Abstract
Multiple lines of evidence implicate the serotonin (5-HT) system in social function, including biomarker findings in autism spectrum disorder. In mice, knock-in of a rare Gly56Ala substitution in the serotonin transporter (SERT) causes elevated whole blood 5-HT levels, increased 5-HT clearance in the brain, and altered social and repetitive behavior. To further examine the molecular impact of this variant on social response, SERT Ala56 mutant mice and wildtype littermate controls were exposed to a social or non-social stimulus. We examined the differential activation of the prefrontal cortex, lateral amygdala, and medial amygdala, to social stimuli through RNA sequencing. Differentially expressed genes were enriched in axonal guidance signaling pathways, networks related to nervous system development and function, neurological and psychiatric disorders, and behavior. These identified pathways and networks may shed light on the molecular cascades underlying the impact of altered SERT function on social behavior.
Keywords: Serotonin, social, transcriptome, amygdala, prefrontal cortex, autism spectrum disorder
Background
The serotonin (5-hydroxytryptamine, 5-HT) system has myriad functions in the periphery and brain, ranging from developmental effects to dynamic modulation of cognition and social behavior. Serotonergic function is linked to social function in wildtype mice and in genetic mouse models related to autism [1–4], and 5-HT signaling is important for social reward [5, 6].
While 5-HT is implicated in a number of disorders, the most robust biomarker association is for elevated whole-blood 5-HT in autism spectrum disorder (ASD) [7]. Sibling pair studies in ASD found evidence for linkage to the SLC6A4 gene that encodes the 5-HT transporter (SERT) [8]. Multiple rare variants of SLC6A4 were identified in families with ASD, largely inherited by boys from their unaffected mothers [8, 9]. The most common amino acid variant is a glycine-to-alanine change at position 56 in the SERT protein (Gly56Ala) [8] that leads to increased SERT function in vitro [10]. SERT Ala56 knock-in mice display elevated whole blood 5-HT levels, altered responses to 5-HT receptor agonists, decreased basal firing of dorsal raphe neurons, and altered social and repetitive behaviors [2].
Here, we used RNA sequencing to examine molecular cascades that respond to a social stimulus in SERT Ala56 mutant mice. We previously demonstrated that the lateral amygdala (LA, including the basolateral amygdala), medial amygdala (MA), and prefrontal cortex (PFC) exhibited the greatest changes in neuronal activity in response to a social stimulus compared to a non-social stimulus and that these regions have correlated activity [11], consistent with previous studies showing evidence of connectivity among these regions in the context of social behavior [11–15]. We therefore focused our study on socially-induced differences in gene expression in these brain regions in SERT Ala56 mice.
Methods
Animals
Male SERT Ala56 [2] and wildtype littermate control mice were generated from heterozygous breedings. Only male mice were used because the SERT Ala56 variant only affects males in the human population [8]. Mice were housed with same-sex littermates, two to five per cage with a 12h light/dark cycle and food and water available ad libitum until experimentation. All procedures were approved by the Vanderbilt University Institutional Animal Care and Use Committee, where the experiments took place, and designed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
RNA Sequencing
Animal exposure to stimuli, RNA processing and sequencing, and analysis were done according to [11]. SERT Ala56 mutant and wildtype littermate control mice (SERT Gly56) (n=36, 9 per stimulus per genotype) were housed individually and habituated to a pencil cup for 3 days prior to being exposed to a social stimulus (novel mouse) or non-social stimulus (toy horse, Playmobil) that was placed in the pencil cup for 30 minutes in the test mouse’s home cage. Ten hours after stimulus exposure, following rapid decapitation, brains were extracted. Tissue samples taken from the from the lateral amygdala (LA), medial amygdala (MA), and prefrontal cortex (PFC) were stored at −80°C until total RNA extraction (RNeasy mini kit, Qiagen). Tissue samples were pooled across groups, with three brain samples per pool and three pools per group. Following strand-specific library preparation, paired-end sequencing with 30M reads per library was performed using Illumina HiSeq 2000. Correlations between biological replicates were analyzed using the normalized counts for all reads (Supplemental File 1). One sample in the LA for the mutant mice exposed to the nonsocial stimulus did not reach the required > 0.90 correlation cut-off and was excluded from all analyses.
Analyses of differentially expressed genes
Differential expression (fold change) of genes was determined for comparisons of: (1) the interaction across genotype and condition (social vs non-social in mutants compared to social vs non-social in wildtype mice); (2) social vs nonsocial in wildtype; (3) social vs nonsocial in mutant; (4) mutant vs wildtype in nonsocial; and (5) mutant vs wildtype in social.
Differentially expressed genes (DEGs) were compared against the list of validated autism candidate genes from the Simons Foundation Autism Research Initiative (SFARI) gene database (gene.sfari.org) to assess overlap using a Chi-square analysis (GraphPad Software, San Diego, CA). DEGs (p < 0.05) from each comparison and brain region were examined for enrichment in systems such as networks, pathways, diseases and functions, and links to upstream regulators were examined using Ingenuity Pathway Analysis (IPA, Qiagen), which calculates a p-value of overlap between the DEGs involved in each of the systems and all the molecules known to be involved in that system. Enrichment is considered significant at Benjamini-Hochberg-corrected p (B-H p) < 0.05, calculated based on a 5% false discovery rate. IPA also makes predictions about whether systems associated with the overlapping DEGs will be activated or inhibited by calculating a Z-score from the direction of DEGs expression. Much of the literature has focused on the roles of DEGs in immune response and cancer pathways, which are also highly represented in our results. To narrow the results down to diseases and functions (B-H p-value < 0.05) that are more obviously relevant to the brain, we manually categorized a subset of the data into the following categories: Morphology, Cell Survival, Neurotransmission, Synaptic Function, Behavior, and Clinical Diagnoses.
Gene expression differences between genotypes and stimulus conditions were examined by the interaction between those variables, based on significant DEGs in the social versus non-social conditions in mutants relative to those in wildtypes (Mutant-SvsNS vs Wildtype-SvsNS). Thus, the interaction analysis reveals how the genotypes differentially respond to social versus non-social stimulus. Because of the nature of this analysis, the directionality (or fold change) of the DEGs is difficult to interpret. For example, a DEG up-regulated in the interaction analysis may be annotated as such if the gene is down-regulated for both genotypes but more down-regulated in the wildtype. We therefore do not report the directionality of the DEGs for the interaction analyses.
