Abstract
Background
Lenvatinib inhibits tyrosine kinases, including vascular endothelial growth factor (VEGF) receptor, fibroblast growth factor receptor, platelet-derived growth factor receptor alpha, RET proto-oncogene and KIT proto-oncogene, receptor tyrosine kinase. We assessed the efficacy and safety of lenvatinib in patients with metastatic colorectal cancer after failure of standard chemotherapies.
Patients and methods
This was an open-label, single centre, single-arm, phase 2 study. Eligible patients had unresectable metastatic colorectal adenocarcinoma, refractory or intolerant to fluoropyrimidine, irinotecan, oxaliplatin, trifluridine/tipiracil, anti-VEGF therapy and anti-epidermal growth factor receptor therapy (for tumours with wild-type RAS). Patients were treated with oral lenvatinib at 24 mg one time a day in 28-day cycles until disease progression or unacceptable toxicity. The primary endpoint was centrally assessed disease control rate. Secondary endpoints included safety, response rate, progression-free survival and overall survival. The planned sample size was 30 patients to expect a disease control rate of 60% with a threshold disease control rate of 35%, one-sided alpha of 5% and power of 80%
Results
Between 24 October 2016 and 23 January 2018, 30 patients were enrolled; 11 (37%) and 19 (63%) had received 3 or ≥4 lines of prior chemotherapy for metastatic disease, respectively. The median number of lenvatinib cycles was 4 (range 1–13). The centrally assessed disease control rate was 70.0% (21/30, 90% CI 53.5% to 83.4%, one-sided p=0.0001); 2 patients had a partial response and 19 had a stable disease. Median progression-free survival was 3.6 months (95% CI 2.6 to 3.7). Median overall survival was 7.4 months (95% CI 6.4 to 10.8). The most common grade ≥3 adverse events were hypertension (53%), thrombocytopenia (10%), increased alanine aminotransferase and anorexia (7% each).
Conclusions
Lenvatinib showed promising clinical activity and was tolerated in patients with metastatic colorectal cancer after failure of standard chemotherapies.
Trial registration number
UMIN-CTR, UMIN000023446 and JAMCCT-CTR, JMA-IIA00261.
Keywords: colorectal cancer, lenvatinib, phase 2, salvage-line
Key questions.
What is already known about this subject?
No studies have previously reported the efficacy and safety of lenvatinib monotherapy in patients with metastatic colorectal cancer refractory to standard chemotherapies.
What does this study add?
Lenvatinib showed promising antitumour activity with acceptable toxicity for heavily pretreated patients with metastatic colorectal cancer refractory to standard chemotherapies.
No unexpected safety signals were observed and toxicities were manageable with dose modification, interruptions and supportive medications.
How might this impact on clinical practice?
Further prospective randomised studies are warranted to evaluate the efficacy of lenvatinib in patients with metastatic colorectal cancer refractory to standard chemotherapies.
Introduction
The combination of cytotoxic chemotherapy with a molecular targeted agent has significantly improved the survival of patients with unresectable metastatic colorectal cancer.1–5 From results of recent clinical trials, trifluridine/tipiracil and regorafenib are recognised as new treatment options for patients with metastatic colorectal cancer refractory or intolerant to standard therapies.6 7 Nevertheless, the prognosis of patients, which are refractory or intolerant to standard chemotherapies, is poor, and there are still an unmet medical needs for these patients, especially for those who are in a good performance status and eligible for further therapies.
Lenvatinib is an oral multitargeted tyrosine kinase inhibitor of the vascular endothelial growth factor receptor (VEGFR) 1–3, fibroblast growth factor receptors 1–4, platelet-derived growth factor receptor alpha, RET and KIT.8 9 Preclinical studies have shown that lenvatinib not only interferes the interaction between cancer cells and endothelial cells but also inhibits tumour growth.10 Several phase 1 trials of patients with solid tumours in the USA,11 Europe12 and Japan13 14 showed that the optimum dosage of lenvatinib was 24 mg one time a day in a 28-day cycle.
