Abstract
The country of Georgia initiated an ambitious national hepatitis C elimination program. To facilitate elimination, a national hospital hepatitis C screening program was launched in November 2016, offering all inpatients screening for HCV infection. This analysis assesses the effectiveness of the first year of the screening program to identify HCV-infected persons and link them to care. Data from Georgia’s electronic Health Management Information System and ELIMINATION-C treatment database were analyzed for patients aged ≥18 years hospitalized from November 1, 2016 to October 31, 2017. We described patient characteristics and screening results and compared linked-to-care patients to those not linked to care, defined as having a test for viremia following an HCV antibody (anti-HCV) positive hospital screening. Of 291,975 adult inpatients, 252,848 (86.6%) were screened. Of them, 4.9% tested positive, with a high of 17.4% among males aged 40–49. Overall, 19.8% of anti-HCV+ patients were linked to care, which differed by sex (20.6% for males vs.18.4% for females; p=.019), age (23.9% for age 50–59 years vs. 10.7% for age ≥ 70 years; p < .0001), and length of hospitalization (21.8% among patients hospitalized for 1 day vs. 16.1% for those hospitalized 11+ days; p = .023). Redundant screening is a challenge; 15.6% of patients were screened multiple times and 27.6% of anti-HCV+ patients had a prior viremia test. This evaluation demonstrates that hospital-based screening programs can identify large numbers of anti-HCV+ persons, supporting hepatitis C elimination. However, low linkage-to-care rates underscore the need for screening programs to be coupled with Effective linkage strategies.
Keywords: Georgia, Hepatitis C, Hospital, Screening, Linkage to care
1. Introduction
Globally, in 2015 an estimated 71 million people were infected with hepatitis C virus (HCV), with approximately 400,000 HCV-attributable deaths (World Health Organization, 2017). Georgia, a lower-middle income Eurasian country with a population of 3.7 million people (The World Bank, 2017) has a high prevalence of HCV infection. Results from a nationally representative seroprevalence survey among Georgian adults (≥18 years) in 2015 found an HCV antibody (anti-HCV) prevalence of 7.7% (equating to approximately 215,000 persons) and a chronic hepatitis C prevalence of 5.4% (HCV RNA positive by PCR) (approximately 150,000 persons) (Hagan et al., 2019).
On April 28, 2015, in collaboration with international partners including technical assistance from U.S. Centers for Disease Control and Prevention (CDC) and a commitment from Gilead Sciences to provide direct-acting antiviral hepatitis C medications (DAAs) free of charge for all persons living with HCV infection in the country, Georgia launched an ambitious national hepatitis C elimination program (Gvinjilia et al., 2016; Nasrullah et al., 2017a; Nasrullah et al., 2017b). The country set a goal of 90% reduction in hepatitis C prevalence by 2020 with the following targets: (1) testing 90% of HCV-infected persons, (2) treating 95% of people with chronic HCV infection, and (3) curing 95% of persons treated for HCV infection (Strategic Plan for the Elimination of Hepatitis C Virus in Georgia 2016–2020, 2016). A national hepatitis C treatment database was established to monitor and evaluate program progress.
Screening for hepatitis C began nationally in January 2015, before the launch of the treatment program (Nasrullah et al., 2017a). Rapid anti-HCV testing is provided to Georgian residents at various settings free of charge (Nasrullah et al., 2017a), and a national screening registry was established. By the end of 2017, the treatment program had increased capacity by expanding to 31 sites throughout the country; however, the number of patients entering treatment, after peaking in late 2016, began to decrease with a smaller pool of untreated persons aware of their infection (Nasrullah et al., 2017a). In response, the Georgian government ramped up screening efforts at various locations including antenatal clinics, blood banks, harm reduction centers and prisons (Georgia Ministry of Health, Labour and Social Affairs, 2019). On September 16, 2016, the Ministry of Health released Resolution (N445), which mandated that medical facilities offer and then provide anti-HCV testing to all willing hospital inpatients regardless of diagnosis, and record both positive and negative results in their electronic Health Management Information System (HMIS) established in 2011. The only exceptions to these provisions were for inpatients with documentation of screening within 6 months or ongoing/past hepatitis C antiviral treatment. On November 1, 2016, the national hospital hepatitis C screening program launched nationwide.
We analyzed retrospective data from the hospital screening program and the national treatment program to assess the effectiveness of the hospital program in screening and linkage to care over its first year of implementation.
2. Methods
2.1. Data source
When the elimination program launched, a treatment database (STOP-C) was developed, and was upgraded in June 2016 (ELIMINATION-C) to meet the growing demands of the program (Mitruka et al., 2015). The database was designed to monitor patients enrolled in the treatment program, from confirmation of active HCV infection (with HCV RNA or core-antigen testing), through treatment outcome, including testing for cure (i.e. sustained virologic response [SVR]).
In 2011, Georgia implemented its HMIS for all hospitals in the country. Pursuant to the government decree on screening, results from inpatients’ rapid anti-HCV test and/or enzyme assay are entered into the HMIS (Health Management Information System (HMIS) Georgia, n.d.). Two fields were added to the HMIS to indicate: (1) whether HCV screening was performed (Yes/No) and (2) HCV screening result (Positive/Negative).