Results
Differentially expressed genes identified by RNA sequencing
The list of DEGs for each comparison and each brain region, and the overlap for these comparisons, are provided in Supplemental Files 2–5. The number of DEGs genotype, stimulus condition, and their interactions is summarized in Table 1 and the top 10 most common DEGs in the mutant versus wildtype or social versus non-social comparisons are shown in Supplemental File 6. The majority of the interaction DEGs, which reveal the genes sensitive to both genotype and stimulus condition, were found in the MA. We found significant overlap between the DEGs for mutant versus wildtype mice and genes related to ASD in all three brain regions (Table 1 and Supplemental File 7). The social versus nonsocial and interaction comparisons identified significant overlap between DEGs and ASD candidate genes only in the MA and LA.
Table 1:
Overlap of differentially expressed genes (p ≤ 0.05) with SFARI autism genes.
| MvsWT NS | MvsWT S | SvsNS WT | SvsNS MU | Interaction | ||
|---|---|---|---|---|---|---|
| MA | DEGs | 384 | 614 | 1117 | 616 | 537 |
| SFARI overlap | OR = 2.61 | OR = 2.35 | OR = 3.68 | OR = 0.68 | OR = 3.30 | |
| p = 0.01 | p < 0.01 | p < 0.0001 | p = 0.50 | p < 0.0001 | ||
| LA | DEGs | 381 | 672 | 1710 | 961 | 230 |
| SFARI overlap | OR = 3.05 | OR = 3.09 | OR = 3.11 | OR = 3.54 | OR = 2.77 | |
| p = 0.01 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.001 | ||
| PFC | DEGs | 1048 | 1059 | 319 | 271 | 159 |
| SFARI overlap | OR = 2.91 | OR = 4.45 | OR = 1.31 | OR = 2.07 | OR = 0 | |
| p < 0.0001 | p < 0.0001 | p = 0.65 | p = 0.14 | p = 0.28 |
OR = odds ratio. p-values are calculated from Chi-square analysis for overlap with the SFARI list of ASD-related genes. p < 0.05 is considered significant.
Enriched canonical pathways and functions
IPA revealed significant enrichment of the interaction DEGs present in several canonical pathways. The top pathways for each comparison are listed in Supplemental File 8. Consistent with the majority of interaction DEGs being found in the MA, significantly enriched pathways were found only in the MA Interaction analysis (30 pathways). The top pathways that were found to be significant across multiple analyses were associated with development, axonal guidance, and Wnt/β-Catenin signaling (Figure 1). Additionally, several pathways related to neuronal signaling were identified in the interaction analysis for the MA: dopamine-DARPP32 feedback in the cAMP signaling pathway (B-H p = 0.001), GABA receptor signaling (B-H p = 0.001), three glutamate-related pathways (glutamate receptor signaling, B-H p = 0.001; glutamate degradation III, B-H p = 0.004; glutamate-dependent acid resistance, B-H p = 0.01), and synaptic long-term depression (B-H p = 0.03). These pathways reflected differential gene expression in dopamine receptor 2 and 5 (DRD2/5), GABA receptor, glutamate receptor, and glutamate synthesizing/degrading enzyme genes.
Figure 1.
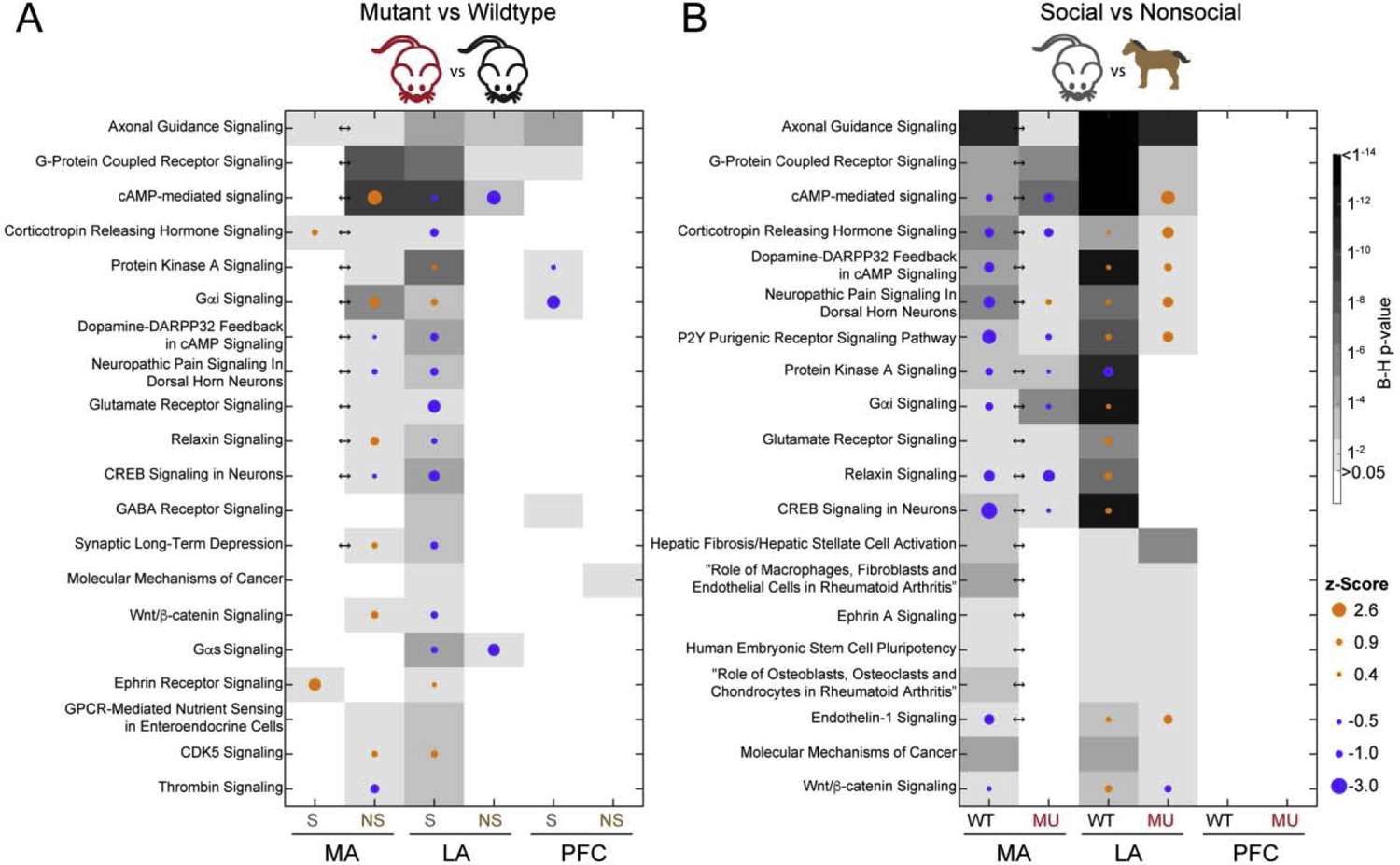
The top 20 canonical pathways enriched for DEGs in A) mutant versus wildtype and B) social versus nonsocial comparisons. The intensity of the grayscale corresponds to the B-H p-value, indicated by the scalebar. Arrows indicate a significant interaction effect (B-H p < 0.05) for that brain region. The dots indicate a z-Score (B-H p-value) that predicts activation (orange) or inhibition (blue) of the pathway and the magnitude is indicated by the size.