A total of 195 patients were enrolled in four phase 1 studies of lenvatinib monotherapy, 28 of whom had colorectal cancer. Disease control rate (DCR) was achieved in 17 out of 28 (61%) patients, including one with a partial response which continued for 30 weeks (2 mg two times a day for 2 weeks of a 3-week cycle). Grade 3 palmar-plantar erythrodysesthesia was reportedly much lower in 3% of patients treated with lenvatinib for thyroid cancer in a Japanese population of the SELECT trial than that of 28% reported in a Japanese population of CORRECT trial using regorafenib for metastatic colorectal cancer.15 16 These results suggested that lenvatinib may have a potential for improving the outcomes of patients with unresectable metastatic colorectal cancer who have already received conventional chemotherapy with a fluoropyrimidine, irinotecan and oxaliplatin.
We conducted a single-centre, phase 2 study to evaluate efficacy and safety in patients with metastatic colorectal cancer failing to standard therapies.
Patients and methods
Study design and patients
This study was a single-arm, phase 2 study, conducted at National Cancer Center Hospital, Tokyo, Japan. The inclusion criteria were: histological diagnosis of colorectal adenocarcinoma (excluding carcinoma of the appendix and the anal canal), unresectable metastatic disease, an Eastern Cooperative Oncology Group performance status of 0 or 1, an age of 20–79 years, no previous treatment with regorafenib or lenvatinib, sufficient oral intake, adequate organ and bone marrow function, at least one measurable lesion in accordance with the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, refractory or intolerant to fluoropyrimidine, irinotecan, oxaliplatin, trifluridine/tipiracil, anti-VEGF therapy, and antiepidermal growth factor receptor therapy (for tumours with wild-type RAS), and no systemic therapy for at least 2 weeks (4 weeks if any investigational drug had been administered) before study enrolment. The exclusion criteria were provided in the online supplementary material.
esmoopen-2020-000776supp001.pdf (40.3KB, pdf)
All patients provided written informed consent.
Procedures
Patients received lenvatinib at 24 mg one time a day in 28-day cycles orally until disease progression or unacceptable toxicity. The dose was reduced to 20 mg, 14 mg, 10 mg, 8 mg and 4 mg if a patient had an intolerable grade 2 or grade 3 adverse event. Treatment was discontinued if a dose interruption was required for more than 42 consecutive days.
Tumour response was assessed by the independent radiological review committee based on the CT or MRI performed at baseline, every 4 weeks for 8 weeks, and every 8 weeks thereafter until confirmed objective disease progression. Safety assessments including laboratory tests were done at screening, days 1, 8 and 15 of cycle 1, and days 1 and 15 of the subsequent cycles. Urinalysis, thyroid function, prothrombin time-international normalized ratio (PT-INR) and tumour markers (both carcinoembryonic antigen and carbohydrate antigen 19–9) were measured at screening and on day 1 of each treatment cycle. Adverse events were recorded from the first day of the protocol treatment to 30 days after the last dose of study medication, and graded using the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.
Blood sampling for biomarker analyses was done at baseline, on days 15 and 29, and at the end of treatment. Plasma levels of angiopoietin-2 were measured by the Human Angiopoietin-2 Quantikine ELISA Kit (R&D Systems, Minneapolis, USA).
Outcomes
The primary endpoint was centrally assessed DCR, which was defined as the proportion of patients with a complete response, partial response or stable disease persisting for more than 6 weeks from the initiation of study treatment according to RECIST version 1.1. A complete response and partial response were needed to be confirmed.
The secondary endpoints were the objective response rate (ORR, proportion of patients who had a complete response or partial response), progression-free survival (PFS, time from the enrolment until investigator-assessed disease progression or death), overall survival (OS, time from the enrolment until death due to any cause) and adverse events. The incidence of adverse events was calculated based on the information of the worst grade of each adverse event experienced in each patient. Relative dose intensity, which is unprespecified outcome, was calculated as the proportion of the actual cumulative dose divided by planned cumulative dose (24 mg times treatment days).
Statistical analysis
For this single-arm study, the required sample size of 28 patients provided 80% power to reject the null hypothesis of DCR ≤35% with expectation that 60% of patients would have a disease control (one-sided α of 0.05). Considering the possibility of a few ineligible patients, we planned to recruit 30 patients.
