Summary
In paroxysmal nocturnal haemoglobinuria (PNH), chronic destruction of PNH red blood cells (RBCs) by complement leads to anaemia and other serious morbidities. Eculizumab inhibits terminal complement-mediated PNH RBC destruction by targeting C5. In the phase III, double-blind, placebo-controlled, TRIUMPH study, eculizumab reduced haemolysis, stabilized haemoglobin levels, reduced transfusion requirements and improved fatigue in patients with PNH. Herein, we explored the effects of eculizumab on measures of anaemia in patients from the TRIUMPH study and the open-label SHEPHERD study, a more heterogeneous population. Eculizumab reduced haemolysis regardless of pretreatment transfusion requirements and regardless of whether or not patients became transfusion-dependent during treatment (P < 0 001). Reduction in haemolysis was associated with increased PNH RBC counts (P < 0–001) while reticulocyte counts remained elevated. Eculizumab-treated patients demonstrated significantly higher levels of haemoglobin as compared with placebo in TRIUMPH and relative to baseline levels in SHEPHERD (P < 0 001 for each study). Eculizumab lowered transfusion requirement across multiple pretreatment transfusion strata and eliminated transfusion support in a majority of both TRIUMPH and SHEPHERD patients (P < 0 001). Patients who required some transfusion support during treatment with eculizumab showed a reduction in haemolysis and transfusion requirements and an improvement in fatigue. Eculizumab reduces haemolysis and improves anaemia and fatigue, regardless of transfusion requirements.
Keywords: paroxysmal nocturnal haemoglobinuria, haemoglobin, haemolysis, transfusion
Paroxysmal nocturnal haemoglobinuria (PNH) is a rare, debilitating and life-threatening acquired haemolytic anaemia. Mutations in the phosphatidylinositol glycan complementation class A gene (PIGA) result in a decrease in or total deficiency of glycosylphosphotidylinositol (GPI)-anchored proteins (Takeda et al, 1993; Bessler et al, 1994). Absence of the GPI-anchored complement inhibitory protein CD59 from the surface of PNH red blood cells (RBCs) renders them susceptible to terminal complement-mediated lysis (Yamashina et al, 1990; Motoyama et al, 1992). Chronic intravascular haemolysis, as found in PNH, appears to be responsible for a series of clinical complications. The inability to maintain endogenous haemoglobin levels leads to anaemia, weakness, pallor and dyspnoea on exertion. Patients may require frequent transfusions to support tolerable haemoglobin levels. Fatigue, which can be disabling, is related to the chronic haemolysis and is disproportionate to anaemia (Rosse, 2000; Caocci et al, 2007). Depletion of plasma nitric oxide by free haemoglobin leads to complications associated with smooth muscle dystonias including abdominal pain, dysphagia, pulmonary hypertension and erectile dysfunction (Rosse, 2000; Parker et al, 2005; Rother et al, 2005). Nitric oxide also functions as a coagulation regulator and its consumption renders patients with PNH at higher risk for life-threatening thrombosis (Rosse & Nishimura, 2003; Rother et al, 2005).
Until recently, treatment options for patients with PNH were generally supportive and directed towards the palliation of clinical symptoms rather than the treatment of the underlying disease process. Eculizumab (SOLIRIS®; Alexion Pharmaceuticals Inc., Cheshire, CT, USA) is a humanized monoclonal antibody that was approved in March 2007 by the Food and Drug Administration and in June 2007 by the European Commission for the treatment of patients with PNH. Eculizumab binds to the human complement component C5 and inhibits terminal complement activation, thereby preventing complement-mediated destruction of PNH RBCs (Thomas et al, 1996). Previously, results from the phase III double-blinded, placebo-controlled TRIUMPH study demonstrated that eculizumab reduced haemolysis and transfusion requirements, and improved fatigue in patients with PNH (Hillmen et al, 2006). Similar benefits of eculizumab were observed in the SHEPHERD study; an open-label, safety and efficacy trial that enrolled a more heterogeneous population of patients with PNH, including those with significant thrombocytopenia and minimal transfusion requirements, compared with TRIUMPH (Brodsky et al, 2008). To fully understand the benefits of reducing haemolysis in patients with PNH, we further analysed data from the TRIUMPH and SHEPHERD studies to determine the effects of eculizumab treatment on anaemia parameters including endogenous PNH RBC count, haemoglobin levels and transfusion requirements.
Methods
Patients – TRIUMPH
Patients ≥18 years of age were eligible for TRIUMPH (A Hemoglobin Stabilization and Transfusion Reduction Efficacy and Safety Clinical Investigation, Randomized, Multi-Center, Double-Blind, Placebo-Controlled, Using Eculizumab in Paroxysmal Nocturnal Hemoglobinuria Patients) if they had a PNH type III RBC proportion of ≥10% with intravascular haemolysis, as indicated by lactate dehydrogenase (LDH) levels >1·5 × upper limit of the normal range. In addition, eligible patients had received at least four transfusions during the previous 12 months and had a platelet count of at least 100 × 109/l prior to entering the trial.
Patients were excluded if they had active bacterial infections, a history of meningococcal disease, or had undergone bone marrow transplantation. Concomitant medications were permitted. All patients were vaccinated against Neisseria meningitides 2 weeks prior to treatment initiation. All patients provided written informed consent and the protocol was approved by the Institutional Review Board at each study centre.
Study design and treatment – TRIUMPH
Details of the TRIUMPH study design are described in an earlier report (Hillmen et al, 2006). Briefly, patients were eligible for randomization if they received a ‘qualifying’ transfusion during the observation period of 13 weeks or less. The haemoglobin level at which the qualifying transfusion was administered was individualized for each patient (individual set point). Patients were randomized to receive either placebo or eculizumab within 10 d after the qualifying transfusion. Randomization was performed with stratification according to the number of units of packed RBCs transfused during the 12- month period prior to treatment. This ensured that each treatment group had a similar number of patients in each pretreatment transfusion strata. Treatments were administered via a 25- to 45-min intravenous infusion as an induction phase of 600 mg once every week for 4 weeks, followed by a 900 mg dose 1 week later, followed by a 900 mg dose every 2 weeks for a treatment duration of 26 weeks.
