplify T and B cell proliferative response.[5] In recent years, abundant studies provided strong evidences that OX40-OX40L interaction is critical for the progression of autoimmune diseases. For example, Jiang et al demonstrated that OX40 and OX40L in serum and in synovial fluid were highly expressed in patients with rheumatoid arthritis (RA) and were positively correlated with clinicopathological characteristics. Nevertheless, OX40 blockade inhibited the pro-inflammatory responses and ameliorated RA.[6] Moreover, Cortini et al reported that OX40 knockout mice showed the decrease of splenic T follicular helper (Tfh) cells in patients with systemic lupus erythematosus (SLE).[7] However, the clinical significance of OX40L in the pathogenesis of pSS remains unclear.
Progranulin (PGRN) is a autocrine growth factor implicated in neurons, lymphocytes, epithelial cells, and chondrocytes and immunocytes.[8,9] A large quantity of evidences has demonstrated that it plays a vital role in early embryonic development, inflammation, tissue reconstruction, and immune response.[8,10] Emerging evidences indicate that PGRN is associated with autoimmune diseases, including systemic lupus erythematous, systemic sclerosis, multiple sclerosis, and pSS.[11] Meanwhile, PGRN was previously reported to be an endogenous antagonist of TNF-α through competitively binding to TNFR, which in turn mediated anti-inflammatory activity in various inflammatory diseases including rheumatoid arthritis (RA).[11,12] Serum PGRN is up-regulated in patients with pSS,[13] but its association with OX40L needs more researches to confirm.
On the basis of aforementioned studies, in the present study, serum PGRN, serum soluble OX40L (sOX40L) levels in patients with pSS were determined to investigate the relationship between them, and to explore the expression differences among different types of patients.
2. Material and methods
2.1. Study population
This prospective study included a total of 68 pSS patients who were diagnosed in our hospital during January 2015 to December 2016. Diagnosis was made if any three of the four objective criteria were present (that is items 3–6):
-
1.
ocular symptoms;
-
2.
oral symptoms;
-
3.
ocular signs;
-
4.
histopathology;
-
5.
salivary gland involvement; and
-
6.
autoantibodies.
We subdivided pSS patients into 4 groups according to EULAR Sjögren's syndrome disease activity index (ESSDAI) values[14]: stage I, subjects with ESSDAI 1 to 2 (n = 11); stage II, subjects with ESSDAI 3 to 5 (n = 31); stage III, subjects with ESSDAI 6 to 8 (n = 14); stage IV, subjects with ESSDAI ≥9 (n = 12). Meanwhile, all patients were likewise were randomized to 2 subgroups according to clinical characteristics: subjects with multiple system damage (n = 29) or simple exocrine gland injury (n = 39). To understand the relationships between clinical characteristics and serum PGRN or sOX40L levels, all pSS patients were grouped according to the serum levels of PGRN (high PGRN group: PGRN ≥11.98 pg/L; low PGRN group: PGRN < 11.98 pg/L) or sOX40L (high sOX40L group: PGRN ≥7.34 ng/mL; low sOX40L group: sOX40L < 7.34 ng/mL). In addition, a total of age- and gender-matched healthy subjects (n = 50) were recruited as controls. Patients who received radiation treatment or anticholinergic drugs or had acquired immunodeficiency disease syndrome (AIDS) or graft-versus-host disease (GVHD) were excluded. All experimental protocols were approved by the Ethics Committee of the Second Hospital of Hebei Medical University. Informed consent was obtained from all subjects.
2.2. Data collection
Patients’ characteristics included demographics such as age and sex and disease duration were recorded. Furthermore, fluorescence-enzyme immunoassay was carried out for serological analysis of La/SSB and Ro/SSA autoantibodies. Disease activity was measured according to the EULAR Sjögren's Syndrome Patients Reported Index (ESSPRI) and ESSDAI.
