Abstract
Purpose
Pancreatic ductal adenocarcinomas exhibit a high degree of desmoplasia due to extensive extracellular matrix deposition. Encasement of mesenteric vessels by stroma in locally advanced pancreatic cancer (LAPC) prevents surgical resection. This study sought to determine if the addition of a monoclonal antibody to connective tissue growth factor, pamrevlumab, to neoadjuvant chemotherapy would be safe and lead to improved resectability in this surgically adverse patient population.
Methods
In this phase I/II trial, 37 patients with LAPC were randomised 2:1 to gemcitabine/nab-paclitaxel plus (Arm A, n=24) or minus (Arm B, n=13) pamrevlumab. Those who completed six cycles of treatment were assessed for surgical eligibility by protocol-defined criteria. Resection rates, progression-free and overall survival were evaluated.
Results
Eighteen (75%) patients in Arm A and seven (54%) in Arm B completed six cycles of therapy with similar toxicity patterns. In Arms A and B, carbohydrate antigen 19–9 response, as defined by ≥50% decline from baseline, occurred in 13 (65%) and 5 (42%), respectively. Sixteen (16%) per cent of patients were radiographically downstaged by National Comprehensive Cancer Network criteria (5 in Arm A (21%) and 1 (8%) in Arm B). Positron emission tomography normalised in 9 (38%) vs 3 (23%) of patients in Arm A vs Arm B, respectively, and correlated with surgical exploration. Eligibility for surgical exploration was 17 (71%) vs 2 (15%) (p=0.0019) and resection was achieved in 8 (33%) vs 1 (8%) of patients in Arm A vs Arm B (p=0.1193), respectively. Postoperative complication rates were not different between arms.
Conclusions
Neoadjuvant chemotherapy with pamrevlumab holds promise for enhancing resection rates in patients with LAPC without added toxicity. This combination merits evaluation in a larger patient cohort.
Keywords: Pancreatic ductal adenocarcinoma, desmoplasia, connective tissue growth factor, monoclonal antibody
Key questions.
What is already known about this subject?
Pamrevlumab is anti CTGF-1 inhibitor, highly safe when given to patients with pancreatic cancer and potentially adds to the activity of gemcitabine and erlotinib when given in metastatic disease
What does this study add?
This study examines a) the safety and activity of pamrevlumab when added to gemcitabine and nab-paclitaxel in locally advanced pancreatic cancer b) the impact of this regimen and surgical resection and postoperative safety c) explores the utility of a novel study design for locally advanced pancreatic cancer
How might this impact clinical practice?
This study has the potential to lead to practice changing activity in locally advanced pancreatic cancer via 1) the eventual approval of pamrevlumab for use in this situation 2) the promulgation of a new study design for locally advanced pancreatic cancer and 3) increased potential for surgical resection (and thus, prolonged OS/ curability) in locally advanced pancreatic cancer
Introduction
Pancreatic cancer is currently the third leading cause of cancer death in the USA,1 and by 2020, it will likely become the second leading cause of cancer-related death after lung cancer, surpassing breast and colon cancer.2 Surgical resection is generally necessary for treatment with curative intent or to extend life expectancy.3 However, only 15% of patients have disease amenable to upfront curative resection at the time of diagnosis.4 Approximately 25%–30% of patients are diagnosed with locally advanced disease5 determined surgically unresectable per National Comprehensive Cancer Network (NCCN) guidelines.6 Patients with locally advanced pancreatic cancer (LAPC) have a prognosis similar to those with metastatic disease, with a historical median overall survival (OS) of 9–12 months with recent trials demonstrating median OS of 16 months.7 Recent single-institution, retrospective studies have reported the potential for resection of LAPC with neoadjuvant therapy irrespective of imaging findings, with promising results.8 9 However, these are limited by significant selection bias, lack of strict radiographic classification and chemotherapy standardisation. Current prospective trials have documented resection rates of LAPC in the range of 4%10 to 15%;11 therefore, novel approaches are needed to improve patient outcomes.
The tumour biology inherent to pancreatic ductal adenocarcinoma (PDAC) significantly contributes to the poor outcomes seen in this disease. Notably, PDAC exhibits a high degree of desmoplasia, often in association with elevated connective tissue growth factor (CTGF) expression.12 CTGF appears to play a central role in the biology of pancreatic cancer, affecting both its cellular biology and its extracellular matrix composition. This leads to many simultaneous biological effects that promote pancreatic cancer growth, including increased cellular proliferation and differentiation, increased cellular adherence and migration, antiapoptosis, vascular permeability, angiogenesis and suppression of tumour immunological responses.13 This stroma may also contribute to the radiographic imaging findings of mesenteric vessel involvement or encasement that is used to determine resectability of pancreatic tumours. Executing a pharmacological intervention on the pancreatic cancer stromal environment is therefore a major goal of the development of novel pancreatic cancer therapeutics.
