Abstract
The glial cell line-derived neurotrophic factor (GDNF) family ligands (GFLs) alleviate symptoms of experimental neuropathy, protect and stimulate regeneration of sensory neurons in animal models of neuropathic pain, and restore their functional activity. However, clinical development of GFL proteins is complicated by their poor pharmacokinetic properties and multiple effects mediated by several receptors. Previously, we have identified a small molecule that selectively activates the major signal transduction unit of the GFL receptor complex, receptor tyrosine kinase RET, as an alternative to GFLs, for the treatment of neuropathic pain. We then introduced a series of chemical changes to improve the biological activity of these compounds and tested an optimized compound named BT44 in a panel of biological assays. BT44 efficiently and selectively stimulated the GFL receptor RET and activated the intracellular mitogene-activated protein kinase/extracellular signal-regulated kinase pathway in immortalized cells. In cultured sensory neurons, BT44 stimulated neurite outgrowth with an efficacy comparable to that of GFLs. BT44 alleviated mechanical hypersensitivity in surgery- and diabetes-induced rat models of neuropathic pain. In addition, BT44 normalized, to a certain degree, the expression of nociception-related neuronal markers which were altered by spinal nerve ligation, the neuropathy model used in this study. Our results suggest that the GFL mimetic BT44 is a promising new lead for the development of novel disease-modifying agents for the treatment of neuropathy and neuropathic pain.
Keywords: Glial cell line-derived neurotrophic factor, GDNF family ligands, artemin, receptor tyrosine kinase RET, RET agonist, neuropathy, spinal nerve ligation, diabetes mellitus, neuropathic pain, diabetic neuropathy
Introduction
Lesions or diseases of the somatosensory system may cause chronic neuropathic pain1 which impairs the patient’s quality of life and causes a challenge to the health-care system.2,3 Neuropathic pain affects millions of people worldwide, occurring more often in the elderly. It is difficult to treat, and it is often underdiagnosed and undertreated.4 It can be caused, for example, by injuries, cancer, infections (Herpes Zoster, HIV), and metabolic diseases such as diabetes.5–9 Existing pain therapies control neuropathic pain only in a minority of patients, and they may cause significant side effects.10 Importantly, they fail to normalize the underlying changes in the nervous system. Thus, there is an urgent need for the development of new medications.
The proteins supporting and regenerating neurons such as the glial cell line-derived neurotrophic factor (GDNF) family ligands (GFLs) artemin (ARTN) and GDNF are among the interesting targets for neuropathic pain therapy. These proteins attenuate the signs of neuropathy and stimulate the regeneration of sensory neurons in experimental animals.11–15 However, ARTN and GDNF have poor pharmacokinetic properties, and their transition to clinical use is challenging.16,17 Besides, they are expensive to produce, and it is difficult to maintain a uniform biological activity among batches.
GFLs signal predominantly via a receptor complex consisting of a signal transduction module represented by a receptor tyrosine kinase called RET and a ligand-binding subunit—GDNF family receptor alpha 1–4 (GFRα1-4), which provides binding specificity. Sensory neurons mostly express RET, GFRα1 (the preferred co-receptor for GDNF), and GFRα3 (the preferred co-receptor for ARTN). A few sensory neurons are also positive for GFRα2, which primarily binds another GFL called neurturin (NRTN). The expression of GFRα2, however, is downregulated upon nerve injury and therefore, NRTN administration is considered to be of little significance in NP therapy. In addition to RET, GFLs can also transmit the signal via other receptors such as the neural cell adhesion molecule (NCAM)18 or syndecan-3.16 GFLs are important for the development and maintenance of nociceptive neurons,19 and they have induced thermal hypersensitivity in both experimental animals20 and patients.21,22 However, at least some of the effects of GFLs on temperature perception (e.g., cold pain) might be mediated by other receptors than RET.20
Small molecules with improved pharmacological characteristics selectively activating GFL-like signaling via RET could be a good alternative for clinical use. The first of these candidates, XIB4035, considered a small-molecule agonist for GFRα1, activated RET in mouse neuroblastoma cell line cells23 and alleviated experimental diabetic neuropathy in rodents. However, it was later shown to increase the activity of GDNF or ARTN rather than to directly activate GFL receptors,24 which limits its therapeutic applications.
Previously, we have identified the first chemical compounds BT1325,26 and BT1827,28 which selectively activate RET and its downstream targets in the absence of GFLs and support sensory neurons.
Recently, we developed a third-generation GFL mimetic BT44 with predicted improved properties using computational chemistry methods. In this study, we tested the biological activity of BT44 focusing on the sensory system. We first proved that BT44 selectively activates RET, but has no effect on TrkA and TrkB receptors. We further studied whether BT44 supports sensory neurons in vitro and alleviates behavioral manifestations of experimental neuropathy in vivo, using two animal models: spinal nerve ligation (SNL) and streptozotocin (STZ)-induced diabetes mellitus (DM) (a model of type 1 diabetes). These models were chosen because the activation of GFL/GFRα/RET signaling in these NP models was shown to produce positive effects. We also assessed the ability of BT44 to restore damaged dorsal root ganglion (DRG) neurons in vivo.
Material and methods
Proteins
ARTN and GDNF were purchased from PeproTech (USA).
The design and synthesis of BT44
Earlier, we described the discovery of the first direct RET agonist that supports sensory neurons in vitro and in vivo and alleviates surgery-induced neuropathy in experimental animals in high throughput screening.25 To improve its biological activity, we first developed fragmental quantitative structure-activity relationship (FQSAR) models using known crystal structures of the GFRα1 and GFRα3 co-receptors,29,30 the previously described compound XIB403523 which is able to modulate the biological activity of GFLs24 and related small molecules with demonstrated activity towards RET. Compounds were docked to the GFRα3 using the Autodock 3.0 program.31 Grid maps of 120 × 120 × 120 grid points at a spacing of 0.375 Å were generated around the center of the ligand binding site by the Autogrid program. Kollman’s partial charges were used for heavy atoms and polar H atoms of the protein. Atomic solvation parameters and fragmental volumes were obtained from the Addsol program. Gasteiger charges were assigned to the ligand molecules. The predicted free energies of binding of the ligands to the proteins were calculated by using the scoring functions implemented in the program package AutoDock. Thereafter, FQSAR models were developed for the predicted binding free energies of the compounds as described elsewhere.32 Several ligands with predicted low free binding energies to both co-receptors were found, including compound BT44 were designed. BT44 was synthesized by EvoBlocks (Hungary) with the purity of 98.6% (LCMS).
The structure, integrity, and purity of the resulting compound was confirmed in NMR experiments recorded on a Bruker Avance III HD NMR spectrometer operated at 1H frequency of 850.4 MHz equipped with a cryogenic probe head by NMR facility at the Institute of Biotechnology, University of Helsinki (Supplemental Material).
Luciferase assay
Luciferase assay was performed as described previously.33 MG87RET murine fibroblasts34 stably transfected with PathDetect-Elk1 (Stratagene, USA) and one of the following GFRα1-, GFRα3-expressing plasmids, or pcDNA6 vector33 were plated onto 96-well plates with a density of 20,000 cells/well in DMEM, 10% FBS, 100 µg/ml Normocin™ (Invivogen, USA), 1% DMSO, 15 mM HEPES pH 7.2 the day before the assay. The next day, BT44 was applied to cells in concentrations of 0.1, 1, 5, 10, 25, and 50 μM in DMEM, 15 mM HEPES, 1% DMSO. ARTN and GDNF (100 ng/ml) were used as positive controls for GFRα3/RET and GFRα1/RET-expressing cells, respectively. The following day, media was discarded and 30 µl of NeoLite reagent (Perkin Elmer) diluted 1:1 with PBS was added to the wells. The plate was incubated on the shaker (RT) for 10 min, and luminescence was measured using Victor3 1420 multilabel counter (Perkin Elmer/Wallac). Measurements were done in quadruplicates and the biological activity of the compounds was evaluated in four independent experiments.
Phosphorylation assays
Phosphorylation assays were performed as described previously.35 Phosphorylation of receptor tyrosine kinase RET in cultured cells was assessed by antibodies against phosphorylated tyrosine residues (clone 4G10, Merck Millipore) after immunoprecipitation of RET with RET specific antibodies C-20 (sc-1290, Santa-Cruz Biotechnology Inc., USA). Phosphorylation of Trk receptors was analyzed after immunoprecipitation of Trk with the anti-pan Trk antibody C-14 (sc-11, Santa-Cruz Biotechnology Inc., USA). Phosphorylation of the intracellular signaling protein ERK was determined using specific antibodies against phosphorylated forms of the respective proteins in whole cell lysates (see below). MG87RET, MG87TrkA or MG87TrkB cells were plated onto six-well plates a day before the experiment. MG87RET cells were transfected with 4 µg/well of GFRα3- or GFP- expressing plasmid using Turbofect (Thermo Fisher Scientific). Before the stimulation with the compounds, cells were starved for 4 h in serum-free DMEM supplemented with 1% DSMO, 15 mM Hepes pH 7.2 and stimulated with 7.5, 18, 35, or 75 μM BT44 for 15 min. 100 ng/ml ARTN (250 ng/ml), NGF (200 ng/ml), or BDNF (200 ng/ml) were used as positive controls in MG87/RET/GFRα1, MG87TrkA, and MG87TrkB cells, respectively. After the stimulation with test compounds, the cells were washed once with ice-cold PBS containing 1 mM Na3VO4 and 1 mM NaF and lysed on ice in 500 µl per well of RIPA-modified buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% NP-40, 1% TX-100, 10% glycerol, EDTA-free protease inhibitor cocktail (Roche), 1 mM Na3VO4, 2.5 mg/ml of sodium deoxycholate, 1 mM NaF). Subsequently, 80 μl of the whole cell lysate was removed, mixed with 80 μl of Laemmli loading buffer (4% SDS, 20% glycerol, 10% 2-mercaptoethanol, 0.004% bromophenol blue, 0.125 M tris-HCL pH 6.8) and boiled for 10 min to prepare the samples for the analysis of pERK levels. The remaining lysates were centrifuged for 10 min (10,600g, 4°C, Eppendorf table-top centrifuge) to precipitate cell debris. Supernatants were collected and incubated with 2 µg/ml of RET C-20 antibodies (sc-1290, Santa-Cruz Biotechnology Inc., USA) or 2 µg/ml of Trk C-14 antibodies and Dynabeads Protein G magnetic beads (Thermo Fisher Scientific) overnight onto a rotator at +4°C to immunoprecipitate RET. The following day, the beads were separated from the lysate by using a magnetic holder, washed twice with 1xTBS containing 1% TX-100, mixed with 50 μl of Laemmli loading buffer, boiled for 5 min and centrifuged at 17,900g for 5 min. The whole cell lysate samples were resolved on 12% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE), and proteins, immunoprecipitated by RET or Trk antibodies—on 7.5% SDS-PAGE. The samples were then transferred to a nitrocellulose membrane (GE Healthcare).