Monthly, the Georgia National Centers for Disease Control (NCDC) receives electronically transmitted data for patients of all ages admitted to hospitals the previous month, including: national identification number; basic demographic information (age, sex); discharge diagnoses, comorbidities, and complications (ICD10 codes); discharge/death date; length of hospitalization; HCV screening performed; and HCV screening result.
Data for this analysis was compiled from 4 different sources. Hospital HMIS data from November 1, 2016 to October 31, 2017 was used to determine the number of unique inpatients and those who were screened for hepatitis C. For linkage to care analysis and care continuum results among those linked to care, hospital data were cross-referenced with the ELIMINATION-C treatment database as well as vital statistics from November 1, 2016 to January 31, 2018, to allow a minimum 90 days follow-up for each patient after hospital discharge. Finally, to quantify the national impact of the hospital program, consolidated records were reviewed for all screening venues throughout the country from May 1, 2016 to April 31, 2017. Patients’ encrypted unique identification numbers, which are common to all data sources, allow for cross-referencing and deduplication in screening and treatment records.
2.2. Definitions
Definitions for unique hospital inpatients, patients ever/not HCV screened, anti-HCV positive, not anti-HCV positive, linked to care, and not linked to care are outlined in Table 1. Briefly, linkage to care was defined as receiving HCV viremia testing after hospital discharge. During the evaluation period, all anti-HCV positive patients had to visit a specialized HCV treatment provider site for viremia testing at the patients’ expense, the results of which are all entered into ELIMINATION-C. Patient inclusion/exclusion criteria are depicted in Fig. 1. For hospital diagnosis comparisons, “Liver-related: Any Hepatitis” ICD10 codes included: B15-B17, B18.0-B18.2, B18.8-B18.9, B19.0, B19.9, and K73, while “Liver-related: Non-Hepatitis” ICD10 codes included: B67.0, B67.5, B67.8, C22, I82.0, K70-K72, K74-K77, R17, R18, R16.0, R16.2, T51, T64, and Z20.5 (Supplementary Table S1). All other ICD10 codes in HMIS were included in the “Non-Liver related” category.
Table 1.
Definitions of patient categories for screening and linkage to care.
| Patient Category | Definition | Exclusion criteria |
|---|---|---|
| Unique hospital inpatient | Any adult (≥ 18 years old at time of death or discharge from the hospital) inpatient with at least one discharge or death date documented between November 1, 2016 and October 31, 2017. | Patients <18 years old at the time of their first discharge or death date within the evaluation period (treatment was not available for this population in Georgia at the time of assessment). |
| Ever HCV screened | Any unique inpatient with HCV screened field answer “yes” and a result (positive or negative) entered in the HCV result field. For patients with hospital admissions in the evaluation period, if they had a valid screening result during at least one admission they were counted in the ever HCV screened group. | Any entry with no response in the HCV screening field. Entries with an HCV screened answer “yes” but without a result entered in the HCV result field. |
| Not HCV screened | Any unique inpatient with HCV screening field answer “no” during hospitalization and no result in the HCV result field. | Entries with “no” in the HCV screening field but with a result (positive/negative) in the HCV result field. |
| Anti-HCV positive | Affirmative HCV screening field and a positive anti-HCV result. A patient with at least one valid anti-HCV positive result during the evaluation period was defined as anti-HCV Positive | |
| Not anti-HCV positive | Affirmative HCV screening field and negative anti-HCV results on each screening (if screened multiple times, all results negative). | |
| Linked to care | Any anti-HCV positive patient who had a documented HCV RNA or HCV core antigen test to confirm active infection after the date of hospital discharge, but on or before January 31, 2018. | Patients with documented HCV RNA or core antigen test results in the HCV treatment database dated before the discharge date for the hospital admission in which they screened positive. |
| Not linked to care | Any anti-HCV positive patient who did not have a test for active HCV infection at one of the HCV testing provider sites within the period between hospital discharge and January 31, 2018. | Patients with documented death date within the evaluation period. |
Fig. 1.