The final analysis was planned approximately 12 months after enrolment of the last patient. We included all eligible patients in the efficacy analysis and all patients receiving a least one dose of lenvatinib in the safety analyses. For the primary analysis, binomial test was performed and the centrally assessed DCR was estimated with 90% CI using the Clopper and Pearson method, which corresponds to one-sided α of 0.05. We also estimated the investigator-assessed DCR (a supplementary analysis of the primary endpoint) and ORR with 95% CIs using the same method. We estimated the median time and 6-month and 1-year probability of OS and PFS with the Kaplan-Meier method. The 95% CIs for the median time were calculated using Brookmeyer and Crowley method. The 95% CIs of 6-month and 1-year survival probabilities were calculated based on the Greenwood’s formula. HRs and 95% CIs were estimated by Cox regression. We did subgroup analyses divided by prespecified baseline patient and disease characteristic variables including RAS status for DCR, PFS and OS. We also did a prespecified exploratory analysis of potential predictive biomarkers in blood samples. We did all analyses with SAS V.9.4.
Results
Patient characteristics
Between 24 October 2016 and 23 January 2018, 30 patients with unresectable metastatic colorectal cancer were enrolled. All patients were eligible and received the study medication. Table 1 summarises the baseline characteristics of all 30 enrolled patients. The median number of previous lines of palliative chemotherapy was 4 (range 3–8); 11 (37%) and 19 (63%) patients had received 3 or ≥ 4 prior lines of chemotherapy for metastatic disease, respectively. The data cut-off date was 23 January 2019, with median follow-up of 7.4 months (IQR 5.4–11.8).
Table 1.
Baseline patient characteristics
| Characteristics | Overall (N = 30) |
| Age (years) | |
| Median (range) | 61.5 (42-78) |
| Sex | |
| Male | 20 (67%) |
| Female | 10 (33%) |
| ECOG performance status | |
| 0 | 12 (40%) |
| 1 | 18 (60%) |
| Primary site | |
| Right-sided colon | 3 (10%) |
| Left-sided colorectum | 28 (93%)* |
| Number of metastatic site | |
| 1 | 6 (20%) |
| ≥ 2 | 24 (80%) |
| Metastatic organ | |
| Lung | 26 (87%) |
| Liver | 18 (60%) |
| Lymph node | 17 (57%) |
| Peritoneum | 10 (33%) |
| Time from start of first-line chemotherapy | |
| < 18 months | 5 (17%) |
| ≥ 18 months | 25 (83%) |
| Number of previous palliative chemotherapy | |
| 3 | 11 (37%) |
| ≥ 4 | 19 (63%) |
| Previous chemotherapy and reason for discontinuation | |
| Fluoropyrimidine | 30 (100%) |
| Refractory | 30 (100%) |
| Intolerant | 0 |
| Oxaliplatin | 30 (100%) |
| Refractory | 27 (90%) |
| Intolerant | 3 (10%) |
| Irinotecan | 30 (100%) |
| Refractory | 30 (100%) |
| Intolerant | 0 |
| TAS-102 (trifluridine/tipiracil) | 30 (100%) |
| Refractory | 30 (100%) |
| Intolerant | 0 |
| Angiogenesis inhibitor | 30 (100%) |
| Refractory | 30 (100%) |
| Intolerant | 0 |
| Anti-EGFR inhibitor | 15 (50%)† |
| Refractory | 15/15 (100%) |
| Intolerant | 0 |
| RAS mutational status | |
| Wild type | 14 (47%) |
| Mutant | 16 (53%) |
| BRAF mutational status | |
| Wild type | 23 (77%) |
| Mutant | 0 |
| Unknown | 7 (23%) |
| MSI status | |
| MSS | 7 (23%) |
| Unkown | 23 (77%) |
*There is an overlapping.
†This number includes 14 patients with the RAS wild type and 1 patient with mutant RAS.
ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; MSI, Microsatellite instability; MSS, Microsatellite stable.