2.3. Measurement of serum cytokines
The blood collection and measurement of sOX40L, as well as other serum factors were conducted as reported previously.[15] Briefly, peripheral blood samples (5 mL) were collected in tubes containing ethylenediaminetetraacetic acid (EDTA) to obtain monocytes or did not contain EDTA to obtain serum within 24 h of admission. Isopaque-Ficoll gradient centrifugation was conducted to obtain the peripheral venous blood mononuclear cells and monocytes were harvested and collected in RPMI1640 medium. Non-EDTA blood was immersed in ice and the supernatant was obtained after centrifugation with 1500 × g for 10 min at 4°C and then the supernatants were harvested and stored at −70°C. Enzyme-linked immunosorbent assay (ELISA) was subsequently performed using commercial ELISA kits (BD Biosciences, San Diego, CA) to determine the soluble serum levels of PGRN, sOX40L, IL-4, IL-6, and IL-10 following the manufacturer's protocols.
2.4. Statistical analysis
Statistical analyses were conducted using SPSS version 22.0 (IBM, CA). Differences between groups were analyzed by Student's t test along with Mann–Whitney U test. Spearman's analysis was used to determine the correlation between PGRN and sOX40L. Data were expressed as statistically mean ± standard deviation (SD) or percentage, and P < .05 were considered statistically significant.
3. Results
3.1. Baseline characteristics
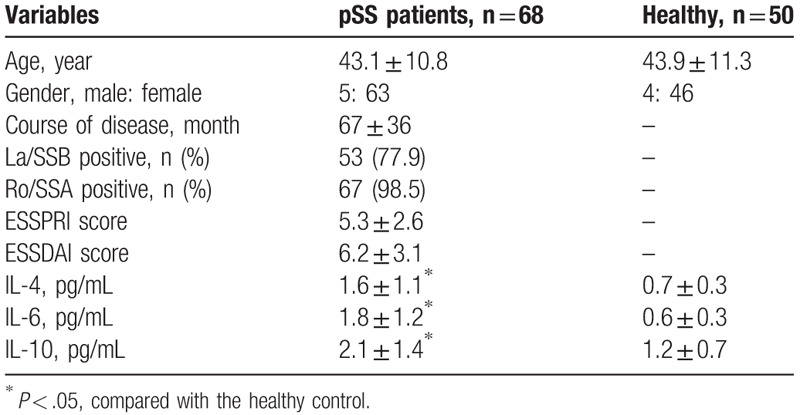
The demographic characteristics of patients’ baseline characteristics are listed in Table 1 showing that inflammatory cytokines IL-4, IL-6, and IL-10 were observably elevated in pSS patients as relative to healthy controls (all P < .05). No significant differences were observed among other clinical parameters.
Table 1.
Basic characteristics for all participants.
3.2. Correlation analysis of serum PGRN and sOX40L levels
As shown in Figure 1A (all P < .05), PGRN and sOX40L levels in pSS patients were significantly increased than those in healthy subjects. Furthermore, Spearman's correlation analysis disclosed pairwise positive correlation between PGRN and sOX40L in pSS patients (Fig. 1B; r2 = 0.3694, P < .001).
Figure 1.
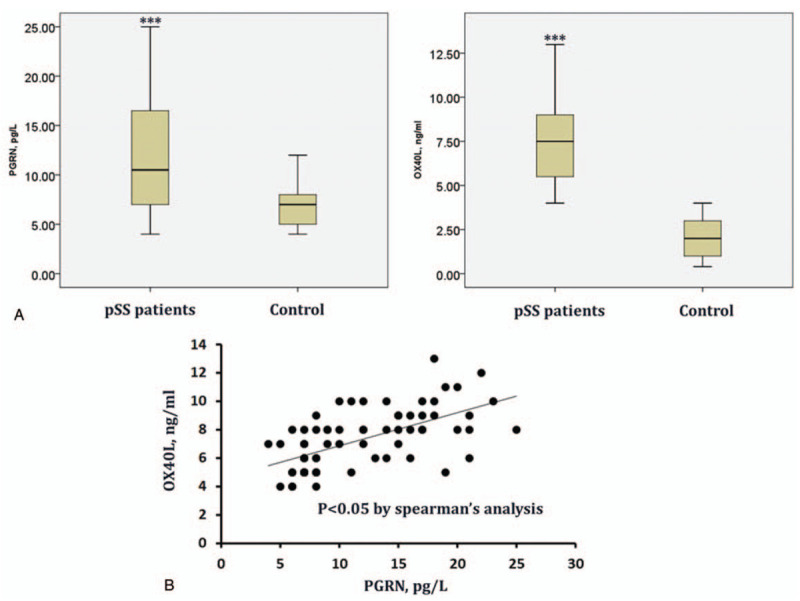
The serum levels of PGRN, sOX40L in patients with pSS (n = 68) and healthy controls (n = 50) determined by ELISA (A) and the correlation analysis between PGRN, sOX40L in patients using Spearman's correlation analysis (B). ∗P < .05 vs the control group.
