Abstract
Rationale:
Previous neurochemical evidence indicates that R(+)-nornicotine is more potent than S(-)-nornicotine in evoking dopamine release in rat nucleus accumbens slices.
Objective:
The current study tested the hypothesis that R(+)-nornicotine is also more potent than S(-)-nornicotine in selectively decreasing intravenous S(-)-nicotine self-administration in rats.
Results:
Following acute pretreatment (1–10 mg/kg for each enantiomer), R(+)-nornicotine was more potent than S(-)-nornicotine in decreasing S(-)-nicotine self-administration; in contrast, within the same dose range, the nornicotine enantiomers were equipotent in decreasing sucrose-maintained responding. This enantioselectivity does not likely reflect a difference in bioavailability, since similar levels of nornicotine were recovered from brain 60 min after injection (5.6 mg/kg for each enantiomer). With repeated pretreatment, tolerance did not develop to the rate-decreasing effect of either nornicotine enantiomer (3 or 5.6 mg/kg) with respect to the decrease in S(-)-nicotine self-administration, although the enantioselectivity dissipated across repeated pretreatments. While both enantiomers acutely produced a similar increase in blood pressure and heart rate, tolerance developed to the blood pressure effects of R(+)-nornicotine, but not to the effects of S(-)-nornicotine, across repeated treatments.
Conclusion:
Both R(+)- and S(-)-nornicotine may have potential utility as a novel tobacco use cessation agents.
Keywords: Nornicotine, Nicotine Self-Administration, Cardiovascular Effects, Tobacco Dependence, Schedule-Controlled Behavior
S(-)-Nicotine, a major alkaloid in tobacco, is accepted generally as being responsible for maintaining smoking behavior (Stolerman and Jarvis 1995). The reinforcing properties of S(-)-nicotine have been demonstrated in laboratory animals using the intravenous self-administration paradigm (Corrigall and Coen 1989; Donny et al. 1995; Goldberg et al. 1981; Rauhut et al. 2003). S(-)-Nicotine engenders self-administration behavior through activation of dopamine (DA) cell bodies in the ventral tegmental area, resulting in increased levels of extracellular DA in the nucleus accumbens (Calabresi et al. 1989; Corrigall et al. 1994; Corrigall et al. 1992; Pontieri et al. 1996).
S(-)-Nicotine replacement therapy (NRT) is the most commonly used pharmacotherapy for tobacco use cessation (Baska et al. 2004; Silagy et al. 2004) and is available as a gum, transdermal patch, nasal spray, inhaler and sublingual formulation (Baska et al. 2004; Benowitz et al. 1998; Fiore et al. 1994; Silagy et al. 2004). While NRT has been shown to be effective as a tobacco use cessation agent, relapse rates continue to be high. In a review of clinical trials, only 18% of individuals using NRT in addition to behavioral therapy showed long term abstinence (Molyneux 2004). Another currently available pharmacotherapy for tobacco dependence is the antidepressant bupropion. Bupropion has been found to reduce the craving and withdrawal associated with smoking cessation (Shiffman et al. 2000), although evidence indicates that the effectiveness of bupropion to promote abstinence may not be long-lasting (Hays et al. 2001). The limited effectiveness of NRT and bupropion indicates that alternative pharmacotherapies are needed to treat tobacco dependence.
RS(±)-Nornicotine, an alkaloid constituent of tobacco and N-demethylated brain metabolite of S(-)-nicotine (Crooks et al. 1997; Ghosheh et al. 1999; Ghosheh et al. 2001), has been suggested to have potential as a tobacco use cessation agent (Crooks and Dwoskin 1997). RS(±)-Nornicotine binds to high affinity nicotinic acetylcholine receptors (nAChRs) thought to be involved in S(-)-nicotine reinforcement (Valette et al. 2003). Similar to S(-)-nicotine, S(-)-nornicotine evokes DA release in a calcium-dependent and mecamylamine-sensitive manner (Dwoskin et al. 1993). RS(±)-Nornicotine also decreases S(-)-nicotine self-administration and is self-administrated weakly in rats (Bardo et al. 1999; Green et al. 2000). Importantly, following intermittent systemic administration of RS(±)-nicotine, significant levels of nornicotine accumulate in rat brain and the brain half-life of nornicotine is 3 times longer then the half-life of nicotine (Ghosheh et al. 1999; Ghosheh et al. 2001). The relatively long residency in brain suggests that RS(±)-nornicotine may produce less intense withdrawal symptoms relative to those associated with the more rapid elimination of S(-)-nicotine. In addition, RS(±)-nornicotine is less potent than S(-)-nicotine in altering blood pressure and heart rate (Dominiak et al. 1985; Risner et al. 1988), suggesting that its clinical use may be tolerated by patients with advanced cardiovascular disease.
Accumulating evidence indicates that the nornicotine enantiomers have different neurochemical and behavioral effects (Dwoskin et al. 1999; Risner et al. 1988). Interestingly, R(+)-nornicotine is more potent than S(-)-nornicotine in stimulating [3H]DA release from rat nucleus accumbens slices; however, R(+)-nornicotine is also less efficacious than S(-)-nornicotine in stimulating rat accumbal [3H]DA release (Green et al., 2001), suggesting that R(+)-nornicotine may be a partial agonist at nAChRs regulating accumbal DA release. In contrast, S(-)-nornicotine appears to be more potent than R(+)-nornicotine in stimulating [3H]DA release from rat striatal slices (Teng et al., 1997), suggesting that different nAChR subtypes mediate these region-specific responses to nornicotine enantiomers. With respect to behavioral effects, S(-)-nicotine and S(-)-nornicotine produce hyperactivity, whereas neither acute nor repeated R(+)-nornicotine produces hyperactivity in rats (Dwoskin et al., 1999). The neurochemical and behavioral profile for the nornicotine enantiomers indicates that they may have potential utility as pharmacotherapies for nicotine dependence.
Based on the greater potency of R(+)-nornicotine to stimulate accumbal [3H]DA release, the current study tested the hypothesis that R(+)-nornicotine would be more potent than S(-)-nornicotine in decreasing intravenous S(-)-nicotine self-administration in rats. To assess whether R(+)- or S(-)-nornicotine alters S(-)-nicotine self-administration specifically, each nornicotine enantiomer was also assessed for its effect on sucrose-maintained responding. The effects of repeated R(+)- and S(-)-nornicotine on S(-)-nicotine self-administration were assessed. Behaviorally relevant doses of R(+)- and S(-)-nornicotine were also assessed for their ability to access the brain. Finally, to begin investigating the safety of R(+)- and S(-)-nornicotine at behaviorally relevant doses, the effects of repeated treatment with each enantiomer on cardiovascular function were determined.
Material and Methods
Subjects
Male Sprague-Dawley rats, weighing 230–300 g at the start of each experiment, were obtained from Harlan Sprague-Dawley Inc (Indianapolis, IN). Rats were acclimated to the colony for 7 days and subsequently were handled daily for 3–5 days before the start of each experiment. Animals had unlimited access to food and water in their home cage, except where noted, and were maintained on a light/dark cycle in which the lights were on from 6:00–20:00 h. All procedures were approved by the University of Kentucky Institutional Animal Care and Use Committee and conformed to the 1996 NIH Guide for the Care and Use of Laboratory Animals and the 2003 Guide for the Care and Use of Mammals in Neuroscience and Behavioral Research.
Testing Apparatus
For the S(-)-nicotine self-administration and sucrose-maintained responding experiments, standard rat operant conditioning chambers (ENV-001; MED Associates, St. Albans, VT) located inside sound-attenuating chambers were used. The front and back walls of the operant chambers were made of aluminum, while the side walls were made of Plexiglas. There was a recessed food tray (5 × 4.2 cm) located in the bottom-center of the front wall. A response lever was located on each side of the recessed food tray on the front wall. A 28-V white cue light was located 6 cm above each response lever. All responses and scheduled consequences were recorded and controlled by a computer interface.