Notably, pathways directly related to 5-HT signaling were not present in the canonical pathways using the IPA, WebGestalt [16], or Mouse Genome Informatics (The Jackson Laboratory, www.informatics.jax.org) platforms. However, similar themes of neuronal transmission and dopamine signaling were prominent through all three platforms.
IPA identified diseases and functions enriched in DEGs (Supplemental File 9), again, the majority of which were found in the interaction analysis for MA (500 significantly enriched diseases and functions), followed by LA (294) and PFC (97). Similar to the development-related canonical pathways, functions associated with neuronal morphology were enriched in all brain regions for the mutant versus wildtype DEGs and in the MA and LA for the social versus nonsocial DEGs (Figure 2). Furthermore, functions related to cell survival and apoptosis, also developmentally-regulated, were enriched predominantly in the MA (Supplemental File 10).
Figure 2.
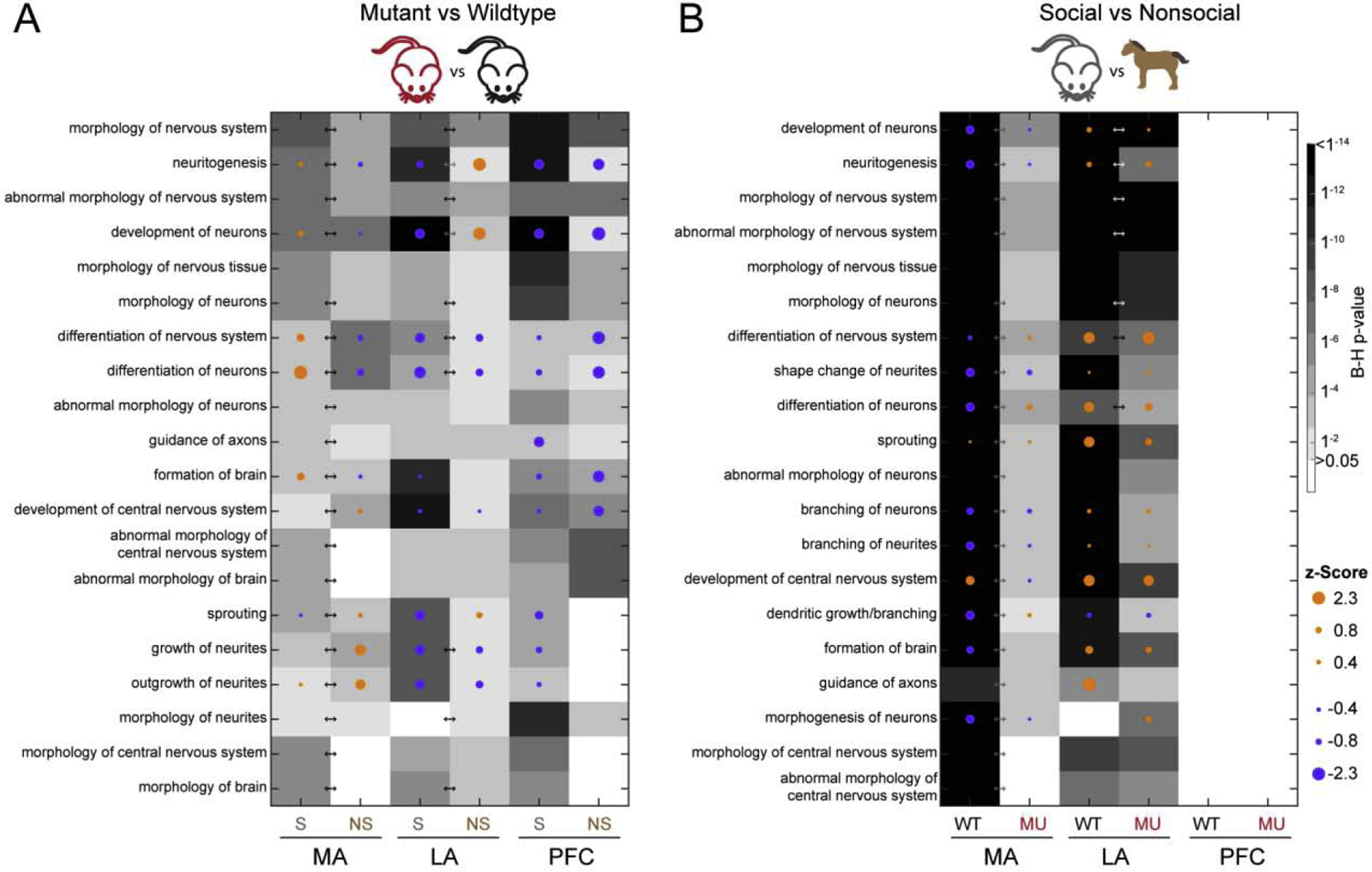
The top 20 functions enriched with DEGs associated with morphology in A) mutant versus wildtype and B) social versus nonsocial comparisons. Arrows indicate a significant interaction effect (B-H p < 0.05) for that brain region.