3.3. Relationship among PGRN and sOX40L and clinical characteristics
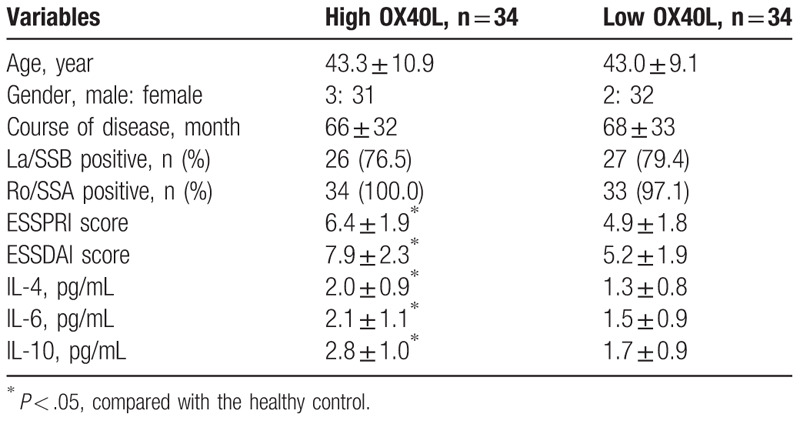
To further determine the association between serum PGRN and sOX40L levels and clinical outcome of patients with pSS, all subjects were divided into PGRN or sOX40L high/low group according to the median levels of serum PGRN or sOX40L. As presented in Tables 2 and 3, patients with elevated PGRN (≥11.98 pg/L) or sOX40L (≥7.34 ng/mL) exhibited higher ESSPRI and ESSDAI scores and IL-4, IL-6, and IL-10 serum levels than those with lower serum levels (all P < .05). However, there were no significant differences in age, sex, disease duration, anti-La/SSB positive rate and anti-Ro/SSA positive rate between groups. These findings suggested that elevated levels of PGRN or sOX40L in pSS patients were associated with higher disease activity.
Table 2.
Clinical outcomes for patients with high/low PGRN.
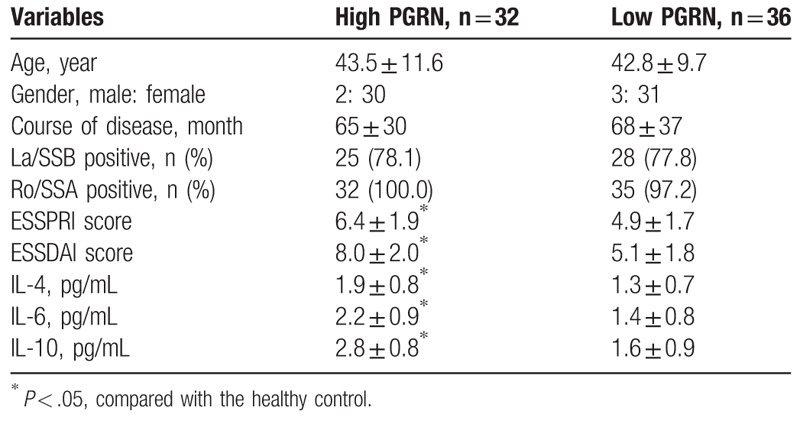
Table 3.
Clinical outcomes for patients with high/low OX40L.