Pamrevlumab is a human monoclonal antibody that targets CTGF. Preclinical studies showed that CTGF overexpression is associated with both desmoplasia and gemcitabine resistance in the KPC pancreatic cancer mouse model.14 When pamrevlumab was used in combination with gemcitabine, sensitivity to gemcitabine was enhanced, which correlated with inhibition of XIAP, an antiapoptotic protein.15 When tested in patients with advanced pancreatic cancer (88% Stage IV and 12% locally advanced Stage III) treated with gemcitabine and erlotinib in a phase I/II study (n=75), pamrevlumab displayed multiple favourable outcomes.16
We hypothesised that through inhibition of the downstream effects of CTGF overexpression on tissue adhesion and other mechanisms, pamrevlumab may influence resectability of PDAC tumours. With this in mind, this novel phase I/II, randomised, multicentre trial was designed to explore the safety and efficacy of pamrevlumab in combination with gemcitabine/nab-paclitaxel in LAPC with special emphasis on surgical eligibility and safety.
Methods
Study design
This was a phase I/II randomised trial of safety and efficacy in patients with LAPC who received gemcitabine and nab-paclitaxel with or without pamrevlumab as neoadjuvant therapy. The randomisation was preplanned and blinded to the investigator. The study was approved by individual institutional review boards at nine US institutions and conducted according to the Declaration of Helsinki. The trial was registered at clinicaltrials.gov as NCT 02210559.
Eligibility
Key protocol eligibility requirements included biopsy-proven diagnosis of PDAC, radiographic staging consistent with locally advanced unresectable disease as defined NCCN guidelines (V.2, 2014), clinical stage confirmed by diagnostic laparoscopy, radiographically measurable disease per Response Evaluation Criteria in Solid Tumors (RECIST) V.1.1, Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, adequate haematological, renal and hepatic function, no prior therapy for PDAC and no concomitant cancer diagnosis within the past 5 years.
Study schema
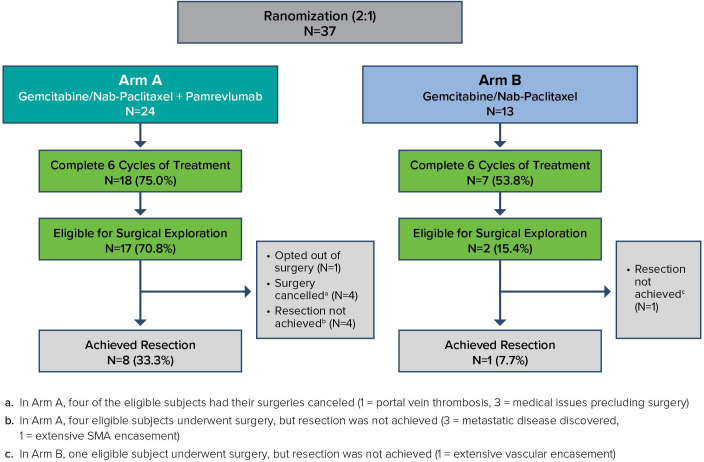
Eligible patients were randomised 2:1 to Arm A or Arm B to receive a total of six treatment cycles (24–28 weeks) of therapy (figure 1). Patients in Arm A received pamrevlumab (35 mg/kg by intravenous infusion on Days 1 and 15 of each 28 day cycle with an additional dose given on Day 8 in the first cycle). Patients in both Arms A and B received gemcitabine (1000 mg/m2 by intravenous infusion on Days 1, 8 and 15 of each 28-day treatment cycle), nab-paclitaxel (125 mg/m2 by intravenous infusion on Days 1, 8 and 15 of each 28-day cycle). Doses for gemcitabine and nab-paclitaxel were modified for haematological and non-haematological toxicity as per standard of care (SOC).15 Patients remained on therapy for six treatment cycles (24–28 weeks) unless they had disease progression, an intolerable adverse event (AE) or toxicity, withdrew consent or were withdrawn at the investigator’s discretion. All patients were followed for drug toxicity until 28 days after the last drug dose. Patients undergoing surgery were followed for 30 days following hospital discharge for surgical complications. CTGF levels were obtained prior to treatment from all patients. Plasma samples for pamrevlumab level determination were obtained from all patients receiving this drug. After all protocol-specified therapy was completed; patients were followed for disease progression, survival and additional oncological therapy. Postoperative complications including 30-day readmissions and 90-day mortality were noted.
Figure 1.
Patient flow and surgery outcomes. In Arm A, four of the eligible subjects had their surgeries cancelled (1=portal vein thrombosis, 3=medical issues precluding surgery). In Arm A, four eligible subjects underwent surgery, but resection was not achieved (3=metastatic disease discovered, 1=extensive SMA encasement). In Arm B, one eligible subject underwent surgery, but resection was not achieved (1=extensive vascular encasement). SMA, superior mesenteric artery.
Response assessment
Patients were evaluated for response by the following measures: carbohydrate antigen (CA) 19–9 measured at baseline, first day of each cycle and end of treatment (EOT), RECIST (V.1.1) read based on full body CT imaging (high-resolution dual phase, fine cut CT imaging) at baseline and every 8 weeks thereafter, fluorodeoxyglucose (FDG)-positron emission tomography (PET) imaging and NCCN (V.2, 2014) resectability criteria at baseline and EOT.
Surgical assessment
Subjects who finished six cycles of combination chemotherapy were evaluated for eligibility for surgical exploration per protocol (PP)-defined criteria. Given that patients included in the trial were determined to be initially unresectable by radiographic imaging and NCCN criteria, objective criteria were developed to standardise attempts at surgical resection.