Consistent with the identified canonical pathways that included dopamine, GABA, and glutamate signaling, several functions related to neurotransmission (Fig. 3A&B) and neuronal signaling/synaptic func-tion (Fig. 3C&D) were enriched. These functions were found in all three brain regions for the mutant versus wildtype DEGs and in the MA and LA for the social versus nonsocial DEGs. Although, as mentioned above, no pathways were found to be significant in 5-HT signaling, “abnormal quantity of 5-hydroxytryptamine” was found as a significant function in the MA in mutants (B-H p = 0.0023) in the social vs nonsocial conditions. This finding was driven by the DEGs nitric oxide synthetase 1 (Nos1), brain derived neurotrophic factor (Bdnf), melanin concentrating hormone receptor 1 (Mchr1, also a GPCR), and nuclear receptor subfamily 4 group A member 2 (Nr4a2).
Figure 3.
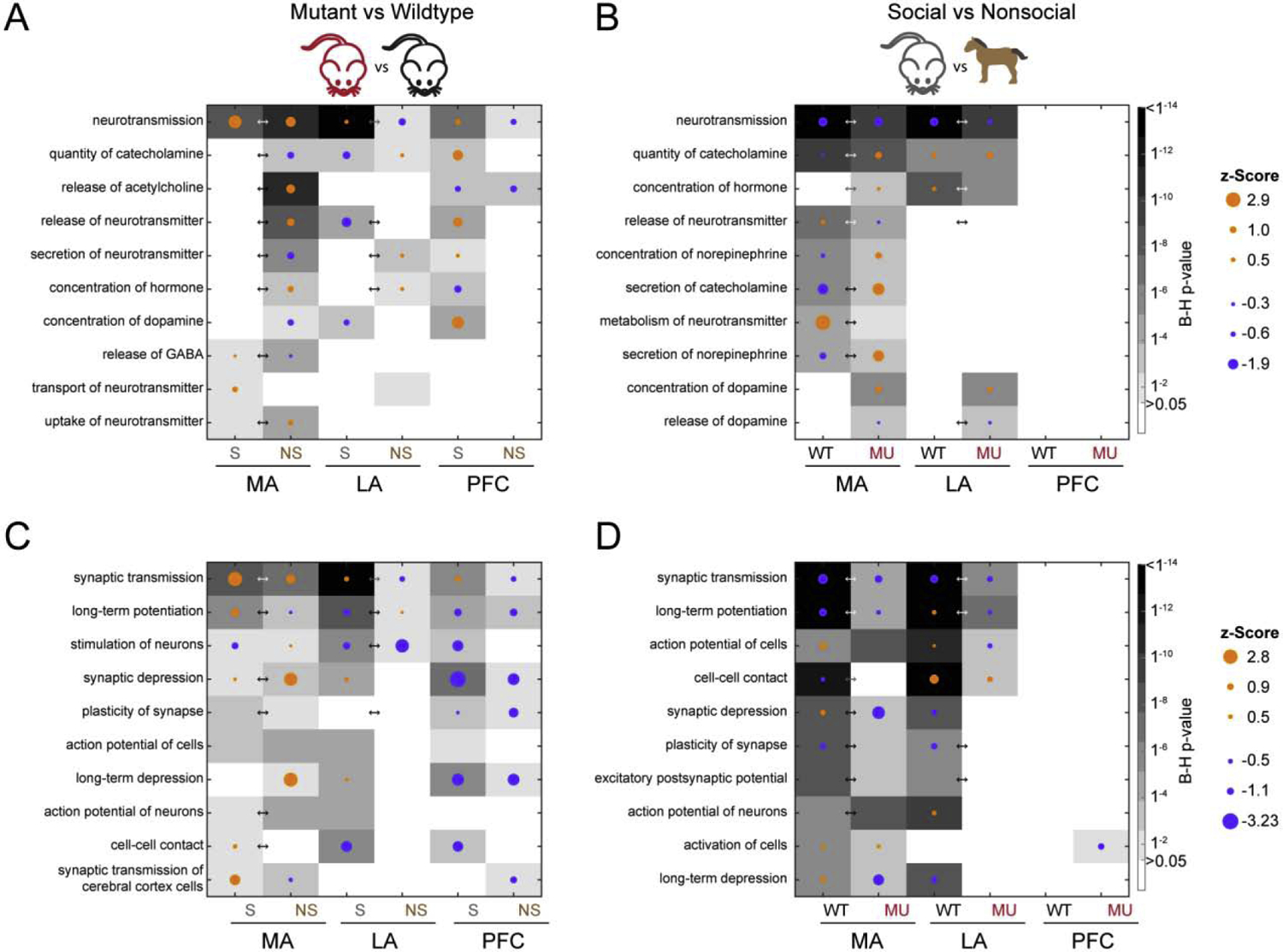
The top 10 functions enriched with DEGs associated with neurotransmission (top) and synaptic function (bottom) in A) mutant versus wildtype and B) social versus nonsocial comparisons. Arrows indicate a significant interaction effect (B-H p < 0.05) for that brain region.
Enriched behaviors and clinical diagnoses
We further classified functions related to behavior (Supplemental File 11) and neurological and psychiatric diagnoses (Supplemental File 12). Functions related to behavior were significantly enriched in all brain regions and included learning, memory and cognition. The DEGs from the mutant versus wildtype comparison also included anxiety and locomotion/movement. In the social versus non-social comparison, anxiety showed significant enrichment in MA and LA for both genotypes, but only appeared in the PFC in mutants. DEGs significantly enriched in locomotion and hyperactive behavior were only present in the MA and LA from social versus non-social conditions. The neurological and psychiatric diagnoses category predominantly included movement and seizure disorders. Genes implicated in schizophrenia were found to be enriched for DEGs in social versus nonsocial conditions in both the MA and LA and in the interaction analyses for both of these regions. Autism was significantly enriched in the MA in the interaction analysis (B-H p < 0.001), as was fragile X syndrome (B-H p < 0.001), a genetic disorder associated with ASD.