3.4. Association between PGRN and sOX40L and disease severity in pSS patients
We further investigated the expressions of serum PGRN and sOX40L in different types of pSS. Compared with these patients with pSS stage of I to II and III to IV, PGRN, OX40, and sOX40L were markedly increased in patients with pSS stage II to IV (Fig. 2A; all P < .05). Additionally, PGRN and sOX40L were dramatically higher in patients with multiple system damage than in patients with simple exocrine gland injury (Fig. 2B; all P < .05). Collectively, our data suggested that the higher serum PGRN and sOX40L were significantly corrected with disease severity, suggesting that PGRN and sOX40L might play crucial roles in the pathogenesis and progression of pSS.
Figure 2.
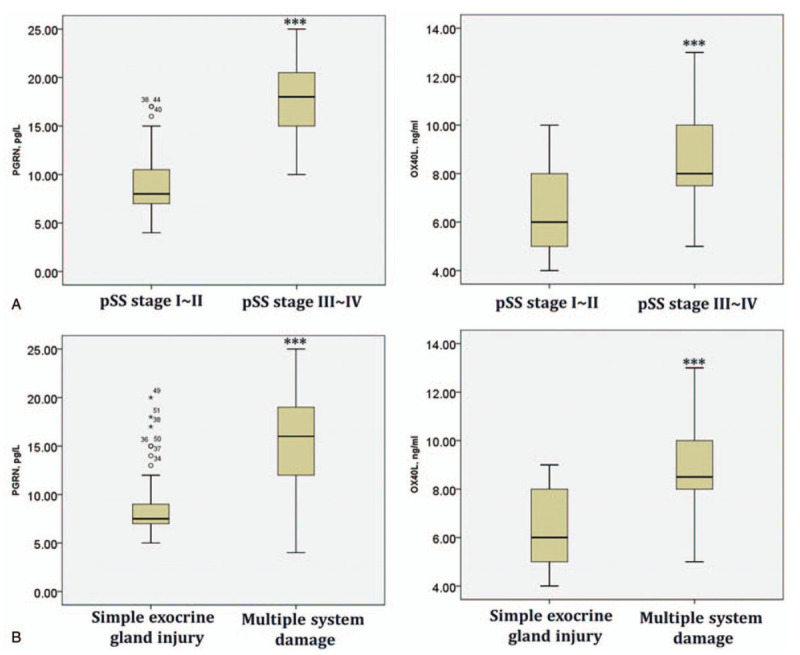
The serum levels of PGRN, sOX40L in patients with stage I to II (n = 42) or II to IV pSS (n = 26) (A); in pSS patients with multiple system damage (n = 29) or simple exocrine gland injury (n = 39) (B). ∗P < .05 vs patients with inactive pSS; #P < .05 vs patients with simple exocrine gland injury.
4. Discussion
The basic pathogenesis of pSS is immune dysfunction, in which exocrine gland duct epithelial cells act as antigen presenting cells (APCs) to facilitate T-lymphocyte proliferation, which in turn produce a large number of immunoglobulin, leading to excessive humoral immune response to release a great deal of pro-inflammatory cytokines.[16] Neurological manifestation including weakness of extremities and sensory dysfunction was present in all pSS patients.[17] In this study, B-lymphocyte derived cytokines IL-4, IL-6, and IL-10 were significantly up-regulated in pSS patients as relative to the healthy controls. To the best of our knowledge, IL-4 is a pleiotropic lymphokine responsible for the proliferation, activation, and survival of B lymphocytes and switching isotypes from IgM and IgD to IgG1 and IgE.[18,19] IL-6 is identified as a B lymphocyte differentiation factor that is essential for B lymphocyte differentiation, whereas, IL-10 promotes the secretion of B lymphocyte stimulator by monocytes, dendritic cells, and macrophages.[20,21] Abundant studies highlighted the anti-inflammatory role of IL-10 in various diseases. For instance, Meng et al[22] showed that the production of IL-10 exerted the anti-inflammatory and antioxidative effects in activated microglia through the inhibition of the NF-κB and STAT1 signaling pathways. However, Anaya et al[23] found that IL-10 concentration was higher in pSS patients than in controls, and were positively correlated with titers of IgA RF, anti-Ro, and anti-La antibodies, as well as focus score. In comparison with patients with low IL-10 production (<9.5 pg/mL), patients producing high IL-10 had significantly more episodes of cutaneous vasculitis. Xu et al reported that NOD/LtJ mice with pSS showed higher levels of IL-4, IL-6, and IL-10 in spleen B lymphocytes and were associated with salivary gland epithelial cell apoptosis.[24] Our findings confirmed that the occurrence of pSS triggered the secretion of inflammatory cytokines, which may exacerbate inflammation.