Membranes were blocked in TBST-T with 10% skimmed milk for 10 min. The membranes were probed with pERK (1:500, E4, Santa-Cruz Biotechnology, Inc) or phospho-tyrosine (pY) (1:1000, clone 4G10, Cat N 05–321, Merck Millipore) antibodies in 3% milk in TBS-T overnight at +4°C. The membranes were washed three times for 10 min with TBS-T and incubated in a solution of a secondary antibody conjugated with horse radish peroxidase in TBS-T (1:3000, anti-mouse, Dako) for 1 h at room temperature. The membranes were washed with TBS-T three times for 15 min. Stained bands were visualized with an ECL reagent (Pierce) using Luminescent Image Analyzer LAS3000 (Fuji-Film). To confirm equal loading, the membranes were stripped and re-probed with anti-RET C-20 (1:500, SantaCruz Biotechnology Inc), anti-Trk C-14 (1:1000, SantaCruz Biotechnology Inc), or anti-GAPDH (1:4000, Millipore) antibodies using a similar protocol to those described above.
Westren blots were quantified using Image Studio 5.2 software (LI-COR Biosciences). Intensities of the bands of the phosphorylated form of RET (MW = 170 kDa) and pERK were normalized to the band intensities of RET and GAPDH respectively. Furthermore, values for pRET/RET ratios and pERK/GAPDH ratios in vehicle and compound treated wells were expressed as percentage of activation with the values in ARTN-treated wells standardized to 100%. The area in which optical density of each band was measured was of the same size for all analyzed membranes.
DRG culture and neurite outgrowth assay
The cultures of DRG neurons were prepared as described by Paveliev et al.36 with small modifications. DRGs were dissected from the spine of the healthy male P30 Wistar rats purchased from The Laboratory Animal Centre of the University of Helsinki. The permission to use experimental animals for research purposes (KEK14-019) was given by The Laboratory Animal Centre of the University of Helsinki. Ganglia were cleaned from nerve fibers in PBS and washed two times with Ca2+ and Mg2+ free HBSS (ThermoFisher Scientific). The ganglia were incubated in HBSS containing trypsin (5 mg/ml) and collagenase/dispase (10 mg/ml) for 30 to 60 min at 37°C, washed once with HBSS-DNase I solution, and triturated in HBSS-DNAase I solution until disappearance of visible tissue pieces. Supernatants were transferred to the fresh tube, the cells were precipitated by centrifugation at 110 g for 5 min, washed two times with neuron culturing media (1xNeurobasal medium, 1xB-27, 100 µg/ml primocin, 25 μM L-glutamine, 0.1% DMSO) and plated on poly-ornithine and laminin-precoated coverslips. Neurons were cultured in the presence of 0.5–10 µM BT44 or ARTN (30 ng/ml) for a positive control for 16–20 h. Afterwards, the DRG neurons were fixed with 4% PFA for 10 min at RT, washed with PBS, and permeabilized with 1xPBS containing 0.1% Triton X-100 (PBS-T). DRG neurons were probed with 1:500 rabbit anti-PGP9.5 antibodies (Enzo Biochem Inc., USA) and visualized with anti-rabbit secondary antibodies conjugated with Alexa488 1:200 (Invitrogen). Mounting was done with Imu-mount (Thermo Scientific). The number of PGP9.5-positive neurons with neurites at least twice as long as the body was quantified under fluorescent microscope (Zeiss5) and normalized to the total number of neurons. The counting in random vision fields on coverslips was carried out by the observer who was blinded to the treatment groups. Experiments were performed at least in triplicates and each concentration of BT44 was evaluated in four to five independent experiments. At least 70 neurons per treatment group were analyzed in each experiment. For the statistical analysis, the data for technical replicates were averaged. For representation purposes, the data were converted to percentages from the values in vehicle-treated wells for each experiment and the results for all concentrations were pooled together.
Experimental animals
Neuropathy was induced by SNL and STZ-induced diabetes. The experiments were performed in male Wistar rats (Envigo Laboratories, Horst, Netherlands). The rats weighed 150 g (SNL) and 200–250 g (STZ and controls) at the beginning of the experiments.
The animals were housed in groups of two to four in plastic cages with a layer of saw dust in ambient temperature (22–24°C) and artificial lighting with a fixed 12-h light–dark cycle. Pellet food and tap water were available ad libitum. The experiments were performed in accordance to the 3 R principles and all efforts were made to limit distress for the animals. The experiments were performed using the minimum number of animals necessary to produce reliable scientific data. The animals were habituated to handling and to the experimental conditions for two to three consequent days before the experiments. The animals were again habituated to test settings for 15 min before the actual measurements. The Experimental Animal Ethics Committee of the Provincial Government of Southern Finland (Etelä-Suomen aluehallintovirasto, Hämeenlinna, Finland; Permission # ESAVI/7929/04.10.07/2014) had approved the methods and the experiments were performed according to the guidelines of the European Parliament and the Council Directive of 22 September 2010 (010/63/EU), International Association for the Study of Pain,37 and the ARRIVE guidelines.38–40
After surgery, the SNL animals were observed by an experienced researcher on daily basis to detect any complications of surgery or motor complications produced by unintended damage of L4 spinal nerve, loss of weight, or any other abnormal signs. Similarly, the STZ-treated animals were monitored on daily basis throughout the experiment to detect weight loss of more than 20% or any signs of suffering in behavior. No complications were observed.
Techniques for producing the SNL model of peripheral neuropathy
Unilateral ligation of two spinal nerves (L5 and L6) was performed under isoflurane anesthesia (Baxter Oy, Helsinki, Finland, diluted in air, 4.5% for induction and 2%–3% for maintenance) as described in detail earlier.41 The left L5 and L6 spinal nerves were exposed by removing a small piece of the paravertebral muscle and part of the left spinous process of the L5 lumbar vertebra. The L5 and L6 spinal nerves were carefully isolated from the L4 spinal nerve and other surrounding tissues and tightly ligated with 6–0 silk (Perma-Hand Seide, Ethicon, Belgium). After ligation, the muscle and the adjacent fascia were closed with sutures (Vicryl-Rapid 3/0, Ethicon, Belgium) and the skin with metal clips.
In the SNL experiments, only animals developing hypersensitivity to mechanical stimulation with digital von Frey test before treatments and with no motor impairment were included in the study.
Animal model of DM
The STZ-induced model of DM is associated with hypersensitivity to various types of pain stimuli.42 DM was induced in a group of animals by intraperitoneal injection of STZ (60 mg/kg; Sigma-Aldrich, St. Louis, MO, USA) in citrate buffer (pH 4.5). The blood glucose levels in the experimental animals were measured first after overnight fasting before the injection of STZ or vehicle and at the end of the experiment using Accu Chek Aviva (Roche, Basel, Switzerland). Non-fasting blood glucose levels were measured at regular intervals during the experiment. STZ-treated animals which had a non-fasting blood glucose level of ≤20 mmol/l the day after the STZ injection were excluded from the study. Consequently, four STZ-treated animals were excluded from the study.
To study any possible long-term effects of BT44 on DM, glycosylation of hemoglobin (HbA1C) levels were measured from the whole blood samples at the end of the experiment with Siemens DCA 2000+ system.
Treatments
The SNL animals received 1, 5, 12.5, or 25 mg/kg of BT44 in two independent experiments. In the first experiment (Figure 5(a)), animals received 12.5 or 25 mg/kg of BT44 dissolved in corn oil containing 5% DMSO and injected subcutaneously (s.c.) every second day for 10 days, starting on the second day after the nerve injury. In the second experiment, SNL animals received 1 or 5 mg/kg of BT44. Vehicle groups received corn oil containing 5% DMSO.
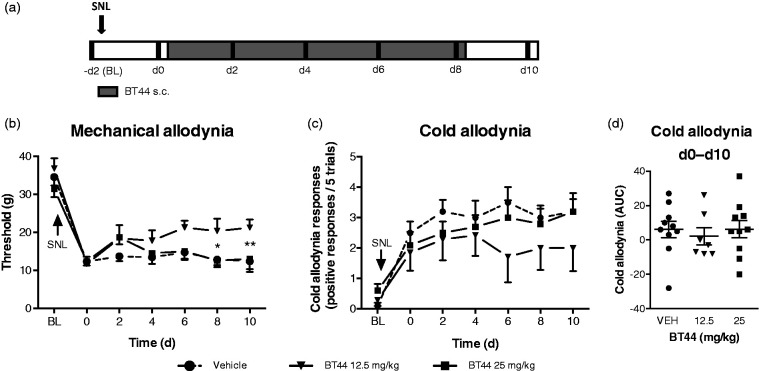
Figure 5.
The effects of BT44 in rats with experimental neuropathy induced by SNL. Baseline was measured before surgery which was performed two days before the first drug administration. Rats received BT44 or vehicle subcutaneously once daily on days 0, 2, 4, 6, and 8 after behavioral tests (black stripes) (a). Panel (b) shows thresholds (g) to mechanical stimulation and panel (c) cold responses to acetone (positive responses/five trials) in the SNL animals treated with vehicle, 12.5 or 25 mg/kg of BT44. Panel (d) shows the area under curve of the cold response–time curve normalized to day 0 values for each group. Data in (b) and (c) represent mean ± SEM. *P < 0.05, **P < 0.01 (two-way ANOVA with Dunnett’s multiple comparison test: reference the corresponding vehicle-group without the BT44 treatment). n = 7–10. d: days; VEH: vehicle; BL: baseline; AUC: area under curve.
The STZ-treated animals received 5 or 12.5 mg/kg of BT44 in two independent experiments (Figure 6(a)). In the first experiment assessing the long-term effects of BT44, the animals received vehicle, 5 or 12.5 mg/kg of BT44 for 42 days. In the second experiment, STZ-treated animals received vehicle or 5 mg/kg of BT44 for 14 days. BT44 was dissolved in propylene glycol and injected s.c. every second day, starting on the next day after the injection of STZ. Vehicle groups received propylene glycol. The vehicle was also administered to healthy control animals. For the statistical analysis and representation purposes, the data from the first and second experiment were pooled together.
Figure 6.