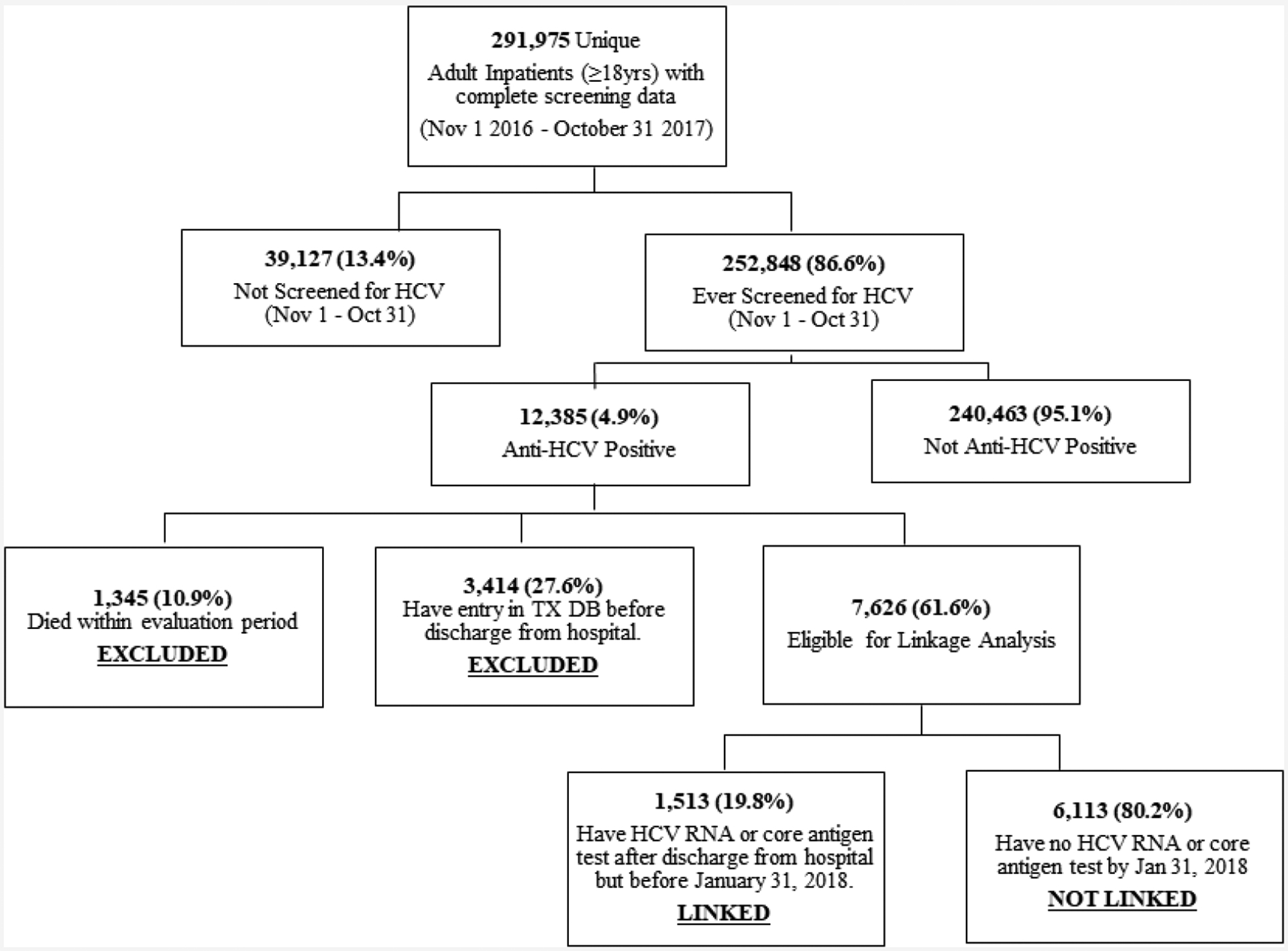
Inclusion and exclusion methodology for data analysis.
2.3. Data Analysis
Descriptive analysis of hospital screening records was performed to elucidate characteristics of patients screened for anti-HCV, patients screening positive, and those linked to care. Patients ever screened were compared to those not screened to assess factors associated with being screened while hospitalized. Likewise, we compared linked-to-care patients to those not linked to care to determine characteristics of anti-HCV positive patients who sought viremia testing following their visit. Statistically significant associations in bivariate analysis were determined using Chi-square test with a significance level of p < .05. All statistical analysis was conducted in SAS version 9.4.
This analysis utilizes data from Georgia’s hepatitis C elimination program, which was determined by Georgia’s NCDC to be a program evaluation and deemed to be a non-research public health program activity.
3. Results
3.1. Screening
Records from 270 out of a total 280 hospitals throughout Georgia were reviewed. Records for 134,641 patients who were <18 at the time of their hospital visit were excluded. Between November 1, 2016 and October 31, 2017 there were 378,552 documented hospital admissions for 300,615 unique adult patients admitted to and discharged from hospitals in Georgia. We excluded from this analysis 8640 patients with missing, incomplete or indeterminate screening results, leaving 291,975 patients from 253 hospitals that were included in this evaluation. Overall, 252,848 (86.6%) inpatients were screened for anti-HCV (Fig. 1) with 12,385 testing positive, for an overall anti-HCV positivity prevalence of 4.9%. The proportion of inpatients screened was lowest in the first month of the program (65.3%) and increased gradually, reaching 91.6% in October 2017 (data not shown). Of those screened, 40,071 (15.6%) were screened more than once; 29,890 (11.8%) were screened twice and 10,181 (4.0%) screened ≥3 times within the evaluation period. Those screened more than once had a median of 2 (IQR: 2, 3) hospital visits during the evaluation period, and the majority (58.7%; n = 23,514) were screened ≥2 times at the same hospital.
The median age of screened patients was 52 years (interquartile range [IQR]: 31, 68), and women (58.8%) were screened more than men (41.2%); more women (n = 170,942) than men (n = 121,033) were hospitalized during the evaluation period (Table 2). Although there were statistical differences (p < .05), screening rates were similar among men (86.0%) and women (87.0%), and among different age groups (range: 85.2% to 87.2%) (Table 2). More than 40,000 women aged 18–29 years were screened, the largest number of any age/sex group (Fig. 2). Screening varied by length of hospital stay, with patients hospitalized 2–10 days being more likely to be screened than those hospitalized for one day, or > 10 days (p < .0001) (Table 2).
Table 2.