Efficacy
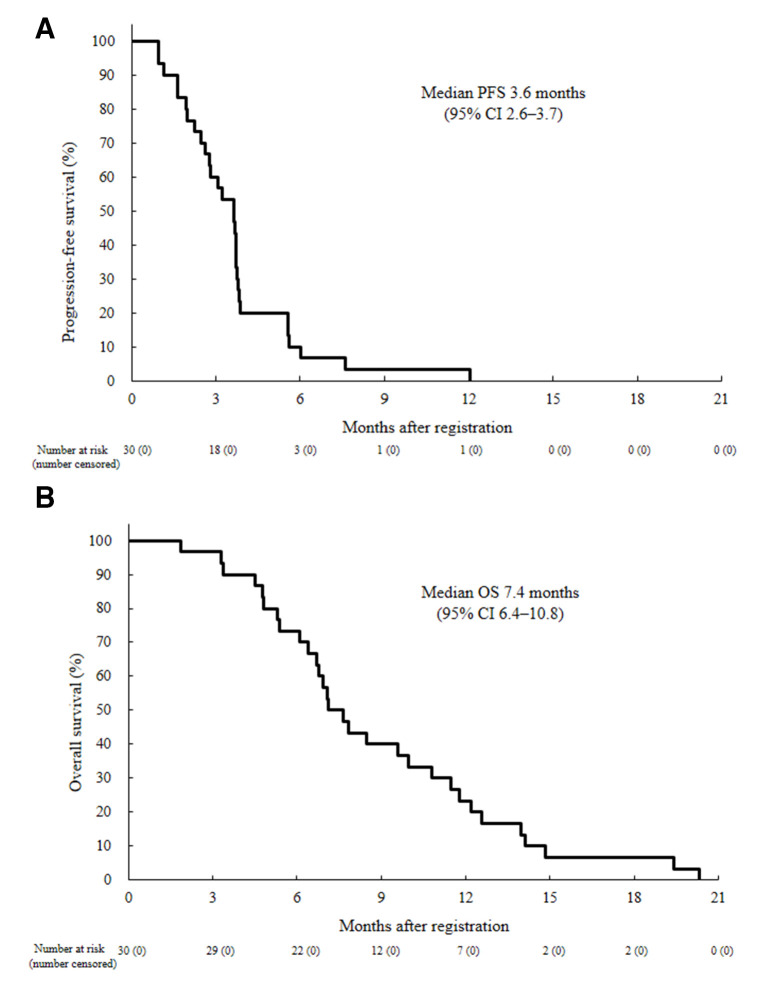
The centrally assessed DCR was 70.0% (21/30, 90% CI 53.5% to 83.4%, one-sided p=0.0001); two patients had a partial response and 19 had a stable disease, including 4 unconfirmed PR (table 2, figure 1). A total of 25/30 (83%) patients had a reduction in target lesion size from baseline (figure 1). Time on treatment for all patients is shown in online supplementary figures 1 and 2. Events for PFS were recorded in all 30 patients, and median PFS was 3.6 months (95% CI 2.6 to 3.7; figure 2). All 30 deaths were recorded, median OS was 7.4 months (95% CI 6.4 to 10.8), with a 6-month and 1-year OS of 73.3% (95% CI 53.7 to 85.7) and 23.3% (95% CI 10.3 to 39.4; figure 2).
Table 2.
Best response to treatment
| Central assessment (n=30) |
Investigator assessment (n=30) |
|
| Complete response | 0 | 0 |
| Partial response | 2 | 1 |
| Stable disease | 19 | 20 |
| Progressive disease | 7 | 7 |
| Not evaluable | 2 | 2 |
| Disease control rate (90% CI) | 70.0% (53.5 to 83.4) | 70.0% (53.5 to 83.4) |
| Response rate (95% CI) | 6.7% (0.8 to 22.1) | 3.3% (0.1 to 17.2) |
Figure 1.
Waterfall plot analysis of maximum percentage change from baseline in measurable target lesions (Response Evaluation Criteria in Solid Tumors version 1.1 central review).
Figure 2.
Kaplan-Meier curves of (A) progression-free survival (PFS) by investigator assessment and (B) overall survival (OS) in all patients (n=30).
esmoopen-2020-000776supp002.pdf (34KB, pdf)
esmoopen-2020-000776supp003.pdf (95.3KB, pdf)
Safety
Patients received the study treatment for four cycles at median (range 1–13). The median relative dose intensity was 65.7% (IQR 57.6–77.5). Dose interruptions and reductions were required in 28 (93%) and 27 (90%) patients, respectively. The major treatment-related adverse events (≥10%) for dose reduction were proteinuria (16 (53%) patients), palmar-plantar erythrodysesthesia (11 (37%) patients), diarrhoea (4 (13%) patients), hypertension (4 (13%) patients), fatigue (4 (13%) patients) and thrombocytopenia (3 (10%) patients). The reasons for treatment discontinuation of all 30 patients were disease progression in 28 (93%) patients and adverse events in 2 (7%) patients; gastrointestinal perforation and grade 3 proteinuria in 1 of each. After treatment with lenvatinib, 16 (53%) patients received a subsequent treatment (online supplementary table 1). Most patients only had mild (grades 1–2) adverse events (table 3). The most common grade ≥3 adverse events were hypertension (16/30 (53%) patients), thrombocytopenia (3/30 (10%) patients), increased alanine aminotransferase and anorexia (2/30 (7%) patients each). No clear relationship was found between the incidence of lenvatinib-associated adverse event of any grade and baseline body surface area (online supplementary table 2). Serious adverse events occurred in four (13%) patients, including five treatment-associated events (anorexia in two, and gastrointestinal perforation, central venous catheter-related bloodstream infection caused by Staphylococcus aureus, and nausea in each one) in each of four (13%) patients; all patients recovered from these adverse events.