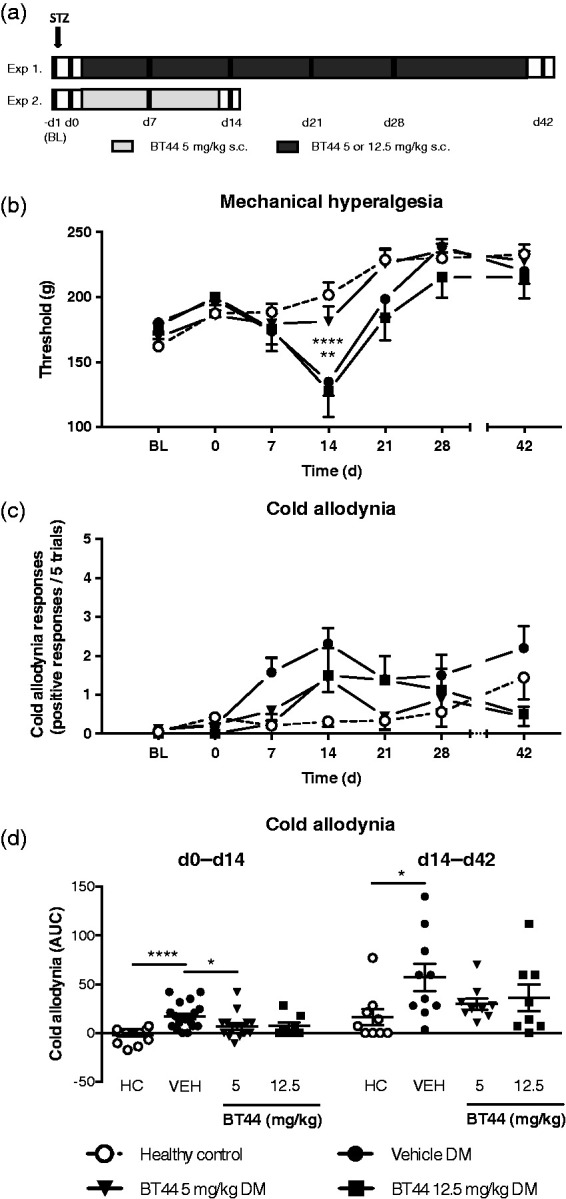
The effect of BT44 in rats with diabetes mellitus. Schemes of the STZ experiments are shown in panel (a). STZ was injected on day −1. The black vertical lines indicate the days when behavioral tests were performed. Rats received BT44 or vehicle subcutaneously once daily every other day for two or six weeks starting the day after the injection of STZ (day 0). Behavioral tests were performed before the injection of STZ (BL), before the first BT44 injection (day 0) and on weeks 1, 2, 3, 4, and 6. BT44 alleviates the mechanical hyperalgesia (b) and the cold allodynia (c and d) in the STZ-treated animals. In (b), thresholds (g) to mechanical stimulation are shown. Panel (c) shows cold allodynia responses to acetone (positive responses/five trials), and panel (d) shows the area under curve of the normalized values of d0–d14 in the acetone test for each group. Data represent mean ± SEM. In (b), **P < 0.01, ****P < 0.0001 (two-way ANOVA with Dunnett’s multiple comparisons test; reference: the corresponding vehicle-group without BT44 treatment). In (d), *P < 0.05, **** P < 0.0001 (Krustal–Wallis test with Dunn’s multiple comparison test). n = 8–19. BL: baseline; VEH: vehicle; d: days; HC: healthy control; DM: diabetes mellitus; AUC: area under curve.
To evaluate the effects of BT44 in healthy control animals, rats were treated with 5 or 12.5 mg/kg of BT44 dissolved in propylene glycol s.c. every second day for two weeks. The vehicle group received propylene glycol.
In all experiments, the animals were randomly assigned to the vehicle and BT44 treatment groups and in the SNL experiments, groups were balanced based on the mechanical nociceptive thresholds measured two days after SNL. Behavioral assessments were made by an observer who was blinded to the treatment.
Behavioral tests
In the SNL animals, mechanical sensitivity (allodynia) was assessed using digital von Frey test and cold allodynia with acetone test before surgery, before the first BT44 injection and every other day after surgery (Figure 5(a)). In the SNL animals, behavioral tests were performed in the ipsilateral and contralateral sides.
In the STZ-treated animals, mechanical sensitivity was assessed using digital von Frey test and/or paw pressure test. Heat and cold sensitivity was assessed using the Hargreaves plantar test and the acetone test, respectively. In the first experiment, all tests were performed before the injection of STZ, before the first BT44 injection, and one, two, three, four, and six weeks after the injection of STZ (Figure 6(a)). In the second experiment plantar, thermal and cold tests were performed before the injection of STZ, before the first BT44 injection, and one and two weeks after the injection of STZ (Figure 6(a)). In the STZ-treated animals, at each time point, the tests were performed at 5 min intervals in the same order. The Hargreaves test was performed first in the left paw, then the digital von Frey test to the right paw, the acetone test to the left paw, and finally the paw pressure test was performed to the right paw.
To assess whether BT44 has any effect in healthy control rats, we assessed mechanical sensitivity with the paw pressure test and cold sensitivity with the acetone test in healthy animals. The nociceptive tests were performed before the first drug administration and once a week for two weeks. To assess the acute effect of BT44 administration, the tests were also performed 1 and 3 h after the first drug administration.
Before the Hargreaves plantar test, the rats were placed on a glass plate and before the mechanical (vonFrey) and cold tests, on a metal mesh covered with a plastic dome to habituate to the test settings for 15 min before the actual measurements.
To measure mechanical sensitivity (mechanical allodynia) in the SNL and STZ-treated animals, we used the von Frey test in which the plantar surface of the paw was touched with a metal monofilament (tip diameter 0.5 mm) attached to a digital force gauge (Imada DPS-1, Northbrook, IL, USA). The paw was slowly approached and contact was made for 1 s to standardize the time-course of the stimulation. The force just sufficient to produce a response was applied, and the pressure leading to the reaction was recorded. Brisk foot withdrawal or lifting and licking of the paw was accepted as a response. At each time point, the threshold was assessed in triplicates three times at 3 min intervals for each hind paw. In the SNL animals, the measurement was done by alternating between the ipsilateral and contralateral side. The mean of the three threshold values at each time point was used in further calculations. In the STZ-treated animals, the test was performed to the right paw.
To assess heat sensitivity in the STZ-treated animals, the Hargreaves plantar test (Ugo Basile, Varese, Italy Comerio, Italy) was performed. The animal was placed on a glass plate, and radiant heat was applied to the left hind paw until spontaneous withdrawal of the hind paw was measured. The time from heat application to withdrawal was considered as the latency of the withdrawal response. The cutoff point was set at 10 s after which heat application was stopped to prevent tissue damage. At each time point, three latency determinations were performed at 2 min intervals. The mean of the three values at each time point was used in further calculations.
To assess cold sensitivity, the acetone test was performed in the SNL, STZ, and healthy animals. Cold sensitivity (cold allodynia) was measured as the number of foot withdrawal responses after application of cold stimuli to the plantar surface of the paw.43 A drop of acetone was gently applied to the plantar surface of the hind paw with a syringe connected to a thin polyethylene tube. A withdrawal response of the foot was considered as a sign of cold allodynia. The test was repeated five times with an interval of approximately 2 min between each test. In the SNL animals, the measurements were done by alternating between the ipsilateral and contralateral side, with a 5-min break between measurements. In the STZ-treated animals, the acetone test was performed in the left paw.
To assess mechanical sensitivity (mechanical hyperalgesia) in the STZ-treated animals and the healthy animals, the paw pressure test was performed with the Ugo Basile paw pressure device (Ugo Basile, Varese, Italy Comerio, Italy). The animals were gently wrapped in a towel during the test, the right hindpaw was placed under a pivot and the force applied to the paw was gradually increased. A mechanical force was applied at a rate of 32 g/s to the hind paw until limb withdrawal or the cut-off force of 250 g was reached. At each time point, three threshold determinations were performed at 2 min intervals. The mean of the three threshold values at each time point was used in further calculations.
Motor performance
To exclude a motor effect of BT44, motor activity of the STZ-treated animals was assessed with a rotarod test using the Ugo Basile (Gemonio, Italy) 47700 Rat RotaRod apparatus, diameter 70 mm. The rats were acclimatized for two consecutive days at a speed of 20 r/min during 60 s. During the testing day (sixth week), an accelerating speed was used. The starting rotation rate of the rod was 10 r/min, and it was increased to 40 r/min over 60 s. The time the animals were able to stay on the rod was calculated. The cut off time was 60 s.
Immunohistochemistry
DRGs (L5 for SNL and lumbar for STZ-induced DM) of the experimental animals were embedded in paraffin, sectioned for 5 µM sections and probed after deparaffinization with isolectin Griffonia simplicifolia (IB4) conjugated with Alexa 488 (1:200; Vector Laboratories, USA) and antibodies against CGRP (1:10 000, Peninsula Laboratory, USA). To visualize bound CGRP primary antibodies, slides were incubated with biotinylated Goat Anti-Rabbit IgG Antibody (Vector Laboratories, USA) and ABC-peroxidase conjugate (VECTASTAIN Elite ABC-Peroxidase Kit antibody, Vector Laboratories, USA) followed by chromogenic visualization with DAB staining kit (Vector Laboratories, USA) according to the manufacturer’s instructions. In the slides labeled with IB4-Alexa488, nuclei were stained with DAPI. The slides were mounted with Immu-Mount (Thermo Scientific, USA) or Coverquick 2000 (VWR, Finland) and imaged with fluorescent (Zeiss Imager M2 Axio, Carl Zeiss, Germany) or bright-field (Olympus BX-61, Olympus, Japan) microscopes. The number of marker-positive neurons in a section of a DRG was calculated manually and normalized to the total number of sensory neurons in this section. The results of the analysis of two to four sections per ganglion were averaged and used in further calculations.
Determination of drug concentrations in the blood of healthy animals
To evaluate the concentration of BT44 in the blood, a separate group of healthy animals was injected s.c. with a single dose of BT44 (25 mg/kg), and venous blood samples were collected at the 0.5, 1, and 3 h time points. The blood samples were let to coagulate on ice after which they were centrifuged at 2000g for 10 min at +4°C. Serum was collected and stored at −80°C.
Homogenized serum was mixed with acetonitrile in a volume ratio of 1:1, and the mixture was vortexed followed by centrifugation (16,110g, 5 min). The BT44 concentration in the supernatants was quantified by Agilent 1100 high performance liquid chromatography (HPLC, Agilent Technologies, USA) with a mobile phase composed of Milli-Q water and acetonitrile (volume ratio 40:60) at a flow rate of 1 ml/min at 25°C. The wavelength used for the BT44 quantification was 230 nm. A Gemini® 3 µm NX-C18 110 Å column (Phenomenex, USA) was used as stationary phase and the injection volume of the samples was 20 μL. This study was conducted in triplicate.
Statistical analysis
Data are presented as mean ± standard error of the mean (SEM). Data analysis was performed using Prism 6.0 software (GraphPad Software, Inc., San Diego, CA, USA).
