Abstract
Early environment can have a major impact on development, with family life known to play an important role. Longitudinal studies can therefore help increase our understanding of variance in cognitive abilities in young animals, as well as over time. We followed 22 marmosets (11 male, 11 female) from infancy through to early adolescence. At 3 months old, the marmosets were trained to reliably touch a rewarded stimulus. At 5 months, behavior was observed within the natal group. At 9 months, the marmosets were given a visual discrimination (VD) task to assess learning ability. Mann Whitney U tests found no sex or family size differences in number of errors at 3 or 9 months. While no significant relationships were found between behavior in the family and learning at 3 months, significant negative correlations were found between duration spent in locomotion and learning errors (p=0.05), as well as between frequency of calm vocalizations and learning errors (p=0.001) at 9 months. A U shape curve was found between amount of social play and learning at 9 months. Positive family interactions, including moderate amounts of play, as well as calm individual behavior, may therefore be important in learning. This study sheds light on cognitive development in much younger marmosets than previously studied, and helps increase understanding of how individual differences in learning may arise.
Keywords: marmoset, cognitive development, family life, longitudinal study
Graphical Abstract
1. Introduction
1.1. Behavioral and cognitive development in the marmoset
Like most primates, common marmosets (Callithrix jacchus) are highly intelligent and have complex social lives, and like humans, are cooperative breeders, with all members of the family helping to raise the young (Saito, 2015). Both captive and wild common marmosets have now been studied in a variety of cognitive topics (captivity: Werdenich and Huber, 2002; wild: Pesendorfer et al, 2009; Gunhold et al, 2014), with these studies finding that marmosets display many higher level cognitive abilities, such as object permanence (Mendes and Huber, 2004), true imitation (Voelkl and Huber, 2000) and problem solving requiring perception of means and ends (parallel string test: Halsey et al, 2006). Cognitive function is well established in adult marmosets (Schultz-Darken et al, 2016), and there are now a small number of cross-sectional studies comparing the abilities of infant and juvenile marmosets with those of adults, which suggest that there may be developmental changes in ability to attend to and acquire information from other group members and novel objects in the environment (Gunhold et al, 2014; Schiel and Huber, 2006). More longitudinal studies in marmosets are therefore important, to increase our understanding of cognitive abilities in younger marmosets and across developmental timelines.
Marmoset behavioral development shows a series of distinct changes from infancy to adulthood (Yamamoto, 1993; Schultz-Daren et al, 2016). Newborns are carried almost continuously for the first three weeks of life, after which caregivers begin to encourage infants to get off, and at four to six weeks, infants display many independent behaviors, including exploration and play (captive: Yamamoto et al, 1993; wild: Stevenson, 1978). Weaning occurs after week eight, and by three months of age, visual and motor systems are fully developed (Missler et al, 1992). By the end of the infant phase, there is a marked decrease in dependence on caregivers (Yamamoto et al, 1993). During the juvenile stage (5–10 months), interactions with siblings, particularly the twin (Box, 1975), are more common and play becomes rougher. Marmosets are now capable of sophisticated hunting techniques and can capture live prey items as well as adults (Schiel et al, 2010). The sub-adult stage (9–15 months) is characterized by the full repertoire of adult behaviors, as well as puberty, and by 15 months, common marmosets have reached adult weight and are capable of reproduction (Yamamoto, 1993). It is important to understand these stages of development when testing cognition at different ages, as there are specific patterns of behavior at certain phases that can influence learning ability (Bastable & Dart, 2007). Methods of evaluating cognition that are age and species appropriate are therefore vital in quantifying normal marmoset neurodevelopment (Schultz-Darken et al, 2016).
1.2. Family interactions: importance of parental style and play
Young marmosets develop within an intimate social group, with family life known to be extremely important for their development (Dettling et al, 2007). Although qualitative aspects of caregiving are similar among groups of marmosets, there can be significant quantitative differences (Arruda et al, 1986; Ingram 1977; Yamamoto, 1993), including the amount of care group members are prepared to give and the amount of care that infants seek. Primate models of maternal behavior have shown marked individual differences in maternal care, along two dimensions of protectiveness and rejection (Maestripieri, 1998). Studies have found that infants reared by highly protective mothers had delayed independence and were more fearful and inhibited (Fairbanks and McGuire, 1993: Cercopithecus aethiops). Meanwhile, infants of more rejecting mothers acquired independence earlier (Bardi and Huffman, 2006: Macaca mulatta and M. fuscata), but tended to be more anxious and impulsive (review by Parker and Maestripieri 2011). Behaviors such as proximity and aggression within the family could therefore play an important role in cognitive development. However, results in Old World monkeys, which are often very maternally bonded, may not be applicable in species where the whole family is responsible. It is therefore important to investigate effects of different family care styles in cooperatively breeding species.
Play is an important part of life for common marmosets (Schiel and Souto, 2017), and essential for development (Ginsberg, 2018) and learning (Whitebread et al, 2009). In marmosets, consistent social play first appears at 5–7 weeks old (Box, 1975), reaching high levels in late infant and juvenile stages (Missler et al, 1992; Yamamoto, 1993), before declining in adulthood (Stevenson and Poole, 1982). Primates in general are exceptionally playful, tending to spend more time playing than most other taxonomic groups (Pellis et al, 2015). This may be due to a functional link between play and the sophisticated cognitive and behavioral abilities of primates (eg. Reader et al, 2011), with positive associations found between amount of play and frequency of extractive foraging, tool use, behavioral innovation and tactical deception (11 primate species: Montgomery, 2014), as well as between play and the relative size of neural systems associated with complex cognition and behavior (cortico-cerebellar system: Kerney et al, 2017). Chalmers & Locke-Haydon (1984) looked at the relationship between amount of play and skill (measured by aperture, wheel and food tests) in marmosets from 6–22 weeks of age, finding that as they got older, they became more agile and accurate, less frustrated and possessed more food, as well as increased their amount of play. However, this study only assessed very young marmosets.
1.3. Family size and sex
The number of helpers in a Callitrichid family group is associated with differences in infant care, such as amount of infant carrying (eg. Ingram, 1977; McGrew, 1988: Saguinus oedipus), as well as infant survival, with the greatest infant survival in groups of 5 or more caretakers (Savage et al. 1996: S. oedipus). In cooperatively breeding birds, individuals living in large groups have better performance in cognitive tasks than those living in small groups, suggesting that the demands of growing up in complex social groups can promote cognitive development (Ashton et al, 2018:Cracticus tibicen dorsalis). However, little is known about the effect of family size on cognitive development in marmosets.
There has been accumulating research looking at sex differences in cognition in the common marmoset, with studies often finding that adult females learn food-acquiring tasks faster and more efficiently than adult males (eg. Yamamoto et al, 2004). However, while Takemoto et al (2015) found no sex differences in visual discrimination and reversal learning in young adult (1–4 year old) marmosets, a recent study in older adult marmosets (4–6 years old) found that females required more trials to learn a visual reversal (VR) task than males. As no differences in motivation or motor skills were found, results suggest that females may have enhanced habit formation (LaClair et al, 2019). This sex difference in cognitive flexibility appears to be related to sex differences in resting-state brain networks (eg. Tunc et al, 2016; LaClair et al, 2019). However, there has been limited research looking at sex differences in infant and adolescent marmosets.
We therefore followed common marmosets throughout infancy and early adolescence, to investigate the effect of sex and family size on performance in cognitive tasks, as well as the relationship between learning and family interactions. Based on previous research suggesting the benefits of large group sizes, we hypothesized that those from larger families would learn faster than those from smaller families. While we hypothesized that females would learn a simple food-related task faster than males at 3 months, it is likely that males will learn more complex tasks faster than females at older ages. We also hypothesized that greater amounts of positive family interactions, particularly play, would be associated with enhanced learning ability.
2. Methods
2.1. Subjects
Twenty-two common marmosets (11 male, 11 female) housed at the Wisconsin National Primate Research Center (WNPRC), University of Wisconsin-Madison (UW-Madison) were studied from June 2017-May 2019. All were born into the colony at the WNPRC, and were family reared from birth. The animals were housed in their natal group, with group sizes ranging from 3–9 (3–5=11; 6–9=11). Table 1 shows sex and group sizes of each animal tested at 3 and 9 months, as well as their learning success at each age. Figure 1 displays the study timeline, with sample sizes at each time point. While all marmosets were included in analysis at 3 months old, 4 animals (1 male, 3 female) were dropped from the study prior to 9 month testing due to health issues. Three additional marmosets did not respond during cognitive testing at 9 months old (ie. they did not reach criterion on initially touching the rewarded object for food reward), and so have no learning data included at this age. Therefore, 15 marmosets (8 male, 7 female) were included in analysis at 9 months.