Table 3.
Treatment-related adverse events occurring in ≥20% patients (N=30)
| Any grade | Grade ≥3 | |
| Treatment-related adverse event | ||
| Hypertension | 24 (80%) | 16 (53%) |
| Proteinuria | 23 (77%) | 1 (3%) |
| Thrombocytopenia | 18 (60%) | 3 (10%) |
| Fatigue | 16 (53%) | 1 (3%) |
| Hypothyroidism | 14 (47%) | 0 |
| Weight loss | 13 (43%) | 0 |
| Hoarseness | 12 (40%) | 0 |
| Palmar-plantar erythrodysesthesia syndrome | 12 (40%) | 0 |
| Anorexia | 11 (37%) | 2 (7%) |
| Diarrhoea | 10 (33%) | 0 |
| Mucositis oral | 6 (20%) | 0 |
| Serum AST increased | 6 (20%) | 2 (7%) |
| Serum creatinine increased | 6 (20%) | 0 |
AST, Aspartate transaminase.
Subgroup analysis
In patients with wild-type RAS, the median PFS was 3.4 months (95% CI 1.6 to 3.8), and that was 3.7 months (95% CI 2.0 to 3.8) in patients with mutant RAS (online supplementary figure 3). In patients with wild-type RAS, the median OS was 6.9 months (95% CI 95% CI 4.5 to 10.8) and 9.0 months (95% CI 6.1 to 11.8) in patients with mutant RAS (online supplementary figure 3).
esmoopen-2020-000776supp004.pdf (298KB, pdf)
Plasma angiopoietin-2 levels were decreased by lenvatinib treatment in almost all patients and increased at the time of treatment discontinuation (online supplementary table 3). With a first quartile cut-off point,17 the eight (26.7%) patients with a first quartile or lower level of angiopoietin-2 had a median OS of 10.2 months (95% CI 3.4 to 12.2 months) compared with 7.0 months (95% CI 5.3 to 10.0) in the 22 patients with higher than a first quartile level of angiopoietin-2 (HR 1.050, 95% CI 0.453 to 2.433, online supplementary figure 4). Patients with a first quartile or less level of angiopoietin-2 had a median PFS of 3.7 months (95% CI 1.1 to 5.6) compared with 3.4 months (95% CI 2.2 to 3.7) in the patients with more than a first quartile level of angiopoietin-2 (HR 1.243, 95% CI 0.546 to 2.831, online supplementary figure 4).
esmoopen-2020-000776supp005.pdf (297.5KB, pdf)
Discussion
Patients with metastatic colorectal cancer with disease progression after three or more lines of therapy have limited treatment options. In this open-label, single-arm, phase 2 study of patients with previously treated metastatic colorectal cancer, lenvatinib demonstrated manageable toxic effects and promising antitumour activity. A total of 21 out of 30 patients (70%) had disease control including with 2 partial responses (7%). Moreover, 25/30 patients (83%) experienced reduction in measurable tumour size. The overall toxicity profiles were similar to that reported for lenvatinib across a spectrum of advanced malignant neoplasms.
Two recent international phase 3 studies reported that regorafenib or trifluridine/tipiracil provided significant improvements in DCR, PFS and OS, compared with placebo, in patients with metastatic colorectal cancer after failure of standard chemotherapies (DCR; 41%, median PFS; 1.9 months, median OS; 6.4 months in the CORRECT study, and DCR; 44%, median PFS; 2.0 months, median OS; 7.1 months in the RECOURSE study).6 7 Interestingly, the present single-arm phase 2 study of lenvatinib revealed favourable DCR and median PFS values in patients with metastatic colorectal cancer, compared with those in the regorafenib or trifluridine/tipiracil study. Moreover, about half of the patients received post study treatment, which led to a favourable OS.