Meanwhile, some other inflammatory markers played crucial roles in pSS progression. For instance, Kim et al indicated that NOD-like receptor protein 3 (NLRP3) inflammasome was upregulated in pSS patients and were associated with the disease activity.[25] Vakrakou et al further found that NLRP3 inflammasome was highly associated with impaired DNA degradation in pSS.[26] Dupire et al illustrated that highly expressed high mobility group box-1 (HMGB1) levels were highly correlated with disease activity of pSS.[27]
Compelling evidence has highlighted the potential role of PGRN in the pathogenesis of several autoimmune diseases.[11] In a collagen-induced mouse model of RA, PGRN exerted the anti-inflammatory effects via TNF/TNFR pathway.[28] In SLE patients, the up-regulated serum PGRN was correlated with the expression levels of pro-inflammatory cytokines including IL-6, TNF-α, and TNFR.[29] In addition, PGRN was prominently elevated in dermal fibroblasts and serum of systemic sclerosis (SSc) patients, while PGRN knockdown exerted the pro-fibrotic effect.[30] Under a collagen-induced arthritis model, PGRN null mice exhibit a higher incidence of arthritis.[28] Recombinant PGRN protein attenuated the degradation of cartilage matrix and protected against osteoarthritis development.[31] PGRN was also reported to be significantly increased in pSS patients before treatment, which was reduced after pSS treatment.[32] In this study, higher levels of serum PGRN were observed in pSS patients as compared with the healthy subjects, which was consistent with results of a previous study.[13]
To the best of our knowledge, sOX40L interaction is activated in response to antigen presentation on multiple APCs and plays the pathogenic role in autoimmune diseases. For instance, Jacquemin et al illustrated that OX40L was significantly up-regulated in myeloid APCs of SLE patients, and were highly correlated with disease activity.[33] In our research, a positive correlation was also found between PGRN and sOX40L, suggesting that PGRN and sOX40L may all involve in the pathogenesis of pSS. However, there are still limitations. For example, the clinical samples are relatively small. There is no control group including other autoimmune disease. Moreover, whether serum levels of PGRN or sOX40L are associated with disease progression were not examined.
In conclusion, in the present study, our findings revealed highly expressed PGRN and sOX40L levels in serum of pSS patients. Additionally, serum PGRN or sOX40L were positively correlated with pSS disease activity indexes. Besides, the levels of serum PGRN and sOX40L were significantly higher in active pSS patients and in patients with multiple system damage. Our study might provide a new line of evidence that PGRN and sOX40L serve as an important markers of pSS.
Author contributions
Xuan Qi and Huifang Guo contributed the central idea, analysed most of the data, and wrote the initial draft of the paper. Hongtao Jin and the remaining authors contributed to refining the ideas, carrying out additional analyses and finalizing this paper.
Footnotes
Abbreviations: AIDS = acquired immunodeficiency disease syndrome, APCs = antigen presenting cells, EDTA = ethylenediaminetetraacetic acid, ELISA = Enzyme-linked immunosorbent assay, ESSDAI = EULAR Sjögren's syndrome disease activity index, ESSPRI = EULAR Sjögren's Syndrome Patients Reported Index, GVHD = graft-versus-host disease, HMGB1 = high mobility group box-1, IL = interleukin, NLRP3 = NOD-like receptor protein 3, PGRN = progranulin, pSS = primary Sjögren's syndrome, sOX40L = soluble Oxford 40 ligand, SD = standard deviation, SSc = systemic sclerosis, Tfh = T follicular helper, TNFR = tumor-necrosis-factor receptor.
