Abstract
Background:
This study aimed to systematically assess the prognostic value of lymphocyte monocyte ratio (LMR) in patients with ovarian cancer through performing a meta-analysis.
Methods:
Web of Science, PubMed, EMBASE, Cochrane Library, and China National Knowledge Infrastructure databases were searched for potentially eligible studies. The baseline characteristics and relevant data were extracted. Hazard ratios with 95% confidence intervals (CIs) were combined to assess the prognostic value of LMR in patients with ovarian cancer.
Results:
Nine studies enrolling 2809 patients were included. The pooled hazard ratios of lower LMR for overall survival and progression free survival in patients with ovarian cancer were 1.71 (95% CI, 1.40–2.09) and 1.68 (95% CI, 1.49–1.88), respectively. Subgroup analysis and sensitivity analysis were also performed. No significant publication bias was found.
Conclusion:
Our results suggested that lower LMR was associated with poorer overall survival and progression free survival in patients with ovarian cancer. The findings may assist prognosis evaluation and future research on therapies based on modulating host immune response in ovarian cancer.
Keywords: lymphocyte monocyte ratio, ovarian cancer, prognosis, survival
1. Introduction
Ovarian cancer (OC) is the most lethal gynecologic cancer and a leading cause of cancer-related death in women.[1,2] In 2018, the estimated new OC cases and deaths worldwide were 295,414 and 184,799, respectively.[2] Since the patients in early-stage are usually asymptomatic and the symptoms in late stage are nonspecific, most patients are diagnosed at an advanced stage.[3] The primary treatment for OC patients was cytoreductive surgery followed by adjuvant chemotherapy.[4] Although the survival period has significantly prolonged in recent years, the 5-year survival rate is still less than 40% in advanced OC patients.[4,5] Several prognostic factors for OC have been suggested, such as, age, histology, and Federation International of Gynecology and Obstetrics stage.[6–8] However, given the poor prognosis of OC, it is worthwhile to find new accurate biomarkers for the prognosis and management of OC.
In recent years, accumulating evidence suggested that inflammation played an important role in cancer growth and metastasis, and had a prognostic significance in a variety of cancers.[9–11] Some inflammatory biomarkers in peripheral blood, such as neutrophil count, lymphocyte count, platelet lymphocyte ratio, neutrophil lymphocyte ratio, and lymphocyte monocyte ratio (LMR) have been proposed to predict cancer outcome.[11–13] LMR has been shown to be related to the prognosis of various cancers, such as hepatocellular carcinoma,[14] esophageal squamous cell carcinoma,[15] Hodgkin lymphoma,[16] urothelial cancers.[17] In recent years, many researchers have studied the prognostic value of LMR in patients with OC. However, the conclusions were controversial. Some researchers found that lower LMR was correlated to poorer survival in OC.[5,18,19] Some researchers concluded that LMR could not be an independent predictor in OC.[8,20] Therefore, this meta-analysis aimed to systematically assess the prognostic value of LMR in patients with OC.
2. Methods
2.1. Search strategy
Since this study was a meta-analysis, ethical approval was waived. We performed this meta-analysis according to the developed guidelines for meta-analyses.[21] Web of Science, PubMed, EMBASE, Cochrane Library, and China National Knowledge Infrastructure databases were searched for potentially eligible studies (the last search was run on May 30th, 2019). The searching keywords included: “ovarian neoplasms” AND (“lymphocyte monocyte ratio” OR “LMR”) AND (“prognosis” OR “survival” OR “outcome” OR “mortality”). The reference lists of relevant articles were also searched for additional records. Languages were restricted to English and Chinese.
2.2. Study selection
The study selection process was independently performed by 2 authors, with any disagreements resolved by discussion. The titles and abstracts of the studies were screened first. Then the rest studies were reviewed in full text. The inclusion criteria included:
-
(1)
the patients were diagnosed with any type of OC by pathological examination;
-
(2)
the routine blood tests of the patients were measured and LMR was calculated;
-
(3)
patients were followed up for survival outcomes;
-
(4)
enough data was reported to evaluate the prognostic role of LMR in patients with OC.