Characteristics of adult patients with complete hepatitis C screening data admitted to the hospital at least once between November 1, 2016 and October 31, 2017, Georgia
| Characteristics | All patients | Patients ever screeneda | Patients not screened | Chi-square p-value | ||
|---|---|---|---|---|---|---|
| n | n | n | ||||
| Overall | 291,975 | 252,848 | 39,127 | |||
| Gender | ||||||
| Female | 170,942 | 148,748 | 22,194 | <.0001 | ||
| Male | 121,033 | 104,100 | 16,933 | |||
| Age Category (years) | ||||||
| 18–29 | 64,288 | 56,033 | 8,255 | <.0001 | ||
| 30–39 | 44,224 | 38,126 | 6,098 | |||
| 40–49 | 31,017 | 26,438 | 4,579 | |||
| 50–59 | 39,062 | 33,566 | 5,496 | |||
| 60–69 | 47,397 | 41,246 | 6,151 | |||
| 70+ | 65,987 | 57,439 | 8,548 | |||
| ICD10 Code (diagnosis, comorbidity, complication)b | ||||||
| Liver-Related: Any Viral Hepatitis | 1,293 | 1,141 | 152 | <.0001 | ||
| Liver-Related: Non-Hepatitis | 2,025 | 1,487 | 538 | |||
| Non-liver related | 288,657 | 250,220 | 38,437 | |||
| Length of Hospital Stay (days)c | ||||||
| 1 | 73,337 | 59,968 | 13,369 | <.0001 | ||
| 2–5 | 172,442 | 152,880 | 19,562 | |||
| 6–10 | 31,698 | 27,991 | 3,707 | |||
| >10 | 14,497 | 12,008 | 2,489 | |||
Patients ever screened (in evaluation period) defined as those patients with HCV screened (yes) and a result in the HCV result field (positive/negative). Patients with multiple admissions who met these criteria at least once included in this group.
Liver-related: any hepatitis ICD10 codes included: B15-B17, B18.0-B18.2, B18.8-B18.9, B19.0, B19.9 and K73. Liver-related: non-hepatitis ICD10 codes included: B67.0, B67.5, B67.8, C22, I82.0, K70-K72, K74-K77, R17, R18, R16.0, R16.2, T51, T64, and Z20.5. All other ICD10 codes found in the 066 system were included in the non-liver related category.
One patient had missing data on length of hospital stay.
Fig. 2.
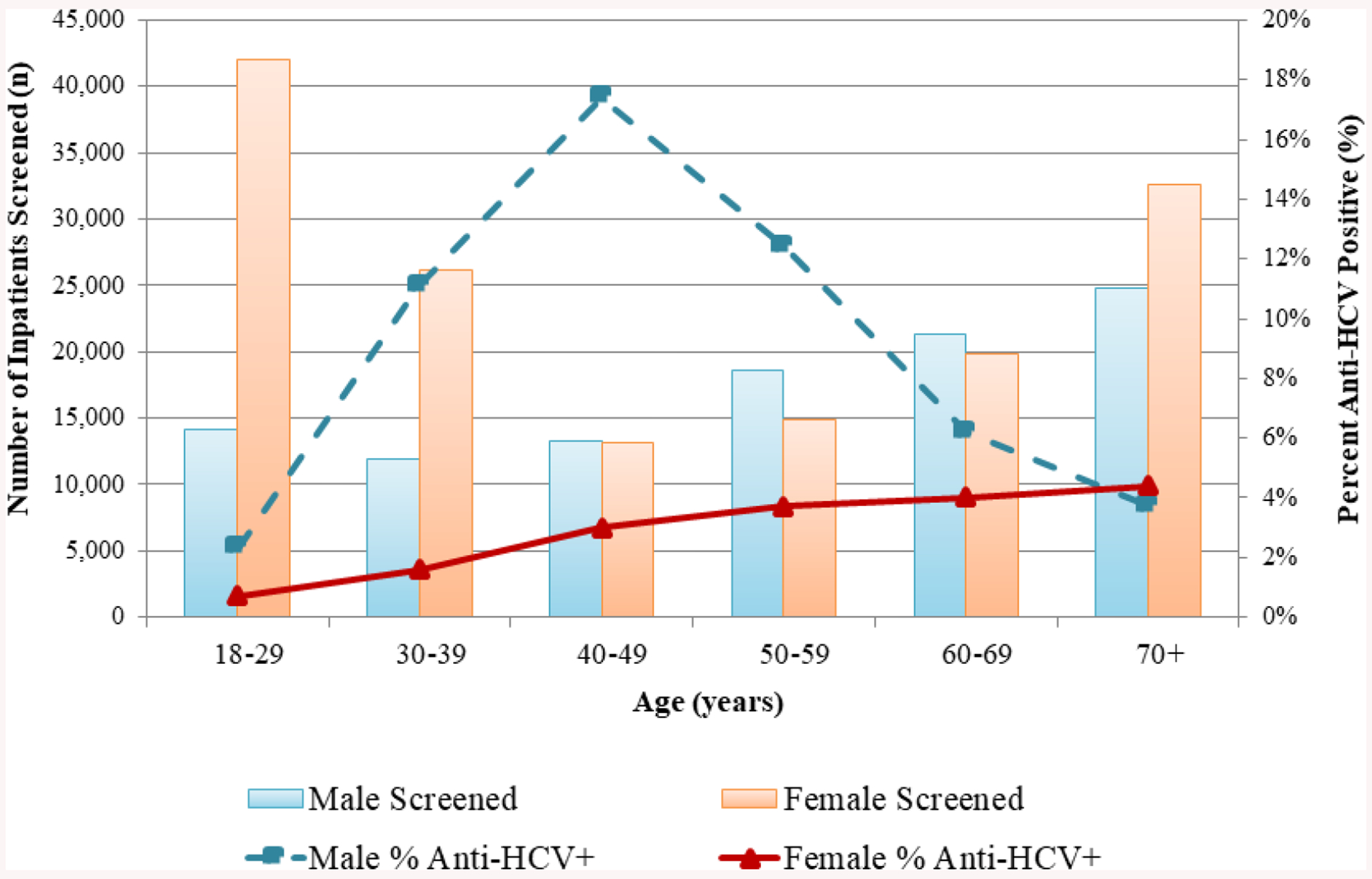
Number of patients screened and percent tested positive for anti-HCV, by age and sex, November 2016 – October 2017 (n = 252,848), Georgia.