How to cite this article: Qi X, Guo H, Sun C, Tian Y, Ding M, Yang Y, Jin H. Clinical significance of progranulin correlated with serum soluble Oxford 40 ligand in primary Sjögren's syndrome. Medicine. 2020;99:18(e19967).
This work was supported by Hebei province key research and development plan self-funded projects to Hongtao Jin.
All authors agreed the submission and the policy of the journal and copyright.
The authors have no conflicts of interest to disclose.
References
- [1].Bezzina OM, Gallagher P, Mitchell S, et al. Subjective and objective measures of dryness symptoms in primary Sjögren's syndrome: capturing the discrepancy. Arthritis Care Res 2016;69:1714–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tong L, Koh V, Thong YH. Review of autoantigens in Sjögren's syndrome: an update. J Inflam Res 2017;10:97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lee SY, Han SJ, Nam SM, et al. Analysis of tear cytokines and clinical correlations in Sjögren syndrome dry eye patients and non-Sjögren syndrome dry eye patients. Am J Ophthalmol 2013;156:247–53. [DOI] [PubMed] [Google Scholar]
- [4].Kang EH, Lee YJ, Hyon JY, et al. Salivary cytokine profiles in primary Sjogren's syndrome differ from those in non-Sjogren sicca in terms of TNF-( levels and Th-1/Th-2 ratios. Clin Exp Rheumatol 2011;29:970–6. [PubMed] [Google Scholar]
- [5].Webb GJ, Hirschfield GM, Lane PJL. OX40, OX40L and autoimmunity: a comprehensive review. Clin Rev Allergy Immunol 2016;50:312–32. [DOI] [PubMed] [Google Scholar]
- [6].Jiang J, Liu C, Mi L, et al. OX40 signaling is involved in the autoactivation of CD4+CD28−T cells and contributes to the pathogenesis of autoimmune arthritis. Arthritis Res Ther 2017;19:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cortini A, Ellinghaus U, Malik TH, et al. B cell OX40L supports T follicular helper cell development and contributes to SLE pathogenesis. Ann Rheum Dis 2017;76:2095–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kuse Y, Tsuruma K, Sugitani S, et al. Progranulin promotes the retinal precursor cell proliferation and the photoreceptor differentiation in the mouse retina. Sci Rep 2016;6:23811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhang K, Li Y, Feng D, et al. Imbalance between TNFα and progranulin contributes to memory impairment and anxiety in sleep-deprived mice. Sci Rep 2017;7:43594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yang D, Wang LL, Dong TT, et al. Progranulin promotes colorectal cancer proliferation and angiogenesis through TNFR2/Akt and ERK signaling pathways. Am J Cancer Res 2015;5:3085. [PMC free article] [PubMed] [Google Scholar]
- [11].Jian J, Li G, Hettinghouse A, et al. Progranulin: a key player in autoimmune diseases. Cytokine 2018;101:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tian Q, Zhao Y, Mundra JJ, et al. Three TNFR-binding domains of PGRN act independently in inhibition of TNF-alpha binding and activity. Front Biosci 2014;19:1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zhang NI, Yang N, Chen Q, et al. Upregulated expression level of the growth factor, progranulin, is associated with the development of primary Sjögren's syndrome. Exp Ther Med 2014;8:1643–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Seror R, Ravaud P, Bowman SJ, et al. EULAR Sjogren's syndrome disease activity index: development of a consensus systemic disease activity index for primary Sjogren's syndrome. Ann Rheum Dis 2010;69:1103–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yan J, Gong J, Chen G, et al. Evaluation of serum soluble OX40 ligand as a prognostic indicator in acute coronary syndrome patients. Clin Chim Acta 2010;411:0–1665. [DOI] [PubMed] [Google Scholar]
- [16].Hillen MR, Ververs FA, Kruize AA, et al. Dendritic cells, T-cells and epithelial cells: a crucial interplay in immunopathology of primary Sjogren's syndrome. Expert Rev Clin Immunol 2014;10:521–31. [DOI] [PubMed] [Google Scholar]