The lenvatinib safety profile in this study was similar to the published safety profiles of lenvatinib for thyroid cancer and hepatocellular carcinoma in the Japanese population.18 19 Moreover, we found no unexpected or off-target safety signals. The most common adverse events were hypertension, proteinuria, thrombocytopenia and fatigue, while the most case of grade 2 or 3 hypertension and proteinuria required treatment interruption and dose reduction. While the target population for thyroid cancer or hepatocellular carcinoma that showed efficacy for lenvatinib was first-line setting,20 21 this study targeted patients receiving salvage-line therapy. Most patients with metastatic colorectal cancer in the salvage-line setting had grade 1 or 2 proteinuria and hypertension at baseline because of the long-term prior treatment with anti-VEGF/VEGFR treatment whereas the occurrence of grade 3 hypertension (53%) was significantly higher compared with that of regorafenib in a similar study population in the CORRECT (7%), CONCUR (11%) and CONSIGN (15%) trials.7 22 23 It was manageable by dose reduction or interruption, but it may be necessary to consider the starting dose in the future. Although palmar-plantar erythrodysesthesia is a not life-threatening toxicity, these adverse events have a significant impact on treatment schedules and quality of life in treated patients. Grade ≥3 palmar-plantar erythrodysesthesia has been observed in 0% and 3% of patients treated with lenvatinib in this study and the SELECT Japanese population,15 respectively, while 28% in patients treated with regorafenib in the CORRECT Japanese population.16 To date, the clear mechanism of palmar-plantar erythrodysesthesia by VEGF receptor tyrosine kinase inhibitors is not known, but it has been reproduced that palmar-plantar erythrodysesthesia by lenvatinib is well tolerated. Overall, it is suggested that lenvatinib might be a favourable treatment option in terms of toxicities.
Several preclinical studies demonstrated that VEGF-targeted treatment affects immune suppression by promoting the expansion of suppressive immune cell populations, such as regulatory T cells and myeloid-derived suppressor cells.24 25 Several clinical studies suggested that modulation of VEGF-mediated immune suppression via angiogenesis inhibition could potentially augment the immunotherapeutic activity of anti-programmed cell death 1 (PD-1) antibody.26 27 Regorafenib and nivolumab showed antitumour activity in patients with metastatic colorectal cancer, including those with microsatellite stable tumours in a phase 1 study.28
Angiopoietin-2, a relatively novel regulator of angiogenesis that acts through the TEK tyrosine kinase, endothelial (Tie2) receptor, has been identified as a potential prognostic biomarker for some types of cancer. Although the baseline Ang-2 level was a predictive biomarker in patients with thyroid cancer in the SELECT trial,17 it did not become a reliable biomarker of lenvatinib response in this study. Prior treatment with anti-VEGF/VEGFR antibodies probably had an effect on baseline angiopoietin-2 levels because the study population was refractory to standard treatment in this study. The decrease in angiopoietin-2 levels was observed after treatment; therefore, it may be an indicator of treatment response.
The limitations of our study include its small size, which could limit the interpretation of the subgroup analyses, and the absence of a comparison group. However, the level of clinical benefit in the form of confirmed responses observed in this study was remarkable in the historical context of other clinical trials done in heavily pretreated patients with metastatic colorectal cancer. Moreover, most of the patients in our study had left sided tumours which were known to have a better prognosis compared with right-sided tumours.
In conclusion, lenvatinib provided promising activity with prolonged survival relative to the anticipated median PFS in heavily pretreated patients with metastatic colorectal cancer. The safety profile of lenvatinib was similar to that in other tumour types, with no new safety signals recorded. Based on these findings, further investigation of lenvatinib with anti-PD-1 antibody or other novel combinations with the potential to build on the benefit of lenvatinib is currently taking place (NCT03797326 and NCT04008797).
Acknowledgments
The authors thank the patients and their families, the members of the Clinical Research Support Office for their support with data collection and running the study, and NAI incorporated for editing a draft of this manuscript.
Footnotes
SI and NO contributed equally.