Patients were deemed eligible for surgery if one or more of the following criteria were met: (1) reduction in plasma CA 19–9 level by ≥50% at EOT compared with baseline; (2) reduction in FDG-PET maximum standardised uptake value (SUVmax) by ≥30% at EOT compared with baseline; (3) radiological tumour response per RECIST 1.1 of partial response (PR) or complete response (CR) at EOT or (4) met the definition of resectable or borderline resectable per NCCN guidelines. Subjects were classified as ineligible for surgical exploration if any of the following occurred: (1) development of distant metastases or local progression on CT scan; (2) tumour anatomy precluding vascular reconstruction (unreconstructible); (3) local complications preventing surgery (eg, portal vein (PV)/splenic vein thrombosis, pancreatitis) or (4) decline in performance status to a Karnofsky score ≤50% or absolute contraindication to surgery from comorbidity (eg, recovery from myocardial infarction or uncontrolled diabetes). The final decision regarding whether resection was to be performed was made by the treating surgeon.
Endpoints
Safety endpoints included serious adverse events (SAE) during neoadjuvant therapy and surgical complications postresection. The efficacy endpoints included: surgical eligibility, R0 resection, R0/R1 resection, median OS, progression-free survival (PFS) and 1-year survival rate. All patients were followed and data analysis was stratified by PP population and intention to treat (ITT) cohort.
Statistical considerations
The comparison between selected clinical characteristics, toxicity profiles and eligibility for surgical exploration or completed surgical resection was performed using the χ² test. Exact 95% CIs for the point estimates as well as the treatment difference were obtained from the SAS PROC FREQ procedure with the EXACT option. The two treatment arms were compared using the Cochran-Mantel-Haentzel test controlling for baseline factors (TNM stage, ECOG, CA 19–9, PET-SUVmax, superior mesenteric artery (SMA) involvement, coeliac abutment and so on, as prespecified in the protocol). All-cause mortality was used in determining OS, which was analysed by the Kaplan-Meier method. Survival status was updated within 1 month before the data-cut-off date. Data from patients who were alive at the cut-off date were censored for survival analysis. All statistical tests were performed at the significance level of α=0.05, using two-sided tests.
Results
Patient characteristics and disposition
Thirty-seven patients were randomised (2:1) to study treatment: 24 to Arm A (pamrevlumab+gemcitabine/nab-paclitaxel) and 13 to Arm B (gemcitabine/nab-paclitaxel alone). Patient characteristics at baseline are summarised in table 1. All patients enrolled were unresectable by NCCN criteria; 30/37 patients (81%) had tumour arterial involvement (SMA encasement >180°, coeliac abutment without gastroduodenal artery involvement or aortic involvement), 2/37 (5%) inferior vena cava invasion and 14/37 (38%) unreconstructible PV/superior mesenteric vein (SMV) occlusion. A higher percentage of patients with SMA encasement >180° were randomised to Arm A (46%) vs Arm B (23%).
Table 1.
Patient characteristics
| Baseline demographics | Arm A (G/NP+P) N=24 |
Arm B (G/NP) N=13 |
Total N=37 |
| Age group | |||
| 18–64 years | 10 (41.7%) | 8 (61.5%) | 18 (48.6%) |
| 65–74 years | 10 (41.7%) | 4 (30.8%) | 14 (37.8%) |
| ≥75 years | 4 (16.7%) | 1 (7.7%) | 5 (13.5%) |
| Median | 67 | 62 | 66 |
| Sex | |||
| Male | 8 (33.3%) | 6 (46.2%) | 14 (37.8%) |
| Female | 16 (66.7%) | 7 (53.8%) | 23 (62.2%) |
| BMI (kg/m2) | |||
| Mean (SD) | 25.27 (4.303) | 26.69 (4.757) | 25.77 (4.455) |
| Median | 25.5 | 28.3 | 25.7 |
| Min, max | (18.4 to 37.5) | (19.3 to 33.8) | (18.4 to 37.5) |
| ECOG | |||
| Grade 0 | 9 (37.5%) | 7 (53.8%) | 16 (43.2%) |
| Grade 1 | 15 (62.5%) | 6 (46.2%) | 21 (56.8%) |
| TNM stage | |||
| T3 N0 M0 | 6 (25.0%) | 0 | 6 (16.2%) |
| T3 N1 M0 | 0 | 2 (15.4%) | 2 (5.4%) |
| T4 N0 M0 | 14 (58.3%) | 7 (53.8%) | 21 (56.8%) |
| T4 N1 M0 | 3 (12.5%) | 4 (30.8%) | 7 (18.9%) |
| T4 NX M0 | 1 (4.2%) | 0 | 1 (2.7%) |
| Location of the tumour in the pancreas* | |||
| Head | 18 (75.0%) | 11 (84.6%) | 29 (78.4%) |
| Body | 7 (29.2%) | 2 (15.4%) | 9 (24·.3%) |
| Tail | 1 (4.2%) | 2 (15.4%) | 3 (8.1%) |
| Median tumour size (mm) | 37 | 38 | 37 |
| Non-resectability per NCCN criterion* | |||
| >180° SMA encasement | 11 (45.8%) | 3 (23.1%) | 14 (37.8%) |
| Any coeliac abutment | 9 (37.5%) | 6 (46.2%) | 15 (40.5%) |
| Inferior vena cava invasion or encasement | 1 (4.2%) | 1 (7.7%) | 2 (5.4%) |
| Unreconstructible SMV/portal occlusion | 9 (37.5%) | 5 (38.5%) | 14 (37.8%) |
| Aortic invasion and encasement | 1 (4.2%) | 0 | 1 (2.7%) |
OK as is
*Not mutually exclusive.
