Abstract
Background
The Sarcopenia Definitions and Outcomes Consortium (SDOC) is a collaborative initiative seeking to develop and evaluate cut-points for low muscle strength and lean mass that predict an increased risk for slowness (usual walking speed <0.8 m/s) among older adults.
Objectives
The goal of the present study was to provide clinicians and researchers with an understanding of the diagnostic implications of employing SDOC variables and cut-points in mobility-limited older adults. Using data from older individuals with specific conditions that render them at increased risk for mobility limitation, we evaluated the performance characteristics (i.e., sensitivity and specificity) of five putative sarcopenia parameters and then compared these values to previously recommended diagnostic criteria for sarcopenia.
Design
Retrospective analysis of six randomized controlled trials enriched in persons at risk for mobility limitation.
Setting
National and international geriatric clinical research centers.
Participants
925 mobility-limited older adults (≥55 y, 58% women) were included in the analysis.
Measurements
The prevalence of low muscle strength and lean mass were assessed using five candidate metrics discriminative of slowness. Analyses of sensitivity and specificity were used to compare muscle weakness criteria to published diagnostics for sarcopenia.
Results
Odds ratios (OR) supported maximal grip strength (Grip max <35.5 and 20.0 in men and women, respectively) as the most discriminative of slowness in both men and women (OR = 3.66 and 3.53, respectively). More men (58%) than women (30%) fell below sex-specific maximal grip cut-points. When applying previously recommended sarcopenia component definitions in our population, we found that fewer individuals met those criteria (range: 6–32%).
Conclusion
A greater number of individuals fall below SDOC Grip max cut-points compared to previous recommendations. Clinicians and researchers working with older adults may consider these thresholds as an inclusive means to identify candidates for low-risk lifestyle, promyogenic and function promoting therapies.
Keywords: aging, muscle, sarcopenia, physical function
INTRODUCTION
Advancing age is accompanied by a progressive loss of skeletal muscle mass and strength that is strongly and independently associated with functional limitation and physical disability.1 Mobility difficulties in older persons contribute to a loss of independence, a fate feared more than death.2 In 2000, an estimated $120 billion were spent on long-term care costs in older persons, a figure that is projected to reach $270 billion by 2030.3 Determination of clinically relevant cut-points for low muscle strength and/or lean mass that predispose older individuals to walk slowly, a key indicator of waning mobility status and increased mortality risk,4, 5 may help to identify candidates for lifestyle, promyogenic and functional promoting therapies in an effort to alleviate mobility limitation and improve quality of life in a rapidly expanding aging demographic.
In 1988, Irwin Rosenberg first highlighted the significance of age-related deficits in skeletal muscle size and strength (i.e., sarcopenia) for the development of physical disability.6 Subsequent investigations by Baumgartner,7 Melton,8 and Newman9 sought to provide an operational definition for this condition based on the statistical distribution of skeletal muscle mass. In an attempt to better predict incident mobility disability, the ensuing decade witnessed the inception of numerous alternative criteria for sarcopenia, most of which were based on expert opinion.10–12 Pooling data from over 25,000 community-dwelling older adults, the first evidence-based cut-points for low muscle strength and lean mass were provided in 2014 by the Foundation for the National Institutes of Health (FNIH) Sarcopenia Project.13–17 However, the study population included in the FNIH analysis consisted of healthy older adults mostly without mobility limitation; therefore, these thresholds may be inadequate for older individuals with pre-existing mobility limitations,18 who are at the greatest risk for incident disability.19
Before sarcopenia can be effectively treated in clinical practice, a unified consensus on how to screen for and diagnose this condition must be established. The Sarcopenia Definitions and Outcomes Consortium (SDOC) aims to develop and assess clinically relevant cut-points for low muscle strength and lean mass in older adults.20, 21 Preceding analyses22 for the outcome of walking speed < 0.8 m/s (i.e., slowness) empirically identified five indices of impaired muscle strength, either alone or scaled to body size or composition, most predictive of mobility limitation (Table 1) in a number of large-scale epidemiologic study cohorts of community-dwelling older adults. To provide clinicians and scientists with an understanding of the diagnostic implications of employing these thresholds, the aim of the present study was to assess the sensitivity and specificity of these candidate metrics in clinical populations with specific conditions (e.g., self-reported mobility limitation, heart failure, osteoarthritis, etc.) that place them at increased risk for mobility disability, a unique and challenging group of individuals to recruit. To gain insight into the performance characteristics (i.e., sensitivity and specificity) of these criteria, we then compared these novel cut-points to those previously proposed by the FNIH Sarcopenia Project13–17 and the European Working Group on Sarcopenia.11 Read and interpreted as a whole, the proceedings of the SDOC20–25 should aid in the development of a consensus definition for sarcopenia acknowledging muscle strength and power, and to identify older individuals most likely to benefit from lifestyle, promyogenic and function-promoting therapies.
Table 1.
| Sarcopenia Variable | Men | Women |
|---|---|---|
| Grip maximal value | <35.5 kg | <20.0 kg |
| Grip/BMI* | <1.05 kg/kg/m2 | <0.79 kg/kg/m2 |
| Grip/TBF | <1.66 kg/kg | <0.65 kg/kg |
| Grip/arm lean mass | <6.08 kg/kg | <3.26 kg/kg |
| Grip/weight | <0.45 kg/kg | <0.34 kg/kg |
Only variable consistently chosen for men and women.
BMI = body mass index; Grip = grip strength; TBF = total body fat.