Contributors: All authors conceived and designed the study, and drafted and revised the manuscript for publication. SI, NO, HS, YH, AT, KK, TH, NB and YY collected data. AK, GO, MK and KN analysed the data and managed data and study progress. All authors interpreted the data and approved the final version of the manuscript.
Funding: The study was supported by the Project Promoting Clinical Trials for Development of New Drugs and Medical Devices (Japan Medical Association) from the Japan Agency for Medical Research and Development (Grant Number JP18lk0201037) and by Eisai Co.
Competing interests: SI has received research grants from Eisai and Merck Biopharma. TH has received research grants from Eisai and honoraria from Merck Serono. YY has received honoraria from Eisai.
Patient consent for publication: Not required.
Ethics approval: The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. The study protocol was approved by the National Cancer Center Institutional Review Board T4329.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request. Proposals should be directed to siwasa@ncc.go.jp. The data will be available for achieving aims in the approved proposal.
References
- 1.Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 2008;26:2013–9. 10.1200/JCO.2007.14.9930 [DOI] [PubMed] [Google Scholar]
- 2.Tabernero J, Yoshino T, Cohn AL, et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (raise): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol 2015;16:499–508. 10.1016/S1470-2045(15)70127-0 [DOI] [PubMed] [Google Scholar]
- 3.Van Cutsem E, Tabernero J, Lakomy R, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol 2012;30:3499–506. 10.1200/JCO.2012.42.8201 [DOI] [PubMed] [Google Scholar]
- 4.Yamazaki K, Nagase M, Tamagawa H, et al. Randomized phase III study of bevacizumab plus FOLFIRI and bevacizumab plus mFOLFOX6 as first-line treatment for patients with metastatic colorectal cancer (WJOG4407G). Ann Oncol 2016;27:1539–46. 10.1093/annonc/mdw206 [DOI] [PubMed] [Google Scholar]
- 5.Price TJ, Peeters M, Kim TW, et al. Panitumumab versus cetuximab in patients with chemotherapy-refractory wild-type KRAS exon 2 metastatic colorectal cancer (ASPECCT): a randomised, multicentre, open-label, non-inferiority phase 3 study. Lancet Oncol 2014;15:569–79. 10.1016/S1470-2045(14)70118-4 [DOI] [PubMed] [Google Scholar]
- 6.Mayer RJ, Van Cutsem E, Falcone A, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med 2015;372:1909–19. 10.1056/NEJMoa1414325 [DOI] [PubMed] [Google Scholar]
- 7.Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (correct): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:303–12. 10.1016/S0140-6736(12)61900-X [DOI] [PubMed] [Google Scholar]
- 8.Matsui J, Yamamoto Y, Funahashi Y, et al. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int J Cancer 2008;122:664–71. 10.1002/ijc.23131 [DOI] [PubMed] [Google Scholar]
- 9.Matsui J, Funahashi Y, Uenaka T, et al. Multi-Kinase inhibitor E7080 suppresses lymph node and lung metastases of human mammary breast tumor MDA-MB-231 via inhibition of vascular endothelial growth factor-receptor (VEGF-R) 2 and VEGF-R3 kinase. Clin Cancer Res 2008;14:5459–65. 10.1158/1078-0432.CCR-07-5270 [DOI] [PubMed] [Google Scholar]
- 10.Wiegering A, Korb D, Thalheimer A, et al. E7080 (lenvatinib), a multi-targeted tyrosine kinase inhibitor, demonstrates antitumor activities against colorectal cancer xenografts. Neoplasia 2014;16:972–81. 10.1016/j.neo.2014.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong DS, Kurzrock R, Wheler JJ, et al. Phase I dose-escalation study of the multikinase inhibitor lenvatinib in patients with advanced solid tumors and in an expanded cohort of patients with melanoma. Clin Cancer Res 2015;21:4801–10. 10.1158/1078-0432.CCR-14-3063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boss DS, Glen H, Beijnen JH, et al. A phase I study of E7080, a multitargeted tyrosine kinase inhibitor, in patients with advanced solid tumours. Br J Cancer 2012;106:1598–604. 10.1038/bjc.2012.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamada K, Yamamoto N, Yamada Y, et al. Phase I dose-escalation study and biomarker analysis of E7080 in patients with advanced solid tumors. Clin Cancer Res 2011;17:2528–37. 10.1158/1078-0432.CCR-10-2638 [DOI] [PubMed] [Google Scholar]