Procedure
S(-)-Nicotine Self-Administration
The S(-)-nicotine self-administration procedure used in the current experiments was similar to that described by Corrigall and Coen (1989), with some modifications; in particular, the length of the signaled timeout (TO) was 20 sec rather than 60 sec and rats were tested during the light portion of the daily cycle rather than the dark portion. Rats were decreased to 85% of their free feeding weight and were placed into the operant conditioning chamber. One lever was designated as the active lever on which responding resulted in the delivery of a reinforcer; the other lever was designated as the inactive lever on which responding had no programmed consequence. Rats initially acquired lever press behavior using sucrose reinforcement (45 mg pellet; P.J. Noyes, Inc., Lancaster, NH). Sucrose pre-training consisted of 5 days in which the schedule of reinforcement was increased from a fixed ratio 1 (FR1) to an FR5. Completion of the ratio requirement resulted in the simultaneous onset of both cue lights and activation of the pellet dispenser. Following sucrose pre-training, rats received unlimited access to standard rat chow in order to regain their free-feeding body weights. Once rats regained their free-feeding weights, they were anesthetized with an i.p. injection of ketamine (80 mg/kg) and diazepam (5 mg/kg). A silastic catheter was inserted into the right jugular vein and threaded subcutaneously with the open end of the catheter exiting the skin and secured to an acrylic head mount, which allowed for the catheter to be connected to an infusion pump. Following surgery, rats received daily i.v. infusions of heparinized saline (Pharmacia, Columbus Ohio; 250,000 IU, 2 mg/ml heparinized saline, 0.1 ml/rat/day).
Following a seven day recovery period, rats were placed into the operant conditioning chamber and received i.v. infusions of S(-)-nicotine at a dose of 0.03 mg/kg/infusion, on an FR1 20-sec signaled TO schedule of reinforcement for 60-min daily sessions. The TO was signaled by the onset of the cue lights above both levers. The 0.03 mg/kg/infusion dose of S(-)-nicotine was chosen based on previous results indicating that this dose engenders near maximal levels of responding (Corrigall and Coen 1989). Rats continued on the FR1 20-sec TO schedule for five consecutive sessions. Following this, the ratio requirement was increased to FR2, FR3, and FR4 for three sessions at each ratio value, until a terminal schedule of FR5 20-sec TO was reached. Rats remained on the FR5 schedule throughout the remainder of the experiment. Prior to the administration of R(+)- or S(-)-nornicotine pretreatments, the following criteria for stable responding on the terminal schedule were required: 1) at least 10 infusions per session, 2) less than 20% variability in number of infusions earned across 3 consecutive sessions, and 3) a minimum of 2:1 (active:inactive lever) response ratio. During the entire duration of the S(-)-nicotine self-administration experiments, rats were restricted to approximately 15 g of rat chow (adjusted to maintain consistent body weight gain), which they received after each daily session. Following completion of each experiment, catheter patency was verified by observing a rapid cataleptic response following i.v. administration of morphine (15 mg/kg). If rats failed the catheter patency check, their data were not included in the statistical analysis.
Sucrose-Maintained Responding.
The procedure for sucrose-maintained responding was similar to that used for S(-)-nicotine self-administration, except that rats did not undergo surgery and the session length was decreased to 15 min in order to avoid satiation with the sucrose pellets used as reinforcers within the session. Also, acquisition to a terminal FR5 schedule occurred over 3 sessions, with FR1 on the first session, FR3 on the second session and an FR5 for the remainder of the experiment. The criteria for stable responding were the same as used in the S(-)-nicotine self-administration protocol.
R(+)- and S(-)-Nornicotine Assay.
Brains were weighed and homogenized in 3 volumes of ice cold 1.15% w/v KCl. Brain samples were then centrifuged at 2,000 g for 5 min at 4°C. Brain supernatants (1 ml) were mixed with 0.33 ml ZnSO4 (400 mg/20 ml) and incubated at 34°C for 60 min. Following incubation, the samples were centrifuged at 30,000 g for 60 min at 4°C and stored at −70° C until assay. R(+)-Nornicotine or S(-)-nornicotine levels were analyzed using gas chromatography-mass spectrometry (GC-MS).
Cardiovascular Function.
Rats were anesthetized with pentobarbital (50 mg/kg, i.p.) and a blood pressure catheter with telemetry transmitter was implanted to record abdominal aortic blood pressure and heart rate. Briefly, a catheter was inserted into the abdominal aorta below the origin of the renal arteries, pointing upstream against the blood flow. A transmitter (TA11PA-C40, Datasciences International, Arden Hills, MN, USA) was placed in the peritoneal cavity and sutured to the abdomen at the incision site. Following surgery, rats were allowed to recover for one week. Each individual animal cage was placed on top of a platform (model RCP-1) equipped with a detector to receive the radiotelemetry signal providing cardiovascular information. Data were acquired and analyzed using software provided by ViiSoftWare (D. Brown, University of Kentucky, Lexington, KY). Mean arterial blood pressure (MAP), systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate were calculated from the pulsatile blood pressure sampled at 500 Hz by an analog-to-digital converter. Prior to the start of the experiment, rats were habituated to the procedure by being placed on the platform for 2 hr. On the next day, each rat was injected with drug or saline and was immediately placed on the platform, where cardiovascular data were recorded for 2 hr. This procedure was repeated on each of 10 consecutive days.
Experiment 1
Dose Effect of Nornicotine Enantiomer Pretreatment on S(-)-Nicotine Self-Administration.
Rats were assigned randomly to one of two different pretreatment groups (n = 8/group). Rats received either R(+)- or S(-)-nornicotine pretreatment 15 min before placement into the operant conditioning chamber for S(-)-nicotine self-administration. Doses of each nornicotine enantiomer (0, 1, 3, 5.6, and 10 mg/kg, s.c.) were administered in a Latin Square design, with each rat assigned randomly to a different dose order. Following each pretreatment session, rats received at least two sessions of maintenance in which there was no pretreatment in order to maintain S(-)-nicotine self-administration. During the maintenance sessions, the rats were required to earn 10 or more infusions before the next pretreatment was administered.
Experiment 2
Dose Effect of Nornicotine Enantiomer Pretreatment on Sucrose-Maintained Responding.
Rats were assigned randomly to one of two pretreatment groups (n = 6/group). Rats received either R(+)- or S(-)-nornicotine pretreatment 15 min before placement into the operant conditioning chamber for sucrose-maintained responding. Doses of each nornicotine enantiomer (0, 1, 3, 5.6, and 10 mg/kg, s.c.) were administered in a Latin Square design, with at least two maintenance sessions (no pretreatment) between each pretreatment session.
Experiment 3
Time Course Effect of Nornicotine Enantiomer Pretreatment on S(-)-Nicotine Self-Administration.
Rats were assigned randomly to one of four pretreatment groups (n = 6–7/group). Rats were pretreated with either R(+)-nornictoine (3.0 or 5.6 mg/kg, s.c.) or S(-)-nornicotine (5.6 or 10 mg/kg, s.c.) at one of the various pretreatment intervals (15, 60, 120, and 240 min; randomized order) prior to being placed into the operant conditioning chamber for S(-)-nicotine self-administration. These pretreatment doses were chosen based on the results from the dose effect experiment showing that 3.0 and 5.6 mg/kg of R(+)-nornicotine showed a similar magnitude of effect compared to 5.6 and 10 mg/kg of S(-)-nornicotine, respectively, in decreasing S(-)-nicotine self-administration. There were at least two maintenance sessions between each pretreatment session.
Experiment 4
Effect of Repeated Nornicotine Enantiomer Pretreatment on S(-)-Nicotine Self-Administration.
Rats were assigned randomly to one of five pretreatment groups (n = 6/group): saline, R(+)-nornicotine (3 or 5.6 mg/kg) or S(-)-nornicotine (3 or 5.6 mg/kg). Rats received saline (s.c.) or one of the nornicotine enantiomers (3.0 or 5.6 mg/kg, s.c.) 15 min prior to being placed into the operant conditioning chamber for S(-)-nicotine self-administration. Pretreatments were repeated for 10 consecutive sessions. Following the 10-day pretreatment period, rats in the R(+)-nornicotine (3 or 5.6 mg/kg) or S(-)-nornicotine (3 or 5.6 mg/kg) pretreatment groups were given two additional S(-)-nicotine self-administration sessions, but were pretreated with saline rather than R(+)- or S(-)-nornicotine, to determine if any effect of either enantiomer was enduring.