METHODS
Participants
We examined baseline data among participants in six randomized controlled trials of community-dwelling older adults enriched with individuals at increased risk for mobility disability. These randomized controlled trials of 6 to 18 months intervention duration were conducted to determine the efficacy of various function promoting therapies in mobility-limited older adults (described below). To be included in the present analyses, the participating studies had to have data on the following measures: 1) Body composition measures by dual x-ray absorptiometry (DXA); 2) Muscle strength by handgrip dynamometry; 3) Usual walking speed determined on a 4 meter course; 4) Self-reported physical limitations and functional status. Written informed consent was obtained from all participants and each study was approved by the institutional review board at the participating institutions. The present study was reviewed and approved by the Tufts University Health Sciences Campus Institutional Review board. Baseline measures from the following cohort studies were included in the analysis.
Lifestyle Interventions and Independence for Elders Pilot study (LIFE-P)
From April 2004 to February 2005, a total of 424 sedentary persons 70–89 years at risk for mobility disability (Short Physical Performance Battery (SPPB) ≤ 9) were recruited at four U.S. clinical centers (Winston-Salem, NC; Pittsburgh, PA; Dallas, TX; Palo Alto, CA). Participants were randomized to either a moderate-intensity physical activity or a successful aging educational intervention for an average of 1.2 years.26, 27 The objective of this multi-center pilot study was to provide preliminary data for a future full-scale trial to determine the efficacy of a long-term physical activity program to combat major mobility disability (defined as the inability to walk 400 meters in 15 minutes) or death. Additional outcomes of interest were lower extremity physical performance and walking speed measured over 4 and 400m. Data on body composition and/or physical performance were available in 269 participants (75 men and 194 women).
Vitality Independence and Vigor Study 2 (VIVE2)
This 6-month trial was a multi-center study that took place in two clinical centers (Tufts University, Boston, MA and Karolinska Institutet, Stockholm, Sweden). Between March 2012 and November 2014, a total of 150 (46.3% women) mobility-limited (SPPB ≤ 9) older adults (≥ 70 years) participated in a moderate-intensity resistance exercise program and were randomized to receive either a high-protein, high-vitamin D nutritional supplement or a placebo.28, 29 The primary research outcome was 400 meters walking speed and secondary measures included other changes in physical function (grip strength and stair climb) as well as muscle size, composition, and function. Data on body composition and/or physical performance were available in 149 participants (80 men and 69 women).
Testosterone in Older Men with Mobility Limitations Trial (TOM Trial)
This parallel-group, randomized control trial (April 2011 to June 2014) conducted in Boston, MA recruited older men (≥ 65 years) who had low total (serum 100–350 ng/dl) or free (< 50 pg/ml) testosterone levels and limitations in mobility (SPPB 4–9 and difficulty walking two blocks on a level surface or climbing 10 steps).30 Participants were stratified by age and randomized to receive either 10g of transdermal gel containing either placebo or 100mg of testosterone. Change in maximal voluntary muscle strength measured on the leg press was the primary outcome measure. Secondary efficacy measures included chest press strength, 50m walking speed, and stair climb to assess changes in physical function. The TOM trial was prematurely terminated due to a greater incidence of cardiovascular events in the testosterone (n = 23) compared to placebo (n = 5) group.30 Data on body composition and/or physical performance were available in 209 participants.
Study of the Effects of Calorie Restriction and Exercise Training (SECRET)
From February 2009 to November 2014, 100 older (≥ 60 y) obese (BMI ≥ 30) individuals residing in Winston-Salem, NC with diastolic heart failure with a preserved ejection fraction were enrolled in the study. Subjects were randomized to one of four experimental arms: exercise, diet (caloric restriction), exercise plus diet, or attention control for 5 months. Dual primary outcome measures included gas exchange monitored maximal exercise testing and disease specific quality of life assessed using the Minnesota Living with Heart Failure Questionnaire (MLHFQ).31 Relevant exploratory outcomes included 6-minute walk distance, body composition, and leg muscle power and quality. Data on body composition and/or physical performance were available in 97 participants (18 men and 79 women).
Pharmacologic Interventions in the Elderly Study (PIE 2)
From April 2005 to June 2009, a total of 80 (80% women) older individuals (≥ 60 y) with diastolic heart failure with a preserved ejection fraction living in Winston-Salem, NC were recruited and randomized into a 9-month treatment of spironolactone 25 mg/d or placebo.32 Primary outcome measures of cardiorespiratory capacity (VO2peak) and MLHFQ-determined quality of life were obtained at 4 and 9-months and supplemented by cardiac imaging procedures and a 6-minute walk test. Data on body composition and/or physical performance were available in 78 participants (18 men and 60 women).
Intensive Diet and Exercise for Arthritis study (IDEA)
Taking place between July 2006 and April 2011, this single center (Winston-Salem, NC) trial randomized 454 community-dwelling older (≥ 55 y) overweight or obese (BMI 27–41) men and women with mild-to-moderate radiographic knee osteoarthritis (Kellegren-Lawrence score of 2–3) to one of three experimental groups: intensive diet-induced weight loss plus exercise, intensive diet-induced weight loss, or exercise alone.33 A total of 399 participants (88%) completed all 18-months of the study. Primary outcome measures were inflammatory biomarkers and knee-joint loads, and half of the participants were assigned to supplemental testing which included MRI, strength tests, and x-rays of the lower extremities. Baseline data on body composition and/or physical performance were available in all participants (N = 454; 129 men and 325 women)
Measures
Body Composition
Total body fat mass and total bone-free lean mass were acquired using whole-body dual-energy x-ray absorptiometry (DXA) scans on Hologic 4500 machines (Waltham, MA) in LIFE-P, VIVE2, TOM, and IDEA, and on Lunar Prodigy machines (Madison, WI) in SECRET and PIE2 using standardized protocols.34, 35 Appendicular lean mass (ALM) was the sum of lean mass from both arms and legs.