All quantitative data were subjected to statistical analysis. To analyze parametric data (mechanical tests, Hargreaves plantar test, CGRP-positive neuron counts, luciferase assay data) significance of the difference between treatment groups was determined by one or two-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test. Neurite outgrowth data were analyzed by repeated measures (RM) ANOVA followed by Dunnett’s multiple comparison test.44,45 Comparisons between two groups were performed with two-tailed Student’s t test. P < 0.05 was considered to represent a significant difference.
To analyze non-parametric data (acetone test results, IB4-positive neuron counts), we used the Mann–Whitney U test. To analyze the effect of the chronic treatment of BT44 (acetone test results), the area under the time–effect curve (AUC) after beginning of the treatment was calculated using the trapezoidal rule. For each animal, the acetone test results were normalized to the values on the day when the BT44 injections started (day 0), subtracting the day 0 value from the response value of each experiment day. AUCs were calculated individually for each animal. Significance of the difference between treatment groups was determined by Kruskal–Wallis test with Dunn’s multiple comparison test.
Results
In vitro biological activity of BT44
BT44 promotes RET phosphorylation and selectively activates downstream cascades in the cells expressing GFL receptors
At first, we tested the ability of BT44 (Figure 1(a)) to activate the luciferase reporter gene controlled by the MAPK-signaling cascade in the cells expressing receptors for GDNF (GFRα1/RET), ARTN (GFRα3/RET) and RET alone in four independent experiments. BT44 increased luciferase activity in the reporter cells expressing GFRα1/RET (F6,18 = 19.85, P < 0.0001; Figure 1(b)) and GFRα3/RET (F6,18 = 13.87, P < 0.0001; Figure 1(c)), but not in the cells expressing RET alone (Figure 1(d)). In GFRα1/RET expressing cells, 10–50 µM of BT44 increased the activity of luciferase reporter by approximately two fold (P < 0.0001, Dunnett’s multiple comparison test). In the GFRα3/RET expressing cells, 5–50 µM of BT44 increased the activity of luciferase reporter by 1.6–1.9 fold (P < 0.001, Dunnett’s multiple comparison test). It is important to note here, that all three reporter cell lines responded to the respective positive controls as expected, but the RET-expressing cells were the least responsive. Thus, GDNF (200 ng/ml) increased luciferase activity in GFRα1/RET-expressing reporter cells by 59 fold; ARTN (200 ng/ml) in GFRα3/RET-expressing reporter cells by 37 fold, and the complex of soluble GFRα1 and GDNF (200 ng/ml) in RET-expressing reporter cells by only seven fold. Therefore, lack of luciferase activation by BT44 in the last cell line could be attributed to its low responsivity to stimulation in general.
Figure 1.
Chemical structure of BT44 (a) and its ability to activate luciferase reporter in the cells expressing GFRα1/RET (b), GFRα3/RET (c), and RET (d). Data represent mean ± SEM. ***P < 0.001, ****P < 0 .0001 (RM one-way ANOVA with Dunnett’s multiple comparison test: reference corresponding vehicle group); n = 4. VEH: vehicle; LCPS: luminescence counts per second.
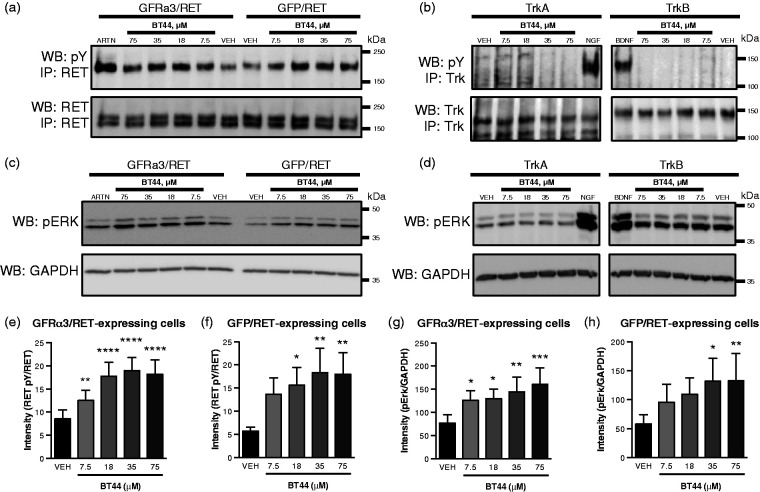
We further tested the ability of BT44 to stimulate phosphorylation of the RET receptor and the downstream ERK signaling cascade using Western blotting with antibodies specific to the phosphorylated forms of the above-indicated proteins. We observed an increase in RET phosphorylation in GFRα3-transfected MG87RET cells (Figure 2(a) and (e)). In addition, BT44 was also able to activate RET in the absence of co-receptor (Figure 2(a) and (f)). RM ANOVA revealed statistically significant differences between the treatments in GFRα3/RET (F4,8 = 45.86;, P < 0.0001) and GFP/RET (F4,8 = 6.781, P = 0.0110) expressing cells. The level of RET phosphorylation increased in GFRα3 and RET-expressing cells treated with BT44 in concentrations of 7.5 µM by 1.5 fold (P = 0.009), 18 µM by 2 fold (P < 0.0001), 35 µM by 2.2 fold (P < 0.0001), and 75 µM by 2.1 fold (P < 0.0001). Similarly, the level of RET phosphorylation in GFP-expressing cells when treated with 18 µM of BT44 increased by 2.7 fold (P = 0.0236), 35 µM by 3.2 fold (P = 0.0064), and 75 µM by 3.1 fold (P = 0.0073, one-way ANOVA with Dunnett’s post hoc test for all comparisons) (Figure 2(e) and (f)). We also evaluated the ability of BT44 to activate ERK, the downstream target of RET. BT44 in concentrations of 7.5–75 µM increased phosphorylation of ERK in both GFRα3/RET and GFP/RET cells (Figure 2(c), (g), and (h)). RM ANOVA of ERK activation also revealed statistically significant differences between the treatments in GFRα3/RET (F4,24 = 6.038, P = 0.016) and GFP/RET (F4,24 = 3.781, P = 0.0160) expressing cells. The level of ERK activation increased in GFR3 and RET-expressing cells treated with BT44 in concentrations of 7.5 µM by 1.6 fold (P = 0.0388), 18 µM by 1.7 fold (P = 0.0259), 35 µM by 1.9 fold (P = 0.0037), and 75 µM by 2 fold (P = 0.0004) (Figure 2(c) and (g)). Similarly, the level of ERK activation in GFP expressing cells treated with BT44 in concentrations of 35 µM increased by 2.2 (P = 0.0107), and 75 µM by 2.3 fold (P = 0.0098, one-way ANOVA with Dunnett’s post hoc test for all comparisons), respectively (Figure 2(c) and (h)). We also evaluated the specificity of the compound analyzing phosphorylation of the receptor tyrosine kinases TrkA and TrkB transmitting signals from nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF), respectively. BT44 failed to activate TrkA, TrkB, or ERK in MG87TrkA and MG87TrkB cells (Figure 2(b) and (d)).
Figure 2.
Selective phosphorylation of RET and activation of downstream signaling in RET-expressing cells by BT44. BT44 increased the level of phosphorylated RET (a) and ERK (c) in MG87RET cells transfected with GFRα3- or GFP-expressing plasmid. BT44 did not increase the level of phosphorylated TrkA or TrkB (b) or ERK (d) in MG87TrkA and MG87TrkB cells. Western blot quantification of RET phosphorylation level (e and f) and ERK phosphorylation levels (g and h). The data are presented as percentages of activation ± SEM, with the values in ARTN-treated well standardized to 100%, n = 3–7 (e to h). All cell lines respond to respective controls (MG87RET/GFRα3 to ARTN (250 ng/ml), MG87TrkA to NGF (200 ng/ml) and MG87TrkB to BDNF (200 ng/ml). IP: immunoprecipitation; WB: Western blotting; NGF: nerve growth factor; BDNF: brain-derived neurotrophic factor; pY: phosphotyrosine; pERK: phosphorylated form of ERK1/2; GAPDH: glyceraldehyde 3-phosphate dehydrogenase, house-keeping protein, loading control; GFP: green fluorescent protein. *P < 0.05, **P < 0.01, ***P< 0.001, ***P < 0.0001, RM ANOVA with Dunnett’s post hoc test.
Based on the data collected in experiments performed in MG87TrkA, MG87TrkB, MG87RET/GFRα3, and MG87RET/GFP cells, we concluded that BT44 is able to stimulate RET and RET downstream signaling and that GFRα co-receptors are dispensable for this process, although the compound seemed to have some degree of preference to GFRα3/RET expressing cells in both reporter gene-based assay (Figure 1) and direct phosphorylation assays (Figure 2). Additionally, the compound is selective to RET. Interestingly, BT44 was able to promote RET phosphorylation in the absence of GFRα3 co-receptor, but it was unable to activate luciferase production in the cells lacking GFRα3 (Figure 1). Higher concentrations of BT44 were necessary to statistically significantly increase pRET and pERK levels in MG87RET cells lacking GFRα3. This together with low level of luciferase activation also by positive control proteins in that particular batch of reporter gene-expressing cells can explain the discrepancy in the results presented in Figures 1 and 2.
BT44 promotes neurite outgrowth from sensory neurons
To evaluate the effect of BT44 in sensory neurons, we treated P30 DRG neurons with different concentrations of this RET agonist. As a positive control we used 30 ng/ml of ARTN. In the first set of five independent experiments, we compared the neurite-outgrowth promoting properties of 0.5–5 µM BT44, ARTN, and vehicle. RM ANOVA showed a significant effect of treatment (F4,16 = 8.05, P = 0.0009). Dunnett’s post hoc multiple comparison test revealed that 5 µM BT44 and ARTN (30 ng/ml) increased the proportion of neurite-bearing DRG neurons in culture (P = 0.03 and P = 0.0008, respectively). In the second set of four independent experiments, we compared the neurite-outgrowth promoting properties of 10 µM BT44, ARTN, and vehicle. RM ANOVA showed that the treatment had a significant effect (F2,6 = 5.950, P = 0.038). Dunnett’s post hoc multiple comparison test revealed that 10 µM BT44 and ARTN (30 ng/ml) increased the proportion of neurite-bearing DRG neurons in culture (P = 0.039 and P = 0.047, respectively). Thus, BT44 dose-dependently stimulated neurite outgrowth from DRG sensory neurons and its efficacy was comparable to that of ARTN (see pooled data in Figure 3). Limited solubility of the compound in cell culture media supplemented with 1% DMSO precluded us from testing higher concentrations of BT44 in this assay.
Figure 3.