Anti-HCV positivity was highest in December 2016 at 5.8% and decreased from February through October 2017 to a low of 3.7% (data not shown). Anti-HCV positivity was higher among men, with 8496/104,100 (8.2%) compared to 3889/148,748 (2.6%) of women testing positive (p < .0001). Patients aged 18–29 years had the lowest anti-HCV positivity (1.1%), while patients aged 40–49 had the highest anti-HCV positivity (10.2%) (p < .0001). Anti-HCV positivity was higher among males aged 40–49 years (17.4%) than any other age/sex group (Fig.2). Positivity among females increased with age, from 0.7% among women aged 18–29 years to 4.4% among women aged ≥ 70 years (Fig. 2).
Nationally, among all hepatitis C screening venues, there was a 3.2-fold increase in screening after the hospital program began; an average of 46,648 unique adults were screened per month during November 2016 – April 2017, compared to an average 14,623 per month between May 2016 – October 2016 (data not shown).
3.2. Linkage to HCV Care
Of the 12,385 patients who tested anti-HCV positive, 3414 (27.6%) had linked to HCV care (i.e. went to a specialized HCV treatment provider site to receive viremia testing) prior to their hospitalization, and an additional 1345 (10.9%) had a recorded death date within the evaluation period −94.0% of whom were hospitalized for non-hepatitis-related conditions – totaling 4759 patients excluded from the linkage to care analysis. The remaining 7626 (61.6%) were eligible for the analysis as they had not been linked to hepatitis C care at the time of their hospitalization. Of those eligible, 1513 (19.8%) were successfully linked, while 6113 (80.2%) were not linked to care within 90 days following their discharge.
When we compared patients linked to care to those not linked to care, men (20.6%) were more likely than women (18.4%) to be linked (p = .019) (Table 3). Linkage rates varied by age (p < .0001) with persons aged ≥ 70 years having the lowest linkage rate (10.7%), although the total number of patients testing positive was highest in this age group. The linkage rate was highest among inpatients hospitalized for one day (21.8%) and decreased to 16.1% among those hospitalized >10 days (p = .023). Length of hospital stay was associated with patient age (p < .0001), with those aged 40–59 years more likely to be hospitalized >10 days (data not shown). Patients with a diagnosis of any viral hepatitis infection were more likely to be linked than patients with non-viral hepatitis, liver-related diagnoses or those with no diagnosis of liver disease (p < .0001) (Table 3).
Table 3.
Characteristics of adult patients who screened anti-HCV positive while admitted to the hospital between November 1, 2016 and October 31, 2017 and linked to care, Georgia
| Characteristic | Anti-HCV positivea | Linked to careb | Not linked to care | Chi-square p-value | ||
|---|---|---|---|---|---|---|
| n | n | n | ||||
| Overall | 7,626 | 1,513 | 6,113 | |||
| Gender | ||||||
| Female | 2,754 | 507 | 2,247 | .019 | ||
| Male | 4,872 | 1,006 | 3,866 | |||
| Age category (years) | ||||||
| 18–29 | 495 | 80 | 415 | <.0001 | ||
| 30–39 | 1,134 | 237 | 897 | |||
| 40–49 | 1,526 | 354 | 1,172 | |||
| 50–59 | 1,605 | 383 | 1,222 | |||
| 60–69 | 1,327 | 295 | 1,032 | |||
| 70+ | 1,539 | 164 | 1,375 | |||
| Length of hospital stay (days) | ||||||
| 1 | 1,369 | 298 | 1,071 | 0.023 | ||
| 2–5 | 4,316 | 859 | 3,457 | |||
| 6–10 | 1,215 | 239 | 976 | |||
| >10 | 726 | 117 | 609 | |||
| ICD 10 code (diagnosis, comorbidity, complication)c | ||||||
| Liver-related: any viral hepatitis | 343 | 123 | 220 | <.0001 | ||
| Liver-related: non-hepatitis | 146 | 41 | 105 | |||
| Non-liver related | 7,137 | 1,349 | 5,788 | |||
Anti-HCV positive patients defined as a patient with screening field “yes” and HCV result field “positive.” Patients with multiple admissions who met these criteria are included in this group. Here n = 8971, which is the sum of patients linked to care and not linked to care. From the original 12,385 anti-HCV positive patients, 3412 were excluded from the linkage to care data/analysis due to entry in ELIM-C treatment database prior to hospitalization and screening date and an additional 1345 were excluded for having died in the analysis period (see inclusion/exclusion flow diagram).
Linked to care patients defined as any anti-HCV positive patient (previously defined) who subsequently received documented HCV RNA or core-antigen testing at one of the diagnostic testing provider sites after date of hospital discharge but before January 31, 2018.