Grip Strength
Grip strength of the dominant hand was measured using a JAMAR handheld dynamometer (Sammons Preston Rolyan, Bolingbrook, IL)36 following a standardized protocol. The test was performed in the seated position, with the shoulder adducted and neutrally rotated, the elbow flexed to 90 degrees, and the forearm and wrist in a neutral position. Participants were instructed to perform two maximal isometric contractions separated by 10 seconds of rest, and the highest value achieved was used for analysis.
Walking Speed
Consistent with the FNIH Sarcopenia Project,17 we chose a usual walking speed of less than 0.8 m/s to identify slowness as our primary outcome measure consistent with previous findings4, 5, 37, 38 and expert opinion. All walking speed measures were determined on a 4-meter course and defined as the length of the walking course divided by the time required for the participants to complete the course at their usual walking speed. All studies used instructions to walk at a usual pace and from a standing start, and the use of a cane or other walking aid was permitted if the subject felt it would be unsafe to perform the test without.39 After an initial practice trial, each subject completed the test twice and the fastest time was recorded.
Statistical Analysis
Statistical analyses were performed using R versions 3.5.1 and later (2018). Analyses were stratified by sex and study. In analyses discussed in companion work presented in this issue, classification and regression trees were used to obtain strength and muscle mass cut-points most predictive of slowness (normal walking speed less than 0.8 m/s).22 The study described in this manuscript applied these thresholds in baseline data from the trials cohorts to operationally define muscle weakness either in terms of maximal grip strength or scaled to various measures of body size and composition (Table 1). We then determined the prevalence of these conditions, as well as their sensitivity and specificity at detecting slow walking speed. Associations between the continua of mass and strength and usual walking speed were summarized using receiver operator curves (ROC) and associated concordance (c-) statistics, equivalent to the area under the ROC curve. Covariate adjustment was facilitated by logistic regression, with associations quantified using estimated odds ratios and associated 95% confidence intervals. The performance characteristics of newly derived cut-points were then compared to those previously proposed by the FNIH17 and European Working Group on Sarcopenia11 by inspection.
RESULTS
Of the 1256 participants considered for inclusion, missing grip strength (n = 298) and DXA (n = 68) measures were the primary reason for exclusion, as walking speed and BMI records were complete in all but a small number of participants (n = 4 and 2, respectively). A total of 925 participants (53% women) met all inclusion criteria (Table 2). The mean (standard deviation; SD) age for men and women was 74 (6) and 72 (8) years, respectively. Participant race was similar among trials, with nonwhites comprising ~20–30% of individuals in each cohort. Twenty-one versus 15 percent of men and women had diabetes, respectively, and the prevalence of COPD (~7%) and cancer (~14%) was similar between sexes. Almost 90% of participating men and women were classified as being overweight (> 25 BMI ˂ 30) or obese (BMI ≥ 30), and on average, men weighed more than women. Similarly, appendicular lean mass (31%) and hand grip strength (39%) were greater in men than women. Usual walking speed was remarkably similar (~0.94 m/s) between older men and women, and almost a third of men and women exhibited a usual walking speed less than 0.8 m/s. The specific recruiting efforts employed by each study resulted in some noteworthy inter-study participant characteristic differences as presented in Table 2. In both genders, participants in LIFE-P and VIVE2 cohorts were ~5–10y older, participants in SECRET had ~20% higher body mass index (BMI), and usual walking speed was ~0.3 m/s faster in participants from the IDEA cohort.
Table 2.
Baseline Participant Characteristics by Study.
|
LIFE-P N = 72M/187W |
VIVE2 N = 80M/64W |
TOM N = 194M/0W |
SECRET N = 16M/71W |
PIE2 N = 17M/40W |
IDEA N = 54M/130W |
Total N = 433M/492W |
|
|---|---|---|---|---|---|---|---|
| Men | |||||||
| Age, y | 76 ± 4 | 78 ± 6 | 74 ± 6 | 68 ± 4 | 70 ± 7 | 67 ± 6 | 74 ± 6 |
| Weight, kg | 91 ± 16 | 85 ± 12 | 88 ± 14 | 108 ± 9 | 98 ± 20 | 106 ± 12 | 91 ± 15 |
| Height, m | 1.74 ± 0.08 | 1.75 ± 0.07 | 1.73 ± 0.07 | 1.73 ± 0.06 | 1.73 ± 0.06 | 1.77 ± 0.07 | 1.74 ± 0.07 |