Conference abstracts, letters, case reports, reviews, unrelated articles, and studies without enough data were excluded. If multiple studies were performed in the same institute and the patients overlapped, the study with the largest sample size and longest follow-up time was included.
2.3. Data extraction
Data of the included studies were independently extracted by 2 authors, with any disagreements resolved by consensus. The primary data included hazard ratio (HR) for overall survival (OS)/progression free survival (PFS) with 95% confidence interval (CI), or survival curves which could be used to calculate the HR and 95% CI. HR with 95% CI calculated from multivariate analyses were extracted over those from univariate analyses. The basic characteristics of the patients and studies included first author, year of publication, country of origin, cancer type, number of samples, number of samples in stage I/II and III/IV, median or mean age of patients, ethnicity of patients, and the cut-off value of LMR.
2.4. Study quality assessment
The quality of each study was assessed by the Newcastle–Ottawa scale (NOS),[22] which assessed the study population, comparability, and outcome. The NOS scores ranged from 0 to 9, with 7 to 9 points indicating high-quality studies.
2.5. Statistical analysis
Log HR and variance calculated from the HR and 95% CI were used for aggregation. Forest plots were outlined to assess the prognostic value of LMR in patients with OC. The pooled HR was regarded statistically significant if the95% CI did not overlap 1 and the P-value was less than .05. Heterogeneity between the studies was evaluated, and P < .10 or I2 > 50% indicates significant heterogeneity. Random effect models were used in combining the date no matter significant heterogeneity exited or not, since the heterogeneity between studies was expected due to the different characteristics of patients and studies. When heterogeneity was present, sensitivity analysis was performed to evaluate the contribution of each study to heterogeneity by excluding each study at a time. Subgroup analyses were also performed according to cancer type, cancer stage, ethnicity of patients, and the cut-off value of LMR. Publication bias was evaluated by Begg test and P < .05 indicates significant publication bias. All the above statistical analyses were performed by STATA 11.0 (STATA Corporation, College Station, TX).
3. Results
3.1. Literature research
The initial database searching retrieved 155 studies. No additional records were identified from other sources. Among the records, 23 were duplicated and were removed. The rest 132 studies were evaluated by titles and abstracts, and 106 records were excluded according to the above inclusion and exclusion criteria. The remaining 26 articles were assessed in full text and 17 studies were excluded due to unrelated, lacking data or other reasons. Finally, 9 studies[5,8,18–20,23–26] fit the inclusion criteria and were included in this meta-analysis. The study selection process was shown in Figure 1.
Figure 1.
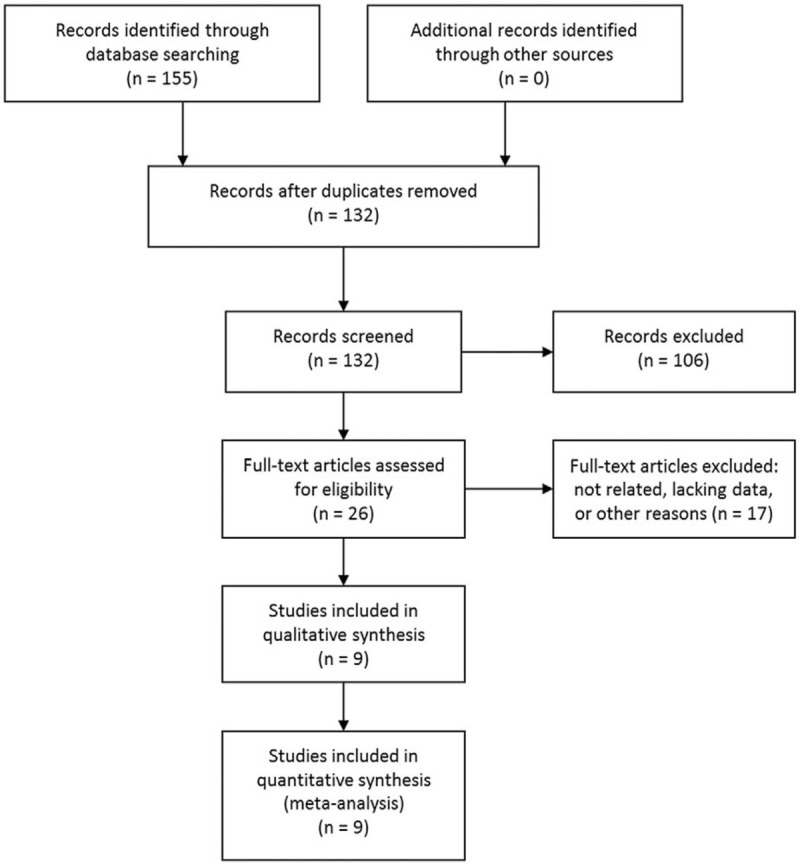
Selection process of studies.