BMI, body mass index; ECOG, Eastern Cooperative Oncology Group; G, gemcitabine; n, number of subjects; NCCN, National Comprehensive Cancer Network; NP, nab paclitaxel; P, pamrevlumab; PV, portal vein; SMA, superior mesenteric artery; SMV, superior mesenteric vein.
Patient disposition is summarised in figure 1. Twenty-four patients in Arm A received gemcitabine/nab-paclitaxel and pamrevlumab; 18/24 patients (75%) completed six treatment cycles. Six patients discontinued treatment early due to progressive disease (three patients), AEs (two patients) or physician decision (one patient). Thirteen patients in Arm B received gemcitabine/nab-paclitaxel; 7/13 patients (54%) completed six treatment cycles. Six patients discontinued treatment early due to progressive disease (two patients), AEs (two patients) or patient/physician decision (two patients).
Safety
SAEs are summarised in table 2. Forty-one per cent (15/37) of patients had a treatment-emergent SAE (38% Arm A, 46% Arm B). No individual toxicity category occurred with >10% frequency except systemic infection (4/37 patients, 11%). There was no demonstrable increase in any toxicity with the addition of pamrevlumab to gemcitabine/nab-paclitaxel chemotherapy.
Table 2.
Summary of treatment-emergent serious adverse events
| System organ class preferred term |
Arm A (n=24) n (%) |
Arm B (n=13) n (%) |
Overall (n=37) n (%) |
| No. (%) of patients with any treatment-emergent SAE | 9 (37.5) | 6 (46.2) | 15 (40.5) |
| Blood and lymphatic disorders | 2 (8.3) | 0 | 2 (5.4) |
| Haemolytic uremic syndrome | 1 (4.2) | 0 | 1 (2.7) |
| Lymphadenopathy | 1 (4.2) | 0 | 1 (2.·7) |
| Cardiac disorders | 0 | 2 (15.4) | 2 (5.4) |
| Cardiac failure | 0 | 1 (7.7) | 1 (2.7) |
| Supraventricular tachycardia | 0 | 1 (7.7) | 1 (2.7) |
| Gastrointestinal disorders | 3 (2.5) | 0 | 3 (8.1) |
| Ascites | 1 (4.2) | 0 | 1 (2.7) |
| Nausea | 1 (4.2) | 0 | 1 (2.7) |
| Pancreatitis | 1 (4.2) | 0 | 1 (2.7) |
| Vomiting | 1 (4.2) | 0 | 1 (2.7) |
| General disorders and administrative site conditions | 2 (8.3) | 1 (7.7) | 3 (8.1) |
| Device occlusion | 0 | 1 (7.7) | 1 (2.7) |
| Drug withdrawal syndrome | 1 (4.2) | 0 | 1 (2.7) |
| Fever | 1 (4.2) | 0 | 1 (2.7) |
| Hepatobiliary disorders | 0 | 2 (15.4) | 2 (5.4) |
| Cholangitis | 0 | 2 (15.4) | 2 (5.4) |
| Hyperbilirubinaemia | 0 | 1 (7.7) | 1 (2.7) |
| Infections | 1 (4.2) | 3 (23.1) | 4 (10.8) |
| Sepsis | 0 | 2 (15.4) | 2 (5.4) |
| Cellulitis | 0 | 1 (7.7) | 1 (2.7) |
| Urinary tract infection | 1 (4.2) | 0 | 1 (2.7) |
| Injury, poisoning and procedural complications | 1 (4.2) | 0 | 1 (2.7) |
| Craniocerebral injury | 1 (4.2) | 0 | 1 (2.7) |
| Respiratory, thoracic and mediastinal disorders | 2 (8.3) | 1 (7.7) | 3 (8.1) |
| Pneumonitis | 1 (4.2) | 1 (7.7) | 2 (5.4) |
| Pulmonary embolism | 1 (4.2) | 0 | 1 (2.7) |
| Skin and subcutaneous disorders | 1 (4.2) | 0 | 1 (2.7) |
| Rash | 1 (4.2) | 0 | 1 (2.7) |
OK as is
Response to therapy
In Arm A, 13/20 (65%) had ≥50% CA 19–9 decline at EOT, 5/24 (21%) response by RECIST (PR), 63% 15/24 (63%)≥30% decline in PET SUVmax and 21% 5/24 (21%) were radiographically downstaged by NCCN criteria. During the treatment period, the median CA 19–9 decline was 93% (4 patients were non-secretors). Seven out of 24 patients (29%) had best objective RECIST response (CR+PR). Some patients had ‘exceptional’ responses defined as normalisation or ≥95% decline of CA 19–9 (6/20 patients, 30%) or normalisation PET SUVmax in 9/24 (38%). In Arm B, 5/12 (42%) had ≥50% CA 19–9 decline at EOT, 23% 3/13 (23%) response by RECIST (PR), 7/12 (54%)≥30% decline in PET SUVmax and 1/13 (8%) were radiographically downstaged by NCCN criteria. Four out of 13 patients (31%) had best objective RECIST response (CR +PR). In Arm B, 4/12 (33%) of patients had an “exceptional” CA 19–9 response and 3/13 (23%) had an ‘exceptional’ PET response, as defined by either ≥95%/ normalized Ca 19.9 response, normalized SUV max, and/or radiographic downstaging post therapy completion.