Experiment 5
Assessment of Brain Levels following Nornicotine Enantiomer Treatment.
Seven days following the completion of Experiment 3, rats that had been given varying pretreatment times of either nornicotine enantiomer (5.6 mg/kg) were injected with either R(+)-nornicotine (5.6 mg/kg, s.c., n = 8) or S(-)-nornicotine (5.6 mg/kg, s.c., n = 7); rats were treated with the same nornicotine enantiomer they had received previously as part of Experiment 3. Following pretreatments, rats were placed into their home cage, and 60 min later, rats were killed by rapid decapitation. Brains were obtained for analysis of R(+)- or S(-)-nornicotine levels.
Experiment 6
Effect of Nornicotine Enantiomers on Cardiovascular Function.
All rats in this experiment had prior history with R(+)- or S(-)-nornicotine treatment as part of an unrelated experiment; however, rats had a minimum of 14 drug-free days prior to assessing cardiovascular function and rats were tested with the same nornicotine enantiomer they had received previously. In the current experiment, rats were administered R(+)-nornicotine (5.6 mg/kg), S(-)-nornicotine (5.6 mg/kg) or saline (n = 3–4 per group). All drug injections were given s.c., and both blood pressure and heart rate were recorded for a 2-hr period following repeated daily treatments.
Drugs
S(-)-Nicotine bitartrate was purchased from Sigma/RBI (Natick, MA). R(+)-Nornicotine and S(-)-nornicotine were obtained in free base form from Yaupon Therapeutics Inc. (Lexington, KY). For i.v. self-administration, S(-)-nicotine was dissolved in 0.9% w/v NaCl (saline) and the solution was adjusted to pH 7 using sodium hydroxide (1 M). For s.c. administration, S(-)-nicotine or the nornicotine enantiomers were dissolved in saline and injected in a volume of 1 ml/kg body weight. All doses of S(-)-nicotine and the nornicotine enantiomers are expressed as the free base weight. Morphine HCl was obtained from NIDA (Bethesda, MD), prepared in saline, and administered i.v. in a volume of 1 ml/kg body weight.
Data Analysis
The number of reinforcers earned, MAP, and heart rate were analyzed using analysis of variance (ANOVA). Contrasts of interests for all experiments were conducted by paired t-tests for within-group and between-group comparisons, and results were deemed significant at p<0.05.
Results
Experiment 1
Dose Effect of Nornicotine Enantiomer Pretreatment on S(-)-Nicotine Self-Administration.
Both nornicotine enantiomers dose-dependently decreased S(-)-nicotine self-administration (Fig 1). A mixed factor ANOVA revealed main effects of dose (F3, 42 = 31.96, p < 0.001) and enantiomer (F1, 14 = 7.46, p < 0.05). The effect of each nornicotine enantiomer was specific to the active lever, as neither enantiomer significantly altered responding on the inactive lever (results not shown). Contrasts using paired sample t-tests revealed that both R(+)-nornicotine and S(-)-nornicotine at the two highest doses tested (5.6 and 10 mg/kg) decreased S(-)-nicotine self-administration, while only R(+)-nornicotine decreased responding following the 3 mg/kg dose. At both the 5.6 and 10 mg/kg pretreatment doses, responding following R(+)-nornicotine was significantly lower than responding following S(-)-nornicotine. When these data were examined for within-session patterns of responding, each nornicotine enantiomer decreased responding throughout the 60-min session (results not shown).
Figure 1. Effects of R(+)-nornicotine and S(-)-nornicotine on S(-)-nicotine self-administration.
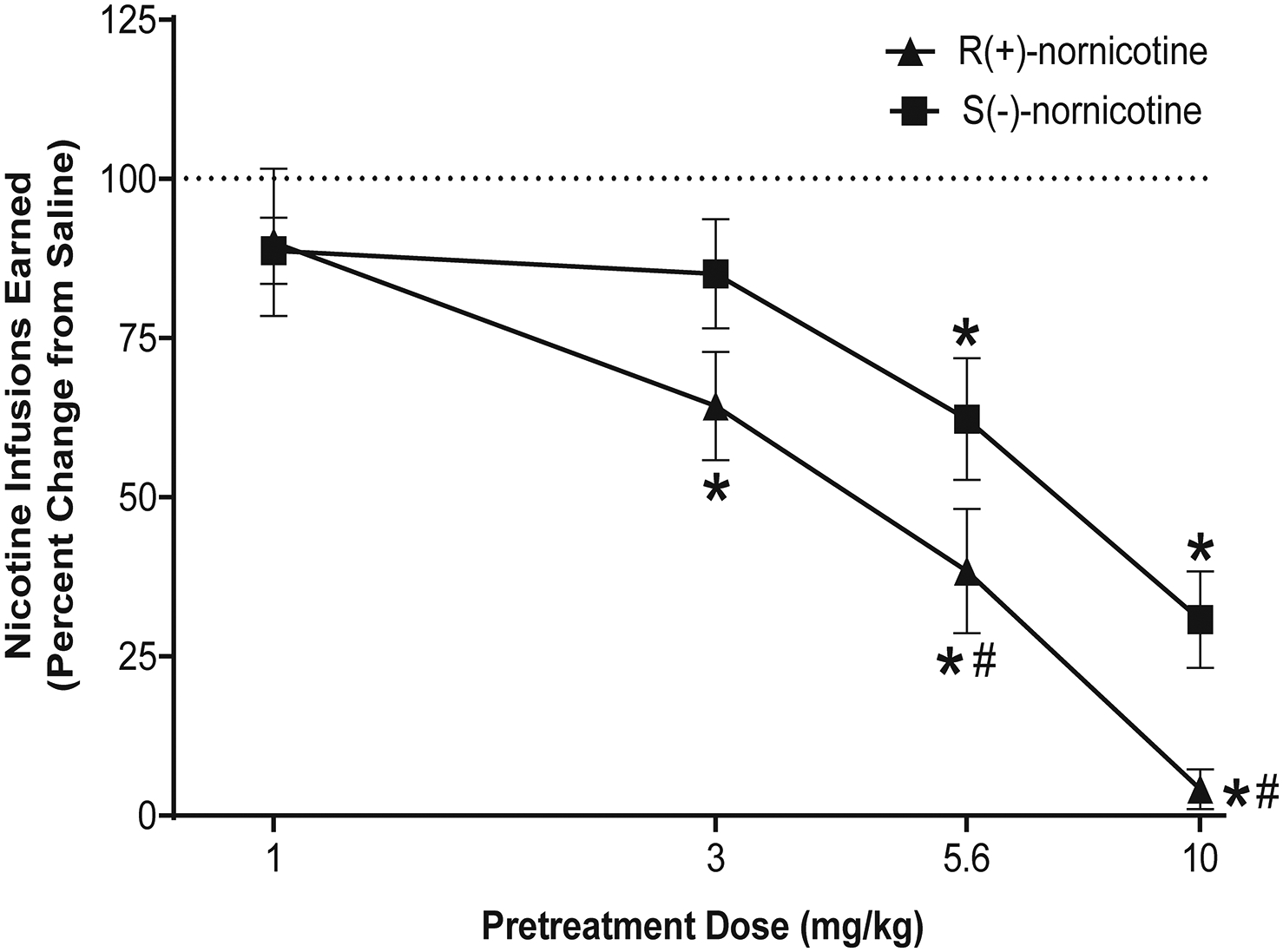
Data are expressed as mean (± SEM) nicotine infusions earned as a percent change from the saline control condition. The x-axis is plotted on a log scale. The mean (± SEM) number of S(-)-nicotine infusions for the saline control condition during the 60 min self-administration session was 12.6 ± 0.4, and 15.6 ± 1.6 for the R(+)-nornicotine and S(-)-nornicotine groups, respectively. The mean (± SEM) number of infusions earned following pretreatment with R(+)-nornicotine at 1, 3, 5.6 and 10 mg/kg doses were 10.6 ± 1.2, 7.6 ± 1.0, 4.6 ± 1.0, 0.5 ± 0.4 respectively. The mean (± SEM) number of infusions earned following pretreatment with S(-)-nornicotine at 1, 3, 5.6 and 10 mg/kg doses were 14.1 ± 1.2, 12.8 ± 1.4, 9.8 ± 1.7, 4.7 ± 1.2 respectively. *denotes a significant difference from saline control, p < 0.05. #denotes a significant difference from S(-)-nornicotine, p < 0.05 (N = 8/group).