Study assessments – TRIUMPH
Endpoints for this subanalysis included haemolysis, measurements of anaemia including PNH RBC counts and haemoglobin levels, transfusion requirements and fatigue.
Haemolysis
To assess the effect of terminal complement blockade with eculizumab on intravascular haemolysis, levels of serum LDH were assessed at baseline and at each scheduled visit.
Measurements of anaemia
Red blood cell counts – RBC counts were assessed and reported as counts X 1012/l. The proportion of PNH RBCs (type II and III) was determined using flow cytometry (Nilsson et al, 1993; Alfinito et al, 1996; Hall & Rosse, 1996; Navenot et al, 1996; Richards & Hillmen, 2001). Counts of PNH RBCs were calculated by multiplying the proportion of PNH RBCs by the total RBC count.
Haemoglobin – RBC
Haemoglobin levels were measured and reported as grams per litre. Stabilization of haemoglobin levels was defined as remaining above the haemoglobin set point for the entire 26-week-treatment period, the haemoglobin level at which the qualifying transfusion was administered, without transfusion. All blood samples were processed according to standard procedures and analysed by a central laboratory.
Transfusion requirements
The number of units of packed RBCs transfused after randomization to 26 weeks was calculated. Transfusion independence was defined as requiring no transfusions during the entire 26-week-treatment period. Patients who discontinued treatment before the end of the study were considered as requiring a transfusion.
Fatigue
The effect of treatment with eculizumab on fatigue levels was assessed using the Functional Assessment of Chronic Illness Therapy – Fatigue (FACIT-Fatigue) instrument (version 4). Scores range from 0 to 52, with higher scores indicating improvement in fatigue (Cella et al, 2002).
Statistical analysis – TRIUMPH
Study treatment randomization was stratified according to the number of packed RBC units transfused during the 12 months prior to screening; 4–14, 15–25 and >25 units. Data were also analysed for the subgroup of patients who received transfusion during eculizumab treatment. Comparison of change in haemoglobin levels, and change in FACIT-Fatigue scores between the two treatment groups were analysed using a mixed model analysis, with baseline scores as the covariate, treatment and time as fixed effects, and the patient identifier as a random effect. Comparisons between the treatment groups for the change in LDH levels, PNH RBC counts and the number of packed RBC units transfused up to week 26 were assessed using a Wilcoxon’s rank sum test. Stabilization of haemoglobin levels and transfusion independence were analysed using Fisher’s exact test. All P-values are 2-sided, were not adjusted for multiple analyses, and were considered significant when <0 05. Mean values are shown ± standard error.
Patients and study design – SHEPHERD
SHEPHERD (Safety in Haemolytic PNH Patients Treated with Eculizumab; A Multi-center Open-label Research Design Study) was an open-label, clinical study designed to investigate the long-term safety and efficacy of eculizumab (Brodsky et al, 2008). The study design consisted of a 2-week screening period and a 52-week treatment period with a prespecified 26-week interim analysis. The study design of SHEPHERD was similar to that of TRIUMPH, except for the following; patients with as few as one transfusion in the past 2 years, and platelet counts as low as 30 × 109/l at one of the two screening visits were permitted. In contrast to the TRIUMPH study design, patients enrolled in the SHEPHERD trial were not required to receive a qualifying transfusion prior to the initiation of eculizumab therapy. The SHEPHERD study was reviewed and approved by the Institutional Review Board at each centre and patients provided written informed consent prior to entering the study.
Study assessments – SHEPHERD
Intravascular haemolysis was assessed by LDH levels. Fatigue was measured using the FACIT-Fatigue instrument. Data were also collected to assess the effects of eculizumab on the PNH RBC clone size, haemoglobin levels and transfusion requirements.
Statistical analysis – SHEPHERD
The change in LDH, units transfused and change in FACIT- Fatigue score were analysed using a signed rank sum test. The changes in haemoglobin levels were analysed using a mixed- model analysis. Transfusion avoidance was evaluated with a Fisher’s exact test. Efficacy assessments were further analysed according to the number of packed RBC units transfused during the 52 weeks prior to screening; 0–4, 4–14, 15–25 and >25 units. In addition, efficacy outcomes were also assessed for the subgroup of patients who received 0 or 1 transfusions in the year prior to the study and those who received transfusion during eculizumab treatment. P-values <0 05 were considered significant.
Results
Patients
A total of 87 patients were randomized in TRIUMPH to receive eculizumab (n = 43) or placebo (n = 44). Demographics and baseline characteristics were similar across both treatment groups (Table I). Patients must have received at least 4 units of packed RBC in the year prior to enrolment to be eligible for the trial. The 97 patients enrolled in SHEPHERD were similar with respect to gender, age and duration of PNH to those enrolled in TRIUMPH. Patients who received <4 units of packed RBC in the year prior to enrolment were eligible for SHEPHERD. Patients enrolled in SHEPHERD had received a median of 8 units of packed red cells transfused during the year prior to screening, compared with approximately 18 units for the patients enrolled in TRIUMPH.
Table I.
Demographics and baseline characteristics.
| TRIUMPH |
SHEPHERD |
||
|---|---|---|---|
| Parameter | Eculizumab (n = 43) | Placebo (n = 44) | (n = 97) |
| Sex, n (%) | |||
| Male | 20 (47) | 15 (34) | 48 (49) |
| Female | 23 (53) | 29 (66) | 49 (51) |
| Median age, years (range) | 41 (20–85) | 35 (18–78) | 41 (18–78) |
| Median duration of PNH, years (range) | 43· (0·9–29·8) | 9·2 (0·5–38·5) | 4·9 (0·1–31·4) |
| Median units packed RBC transfused | 9 (6–12) | 8·5 (7–12·5) | 8·0 (0–66·0) |
| prior to screening,* units (range) | |||
| Units transfused 12 months prior to screening, n (%) | |||
| <4 units | – | – | 21 (22) |
| 4–14 units | 15 (35) | 15 (34) | 47 (48) |
| 15–25 units | 17 (40) | 18 (41) | 15 (15) |
| >25 units | 11 (26) | 11 (25) | 14 (14) |
| Median reticulocyte count, 1012/l (range) | 0·207 (0·040–0·570) | 0·206 (0·045–0·556) | 0·140 (0·036–0·757) |
| Median platelet count, 109/l (range) | 178·0 (72·0–416·0) | 138·5 (59·0–547·0) | 136·0 (23·0–355) |
| Median neutrophil count, 109/l (range) | 2·3 (0·8–11·2) | 2·1 (0·1–11·0) | 2·3 (0·2–7·4) |
| Use of erythropoietin, n (%) | 3 (7) | 0 | 5 (5) |
| Use of folate, iron or B12, n (%) | 5 (12) | 6 (14) | 17 (18) |
| Median haemoglobin set point,* g/l (range) | 77 (61–88) | 77 (62–90) | – |
PNH, paroxysmal nocturnal haemoglobinuria; RBC, red blood cell.