Comparison of MA, LA, and PFC
Although we chose to focus on the pathways and functions that were similarly significant across the most brain regions and comparisons, clear differences emerged across regions and comparisons. Pathways and functions that were most significant in the MA were also typically significant in the LA, with the exception of the functions associated with synaptic transmission. Fewer canonical pathways overlapped between the MA, LA and PFC. Similarly, there was very little overlap of the morphology, synaptic function, and neurotransmission-related functions found in the social vs nonsocial conditions in the PFC, whereas the mutant vs. wildtype comparison had significant enrichment of DEGs in some similar functions as the MA and LA.
Network analysis and upstream regulators
Network analyses provide an overview of how molecules involved in various functions and diseases are connected to one another and interact. The functions associated with the top 10 networks for DEGs from the interaction analyses (Table 2) included cell-to-cell signaling/interaction, related to neurotransmitter functions, which was emphasized in our diseases and functions categories described above and exemplified by the top network in the MA (Supplemental File 13). Finally, the top upstream regulators of DEGs for each comparison are listed in Supplemental File 14.
Table 2:
Diseases and functions related to top 10 networks from interaction analyses
| Score | Brain region | Network functions |
|---|---|---|
| 47 | PFC | Cell Morphology, Cellular Assembly and Organization, Connective Tissue Disorders |
| 44 | MA | Cell-To-Cell Signaling and Interaction, Molecular Transport, Small Molecule Biochemistry |
| 38 | MA | Behavior, Neurological Disease, Psychological Disorders |
| 37 | PFC | Embryonic Development, Organ Development, Organismal Development |
| 36 | LA | Cell Morphology, Cellular Assembly and Organization, Cellular Development |
| 35 | MA | Amino Acid Metabolism, Embryonic Development, Organismal Development |
| 35 | MA | Cell-To-Cell Signaling and Interaction, Nervous System Development and Function, Organismal Injury and Abnormalities |
| 34 | MA | Connective Tissue Disorders, Inflammatory Disease, Organismal Injury and Abnormalities |
| 32 | MA | Auditory and Vestibular System Development and Function, Auditory Disease, Connective Tissue Development and Function |
| 32 | LA | Amino Acid Metabolism, Carbohydrate Metabolism, Drug Metabolism |
All networks contain 35 molecules. The network score, calculated by IPA, is equal to the - log(Fisher’s exact test result) of the chance of getting the number of DEGs from our dataset found within the network versus randomly selecting 35 molecules. For example, in Network 1, 23 of the 35 molecules are significantly differentially expressed in our dataset, resulting in a rank of 47, or a Fisher’s exact test result of 1×10−47. The ranks are calculated separately for each network and the top 10 ranked networks from interaction analyses from each brain region are shown above.
Discussion
In the present study, we used social stimuli to reveal biologically meaningful differences between mutant and wildtype mice that differ at a single amino acid, leading to enhanced and dysregulated function of SERT [2]. We found that the Ala56 variant has notable effects on genes, networks, pathways, and functions that predominantly indicate altered cell signaling. Although the Ala56 variant represents a very minor change in the SERT, in vitro [10] and in vivo [2] studies reveal significant biochemical, physiological and behavioral effects, including modulation of trait domains altered in ASD. In parallel, we observed differential expression of genes related to cell signaling due to the social stimulus. Importantly, this could be seen at the level of pathways related to G protein-coupled receptors (GPCRs) and the neurotransmitters dopamine, acetylcholine, glutamate and GABA, as well as at functional levels related to cell morphology, synaptic function, and neurotransmission.
The axonal guidance signaling pathway had significant enrichment of DEGs in 10 of the 15 comparisons. Axonal guidance signaling is traditionally thought of as a neurodevelopmental process, in which the 5-HT system plays a critical role, in particular in thalamocortical axon development [17]. Additionally, the morphological functions have a developmental undertone and include neurite outgrowth, which 5-HT has also been shown to regulate in vitro [18] and in vivo [19]. However, mice in the present study were assessed in adulthood, so extrapolation of gene expression changes to effects during development are approached with caution. The IPA program relies on established relationships between genes and functions reported in the literature and studies that relate developmentally-relevant genes to adult functions are limited. Indeed, genes associated with developmental processes, like Wnt-signaling, another top canonical pathway, are also involved in amygdala-dependent fear memory consolidation [20] and in plasticity in ASD [21]. Thus, the adult DEGs observed here may represent a signature of neuronal plasticity and ongoing processes dysregulated by the SERT Ala56 variant.
In support of the DEGs being involved in neuronal plasticity, enrichment of DEGs in functions associated with synaptic transmission, long-term potentiation and depression, cell activity, and learning and memory were observed across many comparisons. The presence of these synaptic functions may relate to, or be driven by, the enrichment of DEGs related to neurotransmission which has been proposed to regulate plasticity and cell survival [22].
Furthermore, DEGs were enriched in pathways related to seizures and epilepsy, which may again align with enrichment of DEGs associated with excitability, plasticity, and cell signaling.
DEGs identified in the comparison of SERT Ala56 mutants to wildtype counterparts exhibited significant overlap with the list of SFARI autism genes. Genes from the comparison of social versus non-social stimulus exposure that overlapped with SFARI autism candidate genes were more prevalent in the amygdala regions than the PFC. There was enrichment of DEGs in the MA that were associated with autism and Fragile X syndrome, the latter of which is the leading monogenic cause of autism. Of note, heightened long-term depression in hippocampal slices of Fragile X mental retardation protein (Fmrp) knockout mice can be ameliorated by 5-HT7 receptor stimulation [23].