Experiment 2
Dose Effect of Nornicotine Enantiomer Pretreatment on Sucrose-Maintained Responding.
Both nornicotine enantiomers dose-dependently decreased sucrose-maintained responding (Fig 2). A mixed factor ANOVA revealed a main effect of dose (F3, 30 = 35.51, p < 0.001), but no effect of enantiomer, indicating a lack of enantioselectivity with respect to the dose-dependent decrease in sucrose-maintained responding. The effect of each nornicotine enantiomer was specific to the active lever, as neither enantiomer significantly altered responding on the inactive lever (results not shown). However, since the number of responses on the inactive lever under control conditions was extremely low (<5 per session), the low rate of responding on the inactive lever may not have allowed for detection of a decrease in responding.
Figure 2. Effects of R(+)-nornicotine and S(-)-nornicotine on sucrose-maintained responding.
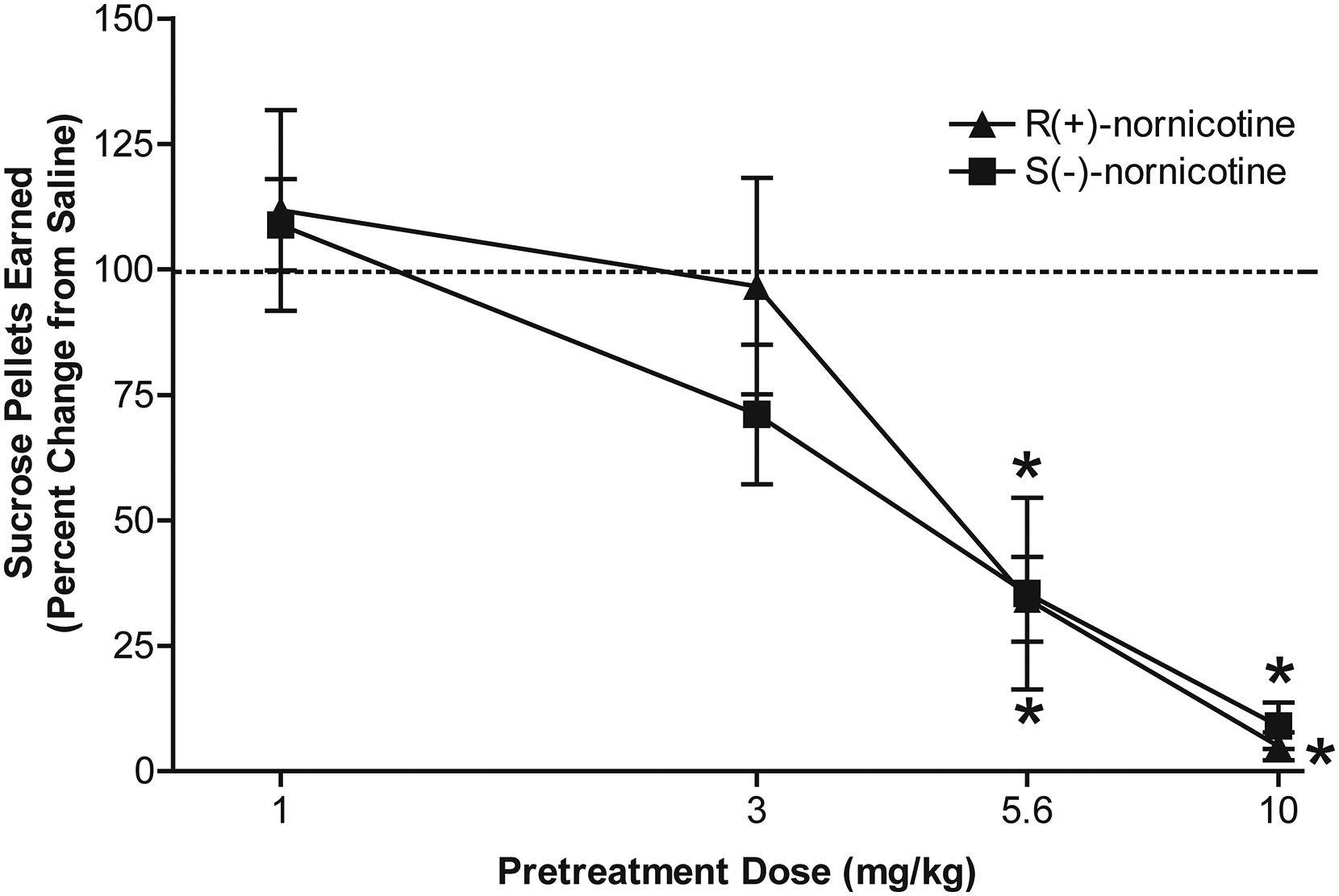
Data are expressed as mean (± SEM) number of sucrose pellets earned as a percent change from the saline control condition. The x-axis is plotted on a log scale. The mean (± SEM) number of sucrose pellets earned during the 15 min session under saline control condition was 88.2 ± 11.8 and 66.3 ± 8.6 for the R(+)-nornicotine and S(-)-nornicotine groups, respectively. The mean (± SEM) number of sucrose pellets earned following pretreatment with R(+)-nornicotine at 1, 3, 5.6 and 10 mg/kg doses were 87.6 ± 6.0, 73.3 ± 8.2, 19.8 ± 6.7, 5.0 ± 2.5 respectively. The mean (± SEM) number of sucrose pellet earned following pretreatment with S(-)-nornicotine at 1, 3, 5.6 and 10 mg/kg doses were 69.2 ± 5.6, 41.7 ± 4.3, 21.0 ± 8.3, 3.0 ± 2.4 respectively. *denotes a significant difference from saline control, p < 0.05 (N = 6/group).
Experiment 3
Time Course Effect of Nornicotine Enantiomer Pretreatment on S(-)-Nicotine Self-Administration.
The 3.0 mg/kg dose of R(+)-nornicotine and 5.6 mg/kg dose of S(-)-nornicotine showed a similar magnitude of effect in decreasing S(-)-nicotine self-administration for up to 60 min (Fig 3). In addition, both the 5.6 mg/kg dose of R(+)-nornicotine and the 10 mg/kg dose of S(-)-nornicotine showed a similar magnitude of effect in decreasing S(-)-nicotine self-administration for up to 240 min. A mixed factor ANOVA revealed main effects for pretreatment interval (F4, 84 = 46.81, p < 0.001) and pretreatment group, (F3, 21 = 18.95, p. <0.001), as well as a significant interaction of pretreatment interval and pretreatment group, (F12, 84 = 3.91, p < 0.001). The significant interaction resulted from a greater initial decrease in S(-)-nicotine self-administration following pretreatments with either 5.6 mg/kg of R(+)-nornicotine or 10 mg/kg of S(-)-nornicotine relative to 3.0 mg/kg of R(+)-nornicotine or 5.6 mg/kg of S(-)-nornicotine. Regardless of enantiomer dose, there was a dose-dependent loss of effect across increasing pretreatment intervals. Direct comparison between enantiomer doses that produced similar magnitudes of effect at 15 min (i.e., 3.0 mg/kg of R(+)-nornicotine vs. 5.6 mg/kg of S(-)-nornicotine; and 5.6 mg/kg of R(+)-nornicotine vs. 10 mg/kg of S(-)-nornicotine) revealed no significant differences at any time interval, indicating that the loss of effect was similar between enantiomers.
Figure 3. Time course effects of R(+)-nornicotine (3.0 and 5.6 mg/kg) and S(-)-nornicotine (5.6 and 10 mg/kg) pretreatment given either 15, 60, 120 or 240 min prior to the S(-)-nicotine self-administration session.