Qualifying transfusion. Data for TRIUMPH represents units transfused during 12 months prior to screening normalized to a 6-month equivalent.
Data for SHEPHERD represents units transfused during 12 months prior to screening.
Reduction in haemolysis
In the previous TRIUMPH study, eculizumab treatment of PNH patients resulted in a rapid and sustained reduction in haemolysis (Hillmen et al, 2006). This reduction in haemolysis was observed for all eculizumab-treated patients, regardless of pretreatment transfusion requirements (Table II, P < 0 001 for each stratum). Similarly, significant reductions in LDH levels were observed with eculizumab treatment in the 12-month SHEPHERD study for the overall population and for patients in each pretreatment transfusion strata including patients receiving <4 transfusions in the year prior to the study (Table II; P < 0 001 for each stratum). Moreover, in 22 patients from SHEPHERD with minimal pretreatment transfusion support (0 or 1 transfusions in the year prior to treatment), eculizumab treatment was associated with a comparable reduction in LDH levels for the 52-week treatment period (median of 1992 units/l at baseline vs. 254 units/l at 52 weeks; P < 0 001). Haemolysis was also significantly reduced in patients regardless of whether they achieved transfusion-independence during the two studies. Patients still requiring transfusions during eculizumab treatment in TRIUMPH showed a significant reduction in LDH levels at 26 weeks (median of 2167 units/l for placebo vs. 252 units/l for eculizumab; P < 0 001). Similarly, patients still requiring transfusions during eculizumab treatment in SHEPHERD also experienced a significant reduction in LDH levels (median of 2163 units/l at baseline vs. 273 units/l at week 52; P < 0–001).
Table II.
Effect of eculizumab on haemolysis in patients with paroxysmal nocturnal haemoglobinuria by pretreatment transfusion strata.
| TRIUMPH |
SHEPHERD |
|||||||
|---|---|---|---|---|---|---|---|---|
| Median (mean ± SE) |
Median (mean ± SE) |
|||||||
| Pretreatment transfusion strata | Baseline |
Week 26 |
Baseline |
Week 52 |
||||
| Placebo | Eculizumab | Placebo | Eculizumab | P-value* | Pretreatment | Eculizumab | P-value† | |
| Overall | 2235 (2258 ± 155) | 2032 (2200 ± 158) | 2167 (2419 ± 140) | 239 (327 ± 68) | <0·001 | 2051 (2201 ± 105) | 269 (297 ± 21) | <0·001 |
| <4 units | – | – | – | – | – | 2030 (2218 ± 211) | 264 (352 ± 89) | <0·001 |
| 4–14 units | 1642 (2001 ± 212) | 1703 (2353 ± 345) | 2060 (2297 ± 226) | 227 (424 ± 198) | <0·001 | 1876 (2124 ± 144) | 270 (284 ± 14) | <0·001 |
| 15–25 units | 2193 (2312 ± 232) | 1877 (1856 ± 162) | 2111 (2385 ± 231) | 264 (280 ± 22) | <0·001 | 2758 (2492 ± 304) | 286 (285 ± 17) | <0·001 |
| >25 units | 2414 (2519 ± 402) | 2482 (2523 ± 290) | 2805 (2640 ± 293) | 259 (273 ± 25) | <0·001 | 2123 (2119 ± 312) | 219 (262 ± 30) | <0·001 |
Values shown are lactate dehydrogenase levels (units/l). P-values are based on a comparison of the median values.
Wilcoxon’s rank sum test.
Signed rank test.
Increase in endogenous red blood cell mass
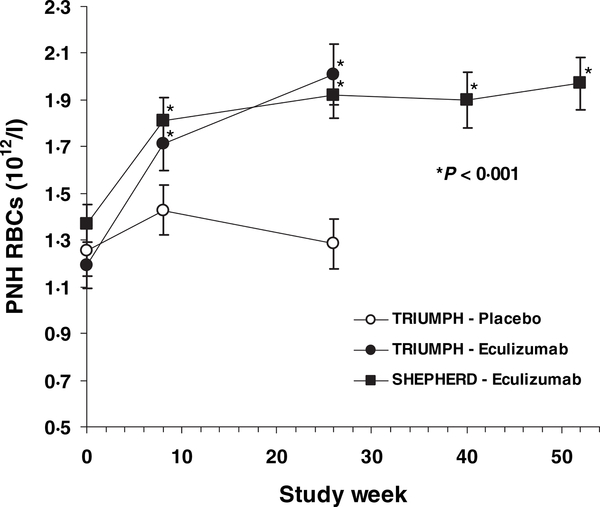
As reported previously, eculizumab treatment significantly increased the proportion of PNH type III RBCs by week 26 (Hillmen et al, 2006). However PNH RBC proportions can dramatically vary because of dilution with transfused packed RBCs. Therefore, to more accurately assess the effect of reducing haemolysis with eculizumab on PNH RBC survival, cell counts for PNH RBCs were assessed. The mean PNH RBC counts plotted over the treatment course from the TRIUMPH and SHEPHERD trials are shown in Fig 1. At baseline, median PNH RBC counts in the TRIUMPH study were 1 00 × 1012/l for the eculizumab group and 1 12 × 1012/l for the placebo group. By 26 weeks, median PNH RBC count had increased by 100% for the eculizumab group compared with 3% for the placebo group (P < 0 001, Wilcoxon’s rank sum test), with values at week 26 of 2 05 × 1012/l vs. 1 15 × 1012/l respectively. Median PNH RBC counts in the SHEPHERD study were 1 27 × 1012/l at baseline. By week 8 the PNH RBC counts had significantly increased and this was sustained for the duration of the treatment period with a value of 1 93 × 1012/l at 52 weeks. Reticulocyte counts remained elevated at 26 weeks in both placebo and eculizumab-treated patients in TRIUMPH (0 280 and 0 237 × 1012/l respectively) with no significant change from baseline values or between treatment groups. Likewise, reticulocyte counts at 52 weeks in SHEPHERD patients remained elevated (0 146 × 1012/l) and did not change significantly from baseline values. Further, platelet and neutrophil counts remained stable throughout the TRIUMPH and SHEPHERD studies (data not shown).