Table 1:
Sex and group sizes, as well as learning success of each animal, at 3 and 9 months old
| Animal ID | Sex | Family size at 3 months | Family size at 9 months | Learned task at 3 months | Learned task at 9 months |
|---|---|---|---|---|---|
| cj1 | F | 3 | 3 | Yes | No |
| cj2 | M | 4 | 4 | Yes | Yes |
| cj3 | F | 4 | 4 | Yes | Yes |
| cj4 | M | 4 | 4 | Yes | Yes |
| cj5 | M | 9 | 8 | No | (no learning data) |
| cj6 | M | 9 | 8 | No | No |
| cj7 | F | 9 | * | Yes | * |
| cj8 | M | 6 | 6 | Yes | No |
| cj9 | F | 6 | 6 | Yes | Yes |
| cj10 | F | 4 | 4 | Yes | No |
| cj11 | M | 4 | 4 | Yes | No |
| cj12 | M | 5 | * | Yes | * |
| cj13 | F | 4 | * | Yes | * |
| cj14 | F | 4 | * | Yes | * |
| cj15 | F | 6 | 6 | Yes | No |
| cj16 | M | 6 | 6 | Yes | No |
| cj17 | M | 4 | 4 | Yes | (no learning data) |
| cj18 | F | 4 | 4 | Yes | (no learning data) |
| cj19 | F | 6 | 5 | Yes | No |
| cj20 | M | 6 | 5 | Yes | No |
| cj21 | F | 6 | 5 | Yes | Yes |
| cj22 | M | 6 | 5 | Yes | Yes |
dropped from study at 8 months
No learning data- did not reach criterion touching s+
Fig 1:
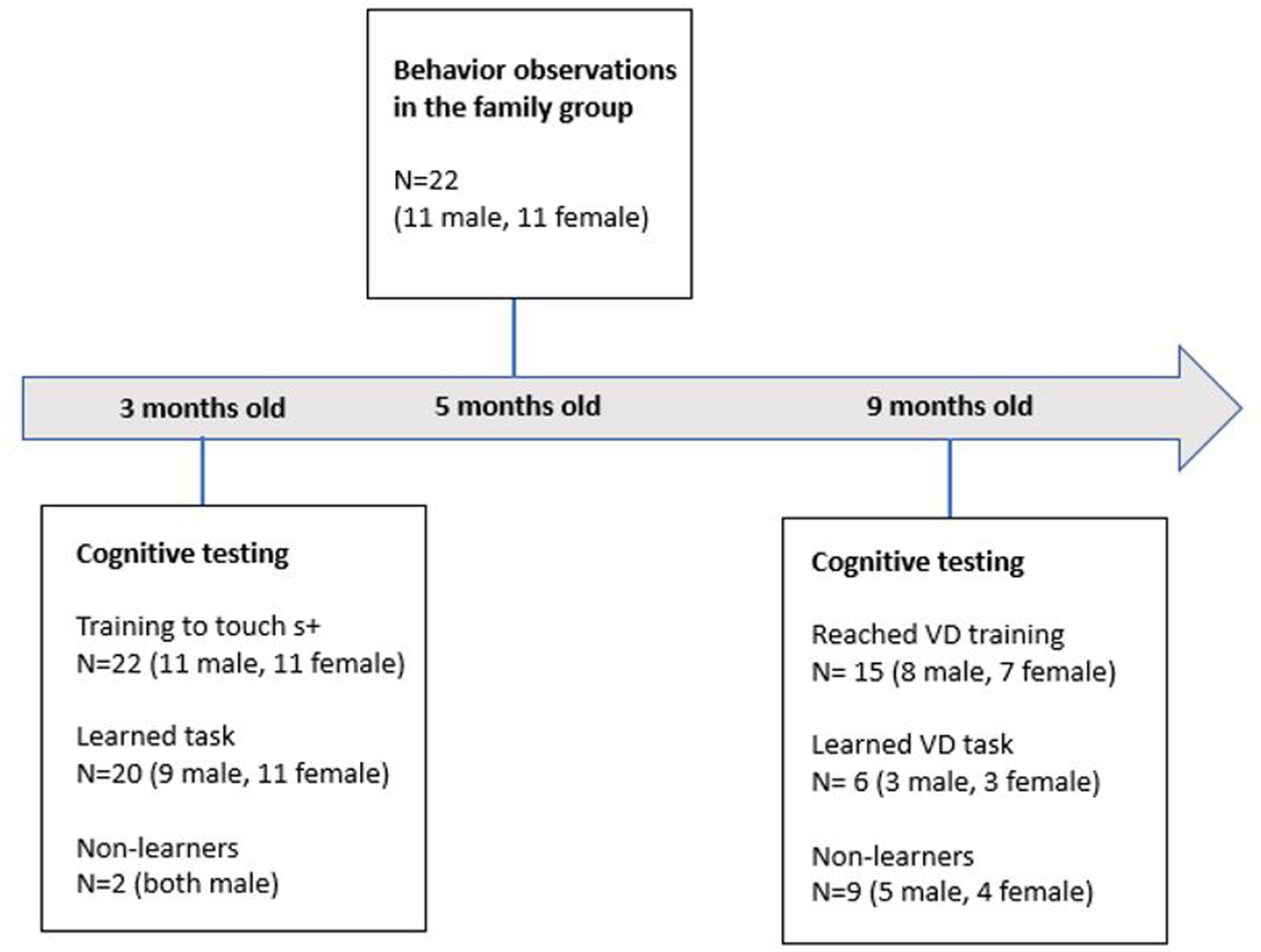
Study timeline, including sample sizes at each age.
2.2. Housing and husbandry
The marmosets were housed in aluminum and wire mesh cages, which contained a variety of cage furnishings, including nest box, platforms, wooden perches and rope ladders, to encourage species-typical behaviors. Cages allowed auditory and olfactory contact with marmosets in other cages, although visual contact was limited. Single cages measured 0.61m × 0.91m × 1.83m (families of 4 or less), while double cages measured 1.22m × 0.91m × 1.83m (families of 5 or more). The marmosets were fed their standard allotment in 2 daily feedings: at approximately 8:00 they received primate pellets (Mazuri 5MI6, Land O’Lakes, Arden Hills, MN) and at 13:00 they received a variety of fruit and vegetables. Extra protein, such as yogurt or nuts, were provided between these times, as well as other enrichment items, such as small parcels of fruit or gum. During cognitive testing periods, the marmosets received their primate pellets as normal, but extra protein and enrichment were withheld until directly after testing, to encourage participation. Water was provided ad libitum. Lighting was maintained on a 12:12 light/dark cycle (lights on at 6:30 to 18:30), with ambient temperature at approximately 27°C and humidity at approximately 50%. Cages were changed and animals weighed every 2 weeks. Novel hanging objects were introduced on a rotational basis.
As part of an ongoing longitudinal study looking at the effect of dietary fat on development of adolescent depression, each marmoset was assigned to a diet condition at 6 months of age (prior to which all were given the standard low-fat colony diet). While there were too few animals per diet group in the current study to adequately look at differences (control n=4, unhealthy saturated fat n=4, healthy unsaturated fat n=7), no differences in weight or metabolic parameters were found between the diet groups through 9 months of age (Colman, in prep).
2.3. Cognitive testing
2.3.1. Apparatus
A custom-made testing box was designed to easily attach to the front of each marmoset homecage. Stimuli were presented outside the test box, on a tray attached to the front. At 3 months and 9 months old, grey plastic horizontal rectangular blocks were presented. Reference blocks (rewarded S+ and unrewarded S−) were 2cm and 10cm long (both 2cm high × 2cm wide). In order to counterbalance the rewarded and unrewarded reference stimuli, half the animals were allocated the largest object and the other half were allocated the smallest object as S+ (Bethell et al, 2012). Prior preference testing (using dichotomous choice tests) revealed that banana cereal or animal crackers would be suitable, low calorie rewards for which the marmosets were willing to work. A small piece of the preferred reward was placed under each stimulus (S+, S−), to avoid olfactory cues.