3.2. Study characteristics
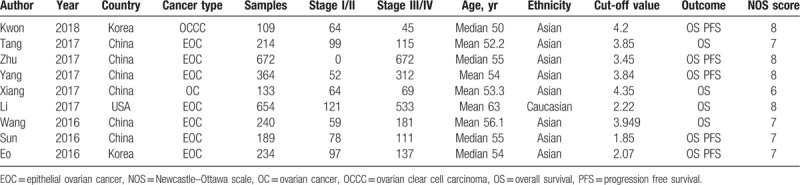
The baseline characteristics of the included studies were shown in Table 1. The studies were published in the latest 3 years and were conducted in 3 different countries. The cancer type included ovarian clear cell carcinoma (OCCC), epithelial ovarian cancer (EOC) and OC. A total of 2809 patients were investigated, with 634 in stage I/II and 2175 in stage III/IV. The mean or median ages were more than 50 years. Eight studies investigated Asian patients and 1 study investigated Caucasian patients. The cut-off values of LMR varied from 1.85 to 4.35. The survival outcomes included OS and PFS. As to the NOS score, 4 scored 8, 4 score 7 and 1 scored 6.
Table 1.
Characteristics of the included studies.
3.3. Overall analysis
All the 9 studies examined the association between LMR level and OS. The combined HR of lower LMR for OS was 1.71 (95% CI, 1.40–2.09) (Fig. 2A). Significant heterogeneity between the studies was observed (I2 = 69.8%, P = .001). Sensitivity analysis was performed, and the heterogeneities were still above 50% when excluding each study at a time. After excluding the study by Tang et al,[19] the heterogeneity dropped to the lowest value of 51.9% and the combined HR remained significant (1.54, 95% CI, 1.32–1.81).
Figure 2.
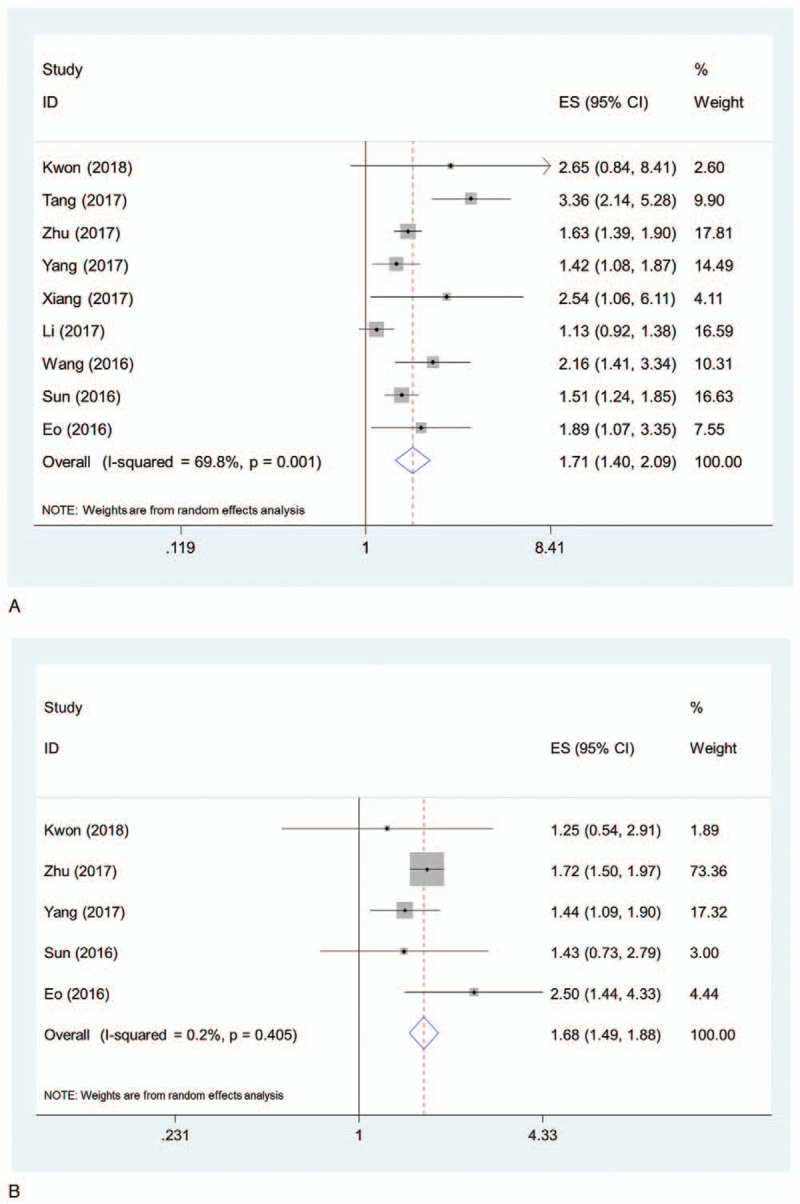