Surgical evaluation
Overall, 19/37 (51%) of the total study patients were eligible for surgical exploration using protocol-defined criteria (17 Arm A, 2 Arm B, p=0.0019). Resection was completed in 9/37 (24%) of the patients (8 Arm A, 1 Arm B, p=0.1193). Details of the nine resected patients are shown in table 3. In Arm A, 17/24 (71%) of the patients were eligible for surgical exploration in the ITT population and 17/18 (94%) of the patients were eligible in the PP population (patients who completed six cycles of treatment). In Arm A, 12 out of 17 eligible patients ultimately underwent surgical exploration for resection (five operations were cancelled; four due to drug toxicity/medical comorbidity (sepsis, gallbladder perforation, declined performance status and fever) and one patient declined). Eight out of 37 patients (33%) in Arm A were resected (4 R0, 4 R1). The remaining four patients who were explored were not resected due to progression or unresectable disease intraoperatively. In Arm B, 2/13 (15%) of the patients were eligible for surgical exploration in the ITT population and 2/7 (29%) were eligible in the PP population. Of the two subjects found to be surgically eligible, only one was resected as the other patient had local progression.
Table 3.
Summary of resected patients
| Site- subject ID |
Treatment arm |
Response to treatment* | NCCN baseline |
NCCN end of treatment |
Resection status |
| 1001–1001 | A | 1, 2, 3 | Unresectable (coeliac) |
Unresectable (coeliac) |
R0 |
| 1001–1004 | A | 1, 2 | Unresectable (SMA, SMV) |
Unresectable (SMA, SMV) |
R1 |
| 1001–1005 | A | 1, 2 | Unresectable (coeliac) |
Unresectable (coeliac) |
R0 |
| 1001–1009 | A | 2, 4 | Unresectable (coeliac) |
Borderline resectable | R0 |
| 1001–1015 | A | 1, 3 | Unresectable (SMV) |
Unresectable (SMV) |
R1 |
| 1001–1017 | A | 1, 2 | Unresectable (SMA) |
Unresectable (SMA) |
R1 |
| 1008–8001 | A | 1, 2 | Unresectable (SMA, SMV, coeliac) |
Unresectable (coeliac) |
R1 |
| 1008–8005 | A | 2 | Unresectable (SMA) |
Unresectable (SMA) |
R0 |
| 1001–1008 | B | 1, 2 | Unresectable (coeliac) |
Unresectable (coeliac) |
R0 |
*Protocol-defined criteria: (1) CA 19–9 decrease >50%; (2) FDG-PET SUVmax decrease ≥30%; (3) RECIST V.1.1 response (PR or CR); (4) NCCN resectable or borderline resectable criteria.
CA, carbohydrate antigen; CR, complete response; FDG, fluorodeoxyglucose; NCCN, National Comprehensive Cancer Network; PET, positron emission tomography; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumors; SMA, superior mesenteric artery; SMV, superior mesenteric vein; SUVmax, maximum standardised uptake value.
Predictors of resection
High CA 19–9 response (≥95% decline and/or normalisation) was contributive to surgical eligibility (37% vs 17%, p=0.3). Normalisation versus non-normalisation of PET SUVmax was predictive of surgical eligibility (53% vs 11%, p=0.013) and successful resection (78% vs 18%, p=0.002). Combining these two criteria was highly predictive for surgical eligibility (79% vs 28%, p=0.003) and completed (100% vs 39%, p=0.002) resection. All nine successful resections were identified by one or both of these criteria. Conversely, radiographic features of response did not correlate with operative potential. Neither RECIST response nor radiographic downstaging per NCCN criteria statistically correlated with completed resection.
Surgical complications
Postoperative complications were summarised according to the Clavien-Dindo classification (posthoc analysis). Ischaemic gastritis and ulceration and right lower lung lobe collapse were reported for one patient in Arm A (Grade II). There was one episode of clinically significant pancreatic leak in each arm (Grade IIIA); no reoperations and no 30-day or 90-day surgical mortality were noted. One patient in Arm B had a delayed gastric perforation following a distal pancreatectomy with coeliac axis resection likely due to thermal injury and was treated non-operatively (Grade IIIB). No wound complications or superficial site infections were noted in either group. Four out of 12 patients (33%) and 1 out of 2 patients (50%) in Arm A and B, respectively, were readmitted within 30 days and the difference between treatment arms was not clinically or statistically significant.