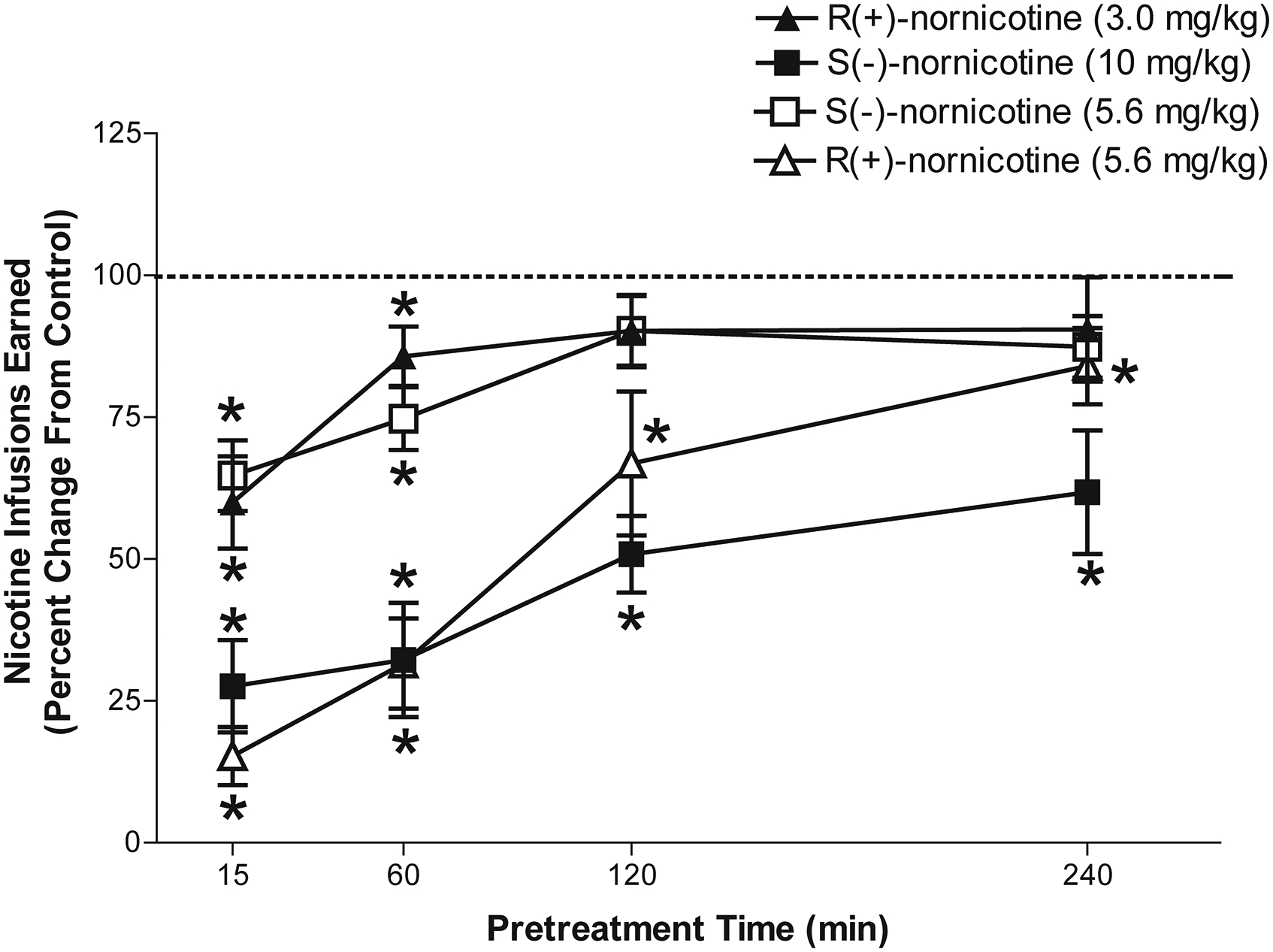
Data are expressed as mean (± SEM) number of S(-)-nicotine infusions as a percent change from the baseline control (no pretreatment). The mean (± SEM) number of S(-)-nicotine infusions for the baseline control was 18.6 ± 1.4 and 16.8 ± 1.7 for the 5.6 mg/kg doses of R(+)-nornicotine and S(-)-nornicotine groups, respectively. The mean (± SEM) number of S(-)-nicotine infusions for the baseline control was 15.9 ± 1.0 and 13.6 ± 1.0 for the 3.0 mg/kg dose of R(+)-nornicotine and the 10 mg/kg dose of S(-)-nornicotine groups, respectively. The mean (± SEM) numbers of nicotine infusions earned following pretreatment with 5.6 mg/kg dose of R(+)-nornicotine at 15, 60, 120 and 240 following pretreatment were 2.4 ± 0.8, 4.8 ± 1.1, 11.4 ± 2.4, 13.6 ± 1.1, respectively. The mean (± SEM) numbers of nicotine infusions earned following pretreatment with 5.6 mg/kg dose of S(-)-nornicotine at 15, 60, 120 and 240 min following pretreatment were 10.3 ± 1.1, 11.9 ± 1.6, 14.3 ± 1.8, 14.3 ± 2.1, respectively. The mean (± SEM) numbers of nicotine infusions earned following pretreatment with 3.0 mg/kg dose of R(+)-nornicotine at 15, 60, 120 and 240 following pretreatment were 9.8 ± 1.6, 13.0 ± 2.4, 13.7 ± 1.8, 14.8 ± 1.4, respectively. The mean (± SEM) numbers of nicotine infusions earned following pretreatment with 10 mg/kg dose of S(-)-nornicotine at 15, 60, 120 and 240 min following pretreatment were 3.7 ± 1.2, 4.3 ± 1.4, 6.7 ± .76, 8.2 ± 1.5, respectively. *denotes a significant difference from control, p < 0.05. (N = 6 – 7/group).
Experiment 4
Effect of Repeated Nornicotine Enantiomer Pretreatment on S(-)-Nicotine Self-Administration.
Each nornicotine enantiomer decreased S(-)-nicotine self-administration dose-dependently across repeated pretreatments (Fig 4). A mixed factor ANOVA revealed a main effect of session, (F12, 324 = 18.69, p < 0.001) and drug pretreatment (F4, 27 = 15.42, p < 0.001). There was also a significant interaction of drug pretreatment and session, (F48, 324 = 4.48, p < 0.001). Paired sample t test comparisons revealed that both doses of each enantiomer decreased S(-)-nicotine self-administration compared to saline across all 10 sessions. With R(+)-nornicotine, a greater decrease was obtained following 5.6 mg/kg than following 3 mg/kg on pretreatment sessions 2–4; with S(-)-nornicotine, a greater decrease was obtained following 5.6 mg/kg than following 3 mg/kg on pretreatment sessions 1–6. More important, on the first pretreatment session, as well as on pretreatment sessions 3 and 6, S(-)-nicotine self-administration was decreased to a greater extent by the 5.6 mg/kg dose of R(+)-nornicotine than by the 5.6 mg/kg dose of S(-)-nornicotine, indicating that enantioselectivity was obtained at this dose. Within each pretreatment group, there was no significant change in infusions earned across the repeated pretreatments, indicating that tolerance did not develop to the decrease in responding with either pretreatment dose of the nornicotine enantiomers. When saline pretreatment was substituted for R(+)- or S(-)-nornicotine pretreatments, the higher pretreatment dose (5.6 mg/kg) of each enantiomer continued to decrease responding significantly on the first session. However, no significant differences were obtained among groups on the second saline pretreatment session, indicating that there was a reversal of the effects produced by repeated nornicotine enantiomer pretreatments.
Figure 4. Effects of repeated pretreatment with R(+)-nornicotine, S(-)-nornicotine or saline pretreatments on S(-)-nicotine self-administration across 10 sessions.