Fig 1.
Mean (SE) paroxysmal nocturnal haemoglobinuria red blood cell mass during the TRIETMPH and SHEPHERD studies. PNH, paroxysmal nocturnal haemoglobinuria; RBC, red blood cell. P-values for the TRIETMPH data were calculated using Wilcoxon’s rank sum test and for the SHEPHERD data using a sign rank test.
Increase in RBC haemoglobin levels
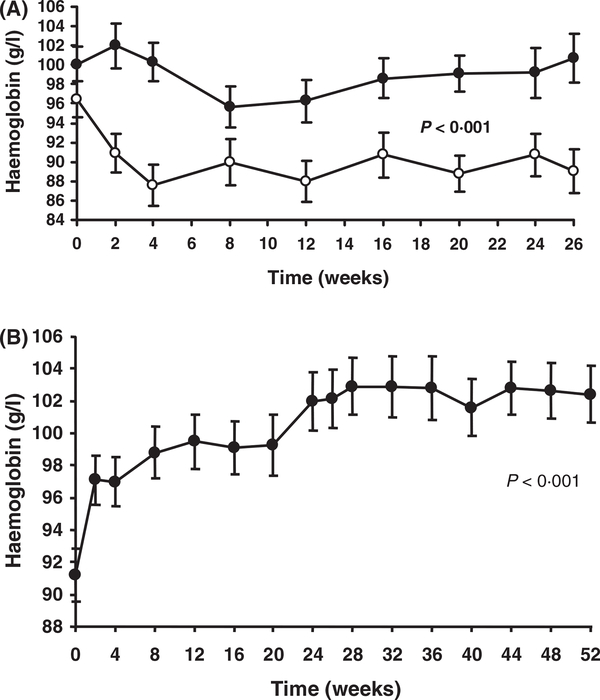
Prior to eculizumab treatment, haemoglobin levels were maintained through regular transfusions of packed RBCs. In the TRIUMPH study, pretreatment baseline mean haemoglobin levels were not significantly different between the two treatment groups (100 ± 1 8 g/1 for patients treated with eculizumab and 97 ± 1 8 g/1 for patients who received placebo). During the study, patients who received eculizumab had a significant increase in haemoglobin levels compared with patients who received placebo (P < 0 001, mixed model analysis; Fig 2A). Following the qualifying transfusion, haemoglobin levels in patients receiving placebo decreased to a mean of 88 ±2 1 g/1 by week 4 and were 89 ± 2 2 g/1 by week 26. In contrast, mean haemoglobin levels in patients treated with eculizumab were maintained during the entire study and were 101 ± 2 5 g/1 at 26 weeks, even though transfusion support significantly decreased during treatment. Patients in the SHEPHERD study experienced a statistically significant increase in haemoglobin levels, from 92 ± 2 0 g/1 at baseline to 102 ± 2 0 g/1 at 52 weeks with eculizumab treatment (P < 0 001; Fig 2B).
Fig 2.
Mean (SE) haemoglobin levels during the TRIUMPH and SHEPHERD studies. (A) Patients enrolled in the TRIUMPH study were treated within 10 d of their qualifying transfusion with either eculizumab (closed circle) or placebo (open circle) for 26 weeks. (B) Patients enrolled in the SHEPHERD trial were treated with ecu- lizumab for 52 weeks. P-values were based on a mixed-model analysis.
Transfusion requirements
We have previously reported that eculizumab treatment of PNH patients in the TRIUMPH study resulted in a significant reduction in transfusion support (Hillmen et al, 2006). This reduction in transfusion support was observed for all eculizumab-treated patients, regardless of pretreatment transfusion requirements (Table III, P < 0 001 for each stratum). Similarly, a reduction in units of packed RBC was observed with eculizumab treatment in the 12-month SHEPHERD study for the overall population and in all pretreatment transfusion strata with three of four strata reaching statistical significance (Table III). Fifty-one percent of patients in both TRIUMPH and SHEPHERD were transfusion-independent during the entire respective treatment periods (P < 0 001); no placebo- treated patients in TRIUMPH achieved this status. Further, transfusion support was also reduced in patients who did not achieve transfusion-independence during the two studies. Patients still requiring transfusion during eculizumab treatment in TRIUMPH showed a significant reduction in units of packed RBC during treatment (median of 10 units for placebo vs. 6 units for eculizumab; P < 0 001). Although not statistically significant, patients still requiring transfusion during eculizumab treatment in SHEPHERD also experienced a reduction in units of packed RBC (median of 12 units pretreatment vs. 7 5 units during treatment).
Table III.