2.3.2. Testing at 3–4.5 months old: Habituation to the test box and stimuli
All training and testing was conducted by a single researcher, in the homecage to avoid removing the animals from their family environment. At three months old, the marmosets were first allowed to familiarize themselves with the apparatus. During the first two-three weeks, they were given access to the testing box with the tray attached, and encouraged to reach out to obtain small pieces of food placed on the tray, while the test box door was closed for increasing amounts of time, to separate them from the rest of the cage and allow for individual testing. A camera was also introduced, which allowed the footage to be watched back to score the responses in each session. At week four, the marmoset was then closed in the test box alone, and encouraged to reach out and touch the block to receive the reward (following Pryce et al, 2004). The rewarded stimulus was presented on 10 consecutive occasions. A five second time limit was given for responses. A new trial began when either the marmoset had obtained the reward or five seconds had passed without a response. The marmoset was considered trained when they were calmly moving around the enclosed space, reliably touching the block to obtain the reward for 80% of the presentations, over three consecutive days.
Sessions were carried out once a day, four days a week (on Mondays, Tuesdays, Thursdays and Fridays) between 10:00–13:00 (between morning and afternoon feeds). If there were more than 10 seconds of persistent escape attempts, the marmoset was allowed to immediately leave the test box. Number of sessions (from first to last day of testing; min= 3 session, max=24 sessions), as well as number of errors to reach criterion (sum of incorrect/no go responses over all sessions; min= 0 errors, max= 240 errors) were recorded as an indication of learning ability. At the end of each session, the monkey was rewarded with a larger piece of food. The session ended after 15 minutes, or if the monkey had completed the full number of trials. Each marmoset was given six weeks to complete the task.
2.3.3. Testing at 9–10.5 months: Visual discrimination learning
The marmosets were first allowed to re-familiarize themselves with the testing box and stimuli, until they reached criterion (same as at three months; 80% trials on three consecutive days) on touching the rewarded object for food reward. Training sessions then involved ‘Go/No go’ trials, in which single stimuli were presented (Burman et al, 2008), with a two second inter-trial interval for responses. Correct ‘Go’ responses to S+ (a touch of the stimulus) were rewarded with access to a treat on a 100% fixed ratio schedule. If there was no response within the two second period, the researcher moved on to the next trial. Correct ‘No go’ responses S- (no touch of the stimulus) to were unrewarded (the treat was inaccessible, i.e. it was not uncovered for access). Incorrect ‘Go’ responses to S- (a touch of the stimulus) were followed by a five second time-out ‘punishment’ (following Pryce et al, 2004). All sessions lasted for 15 minutes maximum, or if the marmoset had earned the maximum number of rewards (10 pieces).
A pseudorandom schedule was used, with 20 trials evenly divided between S+ and S− sizes (10 rewarded, 10 unrewarded). No more than two rewarded or unrewarded sizes were presented consecutively (Burman e al, 2008). The first and last trials were always rewarded. Training in the initial visual discrimination task was considered complete when the marmosets were responding correctly on 80% of S+ trials and 80% of S− trials (Bethell et al, 2012), over three consecutive sessions. Responses were recorded and scored following the session. Number of sessions (from first to last day of testing: min= 3 session, max=24 sessions), and cumulative number of errors to reach criterion (sum of incorrect go/no go responses over all sessions: min= 0 errors; max= 480 errors) were recorded. Again, a six-week learning period was imposed. We made every effort to allow each individual to complete the task, but they were never forced to perform. Three animals who failed to perform the initial familiarization task within the six-week period were excluded at nine months. Those that did perform but failed to learn the VD task in the time were given a ceiling value of 24 for number of sessions. Figure 2 shows a young marmoset inside the testing box performing the cognitive task (inset: stimuli presented during the training task).
Fig 2:

Photograph of a 9-month-old marmoset performing trials in the homecage cognitive testing, with an inset of the stimuli used. He was required to touch the small block to obtain a food reward and refrain from touching the large block.
2.4. Behavioral observations
Starting at 5 months old, infants were observed in their homecage for 15 minutes (following 5 minutes of allowing the animal to habituate to the presence of an observer) twice a week (1x morning, 1x afternoon), for 5 weeks. Continuous focal sampling was utilized, using all occurrence recording on the ‘Behavior Pro’ app. Both frequency and duration of each behavior over the 15 minute observation period were recorded (IRR ranged from 80% to 100% for 8 observers for the 15 behaviors). Behaviors of interest within the family group included: aggression, individual, sexual, social affiliative and submission (see Table 2 for full description of each behavior within these groups).
Table 2:
Behaviors recorded and their definitions
| Behavior | Definition |
|---|---|
| Aggression | |
| Erh erhb | The animal emits low-pitched, staccato chattering (Epple, 1968; Bezerra & Souto, 2008; Stevenson & Poole, 1976). |
| Aggressionb | The animal lunges at (Abbott, 1984), snap-bites (Stevenson & Poole, 1976), chases (Saltzman et al, 1997) cuffs (Stevenson & Poole, 1976) or fights (Abbott, 1984) another animal. |
| Individual | |
| Autogrooma | The animal cleans its own fur or skin with hand or mouth (including scratching- rapidly drawing claws of hand or foot across the fur or skin). |
| Inactivea | The animal remains stationary while alone (either resting, with tail curled around the body, or vigilant to surroundings- including the observer), without engaging in any other behavior (Saltzman et al, 1997). |
| Locomotiona | The animal travels between locations by walking, running, climbing or jumping. |
| Foragea | The animal is engaged in any activity directly related to acquiring or ingesting food. |
| Solitary playa | High activity behavior performed alone, such as hanging or swinging on a rope, chasing tail (Stevenson & Poole, 1976), or investigating objects in the environment by handling, sniffing, gnawing or attending to them whilst walking around them. |
| Othera | The animal has entered the nestbox and so cannot be seen by the observer, or is performing any other behavior not noted here. |
| Sexual | |
| Mountb | The animal climbs/attempts to climb onto another animals back from behind and grips the other animal around the waist, may include pelvic thrusting (Kendrick & Dixson, 1983). |
| Social affiliative | |
| Calm vocalb | The animal emits quiet, birdlike calls, such as ‘trills’ and ‘chirps’ audible to the observer. |
| Proximitya | The animal is stationary, sitting, crouching or lying next to another individual, with some form of physical contact (often torso-torso) for at least 3 secs (huddle- Abbott, 1984). |
| Allogrooma | The animal cleans the fur or skin of another individual with its hands or mouth (Abbott, 1984). |
| Social playa | High activity social interactions involving close, non-aggressive physical contact with other individuals, such as wrestling, chasing, grasping, pouncing, back-hugging, batting, biting or mutual exploration, often accompanied with a play face (open mouth without lip retraction) (Stevenson & Poole, 1976). |
| Submission | |
| Nga ngab | The animal emits a relatively low-pitched, infantile squeal (Epple, 1968). |
| Submitb | The animal moves at least one body length away from another animal within 1 second of the other animal being in proximity (Saltzman et al, 1997). May include the animal flattening their tufts against their head, partially opening their mouth with corners retracted, exposing teeth (facial grimace) or half closing their eyelids (eye slit) (Stevenson & Poole, 1976; Abbott, 1984). |
duration used in analysis;
frequency used in analysis
2.5. Statistical analysis
Due to non-normality of data (based on Kolmogorov-Smirnov tests), non-parametric tests were utilized. Spearman’s rank correlations were conducted between sessions to criterion and total number of errors at three and nine months. Effect of family size (3–5 v. 6–9) and sex (male v. female) on sessions to criterion and total number of errors at 3 and 9 months were analyzed using Mann Whitney U tests. Family size was grouped in this way, as family sizes of 3–5 often included only parents, while family sizes of 6–9 also included older siblings. Spearman’s rank correlations were conducted between each behavior at 5 months and sessions to criterion, as well as total number of errors, at both 3 and 9 months to look at individual differences in learning. Some behaviors were not included in the analysis, due to very low frequencies of occurrence (‘mount’ and ‘submit’). ‘Other’ was also not analyzed, as it was only included to allow accurate measures of relevant behaviors. Frequencies were used for short duration behaviors, while durations were used for long duration behaviors. As six marmosets learned the task at 9 months old and nine marmosets did not learn the task at this age (with sessions to criterion clustering at 24), subjects were grouped into 2 categories (‘learner’ and ‘non-learner’), and Mann Whitney U tests were conducted to look at differences in behavior at 5 months between the groups. All statistical analyses were conducted in SPSS. Level of significance was ≤0.05, although ≤0.1was considered a trend.
2.6. Ethics statement
Housing conditions met the guidelines for nonhuman primates and were approved by the Animal Care and Use Committee (ACUC) of the Office of Vice Chancellor for Research and Graduate Education at the University of Wisconsin, Madison. The UW-Madison ACUC monitors the housing conditions regularly to ensure the welfare of the marmosets. The facility meets AAALAC approval and USDA standards. The study reported here meets the standards and approval of the ACUC, with funding from NIH HD086057, and complies with legal and ethical requirements in the USA. The study adheres to the American Society of Primatologists Principles for the Ethical Treatment of Non-human Primates.