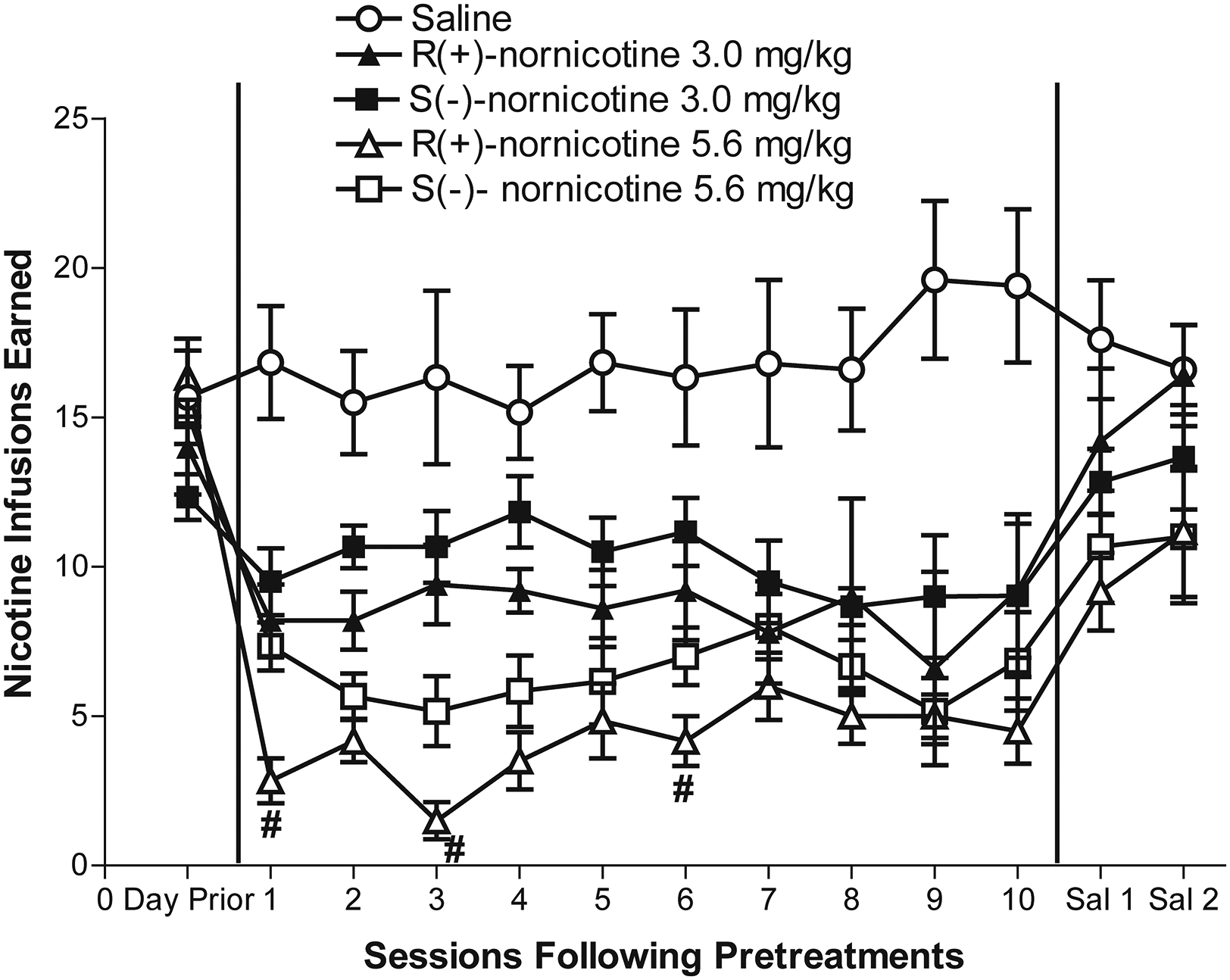
Data are expressed as mean (± SEM) number of S(-)-nicotine infusions earned. All groups received saline pretreatment on sessions 11 and 12. #denotes significant difference from 5.6 mg/kg S(-)-nornicotine group, p < 0.05.
Experiment 5
Assessment of Brain Levels following Nornicotine Enantiomer Treatment.
A paired sample t-test comparing brain levels 60 min after s.c. treatment with the two enantiomers revealed no difference in brain levels between R(+)- and S(-)-nornicotine; mean (± SEM) brain levels of R(+)-nornicotine and S(-)-nornicotine were 161.4 ± 20.5 ng/ml and 214.6 ± 31.1 ng/ml, respectively. The 60-min time point was chosen to correspond to the duration of the self-administration session.
Experiment 6
Effect of Nornicotine Enantiomers on Cardiovascular Function.
Due to a technical error, cardiovascular data from Day 10 of treatment were lost, so only results from Days 1–9 are presented in Figure 5. Both R(+)- and S(-)-nornicotine increased MAP and heart rate acutely. A mixed factor ANOVA among groups treated with S(-)-nornicotine, R(+)-nornicotine or saline revealed a significant interaction between treatment and day for MAP (F16, 64 = 2.59, p < 0.01). While both nornicotine enantiomers increased MAP following acute injection (Day 1), tolerance was evident on Day 9 with R(+)-nornicotine (p < 0.05), but not with S(-)-nornicotine. Paired sample t test comparisons on Day 9 revealed that, relative to the saline control, MAP was elevated by S(-)-nornicotine (p < 0.05), but not by R(+)-nornicotine. A similar pattern of results was obtained on heart rate following repeated nornicotine enantiomer treatment; however, there was greater day-to-day variability on the heart rate measure, and the interaction of treatment and day only approached significance (F16, 64 = 1.64, p = 0.08; Fig 5B). Nonetheless, paired sample t-test comparisons on Day 9 revealed that relative to the saline control, heart rate was elevated by S(-)-nornicotine (p < 0.05), but not by R(+)-nornicotine. Thus, at the dose tested, tolerance developed to repeated R(+)-nornicotine treatment, but not to repeated S(-)-nornicotine treatment.
Figure 5. Effects of nornicotine enantiomers on cardiovascular function.
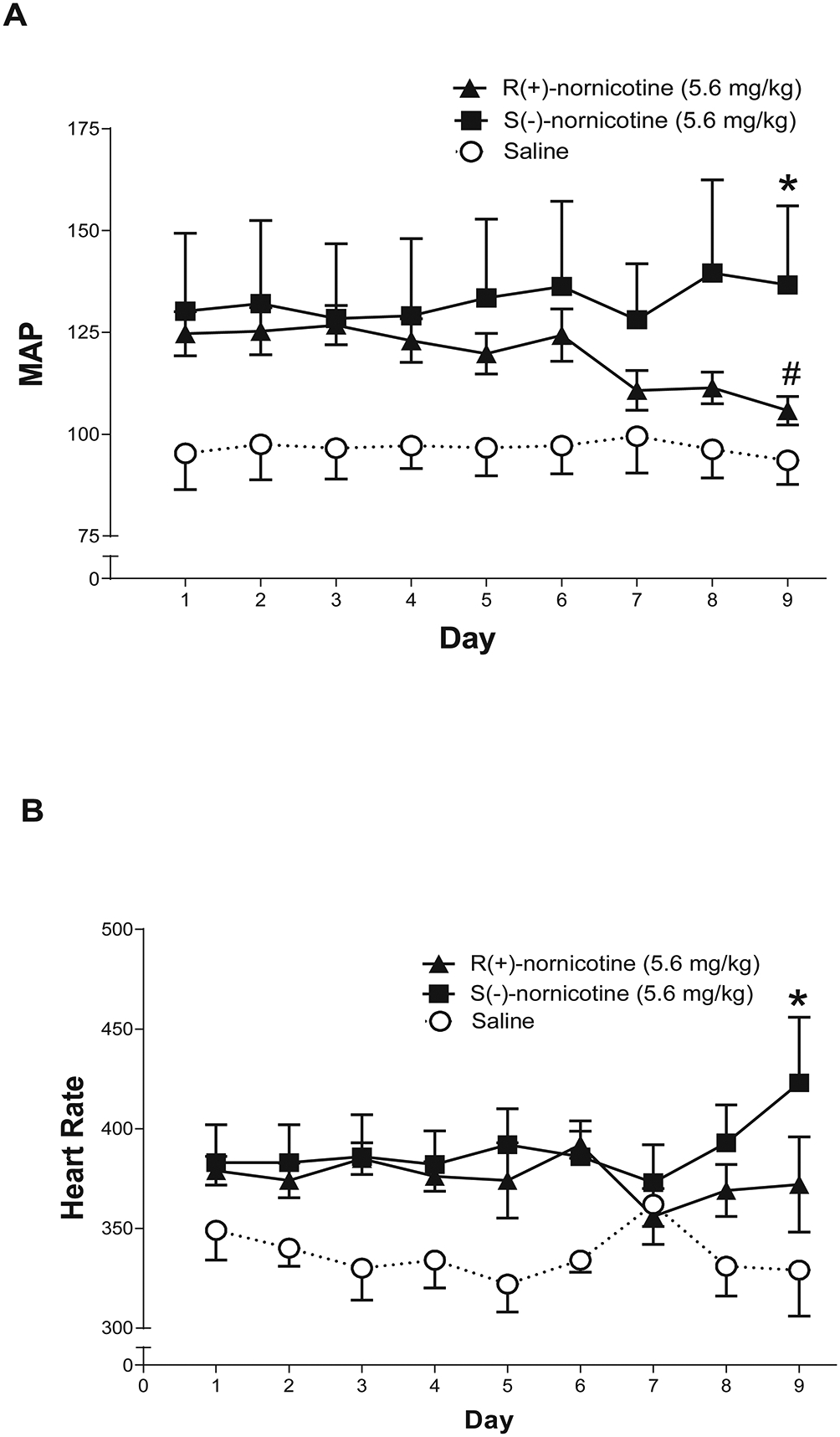
Mean arterial pressure (MAP) for the 2-hr period on days 1–9 following either R(+)-nornicotine, S(-)-nornicotine, or saline (panel A). Heart rate for the 2-hr period on days 1–9 following either R(+)-nornicotine, S(-)-nornicotine or saline (panel B). In both panels, the data are expressed as the mean (± SEM; N = 3–4/group) collapsed across the 2-hr period; no reliable alterations were noted in 15-min intervals across the sessions. On Day 9: *denotes significant difference from saline group, p < 0.05 and # denotes significant difference from day 1, p < 0.05.