- [17].Seeliger T, Prenzler NK, Gingele S, et al. Neuro-Sjogren: Peripheral Neuropathy With Limb Weakness in Sjogren's Syndrome. Front immunol 2019;10:1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Froidure A, Mouthuy J, Durham SR, et al. Asthma phenotypes and IgE responses. Eur Respir J 2016;47:304. [DOI] [PubMed] [Google Scholar]
- [19].Baba Y, Ripley B, Kishimoto T, et al. Cytokine Regulation of B Cell Activation and Differentiation. Encyclopedia of Immunobiology 2016;3:244–52. [Google Scholar]
- [20].Maeda K, Mehta H, Drevets DA, et al. IL-6 increases B-cell IgG production in a feed-forward proinflammatory mechanism to skew hematopoiesis and elevate myeloid production. Blood 2010;115:4699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Saraswathula A, Reap EA, Choi BD, et al. Serum elevation of B lymphocyte stimulator does not increase regulatory B cells in glioblastoma patients undergoing immunotherapy. Cancer Immunol Immunother 2016;65:205–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Meng J, Ni J, Wu Z, et al. The critical role of IL-10 in the antineuroinflammatory and antioxidative effects of rheum tanguticum on activated microglia. Oxidative Med Cell Longevity 2018;2018:1083596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Anaya JM, Correa PA, Herrera M, et al. Interleukin 10 (IL-10) influences autoimmune response in primary Sjogren's syndrome and is linked to IL-10 gene polymorphism. J Rheumatol 2002;29:1874–6. [PubMed] [Google Scholar]
- [24].Xu T, Xie W, Ma Y, et al. Leptin/OB-R pathway promotes IL-4 secretion from B lymphocytes and induces salivary gland epithelial cell apoptosis in Sjögren's syndrome. Oncotarget 2017;8:63417–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kim SK, Choe JY, Lee GH. Enhanced expression of NLRP3 inflammasome-related inflammation in peripheral blood mononuclear cells in Sjögren's syndrome. Clin Chim Acta 2017;474:147–54. [DOI] [PubMed] [Google Scholar]
- [26].Vakrakou AG, Boiu S, Ziakas PD, et al. Systemic activation of NLRP3 inflammasome in patients with severe primary Sjögren's syndrome fueled by inflammagenic DNA accumulations. J Autoimmun 2018;91:23–33. [DOI] [PubMed] [Google Scholar]
- [27].Dupire G, Nicaise C, Gangji V, et al. Increased serum levels of high-mobility group box 1 (HMGB1) in primary Sjögren's syndrome. Scand J Rheumatol 2012;41:120–3. [DOI] [PubMed] [Google Scholar]
- [28].Tang W, Lu Y, Tian QY, et al. The growth factor progranulin binds to TNF receptors and is therapeutic against inflammatory arthritis in mice. Science 2011;332:478–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Feng Q, Song L, Feng D, et al. Expression level of the growth factor progranulin is related with development of systemic lupus erythematosus. Diagn Pathol 2013;8:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ichimura Y, Asano Y, Akamata K, et al. Progranulin overproduction due to Fli-1 deficiency contributes to the resistance of dermal fibroblasts to tumor necrosis factor in systemic sclerosis. Arthritis Rheumatol 2016;67:3245–55. [DOI] [PubMed] [Google Scholar]
- [31].Zhao YP, Tian QY, Frenkel S, et al. The promotion of bone healing by progranulin, a downstream molecule of BMP-2, through interacting with TNF/TNFR signaling. Biomaterials 2013;34:6412–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhang N, Yang N, Chen Q, et al. Upregulated expression level of the growth factor, progranulin, is associated with the development of primary Sjogren's syndrome. Exp Ther Med 2014;8:1643–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Jacquemin C, Schmitt N, Continbordes C, et al. OX40 ligand contributes to human lupus pathogenesis by promoting T follicular helper response. Immunity 2015;42:1159. [DOI] [PMC free article] [PubMed] [Google Scholar]