3. Results
3.1. Learning at 3 and 9 months
Twenty marmosets (9 male, 11 female) learned the task at 3 months, with only 2 males failing to learn the task at this age. Six marmosets (3 males, 3 females) learned the VD task at 9 months old, while nine marmosets (4 female, 5 male) did not learn the task within the allotted time (refer to Fig 1 and Table 1 for full details). There was no significant correlation between sessions to criterion at 3 and 9 months old (r=0.13, p=0.64), nor between total number of errors at 3 and 9 months old (r=−0.14, p=0.62) (Fig 3).
Fig 3:
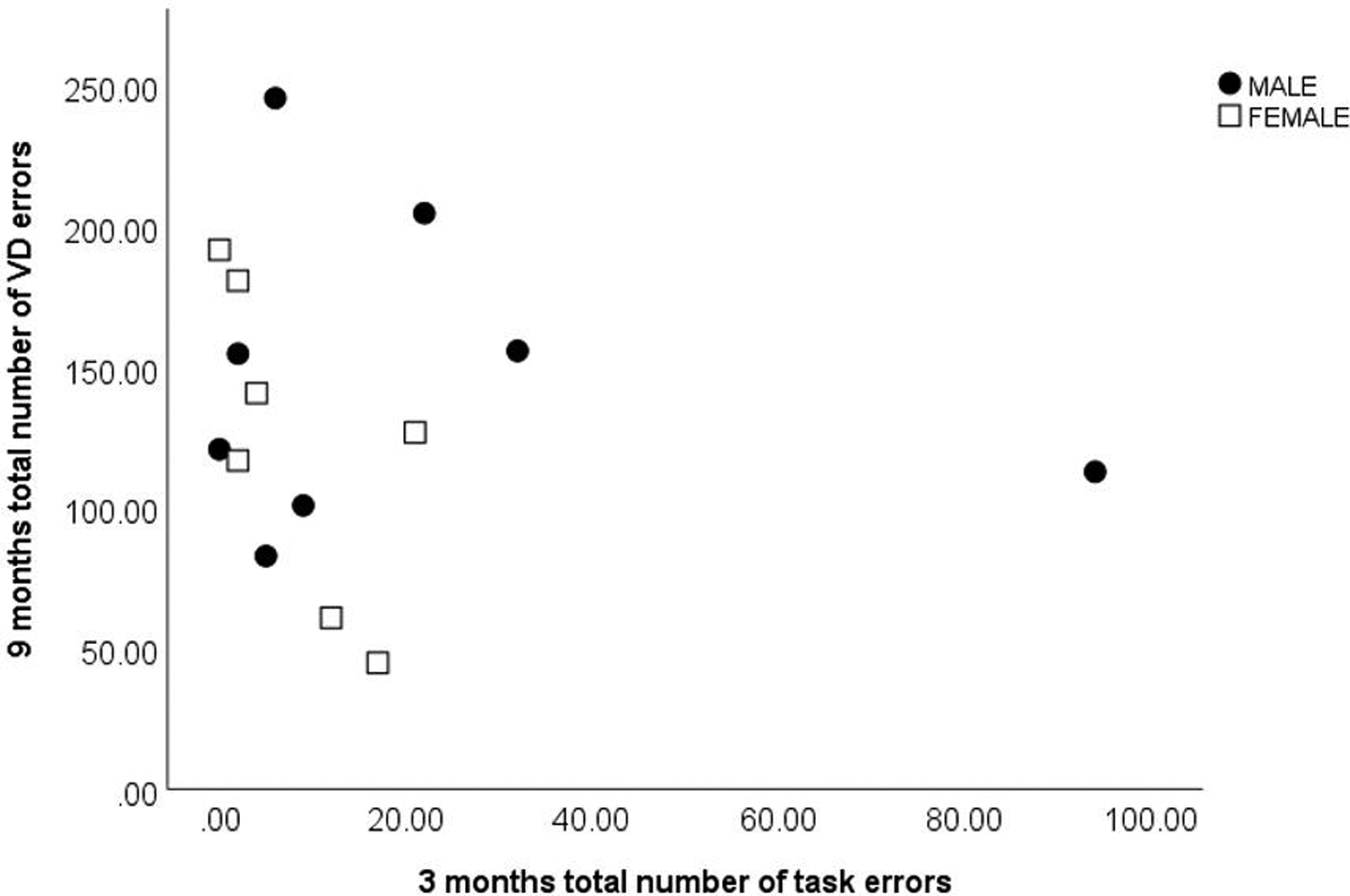
Lack of correlation (p>0.05) between total number of errors at 3 and 9 months old (n=15; 8 male, 7 female).
3.2. Effect of family size and sex on learning at 3 months and 9 months
Mann Whitney U tests found no significant effect of sex (U(1)=45.00, p=0.29), or family size (U(1)=46.00, p=0.32) on sessions to criterion at 3 months. There was also no significant effect of sex (U(1)=47.00, p=0.37) on total number of errors at 3 months, although there was a trend for infants from larger families to have more total errors than infants from smaller families (U(1)=31.00, p=0.051). However, once two outliers were removed (identified using the 1.5x IQR rule), the trend disappeared (U(1)=31.00, p=0.18).
Mann Whitney U tests found no significant effect of sex (U(1)=23.50, p=0.56) or family size (U(1)=19.00, p=0.41) on sessions to criterion at 9 months. There was no significant effect of sex (U(1)=23.00, p=0.56) or family size (U(1)=24.00, p=0.90) on total number of errors at 9 months.
3.3. Relationship between learning and family interactions
Spearman’s rank correlations found a trend for a positive relationship between duration spent in proximity to a family member during family interactions at 5 months and sessions to criterion during cognitive testing at 3 months (r=−0.378, p=0.083), as well as between duration spent in proximity to a family member and total number of errors at 3 months (r=−0.368, r=0.092). However, once 2 outliers were removed (again, identified using the 1.5x IQR rule), the trend disappeared (proximity/sessions to criterion: r=−0.156, p=0.512; proximity/total number of errors: r=−0.285, p=0.223).
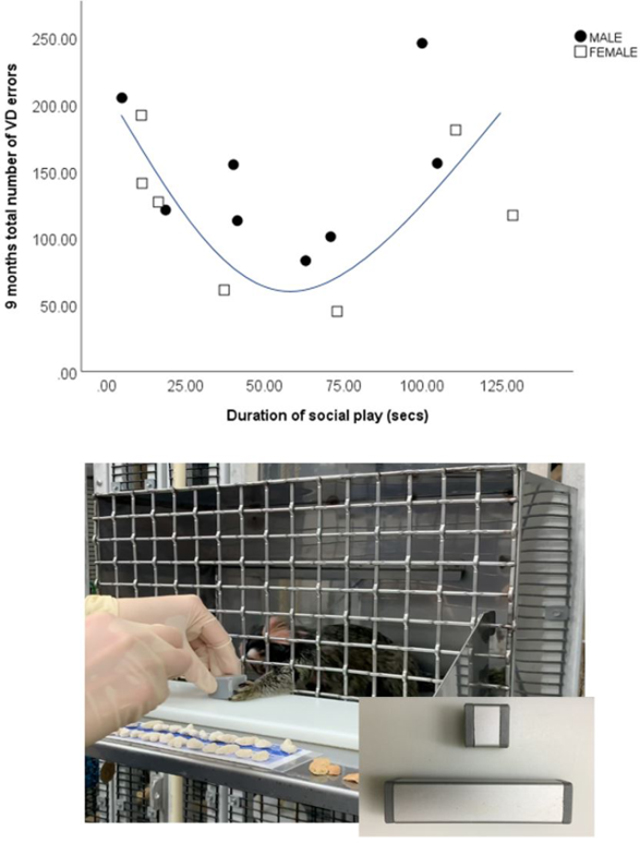
There was a significant negative correlation between duration spent in locomotion and total number of errors at 9 months (r=0.514, p=0.05), as well as between frequency of calm vocalizations and total number of errors at 9 months (r=−0.76, p=0.001) (Fig 4 A and B). While there was no significant linear relationship between social play and learning ability, a U shape curve was found between these variables, with low and high levels of social play at 5 months associated with more errors at 9 months than moderate levels of social play (see Fig 5). Mann Whitney U tests found no significant differences in homecage behavior between learners and non-learners.