Effect of eculizumab on transfusion requirements in patients with paroxysmal nocturnal haemoglobinuria by pretreatment transfusion strata.
| TRIUMPH |
SHEPHERD |
|||||||
|---|---|---|---|---|---|---|---|---|
| Median (mean ± SE) |
Median (mean ± SE) |
|||||||
| Pretreatment transfusion strata | 6-month pretreatment‡ |
Week 26 |
||||||
| Placebo | Eculizumab | Placebo | Eculizumab | P-value* | 1-year Pretreatment | Week 52 Eculizumab | P-value† | |
| Overall | 8·5 (9·9 ± 0·7) | 9·0 (9·5 ± 0·6) | 10·0 (11·0 ± 0·83) | 0·0 (3·0 ± 0·67) | <0·001 | 8·0 (12·3 ± 1·25) | 0·0 (5·9 ± 1·06 | <0·001 |
| <4 units | – | – | – | – | – | 2·0 (1·6 ± 0·31) | 0·0 (1·5 ± 0·69) | 0·42 |
| 4–14 units | 6·5 (6·2 ± 0·4) | 5·5 (5·4 ± 0·3) | 6·0 (6·7 ± 0·72) | 0·0 (0·4 ± 0·29) | <0·001 | 8·0 (7·6 ± 0·44) | 0·0 (4·9 ± 1·39) | 0·002 |
| 15–25 units | 8·8 (8·9 ± 0·5) | 9·5 (9·3 ± 0·3) | 10·0 (10·8 ± 1·17) | 2·0 (4·2 ± 1·14) | <0·001 | 17·0 (19·3 ± 1·01) | 4·0 (9·5 ± 3·10) | 0·008 |
| >25 units | 15·0 (16·3 ± 0·9) | 16·5 (15·6 ± 0·7) | 18·0 (17·0 ± 1·04) | 3·0 (4·5 ± 1·59) | <0·001 | 33·5 (36·6 ± 2·78) | 7·5 (11·8 ± 4·02) | <0·001 |
P-values are based on a comparison of the median values.
Wilcoxon’s rank sum test.
Signed rank test.
Transfusion data obtained during 12 months before treatment were normalized to a value equivalent to the value for a 6-month period.
Stabilization of haemoglobin levels
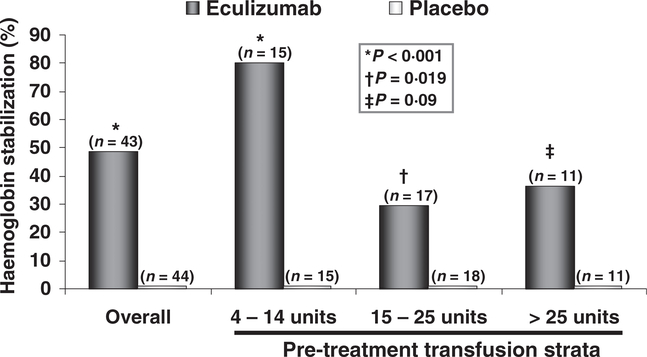
By the end of the 26-week-treatment period, 49% of the patients treated with eculizumab in TRIUMPH maintained haemoglobin levels above the prespecified haemoglobin set point in the absence of transfusions (i.e. stabilized haemoglobin levels). In contrast, none of the patients who received placebo achieved this endpoint. Stabilization of haemoglobin levels was achieved across multiple pretreatment transfusion strata (Fig 3). Eculizumab treatment led to stabilization of haemoglobin levels in 80% of patients in the 4- to 14-unit strata (P < 0 001, Fisher’s exact test), 29% of patients in the 15- to 25-unit strata (P = 0 019) and 36% of patients in the >25 unit strata (P = 0 090).
Fig 3.
Haemoglobin stabilization in patients treated with eculizumab or receiving placebo for 26 weeks by pretreatment transfusion strata. NS, not significant. P-values were calculated using Fisher’s exact test.
Improvement in fatigue
As reported previously (Hillmen et al, 2006), eculizumab was associated with a significant increase (improvement) in FACIT-Fatigue scores from baseline to week 26 of 6 4 ±1 2 points (P < 0 001, mixed-model analysis). In contrast, patients receiving placebo reported a mean decrease (worsening) in FACIT-Fatigue scores from baseline to week 26 of −4–0 ±1 7 points. A significant improvement in fatigue was observed by week 3 in the TRIUMPH study (P = 0 009) and by week 1 in SHEPHERD (P < 0 001). The improvement in fatigue with eculizumab was not limited to patients who achieved transfusion independence; patients in the TRIUMPH study who were transfused during eculizumab treatment also reported a significant improvement in FACIT-Fatigue scores compared with placebo-treated patients (Table IV). Likewise, in SHEPHERD, fatigue was significantly improved regardless of transfusion requirements during the study (Table IV). Fatigue was also improved in patients with minimal or no transfusion requirements prior to starting eculizumab treatment (i.e. patients who underwent 0 or 1 transfusion episodes in the year prior to the trial).
Table IV.
Effect of eculizumab on FACIT-Fatigue scores in patients with paroxysmal nocturnal haemoglobinuria.
| TRIUMPH |
SHEPHERD |
||||
|---|---|---|---|---|---|
| Change from baseline median (mean ± SE) |
Change from baseline median (mean ± SE) |
||||
| Week 26 placebo | Week 26 eculizumab | P-value | Week 52 eculizumab | P-value | |
| Overall | −2·0 (−4·0 ± 1·71) | 4·0 (6·4 ± 1·19) | <0·001 | 10·0 (12·2 ± 1·09) | <0·001 |
| 0 or 1 transfusion episodes during pretreatment year | – | – | – | 11·0 (13·5 ± 2·13) | <0·001 |
| Transfusion independent during study | –* | 3·0 (7·0 ± 1·93) | <0·001 | 8·5 (12·9 ± 1·71) | <0·001 |
| Receiving transfusion during study | −2·0 (−4·0 ± 1·71) | 5·5 (5·7 ± 1·38) | <0·001 | 10·0 (11·5 ± 1·37) | <0·001 |
P-values are based on a signed rank test.
No placebo patients achieved transfusion independence.
Discussion
In patients with PNH, chronic haemolysis leads to anaemia and a dependency on transfusion support to achieve tolerable haemoglobin levels. Few therapeutic options exist for these patients. Transfusions with packed RBCs do not treat haemolysis, and may only transiently improve some anaemia-related symptoms. In addition, corticosteroid and androgen therapy does not provide convincing clinical benefit (Parker et al, 2005). Attempts to increase RBC production via erythropoietin therapy would not be expected to result in a sustainable increase in endogenous RBC mass as the PNH RBCs produced would be continually destroyed by the unopposed activation of terminal complement. A potential therapeutic approach for treating PNH is to protect PNH RBCs from complement- mediated destruction, which would increase the endogenous RBC mass and thereby maintain haemoglobin levels without the typical dependence on transfusion with packed RBCs.