| BMI, kg/m2 | 30 ± 4.7 | 28 ± 3.5 | 30 ± 4.4 | 36 ± 3.4 | 32.9 ± 5.1 | 33.8 ± 3.3 | 30.3 ± 4.6 |
| Gait speed, m/s | 0.83 ± 0.16 | 0.79 ± 0.14 | 0.95 ± 0.20 | 1.06 ± 0.18 | 1.06 ± 0.15 | 1.29 ± 0.18 | 0.95 ± 0.24 |
| <0.8 m/s, % | 34 / 72 (47) | 43 / 80 (54) | 37 / 194 (19) | 2 / 16 (12) | 0 / 17 (0) | 0 / 54 (0) | 116 / 433 (27) |
| Grip strength, kg | 34.3 ± 8.3 | 32.4 ± 7.6 | 31.0 ± 7.2 | 37.7 ± 7.9 | 44.3 ± 8.8 | 41.8 ± 6.4 | 33.9 ± 8.5 |
| Grip/BMI | 1.17 ± 0.32 | 1.17 ± 0.31 | 1.06 ± 0.28 | 1.06 ± 0.26 | 1.37 ± 0.28 | 1.25 ± 0.23 | 1.14 ± 0.29 |
| Grip/TBF | 1.36 ± 0.58 | 1.31 ± 0.50 | 1.23 ± 0.50 | 1.03 ± 0.34 | 1.45 ± 0.50 | 1.33 ± 0.43 | 1.28 ± 0.51 |
| Grip/arm lean mass | 4.79 ± 1.02 | 5.07 ± 1.07 | 4.85 ± 1.05 | 4.74 ± 1.09 | 5.88 ± 1.1 | 4.74 ± 0.86 | 4.90 ± 1.05 |
| Grip/weight | 0.38 ± 0.1 | 0.38 ± 0.09 | 0.36 ± 0.09 | 0.35 ± 0.08 | 0.46 ± 0.1 | 0.40 ± 0.07 | 0.38 ± 0.09 |
| ALM, kg | 25.9 ± 4.0 | 23.8 ± 3.3 | 24.1 ± 3.3 | 28.9 ± 3.5 | 27.6 ± 5.3 | 31.9 ± 3.5 | 25.6 ± 4.4 |
| Women | |||||||
| Age, y | 77 ± 4 | 77 ± 5 | - | 66 ± 6 | 71 ± 7 | 65 ± 6 | 72 ± 8 |
| Weight, kg | 76 ± 16 | 74 ± 11 | - | 100 ± 14 | 80 ± 16 | 88 ± 11 | 83 ± 17 |
| Height, m | 1.6 ± 0.07 | 1.62 ± 0.07 | - | 1.61 ± 0.06 | 1.62 ± 0.05 | 1.62 ± 0.06 | 1.61 ± 0.07 |
| BMI, kg/m2 | 29.7 ± 6.4 | 28.5 ± 3.9 | - | 38.7 ± 5.3 | 30.3 ± 6.2 | 33.5 ± 3.6 | 31.9 ± 6.2 |
| Gait speed, m/s | 0.78 ± 0.15 | 0.75 ± 0.15 | - | 0.99 ± 0.16 | 0.99 ± 0.23 | 1.18 ± 0.18 | 0.93 ± 0.24 |
| <0.8 m/s, % | 110 / 187 (59) | 42 / 64 (66) | - | 10 / 71 (14) | 4 / 40 (10) | 4 / 130 (3) | 170 / 492 (35) |
| Grip strength, kg | 20.4 ± 5.8 | 19.7 ± 4.6 | - | 25.8 ± 6.9 | 26.2 ± 7.4 | 24.4 ± 5.1 | 22.6 ± 6.3 |
| Grip/BMI | 0.71 ± 0.24 | 0.70 ± 0.20 | - | 0.68 ± 0.20 | 0.88 ± 0.26 | 0.74 ± 0.18 | 0.73 ± 0.22 |
| Grip/TBF | 0.71 ± 0.33 | 0.66 ± 0.23 | - | 0.56 ± 0.19 | 0.86 ± 0.40 | 0.67 ± 0.20 | 0.68 ± 0.29 |
| Grip/arm lean mass | 4.92 ± 1.39 | 4.87 ± 1.23 | - | 5.03 ± 1.26 | 5.59 ± 1.34 | 4.85 ± 0.92 | 4.97 ± 1.25 |
| Grip/weight | 0.28 ± 0.09 | 0.27 ± 0.07 | 0.26 ± 0.08 | 0.34 ± 0.09 | 0.28 ± 0.06 | 0.28 ± 0.08 | |
| ALM, kg | 17.7 ± 3.6 | 17.1 ± 2.4 | - | 21.2 ± 3.3 | 19.4 ± 3.6 | 20.7 ± 3.0 | 19.1 ± 3.6 |
M = men; W = women; ALM = appendicular lean mass; BMI = body mass index; IDEA = Intensive Diet and Exercise for Arthritis Study; LIFE-P = Lifestyle Interventions for the Elders Pilot Study; PIE2 = Pharmacologic Interventions in the Elders Study; SECRET = Study of the Effects of Calorie Restriction and Exercise Training; TOM = Testosterone in Older Men with Mobility Limitations Trial; VIVE2 = Vitality Independence and Vigor Study 2.
The prevalence of low muscle strength and lean mass, by sex and by study, as determined by our five candidate metrics is shown in Table 3. Prevalence estimates using SDOC cut-points exhibited a wide range in both men (39–87%) and women (8–79%). In pooled analyses, the number who fell below the threshold was the greatest using grip strength scaled to body weight (~80%). While prevalence estimates using grip strength normalized to body weight were remarkably similar between men and women, grip strength normalized to arm lean mass was highly discrepant (87% in men vs. 8% in women).
Table 3.
Prevalence of Sarcopenia in Study Cohorts Using SDOC or Other Criteria, %.
| Definitions | LIFE-P | VIVE2 | TOM | SECRET | PIE2 | IDEA | Total |
|---|---|---|---|---|---|---|---|
| Men | |||||||
| SDOC | |||||||
| Grip maximal value | 54 | 68 | 74 | 25 | 6 | 15 | 58 |
| Grip/BMI | 39 | 41 | 48 | 31 | 12 | 19 | 39 |
| Grip/TBF | 81 | 82 | 87 | 100 | 71 | 83 | 84 |
| Grip/arm lean mass | 92 | 82 | 89 | 100 | 53 | 91 | 87 |
| Grip/weight | 79 | 78 | 86 | 94 | 53 | 81 | 82 |
| FNIH | |||||||
| Grip Strength | 14 | 14 | 19 | 6 | 0 | 0 | 13 |
| Grip/BMI | 36 | 26 | 40 | 31 | 12 | 11 | 32 |
| EWGSOP | 8 | 20 | 10 | 0 | 0 | 0 | 10 |
| Women | |||||||
| SDOC | |||||||
| Grip maximal value | 41 | 44 | - | 14 | 18 | 19 | 30 |
| Grip/BMI | 68 | 75 | - | 75 | 40 | 64 | 67 |
| Grip/TBF | 50 | 59 | - | 73 | 28 | 52 | 53 |
| Grip/arm lean mass | 11 | 6 | - | 6 | 5 | 5 | 8 |
| Grip/weight | 78 | 83 | - | 83 | 55 | 83 | 79 |
| FNIH | |||||||
| Grip Strength | 19 | 12 | - | 3 | 5 | 4 | 11 |
| Grip/BMI | 24 | 27 | - | 24 | 12 | 15 | 21 |
| EWGSOP | 11 | 11 | - | 0 | 5 | 0 | 6 |
FNIH Grip strength <26 and <16 kg in men and women, respectively; Grip/BMI <1.0 and <0.56 in men and women, respectively; EWGSOP based on low lean mass (ALM/ht2 <7.23 and <5.67 kg/m2 in men and women, respectively) plus low strength (grip strength <30 and <20 kg in men and women, respectively) and/or performance (gait speed <0.8 m/s).