It is important to note that neurons whose cell bodies are located in the MA, LA and PFC do not express SERT in adulthood. Thus, the changes that we observed likely reflect circuit- and systems-level responses to alterations in SERT function and 5-HT signaling during development. In agreement with this perspective, we did not find enrichment of DEGs in pathways, diseases, functions, or networks associated with 5-HT directly. It is surprising that we saw such a distinction between the amygdala regions and the PFC in response to social versus non-social stimulus in the present study because these regions are connected in a circuit relevant to social behavior. Optogenetic manipulations in mice demonstrate that inputs from the basolateral amygdala to the PFC directly affect the level of social interaction of an adult with a novel juvenile mouse [24]. Instead, we predominantly observed differential gene expression in the PFC in the mutants compared to the wild type mice both in the social and non-social conditions. These results indicate that the developmental influence of SERT, which is transiently expressed in the PFC [25], is more important to gene expression than the dynamic influence of social experience.
These findings also have important limitations, including the use of only male mice. While the decision to use only male mice was based on the effects of the G56A mutation in humans [8], exclusion of female mice inhibits our understanding of sex differences in response to social stimuli. Other limitations of our findings include variability within the RNA sequencing data. Of the three brain areas, biological replicates had a high degree of convergence, with the exception of one sample within the LA that was excluded. Additionally, our RNA sequencing approach was focused primarily on pathway and network analyses, with few individual genes reaching significance after correcting for multiple comparisons. We set a threshold of p < 0.05 for individual DEGs to be included in pathway and network analyses in order to increase specificity of DEGs while eliminating false negatives. Adjusting the threshold in either direction could yield different findings and thus individual DEGs should be replicated before targeting specific, individual genes.
Overall, these findings align with previous work implicating the 5-HT system in neurodevelopment and social function. Some observed differences driven by the SERT Ala56 variant, particularly in the PFC, may reflect developmental changes that are not dynamically regulated in response to a social stimulus. Other observed differences, particularly in the MA and LA, suggest that the cascading effects of altered SERT function during development subsequently impact the dynamic response of cells in these regions to a social stimulus. These data open up multiple avenues for further study, including assessment of overlap with other genetic manipulations that impact social function, as well as overlap with other brain regions, such as the nucleus accumbens, where 5-HT signaling and projections have been found to modulate social reward [1, 5, 6]. Future work could also explore developmental versus dynamic manipulations of 5-HT release or uptake in the MA and LA to understand impact on social function.
Supplementary Material
Supplemental File 1. The correlations of mapped reads between biological replicates using normalized counts per million for all reads were high for all comparisons in all brain regions. All p-values < 0.001. MA = medial amygdala, LA = lateral amygdala, and PFC = prefrontal cortex.
Supplemental File 2. Differentially expressed genes in lateral amygdala
Supplemental File 3. Differentially expressed genes in medial amygdala
Supplemental File 4. Differentially expressed genes in prefrontal cortex
Supplemental File 5. The overlapping DEGs are listed for each of the following comparisons: column 1, the mutant versus wildtype in nonsocial condition and the social versus nonsocial in wildtype mice; column 2, mutant versus wildtype in nonsocial condition and the interaction analysis; column 3, social versus nonsocial in wildtype mice and the interaction are shown in the third column; and column 4, all three comparisons. Analyses from each brain region are shown on separate sheets, accessed by the tabs at the bottom.
Supplemental File 6. Top 10 common differentially expressed genes in the Mutant vs. Wildtype and Social vs. Nonsocial conditions. Shaded boxes indicate p < 0.05.
Supplemental File 7. SFARI autism candidate genes overlapping with differentially expressed genes
Supplemental File 8. Up to 10 canonical pathways with significant enrichment of genes that were differentially expressed are shown for each comparison and each brain region. The number of differentially expressed genes that overlap with each pathway, and the B-H p-value are included in the right two columns. Analyses from each brain region are shown on separate sheets, accessed by the tabs at the bottom.
Supplemental File 9. Up to 10 diseases and functions with significant enrichment of genes that were differentially expressed are shown for each comparison and each brain region. The broad categories that the functions fit into, the disease or function itself, the predicted activation state, and the B-H p-value are shown from left to right. The predicted direction of activation or inhibition is not provided for the interaction analysis due to the inability to interpret these predictions. Analyses from each brain region are shown on separate sheets, accessed by the tabs at the bottom.
Supplemental Figure 10. The top 10 functions enriched with DEGs associated with cell survival in the most comparisons are ranked from most to least significance of enrichment in Left) mutant versus wildtype and Right) social versus nonsocial comparisons. The intensity of the grayscale corresponds to B-H p-value, indicated by the scalebar. Arrows indicate a significant interaction (B-H p-value < 0.05). The dots indicate a z-Score (B-H p-value < 0.05) that predicts activation (orange) or inhibition (blue) of the pathway and the magnitude is indicated by the size of the dot.
Supplemental Figure 11. The top 10 functions enriched with DEGs associated with behavior in the most comparisons are ranked from most to least significance of enrichment in Left) mutant versus wildtype and Right) social versus nonsocial comparisons. The intensity of the grayscale corresponds to B-H p-value, indicated by the scalebar. Arrows indicate a significant interaction (B-H p-value < 0.05). The dots indicate a z-Score (B-H p-value < 0.05) that predicts activation (orange) or inhibition (blue) of the pathway and the magnitude is indicated by the size of the dot.
Supplemental Figure 12. The top 10 functions enriched with DEGs associated with neurological or psychological disorders in the most comparisons are ranked from most to least significance of enrichment in Left) mutant versus wildtype and Right) social versus nonsocial comparisons. The intensity of the grayscale corresponds to B-H p-value, indicated by the scalebar. Arrows indicate a significant interaction (B-H p-value < 0.05). The dots indicate a z- Score (B-H p-value < 0.05) that predicts activation (orange) or inhibition (blue) of the pathway and the magnitude is indicated by the size of the dot.
Supplemental File 13. A network with enrichment of differentially expressed genes from the interaction comparison of genotypes and stimuli from the medial amygdala is relevant to cell-to- cell signaling. Direct and indirect interactions of molecules are indicated based on the IPA knowledge base.
Supplemental File 14. Upstream regulator analyses reveal regulatory molecules known to affect a certain network of molecules, overlapping with DEGs in each comparison. The name of the top 10 upstream regulators, the predicted direction of activation or inhibition based on the up/down-regulation of DEGs that overlap with the affected network, and the p-value are shown from left to right. The predicted direction of activation or inhibition is not provided for the interaction analysis due to the inability to interpret these predictions. Analyses from each brain region are shown on separate sheets, accessed by the tabs at the bottom.