Eculizumab is the only treatment option that targets terminal complement-mediated haemolysis in patients with PNH. In this study, we investigated the effect of eculizumab on parameters of anaemia, including changes in endogenous RBC mass and haemoglobin levels. Analyses were also conducted to determine the benefit of eculizumab in multiple subpopulations with varying pretreatment transfusion requirements.
Reductions in haemolysis with eculizumab led to an elimination in or reduction of transfusion requirements. This was observed in multiple patient subgroups from the most heavily transfusion-dependent to those with minimal transfusion requirements leading up to the study. Although approximately 49% of eculizumab-treated patients still required some transfusions, eculizumab was highly efficacious in these patients as evidenced by significantly lower haemolysis, improved fatigue scores and fewer units of packed RBCs transfused. Patients with greater pretreatment transfusion requirements experienced the greatest absolute reduction in transfusion needs with eculizumab treatment but were also more likely to require some occasional transfusions. The need for transfusion even in the presence of terminal complement blockade with eculizumab may be related to distinct conditions separate from terminal complement activation. Underlying bone marrow failure (i.e. cytopenia) may account for the reduction, but not elimination, of transfusion requirements in some patients receiving eculizumab. In the previous phase II study, a more pronounced reduction in transfusions with eculizumab was observed among patients with PNH who did not have cytopenias as compared with those who did (Hill et al, 2005). Addition of erythropoietin treatment has been shown to facilitate transfusion independence in some eculizumab-treated PNH patients with cytopenia (Hill et al, 2005, 2007). In a cohort of eight patients from TRIUMPH and SHEPHERD who were concomitantly receiving erythropoietin-stimulating agents, eculizumab significantly reduced haemolysis (P = 0 008), reduced transfusion requirements (P = 0 031) and improved fatigue (P = 0 016) (Mojcik et al, 2007). It is also possible that some patients may experience residual extravascular haemolysis of PNH RBCs through opsonization by C3b and subsequent removal via C3 fragment receptors on phagocytes, or by complement-independent mechanisms of the immune system. Deposition of C3b on PNH RBCs has been reported (Hill et al, 2006). In addition, infection may lead to enhanced extravascular clearance of PNH RBCs. Further studies are required to understand whether mechanisms unique to subpopulations of patients with PNH can account for continued minor transfusion requirements during treatment with eculizumab.
Eculizumab reduces transfusion requirements by protecting endogenous PNH RBCs, which leads to a greater sustained endogenous RBC mass. In this analysis, baseline reticulocyte counts were substantially increased, supporting the premise that erythropoiesis is elevated in PNH in an attempt to replenish the loss of PNH RBCs to complement-mediated lysis. Interestingly, reticulocyte counts remained elevated during eculizumab treatment, suggesting that consumption of PNH- RBCs was continuing to some degree, and that a reduction in reticulocytes is not a good indicator for the effectiveness of eculizumab in reducing haemolysis; reduction in levels of LDH provides the best measure of drug efficacy. Eculizumab markedly reduced haemolysis and significantly increased the mean PNH RBC count at 26 weeks and 52 weeks over baseline while no such effect was observed with placebo treatment. In eculizumab-treated patients, haemoglobin levels were maintained or increased with 73% fewer units of packed RBCs transfused during the TRIUMPH trial and 52% fewer units transfused during SHEPHERD, further demonstrating that blocking terminal complement-mediated lysis leads to an increase in the endogenous RBC mass.
Controlling haemolysis with eculizumab resulted in a superior outcome with respect to haemoglobin levels as compared with transfusion support alone. Haemoglobin levels remained steady in the eculizumab-treated patients from baseline to week 26 in the TRIUMPH trial. This endpoint was achieved with eculizumab across multiple pretreatment transfusion strata. The finding that haemoglobin levels did not increase significantly from baseline during eculizumab-treatment within the TRIUMPH study was not unexpected, as the baseline measurement in all patients was within 10 d following the qualifying transfusion. In addition, even though placebo-treated patients received continuous transfusions, haemoglobin levels were significantly higher in patients treated with eculizumab (Fig 3) who, by comparison, received 73% fewer units transfused than placebo patients. During the SHEPHERD trial, there was a significant increase in haemoglobin levels for eculizumab-treated patients; the patients in this study did not receive a qualifying transfusion prior to the initiation of dosing. Interestingly, while haemoglobin levels remained constant during eculizumab treatment in the TRIUMPH study, fatigue scores significantly improved in association with the reduction in haemolysis. A direct relationship between haemolysis and fatigue in these patients was identified using univariate and multivariate analysis (Brodsky et al, 2006). These results underscore the importance of reducing the haemolysis rather than just treating the anaemia in the clinical management of fatigue in patients with PNH. Even in patients who either rarely required, or did not require, transfusion (those receiving 0 or 1 transfusion in the year prior to treatment), reducing haemolysis with eculizumab led to a marked improvement in fatigue. This demonstrates that underlying haemolysis directly contributes to the burden of disease in all patients with PNH, that patients requiring minimal or no transfusions have significant morbidities, and that the presence of haemolysis, with or without requirement for transfusion, is the most appropriate sign indicating treatment benefit. The potential role of haemolysis and subsequent depletion of nitric oxide by cell-free plasma haemoglobin in the morbidities of PNH has been described (Rother et al, 2005; Hillmen et al, 2007). Further, the effect of eculizumab on levels of cell-free plasma haemoglobin and nitric oxide consumption has been assessed and will be the subject of further analyses.
Patients who still required transfusion during eculizumab therapy benefited significantly, with a significant decrease in haemolysis and improvement in fatigue noted for patients in both studies. Furthermore, patients receiving transfusion during eculizumab treatment had a 23–44% reduction in the mean units transfused.
Eculizumab improves anaemia and eliminates or reduces transfusion requirements for patients with PNH regardless of historical transfusion needs. By inhibiting terminal complement activation, eculizumab increases the endogenous RBC pool such that the increase in haemoglobin with eculizumab is superior to treatment with transfusion support alone.
Acknowledgments
Financial disclosures
Financial disclosures with Alexion Pharmaceuticals Inc. are as follows: Grant support; Drs. Hillmen, Socié and Muus. Lecture fees; Drs. Schubert, Hillmen and Socié. Advisory committee; Drs. Schubert, Hillmen, Socié and Muus. Consultancy; Drs. Schubert and Hillmen. Drs Rother and Geller are employees of Alexion Pharmaceuticals, Inc. and have equity ownership in the company. Dr Rother has assigned to Alexion his inventions made as an employee and has received no royalties from the company for these inventions.