Pooled HR of lower LMR for OS (A) and PFS (B) in patients with ovarian cancer. HR = hazard ratio, LMR = lymphocyte monocyte ratio, OS = overall survival, PFS = progression free survival.
Five of the 9 studies examined the association between LMR level and PFS. The combined HR of lower LMR for PFS was 1.68 (95% CI, 1.49–1.88) (Fig. 2B). No significant heterogeneity between the studies was observed (I2 = 0.2%, P = .405).
3.4. Subgroup analysis
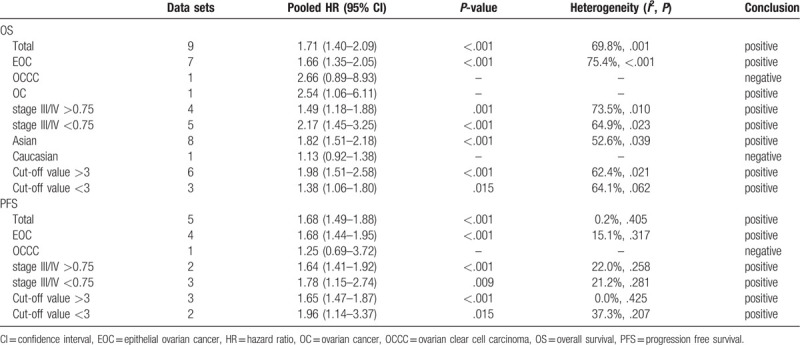
3.4.1. Cancer type
Among the 9 studies, 7 examined EOC, 1 examined OCCC, and 1 examined OC. The pooled HR of lower LMR for OS in EOC was 1.66 (95% CI, 1.35–2.05). The HRs of lower LMR for OS in OCCC and OC were 2.66 (95% CI, 0.89–8.93) and 2.54 (95% CI, 1.06–6.11), respectively.
For PFS, 4 studies examined EOC and 1 examined OCCC. The pooled HR of lower LMR for PFS in EOC was 1.68 (95% CI, 1.44–1.95). The HR of lower LMR for PFS in OCCC was 1.25 (95% CI, 0.69–3.72).
3.4.2. Cancer stage
The patients in stage III/IV accounted for more than 75% in 4 studies (defined as high grade group), and less than 75% in 5 studies (defined as low grade group). The pooled HRs of lower LMR for OS in high grade group and low grade group were 1.49 (95% CI, 1.18–1.88) and 2.17 (95% CI, 1.45–3.25), respectively.
For PFS, 2 studies were in the high grade group and 3 studies were in the low grade group. The pooled HRs of lower LMR for PFS in high grade group and low grade group were 1.64 (95% CI, 1.41–1.92) and 1.78 (95% CI, 1.15–2.74), respectively.