Survival
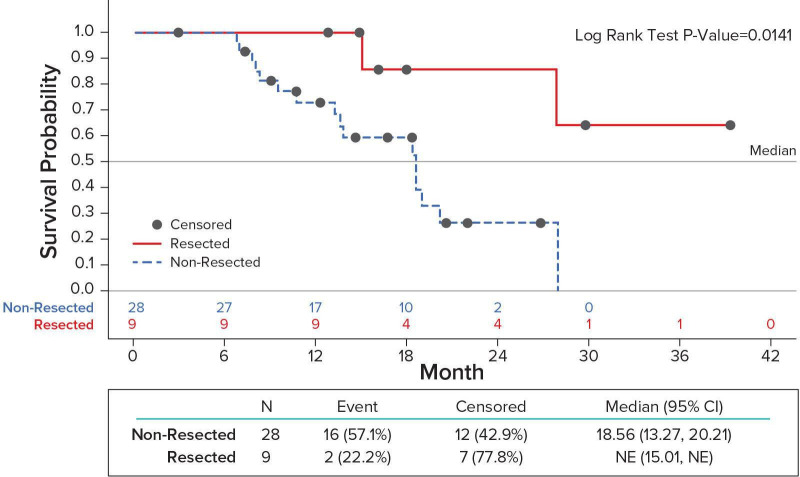
As of the data-cut-off date, 13/37 (35%) of evaluable patients were known to be alive, with a median length of follow-up at approximately 20 months. PFS was 14.1 months (95% CI 8.2 to 18.4) and 11.6 months (95% CI 3.9 to 19) in Arm A and Arm B respectively. One-year survival and median OS were 75% and 19.3 months (95% CI 13.3 to 27.7) in Arm A and 85% and 19.0 months (95% CI 13.2-NR) in Arm B. The median OS for all patients who were eligible for surgical exploration (17 Arm A, 2 Arm B) vs ineligible (7 Arm A, 11 Arm B) was 27.03 months (95% CI 15-NR) vs 18.4 months (95% CI 10.6 to 20.2), p=0.0766). The median OS for resected (8 Arm A, 1 Arm B) vs non-resected patients (16 Arm A, 12 Arm B) was not reached (95% CI 15.01-NR) vs 18.56 months (95% CI 13.2 to 20.2), p=0.0141 (figure 2).
Figure 2.
Overall survival Resected vs. Non-resected patients.
Discussion
The treatment of LAPC with neoadjuvant therapy remains challenging and there is no established SOC. Several high volume centres have reported their single-centre experiences with varying neoadjuvant chemotherapy and chemoradiation strategies.8 9 The combination of more active regimens delivered over an extended period and surgeons’ comfort with resecting and reconstructing major mesenteric vessels has led to an increase in resection rates. A meta-analysis of studies using FOLFIRINOX has demonstrated resection rates ranging from 13% to 43% in LAPC.17 One of the larger studies including 415 patients with LAPC reported a resection rate of 20% that was more dependent on duration of therapy (>4 months) than chemotherapy regimen (FOLFIRINOX or gemcitabine-based).18 Recently, a single institution and single-arm prospective study of neoadjuvant FOLFIRINOX and losartan with selective use of radiation in patients with LAPC reported an R0 resection rate of 61%.19 However, the lack of randomisation makes it difficult to determine what aspect of therapy was responsible for this effect and only 5 of 34 resected patients needed any type of vascular reconstruction perhaps suggesting more favourable outcome than usually seen in locally advanced disease. These retrospective studies contain significant interpretative challenges including selection bias, treatment variables including radiation and the use of different definitions of LAPC.
With respect to gemcitabine-based therapy, a recent large-scale prospective trial of patients with LAPC treated with induction gemcitabine with or without erlotinib followed by radiation, only 8 (4%) patients were able to undergo resection.10 Furthermore, the addition of erlotinib to gemcitabine did not improve any downstaging capacity and there was no difference in survival with the addition of radiation. More recently, the LA-PACT trial examining the role of induction gemcitabine nab-paclitaxel found that only 16 out of 106 patients with LAPC (15%) were able to undergo surgery following neoadjuvant therapy with combination gemcitabine and nab-paclitaxel for six cycles by investigator’s choice.11 Last, although FOLFIRINOX has been the most studied induction combination chemotherapy regimen in this population, recent randomised data from 165 European patients who received neoadjuvant FOLFIRINOX versus gemcitabine/nab-paclitaxel prior to resection20 showed no clear difference with respect to R0/R1 to resection rate (45 vs 31%, p=0.135) or OS (22.5 vs 17.2 months, p=0.268).
Given the anti-CTGF mechanism of action for pamrevlumab, its use in the neoadjuvant setting has the potential to impact tumour regression and modulate the desmoplastic niche and possibly affect tumour margins, allowing for improved resection rates. Previous studies have looked at the ability of gemcitabine/nab-paclitaxel to reduce cancer-associated fibroblasts resulting in a ‘softening’ of tumours by endoscopic ultrasound elastography.21 This stromal depletion also translated into a decrease of SUV uptake on PET.22 In the study reported herein, we combined gemcitabine and nab-paclitaxel with pamrevlumab to explore its effect in terms of therapeutic response, the impact on eligibility for surgical exploration and improved resection rates in locally advanced patients.
The protocol-specified therapeutic response criteria (CA 19–9, PET SUVmax, RECIST and NCCN criteria) were used as criteria to determine eligibility for surgical exploration in LAPC. This is a novel approach specific to the protocol and allows participating patients to be explored for resection when otherwise they may not qualify by current treatment standards (NCCN criteria). For example, by NCCN conversion alone (ie, converted from unresectable to borderline resectable), only 5/24 (21%) of patients in Arm A would have been eligible for surgical exploration. However, by protocol criteria, 17/24 (71%) of patients in Arm A were eligible for surgical exploration. A higher percentage of patients were eligible for surgical exploration by the above criteria in Arm A vs Arm B, 17/24 (71%) vs 2/13 (15%), respectively.