Appendix
In addition to the authors, the following investigators participated in the TRIUMPH study: Australia – Princess Alexandra Hospital, Woolloongabba: Anthony Mills, DR; Queen Elizabeth Hospital, Woodville South: John Norman, MD; Royal Melbourne Hospital, Parkville; Belgium – Ucl St. Luc, Brussels: Eric Van Den Neste, MD; Canada – University of Alberta, Cross Cancer Institute, Edmonton, Alberta: Loree Larratt, MD, Andrew Turner, MD, and Marlene A. Hamilton, MD; Germany – Universitatsklinikum Essen, Essen: Ulrich Dührsen, MD; Medizinische Hochschule Hannover, Hannover: Arnold Ganser, MD; Universitatsklinik Greifswald, Greifswald: Michael Montemurro, MD; Institut für Klinische Transfusionsmedizin and Immungenetik, Universitätsklinikum Ulm, Hubert Schrezenmeier; Saarland University Medical School, Homburg/Saar; France – Hospital De L’Hotel Dieu, Paris: Bernard Rio, MD; Hôpital Saint-Louis, Paris; Ireland – St. James Hospital, Dublin; Italy – Ospedale San Martino, Genova: Andrea Bacigalupo, MD; Azienda Ospedaliera Universitaria Careggi, Firenze: Lucio Luzzatto, MD, Elisabetta Antonioli, MD, Francesco Mannelli, MD, and Alberto Bosi, MD; Ospedale San Bortolo, Vicenza: Francesco Rodeghiero, MD; Universita degli Studi di Napoli, Napoli: Bruno Rotoli, MD and Fiorella Alfinito, MD; Ospedale Maggiore di Milano, Milano: Alberto Zanella, MD; The Netherlands – UMC St. Radboud, Nijmegen; Sweden – Lund University Hospital, Lund: Per-Gunnar Nillson, MD; Umea University Hospital, Umea: Anders Wahlin, MD; Stockholm South Hospital, Stockholm: Jan Samuelsson, MD, PhD, Lars Göran Lundberg, MD, and Patrik Andersson, MD; United Kingdom – St. George’s Hospital, London; Leeds General Infirmary, Leeds: Anita Hill, MB, ChB; Belfast City Hospital, Belfast: Mary Frances McMullin, MD; United States – Washington University School of Medicine, St. Louis, MO: Monica Bessler, MD, PhD, Leslie Andritsos, MD, Morey Blinder, MD, and Steven Devine, MD; The Royal Perth Hospital, Perth WA: Richard Herrmann, MD; Johns Hopkins University Medical Center, Baltimore, MD; Memorial Sloan Kettering Cancer Center, New York, NY: Hugo Castro- Malaspina, MD and David Araten, MD; Stanford University Medical Center, Stanford, CA: Steven Coutre, MD; Duke University Medical Center, Durham, NC: Carlos de Castro III, MD; Cleveland Clinic Florida, Weston, FL: Elizabeth Stone, MD; University of Pennsylvania, Philadelphia, PA: Barbara Konkle, MD; Massachusetts General Hospital, Boston, MA: David Kuter, MD; The Cleveland Clinic Foundation, Cleveland, OH: Alan Lichtin, MD; NYU Clinical Cancer Center, New York, NY: Tibor Moskovits, MD, Bruce Gordon Raphael, MD, Edward Amorosi, MD, Kenneth B. Hymes, MD, and Perry Cook, MD; City of Hope National Medical Center, Duarte, CA; Indiana University Cancer Center, Indianapolis, IN: Robert Nelson, MD; University of California at Los Angeles, Los Angeles, CA: Ronald Paquette, MD; Hartford Hospital, Hartford, CT: Robert Siegel, MD; NIH, National Heart, Blood, and Lung Institute, Bethesda, MD: Bipin Savani, MD. The Authors would like to acknowledge Michael Bombara and Henk-Andre Kroon, MD, MBA for trial oversight, Jason Chan, PhD for statistical analysis and Kerry Quinn-Senger, PhD for technical writing assistance.
Footnotes
In addition to the authors, the following investigators participated in the TRIUMPH study, the list of who is provided in the Appendix.