Fig 4:
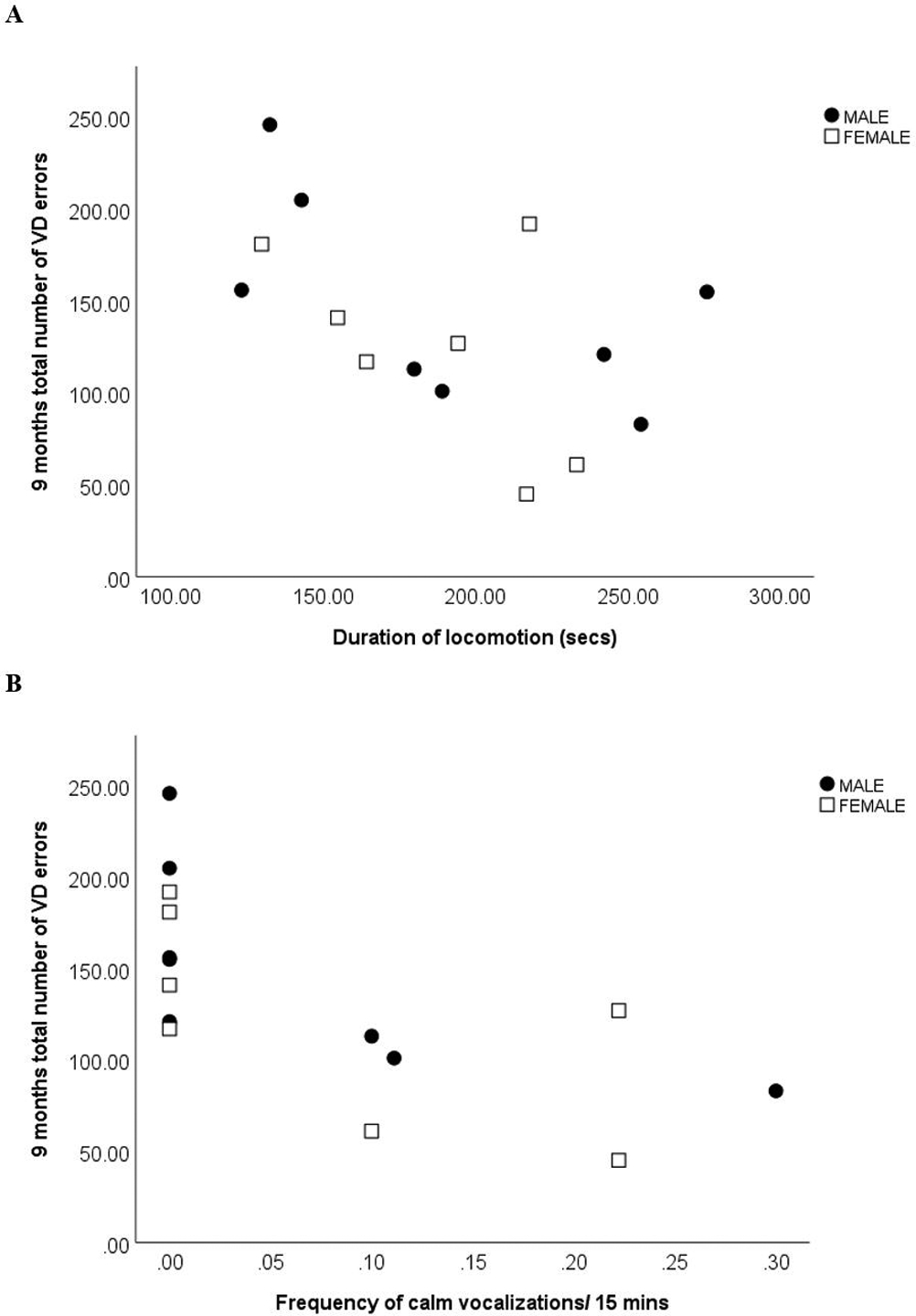
A. Significant negative correlation between total number of errors in visual discrimination (VD) learning at 9 months and duration spent in locomotion per 15 min observation at 5 months (secs) (n=15; 8 male, 7 female). More time locomoting was associated with less errors (p=0.05).
B. Significant negative correlation between total number of errors in visual discrimination (VD) learning at 9 months and frequency of calm vocalizations per 15 min observation at 5 months (n=15; 8 male, 7 female). Higher frequencies of calm vocalizations were associated with less errors (p=0.001).
Fig 5:
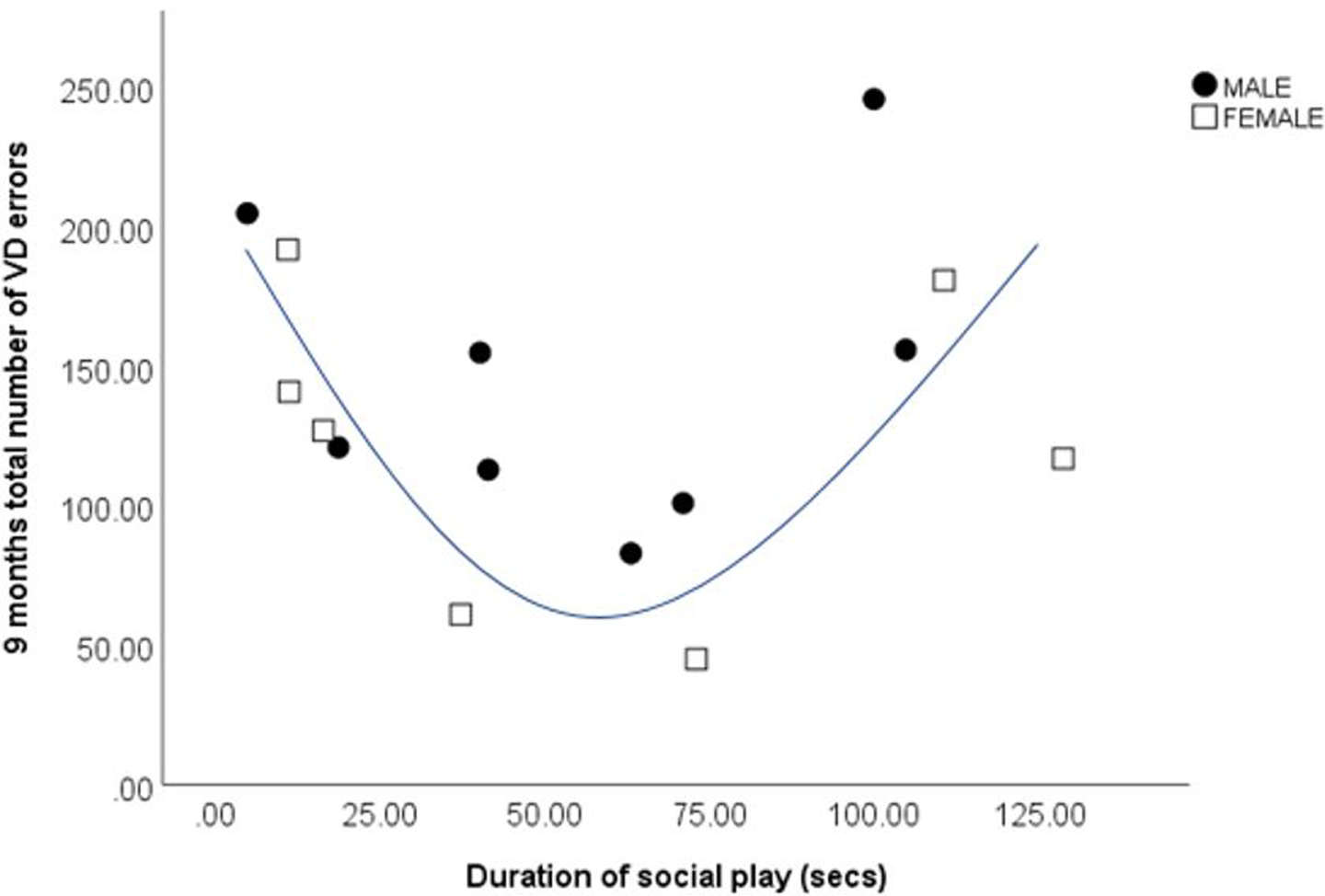
U shape curve between total number of errors in visual discrimination (VD) learning at 9 months and duration of social play per 15 min observation at 5 months (secs) (n=15; 8 male, 7 female). Moderate amounts of social play were associated with less errors.