3.4.3. Ethnicity of patients
Among the 9 studies, 8 investigated Asian patients and 1 investigated Caucasian patients. The pooled HR of lower LMR for OS in Asian patients was 1.82 (95% CI, 1.51–2.18). The HR of lower LMR for OS in Caucasian patients was 1.13 (95% CI, 0.92–1.38).
3.4.4. Cut-off value of LMR
The cut-off value was more than 3 in 6 studies (>3 group) and less than 3 in 3 studies (<3 group). The pooled HRs of lower LMR for OS in >3 group and <3 group were 1.98 (95% CI, 1.51–2.58) and 1.38 (95% CI, 1.06–1.80), respectively.
For PFS, 3 studies were in the >3 group and 2 studies were in the <3 group. The pooled HRs of lower LMR for PFS in >3 group and <3 group were 1.65 (95% CI, 1.47–1.87) and 1.96 (95% CI, 1.14–3.37), respectively.
All the meta-analyses results were summarized in Table 2.
Table 2.
Summary of meta-analysis results.
3.5. Publication bias
No significant publication bias was observed in this meta-analysis. The Begg plots of publication bias of the 9 studies for OS (P = .348) and 5 studies for PFS (P = .806) were shown in Figure 3A and B.
Figure 3.
The Begg plots of publication bias of the studies for OS (A) and PFS (B). S = overall survival, PFS = progression free survival.
4. Discussion
This study aimed to assess the prognostic significance of LMR in patients with OC. A meta-analysis was conducted to summarize the present evidence. A total of 9 studies were included. The results suggested that lower LMR was associated with poorer OS and PFS in patients with OC.
Subgroup analyses were conducted to further explore the value of LMR in patients with OC. As to different cancer types, lower LMR was found to be associated with worse OS in patients with EOC and OC, and associated with worse PFS in patients with EOC. However, LMR was not significantly associated with OS or PFS in patients with OCCC. The results may suggest that the prognostic role of LMR differs among different cancer types. But the difference may also be attributed to the limited number of studies in each subgroup, since only 1 study examined the prognostic role of LMR in OCCC.[20] As to cancer stage, the HRs for OS and PFS in the low grade group were both slightly higher than that in the high grade group. These results suggested that LMR may better predict OS or PFS in low grade OC. As to the ethnicity of patients, we found that lower LMR was associated with poorer OS in Asian patients, but not in Caucasian patients. This difference may also be due to that only 1 study examined the prognostic role of LMR in Caucasian patients. As to the cut-off value of LMR, the pooled HR for OS was higher in the >3 group, but the pooled HR for PFS was slightly higher in the <3 group. Due to the limited number of studies in each subgroup, more studies are needed to determine the best cut-off value of LMR.
The link between inflammation and cancer has been verified previously.[27] It has been found that inflammatory cells and cytokines in tumors could contribute to tumor development, progression, and immunosuppression.[27,28] In recent years, accumulating evidence suggested that systemic inflammatory response played an important role in the prognosis of cancer. Many hematological biomarkers reflecting systemic inflammatory response has been suggested as independent prognostic biomarkers, such as, PLR, NLR, and LMR.[11–13]
Besides, absolute lymphocyte count and absolute monocyte count has also been found to be associated with the prognosis of many solid tumors.[20] Lymphocytes reflect the anti-tumor immune response, and lead to cytotoxic cell death and inhibit cancer cell growth and progression.[29] Tumor-infiltrating lymphocytes in the tumor microenvironments were found to be independent prognostic biomarkers of OC.[30] CD3+ and CD8+ tumor-infiltrating lymphocytes were associated with better OS and PFS in OC.[30] On the other hand, more monocytes were correlated with worse survival in different cancer types, since monocytes in the tumor microenvironments could differentiate into tumor-associated macrophages and promote tumor growth and metastasis.[31] Taken together, more lymphocyte and less monocyte within the tumor microenvironments both predict improved survival in cancers, thus supporting our findings that lower LMR was associated with poorer survival in patients with OC. LMR is cheap and readily accessible, and could serve as a promising prognostic tool in clinical work. Our results also suggest future research on therapies based on modulating host immune response in OC.