Highlights.
Highlights for: A social encounter drives gene expression changes linked to neuronal function, brain development, and related disorders in mice expressing the serotonin transporter Ala56 variant.
A mutation in the serotonin transporter gene introduced to mice affected expression of many genes compared to wildtype mice.
Differentially expressed genes were observed in response to a social stimulus compared to a non-social stimulus in the lateral amygdala and medial amygdala, but fewer differences were seen in the prefrontal cortex.
The differentially expressed genes were associated with pathways and functions relevant to behavior, nervous system development, and neurotransmitter signaling.
Acknowledgements
Studies were performed at VANTAGE and Mouse Neurobehavior Lab facilities at Vanderbilt University. This work was supported by NIH grants MH094604 (JV), MH081066 (JV), MH065215 (TR, RB), and MH016434 (AA, JV). JV has consulted or served on an advisory board for Novartis, Roche Pharmaceuticals and SynapDx; has received research funding from Forest, Novartis, Roche Pharmaceuticals, Seaside Therapeutics, and SynapDx; and has received an editorial stipend from Springer and Wiley. All other authors declare no competing interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Walsh JJ, Christoffel DJ, Heifets BD, Ben-Dor GA, Selimbeyoglu A, Hung LW, Deisseroth K, Malenka RC, 5-HT release in nucleus accumbens rescues social deficits in mouse autism model, Nature 560 (2018) 589–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Veenstra-VanderWeele J, Muller CL, Iwamoto H, Sauer JE, Owens WA, Shah CR, Cohen J, Mannangatti P, Jessen T, Thompson BJ, Ye R, Kerr TM, Carneiro AM, Crawley JN, Sanders-Bush E, McMahon DG, Ramamoorthy S, Daws LC, Sutcliffe JS, Blakely RD, Autism gene variant causes hyperserotonemia, serotonin receptor hypersensitivity, social impairment and repetitive behavior, Proc Natl Acad Sci U S A 109 (2012) 5469–5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Nakatani J, Tamada K, Hatanaka F, Ise S, Ohta H, Inoue K, Tomonaga S, Watanabe Y, Chung YJ, Banerjee R, Iwamoto K, Kato T, Okazawa M, Yamauchi K, Tanda K, Takao K, Miyakawa T, Bradley A, Takumi T, Abnormal behavior in a chromosome-engineered mouse model for human 15q11–13 duplication seen in autism, Cell 137 (2009) 1235–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tamada K, Tomonaga S, Hatanaka F, Nakai N, Takao K, Miyakawa T, Nakatani J, Takumi T, Decreased exploratory activity in a mouse model of 15q duplication syndrome; implications for disturbance of serotonin signaling, PLoS One 5 (2010) e15126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Dolen G, Darvishzadeh A, Huang KW, Malenka RC, Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin, Nature 501 (2013) 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Heifets BD, Salgado JS, Taylor MD, Hoerbelt P, Cardozo Pinto DF, Steinberg EE, Walsh JJ, Sze JY, Malenka RC, Distinct neural mechanisms for the prosocial and rewarding properties of MDMA, Sci Transl Med 11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gabriele S, Sacco R, Persico AM, Blood serotonin levels in autism spectrum disorder: a systematic review and meta-analysis, Eur Neuropsychopharmacol 24 (2014) 919–929. [DOI] [PubMed] [Google Scholar]
- [8].Sutcliffe JS, Delahanty RJ, Prasad HC, McCauley JL, Han Q, Jiang L, Li C, Folstein SE, Blakely RD, Allelic heterogeneity at the serotonin transporter locus (SLC6A4) confers susceptibility to autism and rigid-compulsive behaviors, American journal of human genetics 77 (2005) 265–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Muller CL, Anacker AM, Veenstra-VanderWeele J, The serotonin system in autism spectrum disorder: From biomarker to animal models, Neuroscience 321 (2015) 24–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Prasad HC, Steiner JA, Sutcliffe JS, Blakely RD, Enhanced activity of human serotonin transporter variants associated with autism, Philos Trans R Soc Lond B Biol Sci 364 (2009) 163–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rogers TD, Anacker AMJ, Kerr TM, Forsberg CG, Wang J, Zhang B, Veenstra- VanderWeele J, Effects of a social stimulus on gene expression in a mouse model of fragile X syndrome, Mol Autism 8 (2017) 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Abe N, Suzuki M, Mori E, Itoh M, Fujii T, Deceiving others: distinct neural responses of the prefrontal cortex and amygdala in simple fabrication and deception with social interactions, J Cogn Neurosci 19 (2007) 287–295. [DOI] [PubMed] [Google Scholar]
- [13].Guyer AE, Lau JY, McClure-Tone EB, Parrish J, Shiffrin ND, Reynolds RC, Chen G, Blair RJ, Leibenluft E, Fox NA, Ernst M, Pine DS, Nelson EE, Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety, Arch Gen Psychiatry 65 (2008) 1303–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Rilling JK, Glenn AL, Jairam MR, Pagnoni G, Goldsmith DR, Elfenbein HA, Lilienfeld SO, Neural correlates of social cooperation and non-cooperation as a function of psychopathy, Biol Psychiatry 61 (2007) 1260–1271. [DOI] [PubMed] [Google Scholar]
- [15].Swartz JR, Wiggins JL, Carrasco M, Lord C, Monk CS, Amygdala habituation and prefrontal functional connectivity in youth with autism spectrum disorders, J Am Acad Child Adolesc Psychiatry 52 (2013) 84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Liao Y, Wang J, Jaehnig EJ, Shi Z, Zhang B, WebGestalt 2019: gene set analysis toolkit with revamped UIs and APIs, Nucleic Acids Res 47 (2019) W199–W205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bonnin A, Torii M, Wang L, Rakic P, Levitt P, Serotonin modulates the response of embryonic thalamocortical axons to netrin-1, Nat Neurosci 10 (2007) 588–597. [DOI] [PubMed] [Google Scholar]
- [18].Sikich L, Hickok JM, Todd RD, 5-HT1A receptors control neurite branching during development, Brain Res Dev Brain Res 56 (1990) 269–274. [DOI] [PubMed] [Google Scholar]
- [19].Vitalis T, Cases O, Passemard S, Callebert J, Parnavelas JG, Embryonic depletion of serotonin affects cortical development, Eur J Neurosci 26 (2007) 331–344. [DOI] [PubMed] [Google Scholar]