Liver-related: any viral hepatitis ICD10 codes included: B15-B17, B18.0-B18.2, B18.8-B18.9, B19.0, B19.9, and K73. Liver-related: non-hepatitis ICD10 codes included: B67.0, B67.5, B67.8, C22, I82.0, K70-K72, K74-K77, R17, R18, R16.0, R16.2, T51, T64, and Z20.5. All other ICD10 codes in the 066 system were included in the non-liver related category.
Patients linked to care with a median of 41 (IQR: 12, 116) days between their discharge date and the date of their viremia test. Out of the 1513 patients linked to care, 21.6% (n = 327) had their viremia test within 10 days of hospital discharge, while 31.9% (n = 482) took > 90 days to be linked to care. Time to linkage did not differ significantly by age or sex.
Among the 1513 patients linked to care, 858 (56.7%) initiated HCV treatment by the end of the evaluation period. Of them, 615 (71.7%) had already completed treatment and of 330 eligible (≥12 weeks post treatment completion) and tested for SVR, 326 (98.8%) achieved cure.
4. Discussion
To accelerate identification of HCV infected persons in the country, on November 1, 2016, Georgia launched a program to screen for hepatitis C every patient admitted to any hospital in the country. By analyzing records of nearly 300,000 inpatients, our evaluation reflects great progress made over the first year of the program, and highlights areas in need of improvement. Over a quarter million adult patients were screened for hepatitis C throughout the year, representing nearly 90% of adult inpatients, and monthly national screening rates tripled in the first 6 months of the hospital screening program. Overall, 4.9% of patients screened positive, and 19.8% of eligible anti-HCV positive patients were linked to care. We identified factors associated with linkage to care, which could guide efforts to improve this objective and help Georgia reach its hepatitis C elimination goals.
The proportion of inpatients screened increased as the program progressed, which could be explained by increased access to necessary testing materials at hospitals, and/or increased awareness of the governmental mandate among hospital personnel over time – hospitals could be fined for non-compliance, and automated reminders were built into HMIS to remind personnel to screen patients and document results. Previous studies have identified management guidelines and financial resources (Estevez et al., 2016) as well as physician noncompliance and data errors (Patil et al., 2016) to be barriers to hepatitis C screening among healthcare professionals. Therefore, training and acclimation to new procedures among hospital personnel may have increased over the first year of the screening initiative. We found significant differences in screening rates by age and sex; males were less likely to have been screened than females, and the age group least likely to be screened was patients aged 40–49. This is counterproductive to elimination goals, as these two groups had the highest prevalence of anti-HCV positivity among those screened. Targeted screening could be considered to ensure those most at risk of hepatitis C are screened routinely. Screening men aged 30–59 instead of general screening may increase efficiency, as 13.6% of men aged 30–59 were anti-HCV positive, compared to only 2.5% of females in the same age group.
The proportion of patients screening anti-HCV positive decreased over time. The cause of this is unknown but could be a reflection of the successes of the national HCV treatment program (Gvinjilia et al., 2016; Nasrullah et al., 2017a), which had identified > 45,000 and treated > 40,000 chronically infected Georgians by the end of our evaluation period (Georgia Ministry of Health, Labour and Social Affairs, 2019). Those aware of their status, if hospitalized, may have declined re-testing thereby reducing anti-HCV positivity among those screened. It’s also possible that some providers were still practicing more thorough screening among high-risk patients early in the program, despite the mandate to offer screening to all. Of those who screened positive, 27.6% had received a viremia test prior to their hospital visit, indicating that added scrutiny to prevent redundant screenings could save valuable resources. Many states in the United States require all hospitalized baby boomers (born between 1945 and 1965) to be screened for hepatitis C, and one study in New York state found 63.7% of detected anti-HCV patients had already been diagnosed or treated prior to their admission, more than double our findings (Hung et al., 2016). Furthermore, > 23,000 inpatients were screened multiple times within the same hospital during our evaluation period, indicating that mandatory screening could lead to over-testing. Linkage of the HMIS to the national screening registry and ELIMINATION-C treatment database would allow for real-time determination of a patient’s screening and hepatitis C treatment history. This could facilitate a “flagging” system to help eliminate unnecessary screening of patients already aware of their status.
While identification of anti-HCV positive patients is essential for the success of the hepatitis C elimination program, referral of anti-HCV positive patients for further evaluation and provision of comprehensive treatment services is equally important. At the time of this evaluation, after a patient screened anti-HCV positive, he/she needed to independently seek HCV viremia testing, and subsequent evaluation and treatment at a specialized hepatitis C treatment site. Whereas screening is conducted at a wide range of facilities throughout Georgia, access to hepatitis C evaluation services and treatment was more limited. As of October 2017, treatment was provided at 31 health facilities throughout the country by 139 physician providers (Mitruka et al., 2015). Since the elimination program’s inception in 2015, a substantial proportion of anti-HCV positive patients have failed to seek viremia testing or further evaluation/treatment (Mitruka et al., 2015). Evaluation of the hospital screening program suggests a similar challenge: only 19.8% of patients eligible for linkage to care analysis sought follow-up testing after their hospital discharge. Thus, over four-fifths of the anti-HCV positive patients identified by the hospital program were not linked to care. At the time of this evaluation, there was no systematic method for counseling patients or informing them where to go for further care, but was instead at the hospitals’ discretion, and based on their varying resources and capabilities. Standardized methods for screening and linking patients to care could be considered. Interventions in which hospital personnel assist in coordinating HCV-infected patients’ next steps can significantly improve linkage to care (Deming et al., 2018). Another potential barrier is financial; although screening and treatment are free of charge, the cost of diagnostics, including viremia testing, determination of genotype and degree of liver fibrosis, as well as other testing during treatment, were the responsibility of the patient (Gvinjilia et al., 2016; Nasrullah et al., 2017a). These costs could be significant for persons of low income. In 2017, Georgians’ average monthly nominal earnings were 999 Georgian lari (GEL) (National Statistics Office of Georgia (GEOSTAT), n.d.), and the cost of pre-treatment diagnostic testing ranged from 279 to 335 GEL (Adamia, 2018), or 28–34% of their monthly income. We were unable to assess financial barriers, though other studies in Georgia have shown costs to be a barrier (Averhoff et al., 2019). At the time of this analysis only 57% of linked-to-care patients had initiated treatment, far lower than the 92% reported nationally (Nasrullah et al., 2017a). This proportion is likely to increase as patients have more time to enroll in the program, but could also reflect challenges among persons with possible comorbid conditions that required their hospitalization, in addition to financial barriers.