IDEA = Intensive Diet and Exercise for Arthritis Study; LIFE-P = Lifestyle Interventions for the Elders Pilot Study; PIE2 = Pharmacologic Interventions in the Elders Study; SECRET = Study of the Effects of Calorie Restriction and Exercise Training; TOM = Testosterone in Older Men with Mobility Limitations Trial; VIVE2 = Vitality Independence and Vigor Study 2.
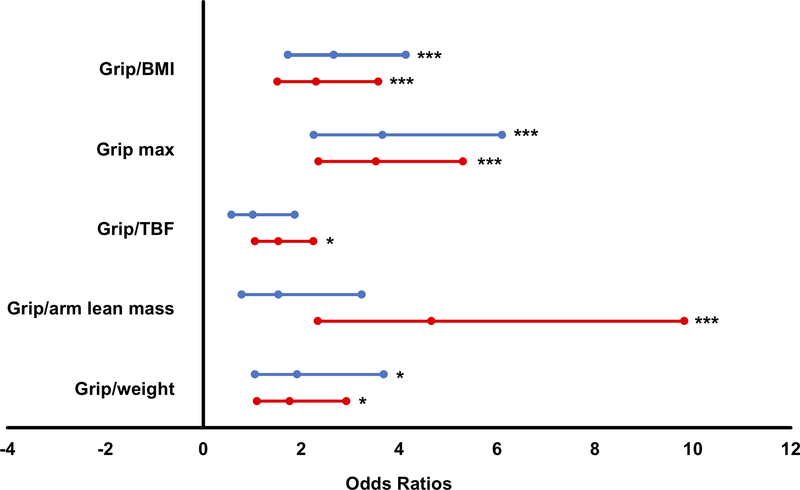
Of the candidate metrics, maximal grip strength and Grip/BMI were most strongly associated with low walking speed (C-statistics 0.65–0.74) in men and women. Odds ratios suggested maximal grip strength (Grip max) to be the most discriminative of slowness in our study cohorts (3.66 [95% CI 2.26, 6.11] and 3.53 [95% CI 2.36, 5.31] for men and women, respectively; Figure 1). Strong associations between Grip/BMI and slowness were also observed (2.67 [95% CI 1.73, 414] and 2.31 [95% CI 1.52, 3.58] for men and women, respectively). Application of Grip max cut-points (<35.5 and <20.0 kg in men and women, respectively) in mobility-limited older adults in the present study (Table 3) resulted in a large percentage (~45%) of participants falling below the threshold, and the number was greater in men (58%) than women (30%). In both men and women alike, fewer participants in SECRET and PIE2 cohorts (6–25%) fell below SDOC Grip max thresholds. Meanwhile, application of FNIH Grip max cut-points (<26.0 and <16 kg in men and women, respectively) detected a far lower prevalence (13% and 11% in men and women, respectively) of muscle weakness that was lower yet using EWGSOP criteria (10% in men and 6% in women).
Figure 1.
Estimated odds ratios (95% confidence intervals) of sarcopenia candidate metrics with low walking speed (< 0.8 m/s) in men (blue lines) and women (red lines). *P<0.05; **P<0.01; ***P<0.001. BMI = body mass index; TBF = total body fat.
To assess the performance characteristics of Grip max and Grip/BMI thresholds established by the SDOC, we compared prevalence and analyses of sensitivity and specificity to previously recommended definitions (Table 4). The prevalence varied widely by definition in both men (10–58%) and women (6–67%) across studies, but the average of these estimates was similar between sexes (~25%). SDOC Grip max cut-points yielded sensitivity and specificity values of 78 and 50% in men, and 48 and 80% in women (Table 4), whereas FNIH Grip max cut-points were comparably less sensitive (~25%) but more specific (>90%). SDOC Grip max thresholds were notably less sensitive in both men (50%) and women (30%) from the SECRET cohort.
Table 4.
Sensitivity/Specificity of the SDOC Criteria and Those of Other Published Criteria in Study Cohorts, %.