4. Discussion
In the current study, marmosets were trained in cognitive tasks at 3 and 9 months old, as well as observed in their natal group at 5 months old, to examine relationships between learning and family behavior in infancy and adolescence, as well as to investigate the effect of family size and sex on task performance. While it was hypothesized that there may be sex and family size differences depending on the task, males and females of small and large family groups behaved similarly during cognitive testing. However, certain individual and social behaviors within the family did seem to be associated with cognition, with results also suggesting some developmental changes in ability and willingness to learn.
4.1. Learning at 3 and 9 months
Although infants are very curious and often enthusiastic learners, their attention span can be short and they may be prone to separation anxiety (Bastable and Dart, 2007). As all but 2 infant marmosets learned the simple task of touching a block to receive a food reward at 3 months old, this Wisconsin General Test Apparatus (WGTA) method proved to be an effective way of encouraging early learning in marmosets. The high level of successful task performance at this age may be due to the infant marmoset’s willingness to interact with their environment, with studies in wild marmosets also finding that infants spend more time close to a novel apparatus (Gunhold et al, 2014) and attending to the foraging behaviour of subadult and adult group members (Schiel and Huber, 2006).
Building on their learning at 3 months old, the monkeys then received a visual discrimination (VD) task at 9 months old. All remembered the initial task of touching the rewarded object (although 3 did not consistently respond), despite previous training occurring 6 months prior. While 6 early adolescents learned the VD task, the rest (n=9) participated in the sessions but did not learn the task, instead continuing to touch the unrewarded stimuli as well as the rewarded stimuli. Previous studies have found that juveniles spend more time manipulating a novel apparatus than adults, which may be due to different levels of motivation or reward feedback (Gunhold et al, 2014). Alternatively, while their independence and well-developed cognitive abilities allow them to learn more from their environment than infants (Schiel and Huber, 2006), adolescents may lack cognitive control (Bastable & Dart, 2007), including the ability to pay attention and inhibit habitual responses. Highly practiced responses (such as the touch response learned at 3 months) can become very efficient and inflexible, and so the best evidence for cognitive control is often found in how well an individual can inhibit these strongly associative responses in favor of a more deliberate, flexible one (Beran et al, 2016). Cognitive control could still be developing in 9-month-old marmosets, which may explain why the majority of marmosets in the current study failed to learn the VD task.
While we did expect that if an individual learned quicker at 3 months, they would also learn quicker at 9 months, there was no significant relationship found between learning time and number of errors at 3 and 9 months old. This lack of correlation may be because most infants learned the task quickly at 3 months, again suggesting the success of the method in allowing very young marmosets to learn simple tasks comfortably. This task may, however, be a better representation of motivation, rather than learning per se (eg. operant response tasks: Pryce et al, 2005). Individuals may be more consistent in their response to learning tasks at later ages. As we will continue to follow these marmosets up to 2 years of age, we will better be able to pinpoint when abilities are formed in relation to other developmental milestones (eg. Missler et al, 1992; Yamamoto et al, 1993), and how they change over time. The current results, together with previous work, do however suggest that there are differences in willingness and ability to learn at different ages in marmosets, with individuals varying greatly in their social and cognitive development during the adolescent period (humans: Bastable & Dart, 2007).
4.2. Effect of sex and family size
In the current study, contrary to predictions, no differences were found between males and females in learning ability at 3 and 9 months old. The lack of sex differences in early life is however similar to previous results in adolescent and young adult marmosets (Takemoto et al, 2015). While no sex differences in initial discriminations have been found in middle-aged marmosets, adult males have been found to be more successful than adult females in reversal tasks (LaClair, 2019). As we will continue to follow these marmosets throughout their early life to adulthood, there may be more apparent sex differences in older marmosets given more complex cognitive tasks involving reward and punishment, which could be related to effects of reproductive hormones.
There were also no differences found in learning ability between marmosets from small and large family groups (once 2 outliers from large families with particularly high numbers of errors were removed). As most of our captive families were relatively small and stable (between 4 and 6 individuals), it is likely that family dynamics were similar between groups (eg. Box, 1975; Yamamoto et a, 1993), and so studying larger differences in group size and composition may reveal more significant influences on cognition. Comparative studies have however questioned the idea that variation in social structure drives differences in cognitive abilities (eg. Holekamp, 2007; DeCasien et al, 2017). Individual factors may also have more of an effect on learning at this age, such as personality, physical predispositions and reactivity towards humans (Bliss-Moreau & Moadab, 2016; Schubiger et al, 2019). However, further tests would be necessary to confirm this.
4.3. Relationships between behavior and learning
Although at 3 months old there was a trend for high durations of proximity to a family member to be associated with more learning errors, supporting studies of parental style in macaques (review by Parker and Maestripieri, 2011), this result was no longer significant once 2 outliers with particularly high numbers of errors were removed. Several significant relationships were however found between homecage behavior and learning at 9 months old. Individuals that spent more time in locomotion and had more calm vocalizations during family interactions at 5 months performed better in the visual discrimination task at 9 months, suggesting that more independent, calm interactions with surroundings may be associated with enhanced learning.
Previous research has found that animals who are more bold or explorative of novelty tend to be more willing to interact with a novel task and so are more able to solve simple food-related problems than more reserved animals (Bowell, 2010: Callithrix jacchus; Carter et al, 2013: Papio ursinus). Active, independent engagement of the environment may therefore be essential for some aspects of cognitive development (Menzel et al, 1963: Pan troglodytes). Alternatively, individuals with calmer responses may be more attentive to changes in the environment (eg. Koolhaas et al, 2007) or more willing to participate in cognitive tasks (Schubiger et al, 2015). Morton et al (2013) found that brown capuchins with low levels of attention during a cognitive task were less vigilant in the homecage, and were rated higher on traits associated with impulsiveness and lack of calm, stable behavior. Alternatively, monkeys with high levels of attention during the cognitive task spent more time vigilant, less time playing and being groomed, and were rated higher on traits indicating pro-social tendencies. Further, more socially tolerant species of macaque (Macaca sylvanus; M. tonkeana) have been found to perform better at a social cognitive task involving cooperation and at an inhibitory control task, compared to less tolerant species (M. mulatta; M. fascicularis) (Pelakanos et al, 2017). These types of behaviors may therefore be most associated with learning. However, an individual’s cognitive performance may be underestimated if they are unwilling to perform a task (Carter et al, 2013). Cognitive ability, or at least willingness to participate, at 9 months may therefore be a reflection of differences in family interactions at younger ages.
The relationship between play and cognition is well established (Whitebread et al, 2009), with play long regarded as a vital element of normal growth and development (Piaget, 1963: humans), allowing young animals to interact with the world around them, overcome fears and practice complex patterns of adult behavior (Box, 1975), as well as develop motor skills (Byer and Walker, 1995) and socio-emotional intelligence (Panksepp & Biven, 2012). While there was no linear relationship between play and learning ability in the current study, a U shape curve was found, with both low and high levels of play associated with more errors than those with moderate amounts of play. While low levels of play may not allow young animals to develop their skills, it is likely that a certain amount of play is beneficial. However, high levels of play may impede learning. Distractibility was a problem in the current study (eg: Schubiger et al, 2015), with subjects spending a large amount of the 9-month training sessions playing. As increased rough play with siblings is very common at 5–10 months (Box, 1975), this may be a characteristic response for juvenile marmosets during our cognitive testing.
4.4. Refined testing of marmoset cognitive development
While previous scales in common marmosets have been developed (eg. Primate postnatal neurodevelopment assessment scale for marmosets (PNAS-M): Braun et al, 2015), they are limited to very early life and generally assess motor function, rather than cognition. As immature animals may need different tests than fully grown adult animals, methods of evaluation that are both age- and species-appropriate are needed to quantify normal marmoset cognitive development (Schultz-Daren et al, 2016). The methods used in the current study therefore offer refined ways to assess cognitive maturation in marmosets over time. Future research will continue to look at differences in learning from infancy to adulthood, and combine this data with methods of quantifying affective state in animals (eg. Harding et al, 2004), as well as novel brain imaging techniques (eg. Silvas et al, 2011), to further increase our understanding of primate cognitive development.
5. Conclusion
Many studies have documented the long-term effect of early experience on development. The current study investigated the effect of sex and family size on learning in infancy and adolescence in common marmosets, as well as the relationship between family behavior and learning. While sex and family size did not have an effect, positive family interactions, including moderate levels of play and calm individual behavior, may be important in aiding cognition. There may also be differences in willingness and ability to learn at different developmental stages. This study therefore sheds light on learning in much younger marmosets than previously studied, and highlights the importance of longitudinal research when investigating individual differences in cognitive development.