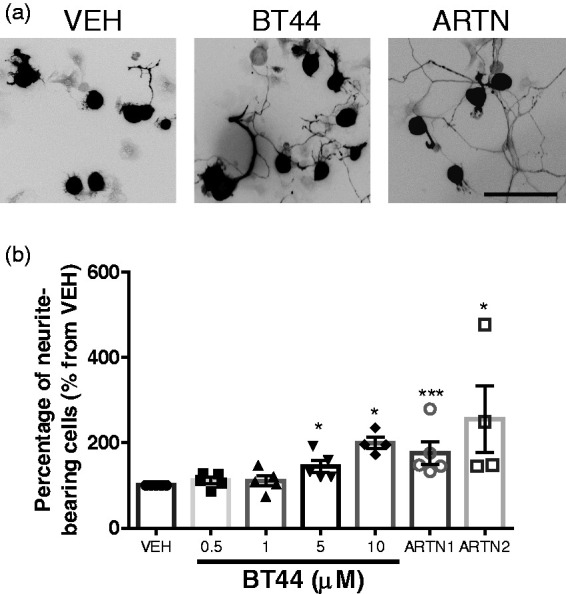
Stimulation of neurite outgrowth from sensory neurons by BT44. Dorsal root ganglion neurons were treated with 0.5–10 µM BT44 or ARTN (30 ng/ml) for 16–20 h. (a) Representative images of PGP9.5-stained DRG neurons. (b) The mean percentage of the neurons with neurites of at least two times longer than neuron body was calculated using the data from four to five independent experiments per concentration. For representation purposes, the data are expressed as mean ± SEM from vehicle-treated well, with the values in vehicle-treated well standardized to 100%, n = 4–5. *P < 0.05, ***P <0.001 (RM one-way ANOVA from independent experiments with Dunnett’s multiple comparison test: reference corresponding vehicle group). VEH: vehicle; ARTN1: ARTN-treated cells in the first set of experiments; ARTN2: ARTN-treated cells in the second set of experiments. Scale bar = 100 µm.
In vivo biological activity of BT44
BT44 concentrations in the serum of healthy rats
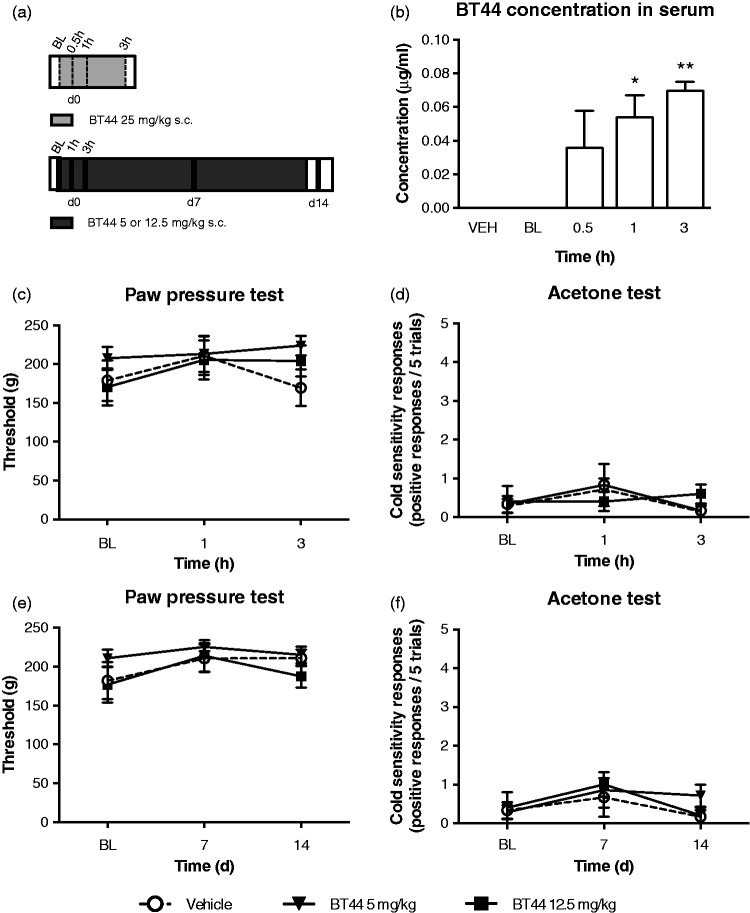
Single s.c. injections of BT44 at the dose 25 mg/kg significantly increased the BT44 concentration in the serum of healthy animals as shown by ANOVA (F4,13 = 5.366, P = 0.0089; Figure 4(b)). Dunnett’s post hoc multiple comparison test revealed that the BT44 concentration increased after 1 h (P = 0.0315) and remained elevated after 3 h (P = 0.0060) of administration indicating that the compound reached systemic circulation after s.c. administration and remained in the body for several hours.
Figure 4.
Acute and chronic effects of BT44 in healthy control rats. Scheme of the experiments (a). In (b), rats received a single injection of BT44 or vehicle subcutaneously (s.c.). Serum was collected before (BL), 0.5, 1, and 3 h after the injections (dashed lines) (a). In (c) to (f), rats received BT44 or vehicle s.c. once daily every second day for two weeks after behavioral tests (black stripes) (a). BT44 at the dose 25 mg/kg increased BT44 serum concentration significantly 1 h and 3 h post administration (b). Mechanical sensitivity was assessed with the paw pressure test (c and e) and cold sensitivity with the acetone test (d and f) in healthy animals which received 5 or 12.5 mg/kg of BT44 acutely (c and d) or every second day for two weeks (e and f). Panels (c) and (e) show thresholds (g) to mechanical stimulation. Panels (d) and (f) show cold sensitivity responses to acetone (positive responses/five trials). Data represent mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 (RM one-way ANOVA with Dunnett’s multiple comparison test: reference corresponding baseline in the healthy controls). n (c–f) = 5–7 and n (b) = 2–4. BL: baseline; VEH: vehicle.
BT44 does not change mechanical and cold sensitivity in healthy rats
In the healthy animals, BT44 at the doses of 5 and 12.5 mg/kg did not affect mechanical sensitivity after acute (Figure 4(c)) or two weeks’ administration (Figure 4(e)). Similarly, cold sensitivity and AUC of the cold response–time curve were not affected by the treatment (Figure 4(d) and (f)).
BT44 alleviates sensory signs in the SNL and STZ models of neuropathic pain
In the first SNL experiment, rats received vehicle or BT44 at doses of 12.5 or 25 mg/kg. Animals developed mechanical allodynia during the first two days following nerve injury (main effect of time: F6,144 = 49.88, P < 0.0001, two-way ANOVA). The compound BT44 alleviated mechanical allodynia in the SNL animals (main effect of treatment: F2,24 = 3.57, P < 0.05, two-way ANOVA). Dunnett’s post hoc multiple comparison test revealed a significant anti-allodynic effect after 12.5 mg/kg, but not after 25 mg/kg of BT44 (Figure 5(b)). The SNL animals also developed cold allodynia during the first two days following nerve injury (Mann–Whitney U test, U = 74, P < 0.0001), but neither 12.5 nor 25 mg/kg of BT44 showed statistically significant effects, and the AUCs of the response–time curve, normalized to day 0 values, did not differ between groups (Figure 5(c) and (d)).
In the second SNL experiment, the animals developed mechanical allodynia during the first two days following nerve injury (main effect of time: F6,144 = 39.15, P < 0.0001), but the lowest doses of BT44 (1 or 5 mg/kg) did not alleviate mechanical allodynia (not shown in the graphs). None of the BT44 doses influenced the responses of the contralateral (to injury) paws in either SNL experiment (not shown).
In the first STZ experiment, the STZ-treated animals received vehicle or BT44 at doses of 5 or 12.5 mg/kg for six weeks. In the second experiment with STZ-treated animals, rats received vehicle or BT44 at the dose of 5 mg/kg for two weeks. Healthy control animals also received vehicle in both experiments (Figure 6(a)).
STZ induced mechanical hyperalgesia, but not mechanical allodynia. In the paw pressure test, the main effects of time and treatment were significant (F6, 332 = 19.22, P < 0.0001 and F3,332 = 3.500, P = 0.0158, respectively). The effect varied between the chronic treatment groups (interaction between time and treatment: F18,332 = 2.331, P = 0.0018). Post hoc analyses using Dunnett’s multiple comparison test indicated that vehicle-treated STZ animals developed mechanical hyperalgesia during the first two weeks following the injection of STZ, as shown by a decrease in the paw pressure test threshold. BT44 treatment with the dose of 5 mg/kg alleviated mechanical hyperalgesia in the STZ-treated animals, while the 12.5 mg/kg dose was not effective (Figure 6(b)).
There were no signs of mechanical allodynia in the STZ-treated animals. Although the main effect of treatment in the von Frey test was significant (F3,224 = 6.134, P = 0.0005), the main effect of time and interaction between time and treatment were not. Moreover, post hoc analyses using Dunnett’s multiple comparison test indicated that mechanical allodynia did not develop during the first six weeks following the injection of STZ (Supplemental Figure 1(a)).
Also the vehicle-treated STZ animals developed cold allodynia during the first two weeks following the induction of STZ, which lasted for six weeks, as shown by the increase in the number of responses in the acetone test (Figure 6(c)). Analysis of the AUC of the response–time curve normalized to day 0 values showed that the overall effect of the treatment was significant during the first two weeks (d0–d14) (Kruskal–Wallis test, H = 24.61, P < 0.0001) and the later period of observation (d14–42) (Kruskal–Wallis test, H = 24.61, P < 0.0001). Post hoc analyses using Dunn’s multiple comparison test indicated that BT44 at 5 mg/kg attenuated cold allodynia in the STZ-treated animals during the first two weeks while the effect of 12.5 mg/was not significant (Figure 6(d)). In the later time frame (d14–42), post hoc analyses using Dunn’s multiple comparison test indicated that the increase in cold sensitivity was significant in the vehicle-treated STZ animals but not in the BT44-treated STZ animals (Figure 6(d)).
Moreover, the main effects of time and treatment in the Hargreaves plantar test latency were significant (F6,332 = 8.518, P < 0.0001 and F3,332 = 8.565, P < 0.0001, respectively). However, the effect did not vary with the chronic treatment group. The post hoc analyses using Dunnett’s multiple comparison test indicated that the healthy control animals were sensitized to heat. This sensitization was not seen in any of the STZ-treated animal groups (Supplemental Figure 1(b)).
BT44 did not influence motor activity of the STZ-treated animals (Supplemental Figure 2). STZ attenuated the increase of body weight (F1,18 =19.47, P = 0.0003), and this was not affected by BT44 at the doses of 5 or 12.5 mg/kg (Supplemental Figure 3(a)). Blood glucose and HbA1c levels were increased in the STZ-treated animals (F1,18 =14.51, P = 0.0013 and t(9) =17.02, P < 0.0001, respectively, Supplemental Figure 3(b) and (c)). BT44 did not influence the blood glucose and HbA1c levels of the STZ-treated animals (Supplemental Figure 3(a) to (c)).