- [20].Maguschak KA, Ressler KJ, Wnt signaling in amygdala-dependent learning and memory, J Neurosci 31 (2011) 13057–13067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Oliva CA, Vargas JY, Inestrosa NC, Wnts in adult brain: from synaptic plasticity to cognitive deficiencies, Front Cell Neurosci 7 (2013) 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lipton SA, Kater SB, Neurotransmitter regulation of neuronal outgrowth, plasticity and survival, Trends Neurosci 12 (1989) 265–270. [DOI] [PubMed] [Google Scholar]
- [23].Costa L, Spatuzza M, D’Antoni S, Bonaccorso CM, Trovato C, Musumeci SA, Leopoldo M, Lacivita E, Catania MV, Ciranna L, Activation of 5-HT7 serotonin receptors reverses metabotropic glutamate receptor-mediated synaptic plasticity in wild-type and Fmr1 knockout mice, a model of Fragile X syndrome, Biol Psychiatry 72 (2012) 924–933. [DOI] [PubMed] [Google Scholar]
- [24].Felix-Ortiz AC, Burgos-Robles A, Bhagat ND, Leppla CA, Tye KM, Bidirectional modulation of anxiety-related and social behaviors by amygdala projections to the medial prefrontal cortex, Neuroscience 321 (2016) 197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Narboux-Neme N, Pavone LM, Avallone L, Zhuang X, Gaspar P, Serotonin transporter transgenic (SERTcre) mouse line reveals developmental targets of serotonin specific reuptake inhibitors (SSRIs), Neuropharmacology 55 (2008) 994–1005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental File 1. The correlations of mapped reads between biological replicates using normalized counts per million for all reads were high for all comparisons in all brain regions. All p-values < 0.001. MA = medial amygdala, LA = lateral amygdala, and PFC = prefrontal cortex.
Supplemental File 2. Differentially expressed genes in lateral amygdala
Supplemental File 3. Differentially expressed genes in medial amygdala
Supplemental File 4. Differentially expressed genes in prefrontal cortex
Supplemental File 5. The overlapping DEGs are listed for each of the following comparisons: column 1, the mutant versus wildtype in nonsocial condition and the social versus nonsocial in wildtype mice; column 2, mutant versus wildtype in nonsocial condition and the interaction analysis; column 3, social versus nonsocial in wildtype mice and the interaction are shown in the third column; and column 4, all three comparisons. Analyses from each brain region are shown on separate sheets, accessed by the tabs at the bottom.
Supplemental File 6. Top 10 common differentially expressed genes in the Mutant vs. Wildtype and Social vs. Nonsocial conditions. Shaded boxes indicate p < 0.05.
Supplemental File 7. SFARI autism candidate genes overlapping with differentially expressed genes
Supplemental File 8. Up to 10 canonical pathways with significant enrichment of genes that were differentially expressed are shown for each comparison and each brain region. The number of differentially expressed genes that overlap with each pathway, and the B-H p-value are included in the right two columns. Analyses from each brain region are shown on separate sheets, accessed by the tabs at the bottom.
Supplemental File 9. Up to 10 diseases and functions with significant enrichment of genes that were differentially expressed are shown for each comparison and each brain region. The broad categories that the functions fit into, the disease or function itself, the predicted activation state, and the B-H p-value are shown from left to right. The predicted direction of activation or inhibition is not provided for the interaction analysis due to the inability to interpret these predictions. Analyses from each brain region are shown on separate sheets, accessed by the tabs at the bottom.
Supplemental Figure 10. The top 10 functions enriched with DEGs associated with cell survival in the most comparisons are ranked from most to least significance of enrichment in Left) mutant versus wildtype and Right) social versus nonsocial comparisons. The intensity of the grayscale corresponds to B-H p-value, indicated by the scalebar. Arrows indicate a significant interaction (B-H p-value < 0.05). The dots indicate a z-Score (B-H p-value < 0.05) that predicts activation (orange) or inhibition (blue) of the pathway and the magnitude is indicated by the size of the dot.
Supplemental Figure 11. The top 10 functions enriched with DEGs associated with behavior in the most comparisons are ranked from most to least significance of enrichment in Left) mutant versus wildtype and Right) social versus nonsocial comparisons. The intensity of the grayscale corresponds to B-H p-value, indicated by the scalebar. Arrows indicate a significant interaction (B-H p-value < 0.05). The dots indicate a z-Score (B-H p-value < 0.05) that predicts activation (orange) or inhibition (blue) of the pathway and the magnitude is indicated by the size of the dot.
Supplemental Figure 12. The top 10 functions enriched with DEGs associated with neurological or psychological disorders in the most comparisons are ranked from most to least significance of enrichment in Left) mutant versus wildtype and Right) social versus nonsocial comparisons. The intensity of the grayscale corresponds to B-H p-value, indicated by the scalebar. Arrows indicate a significant interaction (B-H p-value < 0.05). The dots indicate a z- Score (B-H p-value < 0.05) that predicts activation (orange) or inhibition (blue) of the pathway and the magnitude is indicated by the size of the dot.
Supplemental File 13. A network with enrichment of differentially expressed genes from the interaction comparison of genotypes and stimuli from the medial amygdala is relevant to cell-to- cell signaling. Direct and indirect interactions of molecules are indicated based on the IPA knowledge base.
Supplemental File 14. Upstream regulator analyses reveal regulatory molecules known to affect a certain network of molecules, overlapping with DEGs in each comparison. The name of the top 10 upstream regulators, the predicted direction of activation or inhibition based on the up/down-regulation of DEGs that overlap with the affected network, and the p-value are shown from left to right. The predicted direction of activation or inhibition is not provided for the interaction analysis due to the inability to interpret these predictions. Analyses from each brain region are shown on separate sheets, accessed by the tabs at the bottom.