Acknowledgements
We thank the NIH for their funding and support of the project (R01 HD079493 to TEZ and RJC). This publication was made possible in part by NCRR/ORIP grants P51RR000167/ P51OD011106 to the Wisconsin National Primate Research Center (WNPRC), University of Wisconsin-Madison. We are grateful to Bruce Pape for manufacturing the cognitive testing apparatus, as well as all the staff at the WNPRC colony for their assistance and care of the marmosets, particularly Megan Sosa. We also thank Kirsten Solonika and our undergraduate students (Natalie Dukes, Erik Meitz, Hannah Giammarco, Kira Smiley, Margaret Ford, Morgan Edar and Samantha Busch) for their help collecting behavioral data.
Footnotes
Conflict of interests: The authors declare that there are no conflict of interests.
Data Availability Statement: The datasets analyzed in the current study are available from the corresponding author on reasonable request.
References
- Abbott DH (1984). Behavioral and physiological suppression of fertility in subordinate marmoset monkeys. American Journal of Primatology, 6, 169–186. DOI: 10.1002/ajp.1350060305 [DOI] [PubMed] [Google Scholar]
- Arruda MF, Yamamoto ME & Bueno OFA (1986). Interactions between parents and infants and infants-father separation in the common marmoset (Callithrix jacchus). Primates, 27: 215–228. DOI: 10.1007/BF02382600 [DOI] [Google Scholar]
- Ashton BJ, Ridley AR, Edwards EK & Thornton A (2018). Cognitive performance is linked to group size and affects fitness in Australian magpies. Nature, 554, 364–367. DOI: 10.1038/nature25503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardi M, Huffman MA (2006). Maternal behavior and maternal stress are associated with infant behavioral development in macaques. Developmental Psychobiology, 48: 1–9. DOI: 10.1002/dev.20111 [DOI] [PubMed] [Google Scholar]
- Bastable SB & Dart MA (2011). Chapter 5: Developmental stages of the learner In: Health Professional as Educator: Principles of teaching and learning. Burlington: Jones and Bartlett Publishers; (pp 151–197). [Google Scholar]
- Bezerra BM & Souto A (2008). Structure and usage of the vocal repertoire of Callithrix jacchus. International Journal of Primatology, 29, 671–701. DOI: 10.1007/s10764-008-9250-0 [DOI] [Google Scholar]
- Beran MJ, Menzel CR, Parrish AE, Perdue BM, Sayers K, Smith DJ & Washburn DA (2016). Primate cognition: Attention, episodic memory, prospective memory, self-control, and metacognition as examples of cognitive control in nonhuman primates. Wiley Interdisciplinary Reviews: Cognitive Science, 7, 294–316. DOI: 10.1002/wcs.1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss-Moreau E & Moadab G (2016). Variation in behavioral reactivity is associated with cooperative restraint training efficiency. Journal of the American Association for Laboratory Animal Sciences, 55, 41–49. [PMC free article] [PubMed] [Google Scholar]
- Bowell VA (2010). Improving the welfare of laboratory-housed primates through the use of positive reinforcement training: Practicalities of implementation Unpublished PhD thesis. University of Stirling; URI: hdl.handle.net/1893/3442 [Google Scholar]
- Box HO (1975). Quantitative studies of behaviour within captive groups of marmoset monkeys (Callithrix jacchus). Primates, 16: 155–174. DOI: 10.1007/BF02381414 [DOI] [Google Scholar]
- Braun K, Schultz-Darken N, Schneider M, Moore CF, Emborg ME. (2015). Development of a novel postnatal neurobehavioral scale for evaluation of common marmoset monkeys. American Journal of Primatology, 77, 401–417. DOI: 10.1002/ajp.22356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers NR & Locke-Haydon J (1984). Correlations among measures of playfulness and skillfulness in captive common marmosets (Callithrix jacchus jacchus). Developmental Psychobiology, 17, 191–208. DOI: 10.1002/dev.420170209 [DOI] [PubMed] [Google Scholar]
- Cross N & Rogers LJ (2006). Mobbing vocalizations as a coping response in the common marmoset. Hormones and Behavior, 49, 237–245. DOI: 10.1016/j.yhbeh.2005.07.007 [DOI] [PubMed] [Google Scholar]
- DeCasien AR, Williams SA & Higham JP (2017). Primate brain size is predicted by diet but not sociality. Nature Ecology and Evolution, 1, 112 DOI: 10.1038/s41559-017-0112 [DOI] [PubMed] [Google Scholar]
- Dettling AC, Schnell CR, Maier C, Feldon J & Pryce CR (2007). Behavioural and physiological effects of an infant-neglect manipulation in a bi-parental, twinning primate: Impact is dependent on familial factors. Psychoneuroendocrinology, 32: 331–349. DOI: 10.1016/j.psyneuen.2007.01.005 [DOI] [PubMed] [Google Scholar]
- Digby LJ & Barreto CE (1993). Social organization in a wild population of Callithrix jacchus. I. Group composition and dynamics. Folia Primatologica, 61, 123–134. DOI: 10.1159/000156739 [DOI] [PubMed] [Google Scholar]
- Epple G (1968). Comparative studies on vocalization in marmoset monkeys (Hapalidae). Folia Primatologica, 8, 1–40. DOI: 10.1159/000155129 [DOI] [PubMed] [Google Scholar]
- Fairbanks LA & McGuire MT (1993). Maternal protectiveness and response to the unfamiliar in vervet monkeys. American Journal of Primatology, 30: 119–129. DOI: 10.1002/ajp.1350300204 [DOI] [PubMed] [Google Scholar]
- Gunhold T, Whiten A & Bugnyar T (2014). Video demonstrations seed alternative problem-solving techniques in wild common marmosets. Biology Letters, 10, 1–5. DOI: 10.1098/rsbl.2014.0439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halsey LG, Bezerra BM & Souto AS (2006). Can wild common marmosets (Callithrix jacchus) solve the parallel strings task? Animal Cognition, 9, 229–233. DOI: 10.1007/s10071-006-0016-9 [DOI] [PubMed] [Google Scholar]
- Harding EJ, Paul ES & Mendl M (2004). Animal behaviour: cognitive bias and affective state. Nature, 427: 312 DOI: 10.1038/427312a [DOI] [PubMed] [Google Scholar]
- Holekamp KE. (2007). Questioning the social intelligence hypothesis. Trends in Cognitive Science, 11, 65–69. DOI: 10.1016/j.tics.2006.11.003 [DOI] [PubMed] [Google Scholar]
- Ingram JC (1977). Interactions between parents and infants, and the development of independence in the common marmoset (Callithrix jacchus). Animal Behaviour, 25, 811–827. DOI: 10.1016/0003-3472(77)90035-5 [DOI] [Google Scholar]
- Kendrick KM & Dixson AF (1983). The effect of the ovarian cycle on the sexual behavior of the common marmoset (Callithrix jacchus). Physiology and Behavior, 30, 735–42. DOI: 10.1016/0031-9384(83)90171-3 [DOI] [PubMed] [Google Scholar]
- Kerney M, Smaers JB, Schoenemann PT & Dunn JC (2017). The coevolution of play and the cortico-cerebella system in primates. Primates, 58, 485–491. DOI: 10.1007/s10329-017-0615-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolhaas JM, de Boer SF, Buwalda B &van Reenen K (2007). Individual variation in coping with stress: a multidimensional approach of ultimate and proximate mechanisms. Brain, Behavior and Evolution, 70, 218–226. DOI: 10.1159/000105485 [DOI] [PubMed] [Google Scholar]
- LaClair M, Febo M, Nephew B, Gervais NJ, Poirier G, Workman K, Chumachenko S, Payne L, Moore MC, King JA & Lacreuse A (2019). Sex differences in cognitive flexibility and resting brain networks in middle-aged marmosets. Cognition and Behavior, 6, 1–19. DOI: 10.1523/ENEURO.0154-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestripieri D (1998). Social and demographic influences on mothering style in pigtail macaques. Ethology, 104: 379–385. DOI: 10.1111/j.1439-0310.1998.tb00076.x [DOI] [Google Scholar]
- Mendes N & Huber L (2004). Object permanence in common marmosets (Callithrix jacchus). Journal of Comparative Psychology, 118, 103–112. DOI: 10.1037/0735-7036.118.1.103 [DOI] [PubMed] [Google Scholar]
- McGrew WC (1988). Parental division of infant caretaking varies with family composition in cotton-top tamarins. Animal Behaviour, 36: 285–286. DOI: 10.1016/S0003-3472(88)80270-7 [DOI] [Google Scholar]