BT44 protects IB4-positive neurons in DRGs of animals with experimental neuropathy
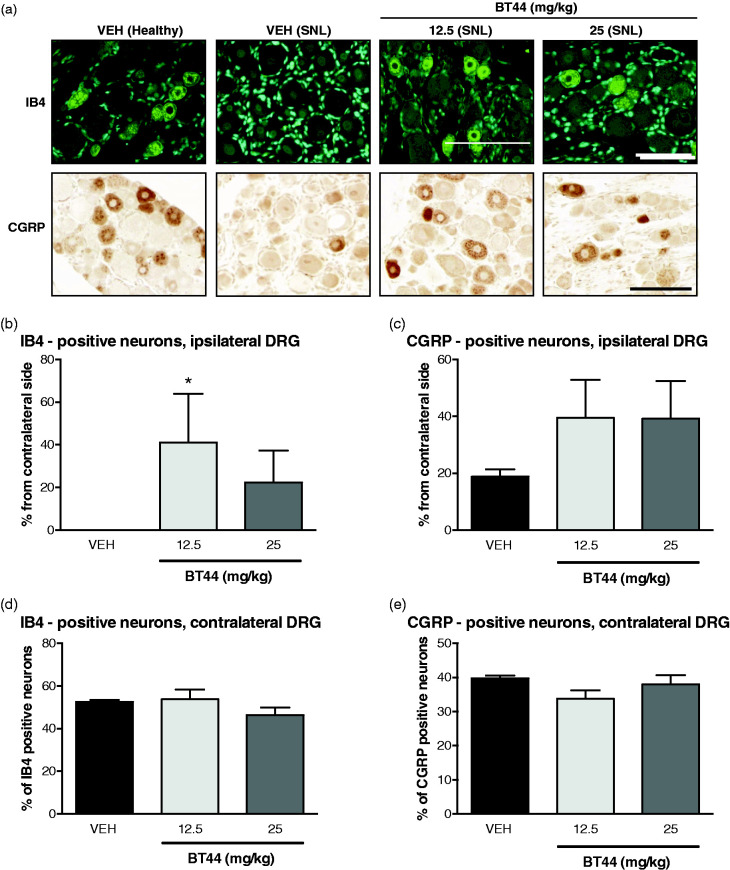
Sensory neurons in DRGs can be broadly divided into two classes: peptidergic, which express the calcitonin-gene related peptide (CGRP) as a marker and non-peptidergic neurons which express carbohydrates binding isolectin B4 (IB4). We analyzed the levels of expression of these markers in DRGs of SNL and diabetic animals treated with vehicle or BT44. In contralateral DRGs of the vehicle-treated rats in the SNL experiment, 39.8% ± 0.79% of the neurons expressed CGRP and 52.5% ± 0.93% of the neurons expressed IB4. SNL led to robust reduction in the number of neurons positive for both of these markers in ipsilateral DRGs. IB4-positive neurons completely disappeared from the ipsilateral DRGs of the animals after SNL. There was a 5.9 fold decrease in the number of CGRP—positive neurons in the ipsilateral DRGs of the SNL animals (Student’s t-test t = 25.37, df = 4, P < 0.0001). Treatment of SNL animals with BT44 led to a significant increase in the number of IB4 expressing neurons in the ipsilateral DRGs (Kruskall–Wallis test, H = 6.114, p = 0.0310). Post hoc analysis with Dunn’s multiple comparison test revealed that the 12.5 mg/kg dose of BT44 protected IB4-positive neurons (P = 0.028) from SNL-induced lesion (Figure 7(a)). We failed to detect statistically significant differences in the number of CGRP-positive neurons in the ipsilateral DRGs of the BT44-treated rats, although there was a two-fold increase in the average numbers (Figure 7(c)). The number of both CGRP- and IB-positive neurons in the contralateral DRGs remained unchanged in response to the treatment with BT44 (Figure 7(d) and (e)).
Figure 7.
Expression of neuronal markers in DRGs of SNL animals treated with BT44. Representative images of IB4 and CGRP—probed DRG sections (a). The number of marker-specific neurons in ipsilateral DRGs was calculated as a percentage of the total number of neurons for each section (two to four per DRG), the data for each DRG were averaged and normalized to the data for the contralateral DRG to reduce interindividual variation (b and c). The number of marker-specific neurons in contralateral DRGs (d and e) was expressed as percentage from total number of neurons. Data represent means ± SEM. *P < 0.05 (Kruskall–Wallis with Dunn’s multiple comparison post hoc test; reference: the corresponding vehicle-group without BT44 treatment). n = 3–5 animals per group. VEH: vehicle. Scale bar = 100 µM.
We quantified IB4- and CGRP-positive cells in the DRGs of the diabetic animals in a pilot experiment two and six weeks post treatment with STZ (one section per animal, three animals per group). We observed no changes in cell counts between healthy and diabetic animals (data not shown) and, therefore, more extensive analysis was not conducted.
Discussion
The novel RET agonist BT44 selectively activated GFRα1/RET and GFRα3/RET receptors leading to activation of intracellular MAPK/ERK signaling pathway at concentrations ≥ 10 µM and 5 µM, respectively. It also increased levels of pRET and pERK in GFRα3/RET expressing cells in concentrations ≥ 10 µM. BT44 (5–10 µM) stimulated neurite outgrowth from cultured DRG neurons, alleviated established mechanical allodynia, and protected IB4-positive neurons in DRGs in a rat SNL model of NP at the dose of 12.5 mg/kg. In the diabetic NP model, BT44 transiently reduced mechanical hyperalgesia and cold allodynia after two weeks of treatment at the dose of 5 mg/kg.
Compared with the parent compound BT13, BT44 had higher potency in immortalized cells and also in the SNL model of NP. It is important to note that in this study, we analyzed the effect of BT44 in animals with established neuropathy, compared with the study conducted with BT13 in which the compound was administered to animals immediately after the surgery.25 As the methods of neurite outgrowth analysis were different for BT13 and BT44, direct comparisons of these data are difficult. However, the efficacy of BT44 to promote neurite outgrowth was comparable to that of ARTN. At the same time, another BT13 analogue, a compound called BT18 in a similar assay to the one used in this study, induced neurite outgrowth in a smaller number of DRG neurons compared with ARTN.27 BT44 activated luciferase reporter in immortalized cells controlled by GFRα1/RET and GFRα3/RET, but not RET alone. BT44 increased also phosphorylation of RET and ERK in a dose-dependent manner, irrespective of the presence of GFRα co-receptor. Somewhat weaker ERK phosphorylation was observed in response to BT44 in the cells lacking GFRα. In silico studies identified a potential binding site for compounds belonging to the BT scaffold on the interface between RET and the GFRα co-receptor.46 Thus, the presence of a co-receptor may change the conformation of the complex and favor the activation of certain pathways in the cells providing a molecular basis for the biased agonism. The discrepancy between ERK phosphorylation assay and luciferase assay data can also be attributed to lower responsivity to stimulation of the reporter cell line lacking GFRα. Taken together, these data indicate that BT44 in contrast to BT13 can have some degree of selectivity to GFRa/RET complex compared with RET alone. This can be beneficial regarding potential side-effects.
In the reporter gene and RET phosphorylation assays, BT44 had higher potency compared with the parent compound BT13, but lower potency and efficacy than GFL proteins.25,26 This can have both positive and negative implications in clinical use of RET agonists. On one hand, it may be difficult to produce a sufficient level of RET activation to elicit neurorestorative events in the sensory system with low potency compounds. On the other hand, high concentrations of GFLs produce undesirable side effects in clinical trials and may result in down regulation of receptors.21,22,47,48 Besides, in the most recent trial in patients with neuropathic pain, a low dose of ARTN produced the highest clinical effect.48 While the treatment with GFLs21,22 and even life-long moderate overexpression of natural ligands49 in the model organisms is generally well-tolerated, RET is a proto-oncogene and its uncontrollable hyperactivation may be detrimental. Therefore, the compounds with moderate biological activity may be the optimal strategy in the development of RET agonists for therapeutic use.
BT44 selectively induced RET phosphorylation and intracellular signaling as it influenced neither TrkA/B phosphorylation nor levels of pERK in the cells expressing Trk receptors. A significant proportion of sensory neurons expresses the TrkA receptor and its activation stimulates nociception and hypersensitivity in vivo.50,51 Thus, inability of BT44 to activate TrkA and Trk-dependent signaling is a beneficial trait since it reduces the potential of the compound to produce pronociceptive effects. BT44 (5 and 10 µM) also increased the number of neurite-bearing DRG neurons in cultures, indicative of its potential to restore sensory neurons.
BT44 attenuated mechanical hypersensitivity in the surgery-induced animal model of neuropathic pain (SNL) at the dose of 12.5 mg/kg. Earlier, we showed that the first direct RET agonist, BT13, delivered to the animals right after the surgery, decreased mechanical hypersensitivity in the rat SNL model of NP.25 Similar attenuation of neuropathy is seen after ARTN and GDNF administration.11,12 Importantly, here we administered BT44 two days post SNL when the animals had already developed hypersensitivity and neuropathy. Thus, for the first time, we were able to demonstrate that a RET agonist can reverse established mechanical allodynia, even though its effect is delayed and needs several days to manifest.
A non-linear dose–response curve was observed in the effect of BT44 on mechanical hypersensitivity. This phenomenon is often observed with a growth factor-related interventions in vitro.52 In addition, a non-linear dose–response curve was seen in a clinical trial with ARTN protein in neuropathic pain patients.48 These data are difficult to explain. Potential reasons can include a mechanistic and/or pharmacokinetic basis. The activation level of RET can decrease in the presence of high doses of the ligand, because the receptor fails to dimerize and signal when each monomer is bound to the co-receptor/ligand complex53 or as a result of increased phosphatase activity.54 We did not see a biphasic dose–response in the biochemical assays, but in immortalized cells, we overexpressed RET and GFRα3. Therefore, the stoichiometry and availability of GFL receptor complex components’ in immortalized cells and neurons may differ. Alternatively, poor solubility of BT44 could have resulted in the precipitation of the highest dose of BT44 in animal experiments.
Differences in the effective dose of BT44 in vivo could be explained by differences in the distinct pathophysiological mechanisms of the NP animal models used. Direct neuronal damage to the nerve in the SNL model of NP induces rapid development of allodynia and hyperalgesia12,15,41,43 which may need higher doses of BT44 to recover than pain sensitivity changes in the diabetic NP model. STZ selectively destroys pancreatic β-cells and chronic hyperglycemia induces neuropathy which may need a longer time to develop.42,55,56 This could also explain why BT44 attenuated cold allodynia in STZ animals but not in SNL animals. It should be noted that in the STZ animals, the effect of BT44 was seen on mechanical hyperalgesia which was assessed with the paw pressure test. In contrast, in the SNL animals, the effect of BT44 was seen on mechanical allodynia which was assessed with the digital von Frey test. We did not see the development of mechanical allodynia in the STZ animals. BT44 did not have any effect on nociceptive responses tested with the digital von Frey test in the STZ animals or in healthy animals.