- 14.Nakamichi S, Nokihara H, Yamamoto N, et al. A phase 1 study of lenvatinib, multiple receptor tyrosine kinase inhibitor, in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol 2015;76:1153–61. 10.1007/s00280-015-2899-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiyota N, Schlumberger M, Muro K, et al. Subgroup analysis of Japanese patients in a phase 3 study of lenvatinib in radioiodine-refractory differentiated thyroid cancer. Cancer Sci 2015;106:1714–21. 10.1111/cas.12826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshino T, Komatsu Y, Yamada Y, et al. Randomized phase III trial of regorafenib in metastatic colorectal cancer: analysis of the correct Japanese and non-Japanese subpopulations. Invest New Drugs 2015;33:740–50. 10.1007/s10637-014-0154-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tahara M, Schlumberger M, Elisei R, et al. Exploratory analysis of biomarkers associated with clinical outcomes from the study of lenvatinib in differentiated cancer of the thyroid. Eur J Cancer 2017;75:213–21. 10.1016/j.ejca.2017.01.013 [DOI] [PubMed] [Google Scholar]
- 18.Takahashi S, Kiyota N, Yamazaki T, et al. A Phase II study of the safety and efficacy of lenvatinib in patients with advanced thyroid cancer. Future Oncol 2019;15:717–26. 10.2217/fon-2018-0557 [DOI] [PubMed] [Google Scholar]
- 19.Yamashita T, Kudo M, Ikeda K, et al. REFLECT-a phase 3 trial comparing efficacy and safety of lenvatinib to sorafenib for the treatment of unresectable hepatocellular carcinoma: an analysis of Japanese subset. J Gastroenterol 2020;55:113–22. 10.1007/s00535-019-01642-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018;391:1163–73. 10.1016/S0140-6736(18)30207-1 [DOI] [PubMed] [Google Scholar]
- 21.Schlumberger M, Tahara M, Wirth LJ, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med 2015;372:621–30. 10.1056/NEJMoa1406470 [DOI] [PubMed] [Google Scholar]
- 22.Li J, Qin S, Xu R, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (concur): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2015;16:619–29. 10.1016/S1470-2045(15)70156-7 [DOI] [PubMed] [Google Scholar]
- 23.Van Cutsem E, Martinelli E, Cascinu S, et al. Regorafenib for patients with metastatic colorectal cancer who progressed after standard therapy: results of the large, single-arm, open-label phase IIIB CONSIGN study. Oncologist 2019;24:185–92. 10.1634/theoncologist.2018-0072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ott PA, Hodi FS, Buchbinder EI. Inhibition of immune checkpoints and vascular endothelial growth factor as combination therapy for metastatic melanoma: an overview of rationale, preclinical evidence, and initial clinical data. Front Oncol 2015;5:202. 10.3389/fonc.2015.00202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kato Y, Tabata K, Kimura T, et al. Lenvatinib plus anti-PD-1 antibody combination treatment activates CD8+ T cells through reduction of tumor-associated macrophage and activation of the interferon pathway. PLoS One 2019;14:e0212513. 10.1371/journal.pone.0212513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor MH, Lee C-H, Makker V, et al. Phase Ib/II trial of lenvatinib plus pembrolizumab in patients with advanced renal cell carcinoma, endometrial cancer, and other selected advanced solid tumors. J Clin Oncol 2020;38:1154–63. 10.1200/JCO.19.01598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Makker V, Rasco D, Vogelzang NJ, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer: an interim analysis of a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol 2019;20:711–8. 10.1016/S1470-2045(19)30020-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fukuoka S, Hara H, Takahashi N, et al. Regorafenib plus nivolumab in patients with advanced gastric (GC) or colorectal cancer (CRC): an open-label, dose-finding, and dose-expansion phase 1B trial (REGONIVO, EPOC1603). JCO 2019;37:2522 10.1200/JCO.2019.37.15_suppl.2522 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2020-000776supp001.pdf (40.3KB, pdf)
esmoopen-2020-000776supp002.pdf (34KB, pdf)
esmoopen-2020-000776supp003.pdf (95.3KB, pdf)
esmoopen-2020-000776supp004.pdf (298KB, pdf)
esmoopen-2020-000776supp005.pdf (297.5KB, pdf)