| LIFE-P | VIVE2 | TOM | SECRET | PIE2 | IDEA | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sarcopenia Criteria | Sens. | Spec. | Sens. | Spec. | Sens. | Spec. | Sens. | Spec. | Sens. | Spec. | Sens. | Spec. | Sens. | Spec. |
| Men | ||||||||||||||
| SDOC | ||||||||||||||
| Grip Strength | 71 | 61 | 72 | 38 | 95 | 31 | 50 | 79 | - | 94 | - | 85 | 78 | 50 |
| Grip Strength/BMI | 56 | 76 | 42 | 59 | 73 | 58 | 100 | 79 | - | 88 | - | 81 | 57 | 67 |
| FNIH | ||||||||||||||
| Grip Strength | 24 | 95 | 14 | 86 | 38 | 86 | 50 | 100 | - | 100 | - | 100 | 25 | 91 |
| Grip Strength/BMI | 53 | 79 | 26 | 73 | 73 | 68 | 100 | 79 | - | 88 | - | 89 | 50 | 75 |
| EWGSOP | 18 | 100 | 26 | 86 | 24 | 93 | 0 | 100 | - | 100 | - | 100 | 22 | 95 |
| Women | ||||||||||||||
| SDOC | ||||||||||||||
| Grip Strength | 46 | 66 | 50 | 68 | - | - | 30 | 89 | 75 | 89 | 75 | 83 | 48 | 80 |
| Grip Strength/BMI | 73 | 38 | 86 | 45 | - | - | 90 | 28 | 100 | 67 | 100 | 37 | 78 | 39 |
| FNIH | ||||||||||||||
| Grip Strength | 28 | 94 | 14 | 91 | - | - | 0 | 97 | 25 | 97 | 25 | 97 | 23 | 96 |
| Grip Strength/BMI | 32 | 87 | 36 | 91 | - | - | 30 | 89 | 75 | 89 | 75 | 83 | 36 | 87 |
| EWGSOP | 15 | 96 | 7 | 82 | - | - | 0 | 100 | 25 | 97 | 0 | 100 | 12 | 98 |
SDOC – Grip strength <35.5 and 20.0 kg in men and women, respectively; Grip/BMI <1.05 and <0.79 in men and women, respectively; FNIH Grip strength <26 and <16 kg in men and women, respectively; Grip/BMI <1.0 and <0.56 in men and women, respectively; EWGSOP based on low lean mass (ALM/ht2 <7.23 and <5.67 kg/m2 in men and women, respectively) plus low strength (grip strength <30 and <20 kg in men and women, respectively) and/or performance (gait speed <0.8 m/s).
IDEA = Intensive Diet and Exercise for Arthritis Study; LIFE-P = Lifestyle Interventions for the Elders Pilot Study; PIE2 = Pharmacologic Interventions in the Elders Study; SECRET = Study of the Effects of Calorie Restriction and Exercise Training; TOM = Testosterone in Older Men with Mobility Limitations Trial; VIVE2 = Vitality Independence and Vigor Study 2.
DISCUSSION
The purpose of this study was to evaluate cut-points for clinically relevant low muscle strength and lean mass in clinical populations at increased risk for mobility disability. In doing so, we hope to provide clinicians and researchers with useful information pertaining to the diagnostic implications of applying SDOC variables and cut-points in clinical populations. Our results demonstrated that Grip strength, both absolute and relative to BMI, was the most discriminative of slow walking speed, an excellent indicator of disability and mortality risk in older men and women.4, 5 Consistent with companion epidemiologic studies,23–25 we selected maximal grip strength (Grip max) as our primary variable of interest due to its repeated recognition as a key determinant of slow walking speed by Classification and Regression Tree (CART) analysis.21, 22 Application of sex-specific Grip max cut-points (<35.5 and <20.0 in men and women, respectively; Table 1) in mobility-limited older adults from clinical cohorts identified ~50% of patients as falling below the threshold, compared with 6–32% when using previously recommended metrics or components of sarcopenia. Compared to prior definitions, SDOC Grip max thresholds proved to be a sensitive but not overly specific indicator of slowness in this population of older adults enrolled in clinical trials. Researchers and clinicians working with clinical populations or mobility-limited older individuals are encouraged to consider SDOC Grip max thresholds as an inclusive means to identify candidates for lifestyle, promyogenic and function promoting therapies.
In 2014, the FNIH Sarcopenia Project published findings from the first systematic effort to identify muscle strength and lean mass cut-points that predict mobility disability in large cohorts of community-dwelling older adults.13–17 To broaden application of these thresholds, the SDOC lead a second initiative to formulate muscle strength and lean mass cut-points, this time including clinical populations at high risk for incident disability. The efficacy of our efforts to include in our analysis older adults at high risk for mobility disability is demonstrated by the large proportion of participants (~30%) that exhibited slow walking speed (<0.8 m/s) during their baseline testing visit. When interpreting our findings, it should be kept in mind that this percentage of slow-walking individuals is more than twice what was seen in epidemiologic cohorts where the variables and cut-points tested here were generated (13.5%).21, 22 A potential limitation to our study is its cross-sectional nature, impeding designation of causality (i.e., whether slow walking speed was a consequence of insufficient muscle strength and/or lean mass). Moreover, although all participants were experiencing some form of mobility limitation, the underlying clinical conditions and thus pathological features contributing to these mobility impairments (e.g., cardiovascular, musculoskeletal, etc.) varied among the cohorts. The diverse array of clinical conditions represented in our mobility-limited cohorts may also be viewed as a strength of our study, as real-world mobility impairments do not stem from a single etiology. Furthermore, although baseline data were used, all participants were enrolled in some form of randomized controlled trial, portending to a likely greater than average desire of these participants to improve health status. Future longitudinal assessments in large populations of older adults where changes in muscle strength and walking speed can be observed simultaneously are needed. Furthermore, future studies are encouraged to inspect for harmony and/or discordance between various sarcopenia definitions.18
Given the success of our efforts to analyze clinical participants at high-risk for mobility disability, we anticipated a large proportion of individuals would fall below the putative muscle strength and lean mass thresholds. Indeed, nearly half of the study participants fell below maximal grip strength and Grip/BMI cut-points, and this number may have been even greater were it not for possible selection bias due to the exclusion of missing and/or incomplete data. Intriguingly, initial efforts by the FNIH Sarcopenia Project also identified Grip max and Grip/BMI as significant indicators of clinically relevant muscle weakness,14, 17 but these cut-points yield a much lower prevalence (<20%) in our clinical sample. This discrepancy highlights an important issue concerning the screening for and diagnosis of sarcopenia; identifying a “single” or “best” definition for sarcopenia in such a dynamic environment may be not only challenging but impractical. To circumvent this issue, the EWGSOP240 has recently proposed a more dynamic model considering both objective (e.g., muscle size and/or strength) and subjective (e.g., participant reports) measures. However, a major limitation of the EWGSOP2 model is that the same person at a single point in time could be differentially classified as both having sarcopenia, or not having sarcopenia, based on what measures are employed in the algorithm. For example, muscle strength may be evaluated using grip strength cut-points that are notably similar to that of the FNIH, or chair stands. This flexibility may lead to confusion and misinterpretation of results. Thus, a more objective and concrete definition, such as ours, may help to obviate interregional differences and yield a more uniform diagnosis of sarcopenia. Comparisons of SDOC variables and cut-points to previously proposed sarcopenia definitions provides insight into the utility of implementing these thresholds in clinical practice. More specifically, when screening candidates for higher risk/cost interventions (e.g., drug trials), more conservative thresholds in components targeted to the mechanism of action of the intervention should be employed to protect against type-I error (i.e., false positives). However, the greater sensitivity of SDOC cut-points may be preferred when assessing older adults for lower risk (e.g., lifestyle or exercise training) promyogenic and function promoting therapies. Though there may be deliberation surrounding the desirability of a criterion preferencing sensitivity over specificity, in the context of our study population, which consisted of older individuals at elevated risk for mobility limitation, a bias for sensitivity may be preferred.