Similarly to ARTN,12,57 BT44 partially normalized expression of the neuronal marker IB4 in rat DRGs in vivo. We observed only a trend to an increase in the number of CGRP-positive neurons in the DRGs of SNL animals, although the average number of CGRP-positive neurons was approximately two fold higher after BT44 treatment. These data differ from the results seen for ARTN12 and the first generation RET agonist BT13,25 both of which protected CGRP-positive neurons in rats with SNL. However, BT13 was delivered to rats 1 h after SNL, while BT44 was administered two days post surgery. Importantly, in healthy animals, the major population of sensory neurons expressing RET are IB4-positive neurons, while among the CGRP-positive neurons only 20% are also RET-positive. Therefore, IB4-positive neurons represent a major target for RET-specific compounds. RET agonists may influence only a subset of CGRP-positive neurons producing a small effect which is difficult to detect statistically. Indeed, BT44 seems to affect approximately 20% of CGRP-positive neurons in average supporting the proposed explanation.
Finally, the published data indicate that a nerve lesion can alter the expression of RET and GFL co-receptors in sensory neurons,11,25,58 but the time-dependence of this alteration is not well established. Both ARTN and BT13 were delivered soon after lesion, therefore, transient activation of RET expression can result in biological effects of these compound in CGRP-positive neurons. BT44 was injected two days after lesion when RET expression in CGRP neurons can already be diminished.
Here, we showed that BT44 attenuated mechanical sensitivity and cold allodynia in the diabetes-induced rat model of neuropathic pain. This is in line with a previous study where topical application of the GDNF signaling agonist, XIB4035, alleviated experimental diabetic neuropathy in rodents.24 It remains unclear whether BT44 can influence STZ-induced neuronal degeneration in the diabetic model as the expression of neuronal markers in the DRGs of the STZ-treated animals remained unchanged during the study. In contrast to the SNL model which is characterized by a quick reduction in the expression of neuronal markers in DRGs,12,15 neuronal degeneration in models of diabetic neuropathy is slow.55,56 Unfortunately, we were not able to monitor diabetic animals for longer periods, because of ethical considerations. Importantly, BT44 did not affect motor performance in the diabetic rats or mechanical sensitivity in non-neuropathic rats indicating that its antiallodynic and antihyperalgetic effects were specific for neuropathy and not caused by sedation or impaired coordination of the animals.
Interestingly, one of the GFL family members, protein NRTN, prevented the development of hyperglycemia and protected pancreatic beta cells in the Zucker diabetic fatty rats, but failed to influence sustained hyperglycemia in these animals.59 BT44 did not influence glucose levels in STZ animals, possibly because of the administration schedule (after the lesion) and the aggressiveness of the STZ-induced degeneration of beta-cells. It is of interest to assess the ability of BT44 to prevent hyperglycemia and the development of hypersensitivity in other models of diabetic neuropathy.
We showed that BT44 transiently attenuated cold allodynia in a diabetes-induced rat model of neuropathic pain at the dose of 5 mg/kg. The lack of effect of BT44 to cold allodynia in SNL animals can also be explained by its selectivity to RET and inability to influence other GFL receptors than RET.20
Although classification of sensory neurons to CGRP- and IB4-positive is a crude division, it still allows distinguishing of largely non-intersecting neuronal populations. New sophisticated methods, such as single cell sequencing,50 helped identify 11 distinct classes of DRG neurons mediating responses to different types of stimuli. Interestingly, RET expression was largely associated with mechanoreceptors, which is in line with our behavioral data. Among other classes, RET is expressed in so-called NP1-NP3 sensory neurons which are likely to participate in pruritus and inflammatory itch initiated signaling. This fact can be predictive of side effects caused by RET-targeting treatments. Indeed, in clinical trials with ARTN, pruritus was one of the adverse effects associated with administration of this protein.21,22,58
Since ARTN has induced cold allodynia and thermal hyperalgesia in rodents,19,20 we assessed the effect of BT44 on cold sensitivity also in healthy rats. Unlike ARTN, BT44 did not increase sensitivity to cold stimuli, which might be explained by the specificity of the small molecule agonist to the RET receptor. The TRPM8-positive neurons, which were negative for RET50 are critical for the hyperalgesic effect of GFLs in response to cold stimuli.20 Therefore, an altered response to cold in GFL-treated animals is likely to be mediated by an alternative receptor, for example, NCAM18,60 and N-syndecan.16
Other major adverse effects of ARTN seen in clinical trials include rash and thermal hypersensitivity.21,22,58 In this study, BT44 failed to influence heat sensitivity in the diabetes-induced rat model of NP and also cold sensitivity in healthy rats similar to our previous experiment in healthy animals,25 in which a RET agonist failed to influence thermal sensitivity. Thus, compounds selectively targeting RET receptors might be free from this liability.
Taken together, we discovered and characterized the small molecule GFRα/RET agonist, BT44, the biological effects of which are similar to those of ARTN and GDNF, both in vitro and in vivo. It activates the GFL receptor RET in cells, stimulates neurite outgrowth from cultured DRG neurons, alleviates NP manifestations in animal models, and reverts surgery-induced changes in the expression of IB4 in DRG neurons in a rat model of experimental neuropathy. In contrast to GFLs which activate several receptors in neurons, BT44 and related compounds seem to be selective to RET26 and to stimulate only RET-mediated processes. Therefore, it can offer advantage for clinical translation compared with GFLs by reducing the number of adverse effects. Further modifications of BT44 aiming at improvement of its pharmacological properties and biological activity may eventually lead to the development of unique disease-modifying drugs against NP, selectively targeting the most important clinical problems namely mechanical hyperalgesia and cold allodynia.
Supplemental Material
Supplemental material, sj-pdf-1-mpx-10.1177_1744806920950866 for Novel RET agonist for the treatment of experimental neuropathies by Hanna Viisanen, Ulpukka Nuotio, Oleg Kambur, Arun Kumar Mahato, Viljami Jokinen, Tuomas Lilius, Wei Li, Hélder A Santos, Mati Karelson, Pekka Rauhala, Eija Kalso and Yulia A Sidorova in Molecular Pain
Supplemental material, sj-pdf-2-mpx-10.1177_1744806920950866 for Novel RET agonist for the treatment of experimental neuropathies by Hanna Viisanen, Ulpukka Nuotio, Oleg Kambur, Arun Kumar Mahato, Viljami Jokinen, Tuomas Lilius, Wei Li, Hélder A Santos, Mati Karelson, Pekka Rauhala, Eija Kalso and Yulia A Sidorova in Molecular Pain
Supplemental material, sj-pdf-3-mpx-10.1177_1744806920950866 for Novel RET agonist for the treatment of experimental neuropathies by Hanna Viisanen, Ulpukka Nuotio, Oleg Kambur, Arun Kumar Mahato, Viljami Jokinen, Tuomas Lilius, Wei Li, Hélder A Santos, Mati Karelson, Pekka Rauhala, Eija Kalso and Yulia A Sidorova in Molecular Pain
Acknowledgments
The authors are grateful to Päivi Leinikka, Lahja Eurajoki, Jenni Montonen, and Ilida Suleymanova for their excellent assistance with the experimental procedures. The Nuclear Magnetic Resonance facility at the Institute of Biotechnology, University of Helsinki supported by Biocenter Finland and Helsinki Institute of Life Science (HiLIFE) is acknowledged for verification of BT44 structure.
Author Contributions
HV, OK, MK, PR, EK, and YAS designed the study; MK designed BT44; HV, UN, OK, AKM, VJ, and TL performed experiments; HV, UN, OK, AKM, VJ, TL, PR, EK, and YS analyzed and interpreted data; HV and YAS drafted the manuscript; all authors contributed to the writing of the manuscript and approved its final version; YAS conceived the study.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MK is an inventor in composition of matter patents of BT compounds (US Patent, No 8,901,129 B2 and European Patent, No 2509953) and in a patent application WO 2014041179 A1 on the treatment of peripheral neuropathy using GFRα3 type receptor agonists.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by funding from the European Union Seventh Framework Programme (FP7/2007–2013) under grant agreement no 602919, Finnish Medical Society (Finska Läkaresällskapet), University of Helsinki 375th Anniversary Grant, the Academy of Finland (grants 1315043 and 1325555), GeneCode Ltd, Centre of Excellence in Molecular Cell Engineering, Estonia, 2014–2020.4.01.15–013, and grant PUT582 from the Estonian Research Council.
ORCID iD
Yulia A Sidorova https://orcid.org/0000-0001-8230-0530
Supplemental Material
Supplemental material for this article is available online.