There are some limitations in this meta-analysis. First, the number of included studies was limited, and the number of studies was smaller in the subgroups. Therefore, the results, especially the difference in the subgroup analyses, should be interpreted with caution and more studies are warranted. Second, the baseline characteristics of the patients and studies were different among the studies, such as cancer type, cancer stage, ethnicity, and cut-off values of LMR. Thus, we performed subgroup analyses according to these characteristics. However, due to the clinical and statistical heterogeneity between the studies, more studies are still needed in the future. Furthermore, significant heterogeneity between the studies was observed when pooling the HRs for OS. Sensitivity analysis identified that the study by Tang et al contributed greatly to heterogeneity. After excluding that study, the combined HR for OS remained significant. Besides, no significant publication bias was found in this study, but publication bias should not be excluded completely.
In conclusion, our results suggested that lower LMR was associated with poorer OS and PFS in patients with OC. The findings may assist prognosis evaluation and future research on therapies based on modulating host immune response in OC. However, much more studies are warranted to verify our results, to determine the optimal cut-off value for LMR, and to examine the study methods to determine if manipulation of the LMR can affect the survival outcome.
Acknowledgment
The authors would like to thank Dr. Jing Zhang from West China Hospital, Sichuan University for his assistance in this research.
Author contributions
Conceptualization: Linrui Cai, Yanlin Song, Xia Zhao.
Data curation: Linrui Cai, Yanlin Song.
Formal analysis: Linrui Cai, Yanlin Song, Xia Zhao.
Investigation: Linrui Cai.
Methodology: Linrui Cai, Yanlin Song.
Project administration: Linrui Cai.
Resources: Linrui Cai, Yanlin Song.
Software: Linrui Cai, Yanlin Song.
Supervision: Xia Zhao.
Validation: Linrui Cai, Yanlin Song.
Visualization: Linrui Cai, Yanlin Song.
Writing – original draft: Linrui Cai, Yanlin Song, Xia Zhao.
Writing – review & editing: Linrui Cai, Yanlin Song, Xia Zhao.
Footnotes
Abbreviations: CI = confidence interval, EOC = epithelial ovarian cancer, HR = hazard ratio, LMR = lymphocyte monocyte ratio, NOS = Newcastle–Ottawa scale, OC = ovarian cancer, OCCC = ovarian clear cell carcinoma, OS = overall survival, PFS = progression free survival.
How to cite this article: Cai L, Song Y, Zhao X. Prognostic significance of lymphocyte monocyte ratio in patients with ovarian cancer. Medicine. 2020;99:14(e19638).
LC and YS contributed equally to this work.
This work was supported by the China Postdoctoral Science Foundation.
The authors have no conflicts of interest to disclose.
References
- [1].Jelovac D, Armstrong DK. Recent progress in the diagnosis and treatment of ovarian cancer. CA Cancer J Clin 2011;61:183–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [3].Polterauer S, Vergote I, Concin N, et al. Prognostic value of residual tumor size in patients with epithelial ovarian cancer FIGO stages IIA-IV: analysis of the OVCAD data. Int J Gynecol Cancer 2012;22:380–5. [DOI] [PubMed] [Google Scholar]
- [4].Cannistra SA. Cancer of the ovary. N Engl J Med 2004;351:2519–29. [DOI] [PubMed] [Google Scholar]
- [5].Zhu JY, Liu CC, Wang L, et al. Peripheral blood lymphocyte-to-monocyte ratio as a prognostic factor in advanced epithelial ovarian cancer: a multicenter retrospective study. J Cancer 2017;8:737–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lewin SN, Wright JD. Comparative performance of the 2009 International Federation of Gynecology and Obstetrics’ Staging System for uterine corpus cancer. Obstet Gynecol 2011;117:1226. [DOI] [PubMed] [Google Scholar]
- [7].Winter WE, 3rd, Maxwell GL, Tian C, et al. Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol 2007;25:3621–7. [DOI] [PubMed] [Google Scholar]