- Menzel EW, Davenport RK & Rodgers CM (1963). The effects of environmental restriction upon the chimpanzee’s responsiveness to objects. Journal of Comparative and Physiological Psychology, 56: 78–85. DOI: 10.1037/h0047360 [DOI] [PubMed] [Google Scholar]
- Missler M, Wolff JR, Rothe H, Merker HJ, Treiber A, Schneider R & Crook GA (1992). Developmental biology of the common marmoset: Proposal for a “postnatal staging”. Journal of Medical Primatology, 21, 285–298. [PubMed] [Google Scholar]
- Montgomery SH (2014). The relationship between play, brain growth and behavioural flexibility in primates. Animal Behaviour, 90, 281–286. DOI: 10.1016/j.anbehav.2014.02.004 [DOI] [Google Scholar]
- Morton BF, Lee PC Buchanan-Smith, Brosnan SF, Thierry B, Paukner A, de Waal FBM, Widness J, Essler JL & Weiss A (2013). Personality structure in brown capuchin monkeys (Sapajus Apella): comparisons with chimpanzees (Pan Troglodytes), orangutans (Pongo Spp.) and rhesus macaques (Macaca Mulatta). Journal of Comparative Psychology, 127, 282–298. DOI: 10.1037/a0031723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp J & Biven L (2012). The archaeology of mind: neuroevolutionary origins of human emotions. New York: Norton. [Google Scholar]
- Parker KJ & Maestripieri D (2011). Identifying key features of early stressful experiences that produce stress vulnerability and resilience in primates. Neuroscience and Biobehavioural Reviews, 35: 1466–1483. DOI: 10.1016/j.neubiorev.2010.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellis SM, Burghardt GM, Palagi E & Mangel M (2015) Modeling play: distinguishing between origins and current functions. Adaptive Behaviour, 23,331–339. DOI: 10.1177/1059712315596053 [DOI] [Google Scholar]
- Pesendorfer MB, Gunhold T, Schiel N, Souto A, Huber L & Range F (2009). The maintenance of traditions in marmosets: Individual habit, not social conformity? A field experiment. PLoS ONE, 4: e4472 DOI: 10.1371/journal.pone.0004472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piaget J (1963). The origins of intelligence in children. New York: Norton. [Google Scholar]
- Pryce CR, Dettling AC, Spengler M, Schnell CR & Feldon J (2004). Deprivation of parenting disrupts development of homeostatic and rewards systems in marmoset monkey offspring. Biological Psychiatry, 56, 72–79. DOI: 10.1016/j.biopsych.2004.05.002 [DOI] [PubMed] [Google Scholar]
- Pryce CR, Ruedi-Bettschen D, Dettling AC, Weston A, Russig H, Ferger B & Feldon J (2005). Long-term effects of early-life environmental manipulations in rodents and primates: Potential animal models in depression research. Neuroscience and Biobehavioural Reviews, 29: 649–674. DOI: 10.1016/j.neubiorev.2005.03.011 [DOI] [PubMed] [Google Scholar]
- Reader SM, Hager Y & Laland KN (2011). The evolution of primate general and cultural intelligence. Philosophical Transections of the Royal Society of London B: Biological Sciences, 366, 1017–1027. DOI: 10.1098/rstb.2010.0342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito A (2015). The marmoset as a model for the study of primate parental behavior. Neuroscience Research, 93, 99–109. DOI: 10.1016/j.neures.2014.12.011 [DOI] [PubMed] [Google Scholar]
- Saltzman W, Severin JM, Schultz-Darken NJ & Abbott DH (1997). Behavioral and social correlates of escape from suppression of ovulation in female common marmosets with the natal family. American Journal of Primatology, 41, 1–21. DOI: [DOI] [PubMed] [Google Scholar]
- Savage A, Snowdon CT, Giraldo H, & Soto H (1996). Parental care patterns and vigilance in wild cotton-top tamarins (Saguinus oedipus) In Norconk M, Rosenberger A, & Garber PA (Eds.), Adaptive radiations of neotropical primate. New York: Plenum Press; (pp. 187–199). [Google Scholar]
- Schiel N & Huber L (2006). Social influences on the development of foraging behavior in free-living common marmosets (Callithrix jacchus). American Journal of Primatology, 68, 1150–1160. DOI: 10.1002/ajp.20284 [DOI] [PubMed] [Google Scholar]
- Schiel N, Souto A, Huber L & Bezerra BM (2010). Hunting strategies in wild common marmosets are prey and age dependent. American Journal of Primatology, 72, 1039–1046. DOI: 10.1002/ajp.20860 [DOI] [PubMed] [Google Scholar]
- Schiel N & Souto A (2017). The common marmoset: An overview of its natural history, ecology and behavior. Developmental Neurobiology, 77, 244–262. DOI: 10.1002/dneu.22458 [DOI] [PubMed] [Google Scholar]
- Schubiger MN, Kissling A & Burkart J (2019). Does opportunistic testing bias cognitive performance in primates? Learning from drop-outs. Plos One, 14, 1–22. DOI: 10.1371/journal.pone.0213727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubiger MN, Wustholz FL, Wunder A & Burkart JM (2015). High emotional reactivity toward an experimenter affects participation, but not performance, in cognitive tests with common marmosets (Callithrix jacchus). Animal Cognition, 18, 701–712. DOI: 10.1007/s10071-015-0837-5 [DOI] [PubMed] [Google Scholar]
- Schultz-Darken N, Braub KM & Emborg ME (2016). Neurobehavioral development of common marmoset monkeys. Developmental Psychobiology, 58, 141–158. DOI: 10.1002/dev.21360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvas AC, Liu JV, Hirano Y, Leoni RF, Merkle H, Mackel JB, Zhang XF, Nascimento GC & Stefanovic B (2011). Longitudinal functional magnetic resonance imaging in animal models. Methods in Molecular Biology, 711, 281–302. DOI: 10.1007/978-1-61737-992-5_14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson MF (1978). The behaviour and ecology of the common marmoset (Callithrix jacchus jacchus) in its natural environment In: Rothe H, Wolters HJ & Hearn JP (Eds.) Biology and Behaviour of Marmosets. Göttingen: Eigenverlag H. Rothe; (pp. 298). [Google Scholar]
- Stevenson MF & Poole TB (1976). An ethogram of the common marmoset (Callithrix jacchus jacchus): General behavioral repertoire. Animal Behaviour, 24, 428–451. DOI: 10.1016/s0003-3472(76)80053-x [DOI] [PubMed] [Google Scholar]
- Stevenson MF & Poole TB (1982). Playful interactions in family groups of the common marmoset (Callithrix jacchus jacchus). Animal Behaviour, 30, 886–900. DOI: 10.1016/S0003-3472(82)80163-2 [DOI] [PubMed] [Google Scholar]
- Takemoto A, Miwa M, Koba R, Yamaguchi C, Suzuki H & Nakamura K (2015). Individual variability in visual discrimination and reversal learning performance in common marmosets. Neuroscience Research, 93, 136–143. DOI: 10.1016/j.neures.2014.10.001 [DOI] [PubMed] [Google Scholar]
- Tunç B, Solmaz B, Parker D, Satterthwaite TD, Elliott MA, Calkins ME, Ruparel K, Gur RE, Gur RC & Verma R (2016). Establishing a link between sex-related differences in the structural connectome and behaviour. Philosophical Transactions of the Royal Society B Biological Sciences, 371: 20150111 DOI: 10.1098/rstb.2015.0111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelkl B & Huber L (2000). True imitation in marmosets. Animal Behavior, 60, 195–202. DOI: 10.1006/anbe.2000.1457 [DOI] [PubMed] [Google Scholar]
- Werdenich D & Huber L (2002). Social factors determine cooperation in marmosets. Animal Behaviour, 64, 771–781. DOI: 10.1006/anbe.2002.9001 [DOI] [Google Scholar]
- Whitebread D, Coltman P, Jameson H & Lander R (2009). Play, cognition and self-regulation: What exactly are children learning when they learn through play? Educational and Child Psychology, 26, 40–52. Corpus ID: 150255306 [Google Scholar]
- Yamamoto ME (1993). From dependence to sexual maturity: the behavioural ontogeny of Callitrichidae In Rylands AB (Ed.) Marmosets and Tamarins: Systematics, behaviour, and ecology New York: Oxford University Press. [Google Scholar]
- Yamamoto ME, Domeniconi C & Box H (2004). Sex differences in common marmosets (Callithrix jacchus) in response to an unfamiliar food task. Primates, 45, 249–254. DOI: 10.1007/s10329-004-0088-6 [DOI] [PubMed] [Google Scholar]