Discussion
The current results demonstrate that acute pretreatment with either R(+)-nornicotine or S(-)-nornicotine dose-dependently decreases S(-)-nicotine self-administration in rats, with acute R(+)-nornicotine being about 2-fold more potent than acute S(-)-nornicotine in decreasing S(-)-nicotine self-administration. The acute effect of R(+)-nornicotine (5.6 mg/kg) on S(-)-nicotine self-administration was greater in magnitude and longer in duration compared to S(-)-nornicotine (5.6 mg/kg). However, when doses that were equally effective at decreasing S(-)-nicotine self-administration were tested, the duration of effect was similar between the two nornicotine enantiomers. Importantly, the nornicotine enantiomers were equipotent at decreasing sucrose-reinforced behavior, indicating that enantioselectivity was specific to S(-)-nicotine-reinforced behavior. The greater potency of R(+)-nornicotine compared to S(-)-nornicotine in decreasing S(-)-nicotine self-administration was not likely due to differences in bioavailability, as similar brain concentrations of each enantiomer were obtained 60 min after treatment.
In addition to the nornicotine enantioselectivity observed on S(-)-nicotine self-administration, comparison across experiments also indicated that R(+)-nornicotine may be more potent in decreasing S(-)-nicotine self-administration than sucrose-maintained responding. That is, following pretreatment with 3 mg/kg of R(+)-nornicotine, there was a significant decrease (~40%) in S(-)-nicotine self-administration, but no change in sucrose-maintained responding. Although baseline responding was higher in the sucrose-maintained responding experiment compared to the S(-)-nicotine self-administration experiment, it is unlikely that the difference was due solely to rate-dependency because high rates of responding are generally more sensitive to disruption than low rates of responding (Dews 1958; Kelleher and Morse 1968). Regardless, from a drug development perspective, the demonstration of a specific decrease in S(-)-nicotine self-administration in the absence of a change in sucrose-maintained responding may not be an essential criterion for identifying a potentially useful smoking cessation pharmacotherapy in preclinical studies. As a case in point, although S(-)-nicotine is a useful treatment in humans, pretreatment doses of S(-)-nicotine that decrease S(-)-nicotine self-administration in rats also decrease food-maintained responding under similar schedule contingencies (Corrigall and Coen 1989; Goldberg et al. 1989).
The greater potency of acute R(+)-nornicotine compared to S(-)-nornicotine in decreasing S(-)-nicotine self-administration dissipated across repeated pretreatments. In addition, tolerance did not develop across repeated pretreatment with either R(+)- or S(-)-nornicotine, suggesting that repeated use of either enantiomer may have clinical efficacy. Further, termination of repeated pretreatment of either nornicotine enantiomer re-established the rate of S(-)-nicotine self-administration to baseline levels, indicating that the effect of each enantiomer was reversible. The decrease in nicotine self-administration found with repeated nornicotine enantiomer pretreatment was not likely due to a general decrease in activity, since the hypoactive effect of each nornicotine enantiomer tolerates rapidly following repeated treatment (Dwoskin et al. 1999).
The effects of the nornicotine enantiomers on S(-)-nicotine self-administration are generally consistent with those reported previously using RS(±)-nornicotine (Green et al. 2000). Both the racemate and the pure enantiomers of nornicotine dose-dependently decreased S(-)-nicotine self-administration and sucrose-maintained responding following acute administration. Also, both the racemate and the pure enantiomers of nornicotine resulted in similar decreases in S(-)-nicotine self-administration, and tolerance did not develop to this effect following repeated injections, suggesting potential clinical utility in the long-term maintenance of smoking cessation.
Although the current study is the first to investigate the effects of pure nornicotine enantiomers on S(-)-nicotine self-administration, previous research has examined the effects of nornicotine enantiomers on schedule-controlled behavior using food reward (Goldberg et al. 1989; Risner et al. 1988; Risner et al. 1985). Two of the previous studies investigated the effects of the nornicotine enantiomers on a multiple fixed-interval, FR schedule (Multi FI FR) in both dogs and monkeys (Risner et al. 1988; Risner et al. 1985), and the third study used a Multi FI FR in rats to investigate the effects of the nornicotine enantiomers (Goldberg et al. 1989). All three studies found that both nornicotine enantiomers produced dose-dependent decreases during the FR component of the schedule, but there was no enantioselectivity observed on food-reinforced behaviors (Goldberg et al. 1989; Risner et al. 1988; Risner et al. 1985). These previous findings are consistent with the results from the current study showing that there is no enantioselective effect of nornicotine on sucrose-maintained responding under FR performance.
The observed nornicotine enantioselectivity with respect to the decrease in S(-)-nicotine self-administration is consistent with the previously observed neuropharmacological profiles of R(+)- and S(-)-nornicotine. Specifically, R(+)-nornicotine has been shown to be more potent than S(-)-nornicotine in stimulating DA release from superfused rat nucleus accumbens slices; however, R(+)-nornicotine is also less efficacious at stimulating DA release (Green et al. 2001), suggesting that R(+)-nornicotine may be functioning as a partial agonist at nAChRs mediating accumbal DA release. In contrast to results from the nucleus accumbens, however, R(+)-nornicotine has also been shown to be less potent than S(-)-nornicotine in stimulating DA release from rat striatal slices (Teng et al. 1997), suggesting a regional difference in nAChRs involved. Regardless of the mechanism, however, the greater potency of R(+)-nornicotine to release DA from the reward-relevant nucleus accumbens and to decrease S(-)-nicotine self-administration suggests that R(+)-nornicotine may serve as a selective therapeutic agent for smoking cessation, although S(-)-nornicotine may also be efficacious.
While the current experiments indicate enantioselectivity in decreasing the reinforcing properties of S(-)-nicotine, the current studies cannot differentiate between the effects on the pharmacological actions of S(-)-nicotine and the effects on the stimuli associated with S(-)-nicotine delivery. Prior research has illustrated the importance of environmental cues associated with S(-)-nicotine delivery at promoting the acquisition and maintenance of S(-)-nicotine self-administration (Caggiula et al. 2002). Future experiments investigating the effects of nornicotine enantiomers on S(-)-nicotine self-administration in the absence of a concurrent nonpharmacological stimulus are needed to elucidate the effect of nornicotine specifically on the reinforcing effect of S(-)-nicotine.