In summary, we have evaluated cut-points for low muscle strength and lean mass in clinical cohorts of community-dwelling older adults. Consistent with previous attempts to define clinically relevant thresholds for low muscle strength and lean mass,14, 17 we identified maximal grip strength, both absolute and normalized to BMI, to be highly discriminative of slow walking speed (<0.8 m/s). Newly recommended SDOC Grip max and Grip/BMI cut-points result in a more liberal operational definition of muscle weakness compared to what has been previously proposed, identifying over half of our clinical participants as exhibiting clinically relevant muscle strength and lean mass deficits. Clinicians and researchers seeking to evaluate older adults for low-risk myogenic and/or function promoting therapies are strongly advised to consider using highly sensitive SDOC muscle strength cut-points. The comparative prognostic value of these cut-points for predicting adverse health-outcomes is provided in a companion paper21 and worthy of future inquiry.
ACKNOWLEDGEMENTS
Conflicts of Interest
P.M.C reports institutional grants from Abbott and Nestle. J.M. reports serving on the advisory board and as a consultant for American Orthopaedic Association Norvatis, Pluristem, Viking, outside the submitted work. E.F.B. reports grants from Astellas Pharmaceuticals, consulting fees from Pfizer, outside the submitted work. S.B. reports grant support from PCORI, Abbvie, Transition Therapeutics, Abbott, Metro International Biotechnology, LLC, and Alivegen, consulting fees from AbbVie and OPKO, and equity in FPT, LLC, outside the submitted work. R.A.F. reports grants, personal fees and other form Axcella Health, other form Inside Tracker, grants and personal fees from Biophytis, grants and personal fees from Astellas, personal fees from Cytokinetics, personal fees from Amazentis, grants and personal fees from Nestle, personal fees from Glaxo Smith Kline, outside the submitted work.
Funding
The Sarcopenia Definitions and Outcomes Consortium was supported by a cooperative agreement from the National Institute on Aging (1U01AG051421) and the Foundation for National Institutes of Health (FNIH grant numbers BHAS16SARC2 and CAWT16SARC2). The Sarcopenia Definitions and Outcomes Consortium Position Statement Conference was supported by a conference grant (1R13AG060712-01) from the National Institute on Aging and by the Foundation for National Institutes of Health, and the Aging in Motion Coalition. Addition Support for the Position Statement Conference was provided by Abbott Nutrition, Astellas Corporation, and Cytokinetics. Additional support was provided by National Institutes of Health contracts: AGR0118915, AGR0112257, P30AG021332, P30AG024827. This work was also supported by the U.S. Department of Agriculture (USDA), under agreement No. 58-1950-4-003 and the Boston Claude D. Pepper Older Americans Independence Center (OAIC; 1P30AG031679). Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the USDA.
Footnotes
Sponsor’s Role
The sponsor and funding agency played no role in the design, methods, data collections, analysis, and preparation of this article.