Linkage-to-care varied by age, with patients aged ≥70 years obtaining viremia testing at substantially lower rates than other age groups. Although our analysis could not assess the reasons for this, it could be related to costs, mobility and access to treatment sites, comorbid conditions, or other social and behavioral factors. A study of inpatient screening among baby boomers at a medical center in the United States (Mehta et al., 2017), in which linkage to care was defined as scheduling a follow-up appointment after RNA confirmation, found linkage rates for that age group slightly less than our analysis (18% for baby boomers vs. 22.2% in our 60–69 year age group). Length of hospital admission also influenced linkage to care and screening; patients with longer hospital stays sought HCV viremia testing and were screened at lower rates. This could suggest that more critical conditions requiring longer hospital admissions may have taken priority over diagnosing past or current HCV infection (Junius-Walker et al., 2010). This finding appears independent of age; the age group least likely to be linked to care (≥70) were less likely than those aged 40–59 year to have an extended hospital stay.
Providing increased access to diagnostic testing and treatment is a priority in the elimination program, and a rollout of decentralization of care began in 2018, whereby HCV-infected individuals can seek treatment at selected primary care and harm reduction sites (Adamia,2018). Several other interventions, such as lowering costs of diagnostics are being implemented (Adamia, 2018). This hospital screening program was expanded by a follow-up governmental decree in May of 2018 to ensure all emergency room patients are offered HCV screening in addition to inpatients. Additionally, in March 2018, Georgia instituted a policy in which hospitals are mandated to obtain and send serum specimen of all patients who screen anti-HCV positive to the national reference laboratory for reflex HCV core antigen testing, free of charge to patients (Averhoff et al., 2019). This change in policy resulted in increased viremia testing, though rates of subsequent hepatitis C treatment initiation decreased among patients diagnosed viremic - the next step in the care continuum that the elimination program must seek to facilitate (Averhoff et al., 2019).
Interventions to improve screening and linkage to care should decrease barriers to the program. However, it is essential to continually monitor and evaluate the care continuum to identify deficiencies and bolster screening and treatment rates. Since the time of this evaluation, hospital screening data was incorporated into a national screening registry, creating a unified database that allows monitoring of the hepatitis C continuum of care at the individual-patient level (Georgia Ministry of Health, Labour and Social Affairs, 2019). This more efficient information system can help prevent unnecessary and repeat screenings, thereby reducing costs.
4.1. Study Limitations
There were several limitations to this evaluation. First, erroneous data entries and entries with missing HCV fields (2.9% of patients) in the HMIS could have affected our findings. Second, the HMIS did not collect information to assess reasons for the screening and linkage-to-care rates observed. The database did not report eligibility criteria (e.g. previous screening results, prior initiation of hepatitis C treatment, patient refusal), nor demographic information such as income or education level; thus, it was impossible to determine reasons for variations in screening rates across different populations. Also, no data was available regarding post-screening counseling to confirm when, how, or if the patient was informed of his/her results, if the patient was counseled about how to seek follow-up diagnostic testing, the importance thereof, or if any potential barriers to linkage were identified. Third, some patients may have had contraindications to hepatitis C treatment, or terminal diseases that would hinder follow-up diagnostics, leading to underestimation of linkage-to-care rates. Finally, anti-HCV positive patients discharged at the end of the evaluation period had only 90 days to seek diagnostic testing, though our analysis found that nearly a third of patients linked to care took > 90 days to do so.
5. Conclusion
Identification of HCV-infected persons, and subsequent care and treatment is essential for the success of Georgia’s hepatitis C elimination program. Our evaluation reports on the first year of the country’s initiative to screen all hospital inpatients for hepatitis C. We highlighted great progress that was made to identify anti-HCV positive patients, as well as some shortfalls that can be addressed to promote screening and linkage to care in the country and can help meet their hepatitis C elimination targets.
Supplementary Material
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ypmed.2020.106153.