To the extent that either R(+)- or S(-)-nornicotine may have potential as a clinically effective smoking cessation pharmacotherapy, the current study also examined the cardiovascular effects of repeated R(+)- or S(-)-nornicotine treatment at a behaviorally relevant dose (5.6 mg/kg). In the only previously published report comparing the cardiovascular effects of R(+)- or S(-)-nornicotine, Risner et al (1988) found that both enantiomers increased heart rate following acute administration in dogs. The current study extends these findings by examining both blood pressure and heart rate across repeated treatments in rats. Although only a single dose of each nornicotine enantiomer was tested, these limited results showed that tolerance developed to the elevation in MAP following R(+)-nornicotine, but not following S(-)-nornicotine; a similar trend was evident with the heart rate measure. Thus, to the extent that increases in MAP and heart rate represent unwanted side effects, repeated use of R(+)-nornicotine may offer a unique advantage over either S(-)-nornicotine or S(-)-nicotine in the treatment of tobacco dependence. Further work is needed to determine if this apparent advantage is retained when these enantiomers are administered via a clinically relevant route of administration such as oral, sublingual or transdermal.
Acknowledgments of Funding:
Supported by USPHS grants DA 016521 to Yaupon Therapeutics and T32 DA DA016176. The University of Kentucky holds a patent for nornicotine as a smoking cessation therapy, and the patent is licensed to Yaupon Therapeutics, Inc. P.A.C, L.P.D. and M.T.B. have financial interests in Yaupon.
References
- Bardo MT, Green TA, Crooks PA, Dwoskin LP (1999) Nornicotine is self-administered intravenously by rats. Psychopharmacology (Berl) 146: 290–6 [DOI] [PubMed] [Google Scholar]
- Baska T, Straka S, Baskova M, Mad’ar R (2004) Economic rewarding of smoking cessation-facilitating drugs--a comparison of over-the-counter and prescribed nicotine replacement therapy. Expert Opin Pharmacother 5: 487–91 [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Zevin S, Jacob P 3rd, (1998) Suppression of nicotine intake during ad libitum cigarette smoking by high-dose transdermal nicotine. J Pharmacol Exp Ther 287: 958–62 [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF (2002) Environmental stimuli promote the acquisition of nicotine self- administration in rats. Psychopharmacology (Berl) 163: 230–7. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Lacey MG, North RA (1989) Nicotinic excitation of rat ventral tegmental neurones in vitro studied by intracellular recording. Br J Pharmacol 98: 135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM (1989) Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology 99: 473–8 [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL (1994) Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res 653: 278–84 [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Franklin KB, Coen KM, Clarke PB (1992) The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology 107: 285–9 [DOI] [PubMed] [Google Scholar]
- Crooks PA, Dwoskin LP (1997) Contribution of CNS nicotine metabolites to the neuropharmacological effects of nicotine and tobacco smoking. Biochem Pharmacol 54: 743–53 [DOI] [PubMed] [Google Scholar]
- Crooks PA, Li M, Dwoskin LP (1997) Metabolites of nicotine in rat brain after peripheral nicotine administration. Cotinine, nornicotine, and norcotinine. Drug Metab Dispos 25: 47–54 [PubMed] [Google Scholar]
- Dews PB (1958) Studies on behavior. IV. Stimulant actions of methamphetamine. Journal of Pharmacology and Experimental Theraputics 122: 137–147 [PubMed] [Google Scholar]
- Dominiak P, Fuchs G, von Toth S, Grobecker H (1985) Effects of nicotine and its major metabolites on blood pressure in anaesthetized rats. Klin Wochenschr 63: 90–2 [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Knopf S, Brown C (1995) Nicotine self-administration in rats. Psychopharmacology (Berl) 122: 390–94 [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Buxton ST, Jewell AL, Crooks PA (1993) S(-)-nornicotine increases dopamine release in a calcium-dependent manner from superfused rat striatal slices. J Neurochem 60: 2167–74 [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Crooks PA, Teng L, Green TA, Bardo MT (1999) Acute and chronic effects of nornicotine on locomotor activity in rats: altered response to nicotine. Psychopharmacology (Berl) 145: 442–51 [DOI] [PubMed] [Google Scholar]
- Fiore MC, Smith SS, Jorenby DE, Baker T (1994) The effectiveness of the nicotine patch for smoking cessation. Jama 271: 1940–1947 [PubMed] [Google Scholar]
- Ghosheh Dwoskin LP, Li WK Crooks PA (1999) Residence times and half-lives of nicotine metabolites in rat brain after acute peripheral administration of [2’-(14)C]nicotine. Drug Metab Dispos 27: 1448–55 [PubMed] [Google Scholar]
- Ghosheh Dwoskin LP, Miller DK Crooks PA (2001) Accumulation of nicotine and its metabolites in rat brain after intermittent or continuous peripheral administration of [2’-(14)C]nicotine. Drug Metab Dispos 29: 645–51 [PubMed] [Google Scholar]
- Goldberg SR, Risner ME, Stolerman IP, Reavill C, Garcha HS (1989) Nicotine and some related compounds: effects on schedule-controlled behaviour and discriminative properties in rats. Psychopharmacology (Berl) 97: 295–302 [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Spealman RD, Goldberg DM (1981) Persistent behavior at high rates maintained by intravenous self-administration of nicotine. Science 214: 573–5 [DOI] [PubMed] [Google Scholar]
- Green TA, Crooks PA, Bardo MT, Dwoskin LP (2001) Contributory role for nornicotine in nicotine neuropharmacology: nornicotine-evoked [3H]dopamine overflow from rat nucleus accumbens slices. Biochem Pharmacol 62: 1597–603 [DOI] [PubMed] [Google Scholar]
- Green TA, Phillips SB, Crooks PA, Dwoskin LP, Bardo MT (2000) Nornicotine pretreatment decreases intravenous nicotine self-administration in rats. Psychopharmacology (Berl) 152: 289–94 [DOI] [PubMed] [Google Scholar]
- Hays JT, Hurt RD, Rigotti NA, Niaura R, Gonzales D, Durcan MJ, Sachs DP, Wolter TD, Buist AS, Johnston JA, White JD (2001) Sustained-release bupropion for pharmacologic relapse prevention after smoking cessation. a randomized, controlled trial. Ann Intern Med 135: 423–33 [DOI] [PubMed] [Google Scholar]
- Kelleher RT, Morse WH (1968) Determinants of the specificity of behavioral effects of drugs. Ergeb Physiol 60: 1–56 [DOI] [PubMed] [Google Scholar]
- Molyneux A (2004) Nicotine replacement therapy. Bmj 328: 454–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Orzi F, Di Chiara G (1996) Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature 382: 255–7 [DOI] [PubMed] [Google Scholar]
- Rauhut AS, Neugebauer N, Dwoskin LP, Bardo MT (2003) Effect of bupropion on nicotine self-administration in rats. Psychopharmacology (Berl) 169: 1–9 [DOI] [PubMed] [Google Scholar]
- Risner ME, Cone EJ, Benowitz NL, Jacob P 3rd, (1988) Effects of the stereoisomers of nicotine and nornicotine on schedule-controlled responding and physiological parameters of dogs. J Pharmacol Exp Ther 244: 807–13 [PubMed] [Google Scholar]
- Risner ME, Goldberg SR, Prada JA, Cone EJ (1985) Effects of nicotine, cocaine and some of their metabolites on schedule-controlled responding by beagle dogs and squirrel monkeys. J Pharmacol Exp Ther 234: 113–9 [PubMed] [Google Scholar]
- Shiffman S, Johnston JA, Khayrallah M, Elash CA, Gwaltney CJ, Paty JA, Gnys M, Evoniuk G, DeVeaugh-Geiss J (2000) The effect of bupropion on nicotine craving and withdrawal. Psychopharmacology (Berl) 148: 33–40. [DOI] [PubMed] [Google Scholar]
- Silagy C, Lancaster T, Stead L, Mant D, Fowler G (2004) Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev: CD000146 [DOI] [PubMed] [Google Scholar]
- Stolerman Jarvis MJ (1995) The scientific case that nicotine is addictive. Psychopharmacology 117: 2–10 [DOI] [PubMed] [Google Scholar]
- Valette H, Bottlaender M, Dolle F, Coulon C, Ottaviani M, Syrota A (2003) Long-lasting occupancy of central nicotinic acetylcholine receptors after smoking: a PET study in monkeys. J Neurochem 84: 105–11 [DOI] [PubMed] [Google Scholar]