REFERENCES
- [1].Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50: 889–896. [DOI] [PubMed] [Google Scholar]
- [2].Aging in Place in America: Seniors fear loss of independence, nursing homes more than death. Volume 2017 Clarity and The EAR Foundation: http://www.marketingcharts.com/demographics-and-audiences/boomers-and-older-2343, 2007. [Google Scholar]
- [3].Knickman JR, Snell EK. The 2030 problem: caring for aging baby boomers. Health Serv Res. 2002;37: 849–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Perera S, Patel KV, Rosano C, et al. Gait speed predicts incident disability: A pooled analysis. J Gerontol A Biol Sci Med Sci. 2016;71: 63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127: 990s–991s. [DOI] [PubMed] [Google Scholar]
- [7].Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147: 755–763. [DOI] [PubMed] [Google Scholar]
- [8].Melton LJ 3rd, Khosla S, Crowson CS, O’Connor MK, O’Fallon WM, Riggs BL. Epidemiology of sarcopenia. J Am Geriatr Soc. 2000;48: 625–630. [PubMed] [Google Scholar]
- [9].Newman AB, Kupelian V, Visser M, et al. Sarcopenia: Alternative definitions and associations with lower extremity function. J Am Geriatr Soc. 2003;51: 1602–1609. [DOI] [PubMed] [Google Scholar]
- [10].Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International Working Group on Sarcopenia. J Am Med Dir Assoc. 2011;12: 249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis Report of the European Working Group on Sarcopenia in Older People. Age and Ageing. 2010;39: 412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Morley JE, Abbatecola AM, Argiles JM, et al. Sarcopenia with limited mobility: An international consensus. J Am Med Dir Assoc. 2011;12: 403–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cawthon PM, Peters KW, Shardell MD, et al. Cutpoints for low appendicular lean mass that identify older adults with clinically significant weakness. J Gerontol A Biol Sci Med Sci. 2014;69: 567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Alley DE, Shardell MD, Peters KW, et al. Grip strength cutpoints for the identification of clinically relevant weakness. J Gerontol A Biol Sci Med Sci. 2014;69: 559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Dam T-T, Peters KW, Fragala M, et al. An evidence-based comparison of operational criteria for the presence of sarcopenia. J Gerontol A Biol Sci Med Sci. 2014;69: 584–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].McLean RR, Shardell MD, Alley DE, et al. Criteria for clinically relevant weakness and low lean mass and their longitudinal association with incident mobility impairment and mortality: The Foundation for the National Institutes of Health (FNIH) sarcopenia project. J Gerontol A Biol Sci Med Sci. 2014;69: 576–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Studenski SA, Peters KW, Alley DE, et al. The FNIH sarcopenia project: Rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69 A: 547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kotlarczyk MP, Perera S, Nace DA, Resnick NM, Greenspan SL. Identifying sarcopenia in female long-term care residents: a comparison of current guidelines. J Am Geriatr Soc. 2018;66: 316–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Vasunilashorn S, Coppin AK, Patel KV, et al. Use of the Short Physical Performance Battery Score to predict loss of ability to walk 400 meters: analysis from the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2009;64: 223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bhasin S, Travison TG, Manini TM, et al. The Position Statements of the Sarcopenia Definition and Outcomes Consortium. J Am Geriatr Soc. 2020. 10.1111/jgs.16372 [DOI] [PubMed] [Google Scholar]
- [21].Cawthon PM, Manini TM, Patel SM, et al. Putative cut-points in sarcopenia components and incident adverse-health outcomes: an SDOC analysis. J Am Geriatr Soc. 2020; In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Manini TM, Patel SM, Newman AB, et al. Identification of sarcopenia components that discriminate slow walking speed: A pooled data analysis from the Sarcopenia Definitions and Outcomes Consortium. J Am Geriatr Soc. 2020; In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Erlandson KM, Travison TG, Zhu H, et al. Application of Selected Muscle Strength and Body Mass Cut-Points for the Diagnosis of Sarcopenia in Men and Women with or at risk for HIV Infection. J Gerontol A Biol Sci Med Sci. 2020; In press.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Orwig D, Travison TG, Zhu H, et al. Application of Selected Cut-Points for Low Muscle Strength and Lean Mass for Recovery of Gait Speed After Hip Fracture. J Gerontol A Biol Sci Med Sci. 2020; In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Patel SM, Duchowny KA, Kiel DP, et al. Performance of Weakness using SDOC Cut Points in Two Nationally Representative Population-Based Cohorts. J Am Geriatr Soc. 2020. 10.1111/jgs.16419 [DOI] [Google Scholar]
- [26].Pahor M, Blair SN, Espeland M, et al. Effects of a physical activity intervention on measures of physical performance: Results of the Lifestyle Interventions and Independence for Elders Pilot (LIFE-P) study. J Gerontol A Biol Sci Med Sci. 2006;61: 1157–1165. [DOI] [PubMed] [Google Scholar]
- [27].Rejeski WJ, Fielding RA, Blair SN, et al. The Lifestyle Interventions and Independence for Elders (LIFE) Pilot study: design and methods. Contemp Clin Trials. 2005;26: 141–154. [DOI] [PubMed] [Google Scholar]
- [28].Kirn DR, Koochek A, Reid KF, et al. The Vitality, Independence, and Vigor in the Elderly 2 Study (VIVE2): Design and methods. Contemp Clin Trials. 2015;43: 164–171. [DOI] [PubMed] [Google Scholar]
- [29].Englund D, Kirn DR, Koochek A, et al. Nutritional supplementation with physical activity improves muscle composition in mobility-limited older adults, the VIVE2 study: A randomized, double-blind, placebo-controlled trial. FASEB J. 2017;31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363: 109–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kitzman DW, Brubaker P, Morgan T, et al. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: A randomized clinical trial. JAMA. 2016;315: 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Upadhya B, Hundley WG, Brubaker PH, Morgan TM, Stewart KP, Kitzman DW. Effect of spironolactone on exercise tolerance and arterial function in older adults with heart failure with preserved ejection fraction. J Am Geriatr Soc. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Messier SP, Legault C, Mihalko S, et al. The Intensive Diet and Exercise for Arthritis (IDEA) trial: design and rationale. BMC Musculoskelet Disord. 2009;10: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Visser M, Fuerst T, Lang T, Salamone L, Harris TB. Validity of fan-beam dual-energy X-ray absorptiometry for measuring fat-free mass and leg muscle mass. Health, Aging, and Body Composition Study--Dual-Energy X-ray Absorptiometry and Body Composition Working Group. J Appl Physiol (1985). 1999;87: 1513–1520. [DOI] [PubMed] [Google Scholar]
- [35].Salamone LM, Fuerst T, Visser M, et al. Measurement of fat mass using DEXA: a validation study in elderly adults. J Appl Physiol (1985). 2000;89: 345–352. [DOI] [PubMed] [Google Scholar]
- [36].Roberts HC, Denison HJ, Martin HJ, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. 2011;40: 423–429. [DOI] [PubMed] [Google Scholar]
- [37].Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13: 881–889. [DOI] [PubMed] [Google Scholar]
- [38].Afilalo J, Eisenberg MJ, Morin JF, et al. Gait speed as an incremental predictor of mortality and major morbidity in elderly patients undergoing cardiac surgery. J Am Coll Cardiol. 2010;56: 1668–1676. [DOI] [PubMed] [Google Scholar]
- [39].Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49: M85–94. [DOI] [PubMed] [Google Scholar]
- [40].Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48: 16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]