Overall, the rate of successful resections in the pamrevlumab treated group 8/24 (33%) was higher than in the control group 1/13 (8%) but this did not reach statistical significance, most probably due to small sample size. Of the nine subjects that were successfully resected in this trial, only one was converted by NCCN criteria to borderline resectable prior to surgical exploration. Despite this phenomenon, the data support the hypothesis that pamrevlumab, a human monoclonal antibody with anti-CTGF mechanism of action, could alter tumour characteristics, allowing resection in otherwise unresectable patients. This hypothesis needs to be confirmed and patients should be stratified by coeliac and/or SMA involvement.
The most common predictive factors for eligibility for surgical exploration and resection were CA 19–9 decline and PET SUV max response, which are indicators of tumour response to treatment. The combination of these two factors proved to be a highly sensitive, objective readout for prediction of potential surgical success. Both the ability of CA 19–9 response and the inability of radiographic response (RECIST and NCCN criteria of resect ability) to predict surgical outcome has been observed by others3 and these observations deserve further examination in subsequent clinical trials. In the MPACT study, both CA 19–9 and PET response correlated to improved survival in metastatic patients treated with gemcitabine and nab-paclitaxel.23 24 Recent surgical series of patients with borderline resectable and LAPC have also corroborated their impact in the localised setting.25 Correlation of clinical response with plasma levels of endogenous CTGF and pamrevlumab exposure as shown in the prior study by Picozzi et al.16 may provide added prognostic and predictive insight.
With regard to safety, no major incremental toxicity in any category was noted with the addition of pamrevlumab to gemcitabine/nab-paclitaxel. In addition, a higher number of patients (18) were able to complete six cycles of the three-drug combination (including pamrevlumab) when compared with gemcitabine/nab-paclitaxel alone (7), Pamrevlumab is well tolerated and considered safe compared with the SOC drugs for patients with PDAC. These observations represent a very favourable attribute when considering (potential) neoadjuvant chemotherapy in a patient population with the frequency of medical problems typically seen in LAPC. In addition, there were no signals of increased surgical morbidity or wound healing problems with CTGF blockade by pamrevlumab. In fact, there were only two clinically significant pancreatic leaks (one in each arm) which is comparable to national outcome data from high volume pancreatic surgery centres. Similarly, readmissions following resection were comparable between arms and reflected the complexity of this challenging patient population.
Finally, while survival data are not yet mature, both patients who were eligible for surgery and those that were ultimately resected had longer PFS and OS highlighting the importance of surgical resection of the tumour. Therefore, more investigation into newer agents targeting LAPC and increased consideration of candidacy for surgery in those patients who do not progress on therapy or suffer toxicity should be of utmost importance to improve outcomes in this disease.
In conclusion, this is the first prospective, randomised, multicentre trial examining the role of neoadjuvant therapy in LAPC with prespecified criteria for surgical exploration. The use of pamrevlumab in combination with gemcitabine and nab-paclitaxel showed a potential to enhance tumour response and increase resection rates. Further evaluation of this drug combination in the neoadjuvant treatment setting for LAPC is warranted and a larger, phase III trial with resection and survival endpoints is ongoing.
Footnotes
Contributors: FibroGen, Inc. was the study sponsor that designed the study in consultation with the Principal Investigator (VP) and surgical co-investigator (FGR). All authors except those of the sponsor contributed patients to the study. FibroGen was responsible for data collection and analysis. All authors reviewed the manuscript and signed off on its accuracy.
Funding: The study was funded by FibroGen, Inc., San Francisco, CA.
Disclaimer: The corresponding author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non-exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd and its Licensees to permit this article (if accepted) to be published in ESMO Open editions and any other BMJPGL products to exploit all subsidiary rights, as set out in our licence.
Competing interests: MC, MZ, SP, EK and EC are employees of FibroGen and hold stock and/or stock options in FibroGen.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available on reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information. Data will be available as presented in this manuscript.