Publisher's Disclaimer: Disclaimer
Publisher's Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
References
- Adamia E, 2018. Decentralization and Integration of HCV Services in Primary Care, Hospitals and Harm Reduction Settings in Georgia, Presented at Decentralization of HCV Diagnostic, Care and Treatment Services Within Georgian National Hepatitis C Elimination Program Workshop, Tbilisi, Georgia, December 5. [Google Scholar]
- Averhoff F, Shadaker S, Gamkrelidze A, Kuchuloria T, Gvinjilia L, Getia V, Sergeenko D, Butsashvili M, Tsertsvadze T, Sharvadze L, Zarkua J, Skaggs B, Nasrullah M. Progress and challenges in a pioneering hepatitis C elimination program in the country of Georgia, 2015–2018. J Hepatol. 2019; pii: S0168–8278(19)30710-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deming R, Ford MM, Moore MS, Lim S, Perumalswami P, Weiss J, et al. , 2018. Evaluation of a hepatitis C clinical care coordination programme’s effect on treatment initiation and cure: a surveillance-based propensity score matching approach. J. Viral Hepat 25, 1236–1243. [DOI] [PubMed] [Google Scholar]
- Estevez J, Kim YA, Le A, Israelski D, Baatarkhuu O, Sarantuya T, et al. Low rates of screening and treatment of chronic hepatitis B, C, D (HBV, HCV, HDV), and hepatocellular carcinoma (HCC), associated barriers, and proposed solutions: results of a survey of physicians from all major provinces of Mongolia. Annals of Global Health. 2016; 82(3): 416 DOI: 10.1016/j.aogh.2016.04.159. [DOI] [Google Scholar]
- Georgia Ministry of Health, Labour and Social Affairs, 2019. National hepatitis C virus elimination progress report Georgia, 2015–2017. Georgia Ministry of Health, Labour and Social Affairs, Tbilisi, Georgia. https://www.moh.gov.ge/uploads/files/2019/Failebi/25.04.2019-1.pdf, Accessed date: 3 March 2020. [Google Scholar]
- Gvinjilia L, Nasrullah M, Sergeenko D, Tsertsvadze T, Kamkamidze G, Butsashvili M, et al. , 2016. National progress toward hepatitis C elimination — Georgia, 2015–2016. MMWR Morb. Mortal. Wkly Rep 65 (41), 1132–1135. [DOI] [PubMed] [Google Scholar]
- Hagan LM, Kasradze A, Salyer SJ, Gamkrelidze A, Alkhazashvili M, Chanturia G, et al. , 2019. Hepatitis C prevalence and risk factors in Georgia, 2015: setting a baseline for elimination. BMC Public Health 19 (Suppl. 3), 480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Management Information System (HMIS) Georgia. Available at: http://www.georgia-ccm.ge/wp-content/uploads/HMIS_Module_Descriptions_ENG.pdf. Accessed on February 20, 2019.
- Hung CK, Demabildo M, Bernstein D, Lee TP, 2016. Inpatient hepatitis C screening at teaching medical centers: an opportunity for improvement. Gastroenterology 150, S1062. [Google Scholar]
- Junius-Walker U, Voigt I, Wrede J, Hummers-Pradier E, Lazic D, Dierks ML, 2010. Health and treatment priorities in patients with multimorbidity: report on a workshop from the European General Practice Network meeting ‘Research on multimorbidity in general practice’. Eur. J. Gen. Pract 16, 51–54. 10.3109/13814780903580307. [DOI] [PubMed] [Google Scholar]
- Mehta A, Down C, Shen NT, Kumar S, 2017. Inpatient hepatitis C screening, health disparities, and inadequate linkage to outpatient care at a large academic medical center. Gastroenterology 152 (5), S1190. [Google Scholar]
- Mitruka K, Tsertsvadze T, Butsashvili M, Gamkrelidze GA, Sabelashvili P, Adamia E, et al. , 2015. Launch of a nationwide hepatitis C elimination program – Georgia, April 2015. MMWR Morb. Mortal. Wkly Rep 64 (28), 753–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrullah M, Sergeenko D, Gvinjilia L, Gamkrelidze A, Tsertsvadze T, Butsashvili M, et al. , 2017a. The role of screening and treatment in national progress toward hepatitis C elimination in Georgia, 2015–2016. MMWR Morb. Mortal. Wkly Rep 66, 773–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrullah M, Sergeenko D, Gamkrelidze A, Averhoff F, 2017b. HCV elimination – lessons learned from a small Eurasian country, Georgia. Nat Rev Gastroenterol Hepatol. 14 (8), 447–448. [DOI] [PubMed] [Google Scholar]
- National Statistics Officeof Georgia (GEOSTAT). Retrieved from: http://www.geostat.ge/index.php?action=page&p_id=149&lang=eng. Accessed on April 19, 2019.
- Patil R, Ona MA, Saikali P, Papafragkakis C, Anand S, 2016. 744a hepatitis C screening barriers in 2016: unusual suspects. Gastroenterology 150 (4), S152. [Google Scholar]
- Strategic Plan for the Elimination of Hepatitis C Virus in Georgia, 2016–2020. Georgia’s Ministry of Labour, Health, and Social Affairs (MoLHSA), Tbilisi: Available at: https://www.moh.gov.ge/uploads/files/2017/akordeoni/failebi/Georgia_HCV_Elimination_Strategy_2016-2020.pdf, Accessed date: 20 February 2019. [Google Scholar]
- The World Bank. The World Bank in Georgia. 2017. Retrieved from: https://www.worldbank.org/en/country/georgia. Accessed on February 19, 2019.
- World Health Organization, 2017. Global Hepatitis Report 2017. Retrieved from: https://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/, Accessed date: 19 February 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