References
- Alfinito F, Del VL, Rocco S, Boccuni P, Musto P & Rotoli B (1996) Blood cell flow cytometry in paroxysmal nocturnal hemoglobinuria: a tool for measuring the extent of the PNH clone. Leukemia, 10, 1326–1330. [PubMed] [Google Scholar]
- Bessler M, Mason PJ, Hillmen P, Miyata T, Yamada N, Takeda J, Luzzatto L & Kinoshita T (1994) Paroxysmal nocturnal haemoglobinuria (PNH) is caused by somatic mutations in the PIG-A gene. EMBO Journal, 13, 110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky RA, Muus P, Duhrsen U, Hill A, Bessler M, Coutre S, De Paz R, Moskovits T, Nakamura R, Van den Neste E, Zanella A, Quinn-Senger K, Kroon HA & Cella D (2006) Effect of the terminal complement inhibitor eculizumab on patient reported outcomes in paroxysmal nocturnal hemoglobinuria (PNH): Phase III TRIUMPH study results. Blood, 108, 16b–17b (Abstract 3770). [Google Scholar]
- Brodsky RA, Young NS, Antonioli E, Risitano AM, Schrezenmeier H, Schubert J, Gaya A, Coyle L, de Castro D, Fu CL, Maciejewski JP, Bessler M, Kroon HA, Rother RP & Hillmen P (2008) Multicenter phase 3 study of the complement inhibitor eculizumab for the treatment of patients with paroxysmal nocturnal hemoglobinuria. Blood, 111, 1840–1847. [DOI] [PubMed] [Google Scholar]
- Caocci G, Baccoli R, Ledda A, Littera R & La NG (2007) A mathematical model for the evaluation of amplitude of hemoglobin fluctuations in elderly anemic patients affected by myelodysplastic syndromes: correlation with quality of life and fatigue. Leukemia Research, 31, 249–252. [DOI] [PubMed] [Google Scholar]
- Cella D, Lai JS, Chang CH, Peterman A & Slavin M (2002) Fatigue in cancer patients compared with fatigue in the general United States population. Cancer, 94, 528–538. [DOI] [PubMed] [Google Scholar]
- Hall SE & Rosse WF (1996) The use of monoclonal antibodies and flow cytometry in the diagnosis of paroxysmal nocturnal hemoglobinuria. Blood, 87, 5332–5340. [PubMed] [Google Scholar]
- Hill A, Hillmen P, Richards SJ, Elebute D, Marsh JC, Chan J, Mojcik CF & Rother RP (2005) Sustained response and longterm safety of eculizumab in paroxysmal nocturnal hemoglobinuria. Blood, 106, 2559–2565. [DOI] [PubMed] [Google Scholar]
- Hill A, Rother RP, Risitano AM, Cole DS, Cullen MJ, Richards SJ, Selleri C, Ricci P, Rotoli B, Luzzatto L & Hillmen P (2006) Blockade of intravascular hemolysis in PNH with the terminal commplement inhibitor eculizumab unmasks low-level hemolysis potentially occurring through C3 opsonization. Blood, 108, 290a (Abstract 972). [Google Scholar]
- Hill A, Richards SJ, Rother RP & Hillmen P (2007) Erythopoietin treatment during complement inhibition with eculizumab in a patient with paroxysmal nocturnal hemoglobinuria. Haematologica, 92, ECR14. [DOI] [PubMed] [Google Scholar]
- Hillmen P, Young NS, Schubert J, Brodsky RA, Socie G, Muus P, Roth A, Szer J, Elebute MO, Nakamura R, Browne P, Risitano AM, Hill A, Schrezenmeier H, Fu CL, Maciejewski J, Rollins SA, Mojcik CF, Rother RP & Luzzatto L (2006) The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. New England Journal of Medicine, 355, 1233–1243. [DOI] [PubMed] [Google Scholar]
- Hillmen P, Muus P, Duhrsen U, Risitano AM, Schubert J, Luzzatto L, Schrezenmeier H, Szer J, Brodsky RA, Hill A, Socie G, Bessler M, Rollins SA, Bell L, Rother RP & Young NS (2007) Effect of the complement inhibitor eculizumab on thromboembolism in patients with paroxysmal nocturnal hemoglobinuria. Blood, 110, 4123–4128. [DOI] [PubMed] [Google Scholar]
- Mojcik CF, Young NS, Luzzatto L, Socie G, Hillmen P & Brodsky RA (2007) Safety and efficacy of eculizumab with concomitant erythropoietin therapy in patients with paroxysmal nocturnal hemglobinuria (PNH). Journal of Clinical Oncology, 25, 125s (Abstract 3032). [Google Scholar]
- Motoyama N, Okada N, Yamashina M & Okada H (1992) Paroxysmal nocturnal hemoglobinuria due to hereditary nucleotide deletion in the HRF20 (CD59) gene. European Journal of Immunology, 22, 2669–2673. [DOI] [PubMed] [Google Scholar]
- Navenot JM, Bernard D, Harousseau JL, Muller JY & Blanchard D (1996) Expression of glycosyl-phosphatidylinositol-linked glycoproteins in blood cells from paroxysmal nocturnal haemoglobinuria patients: a flow cytometry study using CD55, CD58 and CD59 monoclonal antibodies. Leukaemia & Lymphoma, 21, 143–151. [DOI] [PubMed] [Google Scholar]
- Nilsson B, Hagstrom U, Englund A & Safwenberg J (1993) A simplified assay for the specific diagnosis of paroxysmal nocturnal hemoglobinuria: detection of DAF(CD55)- and HRF20(CD59)- erythrocytes in microtyping cards. Vox Sanguinis, 64, 43–46. [DOI] [PubMed] [Google Scholar]
- Parker C, Omine M, Richards S, Nishimura J, Bessler M, Ware R, Hillmen P, Luzzatto L, Young N, Kinoshita T, Rosse W & Socie G (2005) Diagnosis and management of paroxysmal nocturnal hemoglobinuria. Blood, 106, 3699–3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards S & Hillmen P (2001) Advances in the laboratory diagnosis of paroxysmal nocturnal hemoglobinuria. Clinical Applied Immunological Reviews, 1, 315–330. [Google Scholar]
- Rosse W (2000) Paroxysmal nocturnal hemoglobinuria In: Hematology: Basic Principles and Practice (ed. by Hoffman R), pp. 331–342. Churchill Livingstone Inc., New York, NY. [Google Scholar]
- Rosse WF & Nishimura J (2003) Clinical manifestations of paroxysmal nocturnal hemoglobinuria: present state and future problems. International Journal of Hematology, 77, 113–120. [DOI] [PubMed] [Google Scholar]
- Rother RP, Bell L, Hillmen P & Gladwin MT (2005) The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. The Journal of the American Medical Association, 293, 1653–1662. [DOI] [PubMed] [Google Scholar]
- Takeda J, Miyata T, Kawagoe K, Iida Y, Endo Y, Fujita T, Takahashi M, Kitani T & Kinoshita T (1993) Deficiency of the GPI anchor caused by a somatic mutation of the PIG-A gene in paroxysmal nocturnal hemoglobinuria. Cell, 73, 703–711. [DOI] [PubMed] [Google Scholar]
- Thomas TC, Rollins SA, Rother RP, Giannoni MA, Hartman SL, Elliott EA, Nye SH, Matis LA, Squinto SP & Evans MJ (1996) Inhibition of complement activity by humanized anti-C5 antibody and single-chain Fv. Molecular Immunology, 33, 1389–1401. [DOI] [PubMed] [Google Scholar]
- Yamashina M, Ueda E, Kinoshita T, Takami T, Ojima A, Ono H, Tanaka H, Kondo N, Orii T & Okada N (1990) Inherited complete deficiency of 20-kilodalton homologous restriction factor (CD59) as a cause of paroxysmal nocturnal hemoglobinuria. New England Journal of Medicine, 323, 1184–1189. [DOI] [PubMed] [Google Scholar]