References
- 1.American Cancer Society Cancer Facts & Figures, 2018. Available: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2018/cancer-facts-and-figures-2018.pdf
- 2.Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913–21. 10.1158/0008-5472.CAN-14-0155 [DOI] [PubMed] [Google Scholar]
- 3.Reni M, Zanon S, Balzano G, et al. Selecting patients for resection after primary chemotherapy for non-metastatic pancreatic adenocarcinoma. Ann Oncol 2017;28:2786–92. 10.1093/annonc/mdx495 [DOI] [PubMed] [Google Scholar]
- 4.Gillen S, Schuster T, Meyer Zum Büschenfelde C, et al. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med 2010;7:e1000267. 10.1371/journal.pmed.1000267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hidalgo M. Pancreatic cancer. N Engl J Med 2010;362:1605–17. 10.1056/NEJMra0901557 [DOI] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network NCCN clinical practice guidelines in oncology. Available: https://www.nccn.org/professionals/physician_gls/default.aspx
- 7.Malik NK, May KS, Chandrasekhar R, et al. Treatment of locally advanced unresectable pancreatic cancer: a 10-year experience. J Gastrointest Oncol 2012;3:326–34. 10.3978/j.issn.2078-6891.2012.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrone CR, Marchegiani G, Hong TS, et al. Radiological and surgical implications of neoadjuvant treatment with Folfirinox for locally advanced and borderline resectable pancreatic cancer. Ann Surg 2015;261:12–17. 10.1097/SLA.0000000000000867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rangelova E, Wefer A, Persson S, et al. Surgery improves survival after neoadjuvant therapy for borderline and locally advanced pancreatic cancer: a single institution experience. Ann Surg 2019. 10.1097/SLA.0000000000003301 [DOI] [PubMed] [Google Scholar]
- 10.Hammel P, Huguet F, van Laethem J-L, et al. Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: the LAP07 randomized clinical trial. JAMA 2016;315:1844–53. 10.1001/jama.2016.4324 [DOI] [PubMed] [Google Scholar]
- 11.Lacy J, Portales F, Hammel P, et al. Interim results of a multicenter phase II trial of nab -paclitaxel (nab-P) plus gemcitabine (G) for patients (Pts) with locally advanced pancreatic cancer (LAPC). JCO 2017;35:358 10.1200/JCO.2017.35.4_suppl.358 [DOI] [Google Scholar]
- 12.Armstrong T, Packham G, Murphy LB, et al. Type I collagen promotes the malignant phenotype of pancreatic ductal adenocarcinoma. Clin Cancer Res 2004;10:7427–37. 10.1158/1078-0432.CCR-03-0825 [DOI] [PubMed] [Google Scholar]
- 13.Oliver N, Sternlicht M, Gerritsen K, et al. Could aging human skin use a connective tissue growth factor boost to increase collagen content? J Invest Dermatol 2010;130:338–41. 10.1038/jid.2009.331 [DOI] [PubMed] [Google Scholar]
- 14.Hingorani SR, Wang L, Multani AS, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 2005;7:469–83. 10.1016/j.ccr.2005.04.023 [DOI] [PubMed] [Google Scholar]
- 15.Neesse A, Frese KK, Bapiro TE, et al. Ctgf antagonism with mAb FG-3019 enhances chemotherapy response without increasing drug delivery in murine ductal pancreas cancer. Proc Natl Acad Sci U S A 2013;110:12325–30. 10.1073/pnas.1300415110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Picozzi VJ, Pipas JM, Koong AC, et al. FG-3019, a human monoclonal antibody to connective tissue growth factor, combined with gemcitabine and erlotinib in patients with locally advanced or metastatic pancreatic ductal adenocarcinoma, an open label phase II clinical trial. J Cancer Clin Trials 2017;2:123–31. [Google Scholar]
- 17.Suker M, Beumer BR, Sadot E, et al. Folfirinox for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysis. Lancet Oncol 2016;17:801–10. 10.1016/S1470-2045(16)00172-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gemenetzis G, Groot VP, Blair AB, et al. Survival in locally advanced pancreatic cancer after neoadjuvant therapy and surgical resection. Ann Surg 2019;270:340–7. 10.1097/SLA.0000000000002753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy JE, Wo JY, Ryan DP, et al. Total neoadjuvant therapy with Folfirinox in combination with losartan followed by chemoradiotherapy for locally advanced pancreatic cancer: a phase 2 clinical trial. JAMA Oncol 2019;5:1020–7. 10.1001/jamaoncol.2019.0892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kunzmann V, Algül H, Goekkurt E, et al. Conversion rate in locally advanced pancreatic cancer (LAPC) after nab-paclitaxel/gemcitabine- or FOLFIRINOX-based induction chemotherapy (NEOLAP): final results of a multicenter randomised phase II AIO trial. Annals of Oncology 2019;30:v253–324. 10.1093/annonc/mdz247 [DOI] [Google Scholar]
- 21.Miyashita T, Tajima H, Makino I, et al. Neoadjuvant chemotherapy with gemcitabine plus nab-paclitaxel reduces the number of cancer-associated fibroblasts through depletion of pancreatic stroma. Anticancer Res 2018;38:337–43. 10.21873/anticanres.12227 [DOI] [PubMed] [Google Scholar]
- 22.Alvarez R, Musteanu M, Garcia-Garcia E, et al. Stromal disrupting effects of nab-paclitaxel in pancreatic cancer. Br J Cancer 2013;109:926–33. 10.1038/bjc.2013.415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiorean EG, Von Hoff DD, Reni M, et al. Ca19-9 decrease at 8 weeks as a predictor of overall survival in a randomized phase III trial (MPACT) of Weekly nab-paclitaxel plus gemcitabine versus gemcitabine alone in patients with metastatic pancreatic cancer. Ann Oncol 2016;27:654–60. 10.1093/annonc/mdw006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramanathan RK, Goldstein D, Korn RL, et al. Positron emission tomography response evaluation from a randomized phase III trial of Weekly nab-paclitaxel plus gemcitabine versus gemcitabine alone for patients with metastatic adenocarcinoma of the pancreas. Ann Oncol 2016;27:648–53. 10.1093/annonc/mdw020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Truty MJ, Kendrick ML, Nagorney DM, et al. Factors predicting response, perioperative outcomes, and survival following total neoadjuvant therapy for borderline/locally advanced pancreatic cancer. Ann Surg 2019. 10.1097/SLA.0000000000003284 [DOI] [PubMed] [Google Scholar]