- [8].Li Z, Hong N, Robertson M, et al. Preoperative red cell distribution width and neutrophil-to-lymphocyte ratio predict survival in patients with epithelial ovarian cancer. Sci Rep 2017;7:43001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mason M, Maurice C, McNamara MG, et al. Neutrophil-lymphocyte ratio dynamics during concurrent chemo-radiotherapy for glioblastoma is an independent predictor for overall survival. J Neurooncol 2017;132:463–71. [DOI] [PubMed] [Google Scholar]
- [10].Wang W, Bian C, Xia D, et al. Combining carcinoembryonic antigen and platelet to lymphocyte ratio to predict brain metastasis of resected lung adenocarcinoma patients. BioMed Res Int 2017;2017:8076384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Serdarevic M, Kukulj S, Nikolic I, et al. Could neutrophil-to-lymphocyte ratio be predictor of brain metastases in non small cell lung cancer? J Thoracic Oncol 2016;11:S145. [Google Scholar]
- [12].Shaverdian N, Wang J, Levin-Epstein R, et al. Pro-inflammatory state portends poor outcomes with stereotactic radiosurgery for brain metastases. Anticancer Res 2016;36:5333–7. [DOI] [PubMed] [Google Scholar]
- [13].Templeton AJ, McNamara MG, Seruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 2014;106:dju124. [DOI] [PubMed] [Google Scholar]
- [14].Shimizu T, Ishizuka M, Park KH, et al. Preoperative lymphocyte-to-monocyte ratio is useful for stratifying the prognosis of hepatocellular carcinoma patients with a low Cancer of the Liver Italian Program score undergoing curative resection. Ann Gastroenterol Surg 2019;3:325–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Song Q, Wu JZ, Wang S. Low preoperative lymphocyte to monocyte ratio serves as a worse prognostic marker in patients with esophageal squamous cell carcinoma undergoing curative tumor resection. J Cancer 2019;10:2057–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lee SF, Ng TY, Spika D. Prognostic value of lymphocyte-monocyte ratio at diagnosis in Hodgkin lymphoma: a meta-analysis. BMC Cancer 2019;19:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhu X, Wu SQ, Xu R, et al. The evaluation of monocyte lymphocyte ratio as a preoperative predictor in urothelial malignancies: a pooled analysis based on comparative studies. Sci Rep 2019;9:6280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Yang HM, Lou G. The relationship of preoperativelymphocyte-monocyte ratio and the clinicopathological characteristics and prognosis of patients with epithelial ovarian cancer. Zhonghua Zhong Liu Za Zhi 2017;39:676–80. [DOI] [PubMed] [Google Scholar]
- [19].Tang Y, Li J, Xu F, et al. Association between monocyte-to-lymphocyte ratio and prognosis of patients with epithelial ovarian cancer. Chin J Obstet Gynecol Pediat 2017;13:532–8. [Google Scholar]
- [20].Kwon BS, Jeong DH, Byun JM, et al. Prognostic value of preoperative lymphocyte-monocyte ratio in patients with ovarian clear cell carcinoma. J Cancer 2018;9:1127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [23].Xiang J, Zhou L, Li X, et al. Preoperative monocyte-to-lymphocyte ratio in peripheral blood predicts stages, metastasis, and histological grades in patients with ovarian cancer. Transl Oncol 2017;10:33–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wang X, Yuan Z, Qiu H, et al. The relationship between preoperative blood lymphocyte-to-monocyte ratio and the prognostic of epithelial ovarian cancer. Prog Obstet Gynecol 2016;25:654–7. [Google Scholar]
- [25].Sun L, Song Y. Effects of lymphocyte and monocyte ratio on prognosis of epithelial ovarian cancer. Chin Clin Oncol 2016;21:909–12. [Google Scholar]
- [26].Eo WK, Chang HJ, Kwon SH, et al. The lymphocyte-monocyte ratio predicts patient survival and aggressiveness of ovarian cancer. J Cancer 2016;7:289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001;357:539–45. [DOI] [PubMed] [Google Scholar]
- [28].Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140:883–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lin EY, Pollard JW. Role of infiltrated leucocytes in tumour growth and spread. Br J Cancer 2004;90:2053–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Li J, Wang J, Chen R, et al. The prognostic value of tumor-infiltrating T lymphocytes in ovarian cancer. Oncotarget 2017;8:15621–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell 2006;124:263–6. [DOI] [PubMed] [Google Scholar]