References
- 1.Scholz J, Finnerup NB, Attal N, Aziz Q, Baron R, Bennett MI, Benoliel R, Cohen M, Cruccu G, Davis KD, Evers S, First M, Giamberardino MA, Hansson P, Kaasa S, Korwisi B, Kosek E, Lavandʼhomme P, Nicholas M, Nurmikko T, Perrot S, Raja SN, Rice ASC, Rowbotham MC, Schug S, Simpson DM, Smith BH, Svensson P, Vlaeyen JWS, Wang S-J, Barke A, Rief W, Treede R-D; Classification Committee of the Neuropathic Pain Special Interest Group (NeuPSIG). The IASP classification of chronic pain for ICD-11: chronic neuropathic pain. Pain 2019; 160: 53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yawn BP, Wollan PC, Weingarten TN, Watson JC, Hooten WM, Melton LJ. The prevalence of neuropathic pain: clinical evaluation compared with screening tools in a community population. Pain Med 2009; 10: 586–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith BH, Torrance N. Epidemiology of neuropathic pain and its impact on quality of life. Curr Pain Headache Rep 2012; 16: 191–198. [DOI] [PubMed] [Google Scholar]
- 4.Haanpää ML, Treede R-D. Diagnosis and classification of neuropathic pain. PainClinical Updat 2010; 18: 1–6. [Google Scholar]
- 5.Scadding J, Koltzenburg M. Painful peripheral neuropathies In: McMahon S, Koltzenburg M. (eds) Wall Melzack’s textbook of pain. 5th ed London: Elsevier, 2006, pp. 973–999. [Google Scholar]
- 6.Themistocleous AC, Ramirez JD, Shillo PR, Lees JG, Selvarajah D, Orengo C, Tesfaye S, Rice ASC, Bennett DLH. The Pain in Neuropathy Study (PiNS): a cross-sectional observational study determining the somatosensory phenotype of painful and painless diabetic neuropathy. Pain 2016; 157: 1132–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Attal N; Neuropathic Pain Special Interest Group of the International Association for the Study of Pain. Management of neuropathic cancer pain. Ann Oncol 2010; 21: 1134–1135. [DOI] [PubMed] [Google Scholar]
- 8.Dworkin RH, Johnson RW, Breuer J, Gnann JW, Levin MJ, Backonja M, Betts RF, Gershon AA, Haanpää ML, McKendrick MW, Nurmikko TJ, Oaklander AL, Oxman MN, Langston DP, Petersen KL, Rowbotham MC, Schmader KE, Stacey BR, Tyring SK, Wijck AJMv, Wallace MS, Wassilew SW, Whitley RJ. Recommendations for the management of herpes zoster. Clin Infect Dis 2007; 44: S1–S26. [DOI] [PubMed] [Google Scholar]
- 9.Cherry CL, Wadley AL, Kamerman PR. Diagnosing and treating HIV-associated sensory neuropathy: a global perspective. Pain Manag 2016; 6: 191–199. [DOI] [PubMed] [Google Scholar]
- 10.Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, Gilron I, Haanpää M, Hansson P, Jensen TS, Kamerman PR, Lund K, Moore A, Raja SN, Rice ASC, Rowbotham M, Sena E, Siddall P, Smith BH, Wallace M. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 2015; 14: 162–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boucher TJ, Okuse K, Bennett DLH, Munson JB, Wood JN, McMahon SB. Potent analgesic effects of GDNF in neuropathic pain states. Science 2000; 290: 124–127. [DOI] [PubMed] [Google Scholar]
- 12.Gardell LR, Wang R, Ehrenfels C, Ossipov MH, Rossomando AJ, Miller S, Buckley C, Cai AK, Tse A, Foley SF, Gong B, Walus L, Carmillo P, Worley D, Huang C, Engber T, Pepinsky B, Cate RL, Vanderah TW, Lai J, Sah DWY, Porreca F. Multiple actions of systemic artemin in experimental neuropathy. Nat Med 2003; 9: 1383–1389. [DOI] [PubMed] [Google Scholar]
- 13.Takasu K, Sakai A, Hanawa H, Shimada T, Suzuki H. Overexpression of GDNF in the uninjured DRG exerts analgesic effects on neuropathic pain following segmental spinal nerve ligation in mice. J Pain 2011; 12: 1130–1139. [DOI] [PubMed] [Google Scholar]
- 14.Harvey P, Gong B, Rossomando AJ, Frank E. Topographically specific regeneration of sensory axons in the spinal cord. Proc Natl Acad Sci USA 2010; 107: 11585–11590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang R, Rossomando A, Sah DWY, Ossipov MH, King T, Porreca F. Artemin induced functional recovery and reinnervation after partial nerve injury. Pain 2014; 155: 476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bespalov MM, Sidorova YA, Tumova S, Ahonen-Bishopp A, Magalhães AC, Kulesskiy E, Paveliev M, Rivera C, Rauvala H, Saarma M. Heparan sulfate proteoglycan syndecan-3 is a novel receptor for GDNF, neurturin, and artemin. J Cell Biol 2011; 192: 153–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bespalov MM, Saarma M. GDNF family receptor complexes are emerging drug targets. Trends Pharmacol Sci 2007; 28: 68–74. [DOI] [PubMed] [Google Scholar]
- 18.Paratcha G, Ledda F, Ibáñez CF. The neural cell adhesion molecule NCAM is an alternative signaling receptor for GDNF family ligands. Cell 2003; 113: 867–879. [DOI] [PubMed] [Google Scholar]
- 19.Elitt CM, McIlwrath SL, Lawson JJ, Malin SA, Molliver DC, Cornuet PK, Koerber HR, Davis BM, Albers KM. Artemin overexpression in skin enhances expression of TRPV1 and TRPA1 in cutaneous sensory neurons and leads to behavioral sensitivity to heat and cold. J Neurosci 2006; 26: 8578–8587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lippoldt EK, Elmes RR, McCoy DD, Knowlton WM, McKemy DD. Artemin, a glial cell line-derived neurotrophic factor family member, induces TRPM8-dependent cold pain. J Neurosci 2013; 33: 12543–12552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okkerse P, Hay JL, Versage E, Tang Y, Galluppi G, Ravina B, Verma A, Williams L, Aycardi E, Groeneveld GJ. Pharmacokinetics and pharmacodynamics of multiple doses of BG00010, a neurotrophic factor with anti-hyperalgesic effects, in patients with sciatica. Br J Clin Pharmacol 2016; 82: 108–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rolan PE, O'Neill G, Versage E, Rana J, Tang Y, Galluppi G, Aycardi E. First-in-human, double-blind, placebo-controlled, randomized, dose-escalation study of BG00010, a glial cell line-derived neurotrophic factor family member, in subjects with unilateral sciatica. PLoS One 2015; 10: e0125034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tokugawa K, Yamamoto K, Nishiguchi M, Sekine T, Sakai M, Ueki T, Chaki S, Okuyama S. XIB4035, a novel nonpeptidyl small molecule agonist for GFRalpha-1. Neurochem Int 2003; 42: 81–86. [DOI] [PubMed] [Google Scholar]
- 24.Hedstrom KL, Murtie JC, Albers K, Calcutt Na, Corfas G. Treating small fiber neuropathy by topical application of a small molecule modulator of ligand-induced GFRα/RET receptor signaling. Proc Natl Acad Sci USA 2014; 111: 2325–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sidorova YA, Bespalov MM, Wong AW, Kambur O, Jokinen V, Lilius TO, Suleymanova I, Karelson G, Rauhala PV, Karelson M, Osborne PB. A novel small molecule GDNF receptor RET agonist, BT13, promotes neurite growth from sensory neurons in vitro and attenuates experimental neuropathy in the rat. Front Parmacol 2017; 8: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahato AK, Kopra J, Renko J-M, Visnapuu T, Korhonen I, Pulkkinen N, Bespalov MM, Domanskyi A, Ronken E, Piepponen TP, Voutilainen MH, Tuominen RK, Karelson M, Sidorova YA, Saarma M. Glial cell line–derived neurotrophic factor receptor rearranged during transfection agonist supports dopamine neurons in vitro and enhances dopamine release in vivo. Mov Disord 2020; 35: 245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bespalov MM, Sidorova YA, Suleymanova I, Thompson J, Kambur O, Jokinen V, Lilius T, Karelson G, Puusepp L, Rauhala P, Kalso E. Novel agonist of GDNF family ligand receptor RET for the treatment of experimental neuropathy. BioRxiv 2016; ▪: 061820. [Google Scholar]
- 28.Saarma M, Karelson M, Pilv M, Bespalov MM. Methods of facilitating neural cell survival using GDNF family ligand (GFL) mimetics or RET signalling pathway activators. European Patent, No 2509953, US Patent No 8901129B2. USA, Europe: United States Patent and Trademark Office, 2014.
- 29.Wang X, Baloh RH, Milbrandt J, Garcia KC. Structure of artemin complexed with its receptor GFRa3: convergent recognition of glial cell line-derived neurotrophic factors. Structure 2006; 14: 1083–1092. [DOI] [PubMed] [Google Scholar]
- 30.Parkash V, Leppänen V-M, Virtanen H, Jurvansuu JM, Bespalov MM, Sidorova YA, Runeberg-Roos P, Saarma M, Goldman A. The structure of the glial cell line-derived neurotrophic factor-coreceptor complex: insights into RET signaling and heparin binding. J Biol Chem 2008; 283: 35164–35172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, Olson AJ. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J Comput Chem 1998; 19: 1639–1662. [Google Scholar]
- 32.Karelson M, Dobchev DA, Karelson G, Tamm T, Tämm K, Nikonov A, Mutso M, Merits A. Fragment-based development of HCV protease inhibitors for the treatment of hepatitis C. Curr Comput Aided Drug Des 2012; 8: 55–61. [DOI] [PubMed] [Google Scholar]
- 33.Sidorova YA, Mätlik K, Paveliev M, Lindahl M, Piranen E, Milbrandt J, Arumäe U, Saarma M, Bespalov MM. Persephin signaling through GFRα1: the potential for the treatment of Parkinson’s disease. Mol Cell Neurosci 2010; 44: 223–232. [DOI] [PubMed] [Google Scholar]
- 34.Eketjäll S, Fainzilber M, Murray-Rust J, Ibáñez CF. Distinct structural elements in GDNF mediate binding to GFRα1 and activation of the GFRα1-c-RET receptor complex. Embo J 1999; 18: 5901–5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leppänen V-M, Bespalov MM, Runeberg-Roos P, Puurand U, Merits A, Saarma M, Goldman A. The structure of GFRα1 domain 3 reveals new insights into GDNF binding and RET activation. Embo J 2004; 23: 1452–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paveliev M, Airaksinen MS, Saarma M. GDNF family ligands activate multiple events during axonal growth in mature sensory neurons. Mol Cell Neurosci 2004; 25: 453–459. [DOI] [PubMed] [Google Scholar]
- 37.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983; 109–110. [DOI] [PubMed] [Google Scholar]
- 38.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. Osteoarthr Cartil 2012; 20: 256–260. [DOI] [PubMed] [Google Scholar]
- 39.McGrath JC, Drummond GB, McLachlan EM, Kilkenny C, Wainwright CL. Editorial: guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol 2010; 160: 1573–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knopp KL, Stenfors C, Baastrup C, Bannon AW, Calvo M, Caspani O, Currie G, Finnerup NB, Huang W, Kennedy JD, Lefevre I, Machin I, Macleod M, Rees H, Rice ASC, Rutten K, Segerdahl M, Serra J, Wodarski R, Berge O-G, Treede R-D. Experimental design and reporting standards for improving the internal validity of pre-clinical studies in the field of pain: consensus of the IMI-European consortium. Scand J Pain 2015; 7: 58–70. [DOI] [PubMed] [Google Scholar]
- 41.Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain 1992; 50: 355–363. [DOI] [PubMed] [Google Scholar]
- 42.Courteix C, Eschalier A, Lavarenne J. Streptozocin-induced diabetic rats: behavioural evidence for a model of chronic pain. Pain 1993; 53: 81–88. [DOI] [PubMed] [Google Scholar]
- 43.Choi Y, Yoon YW, Na HS, Kim SH, Chung JM. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain 1994; 59: 369–376. [DOI] [PubMed] [Google Scholar]
- 44.Lew M. Good statistical practice in pharmacology problem 2. Br J Pharmacol 2007; 152: 299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lew M. Good statistical practice in pharmacology problem 1. Br J Pharmacol 2007; 152: 295–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ivanova L, Tammiku-Taul J, García-Sosa AT, Sidorova Y, Saarma M, Karelson M. Molecular dynamics simulations of the interactions between glial cell line-derived neurotrophic factor family receptor GFRα1 and small-molecule ligands. ACS Omega 2018; 3: 11407–11414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nutt JG, Burchiel KJ, Comella CL, Jankovic J, Lang AE, Laws ER, Lozano AM, Penn RD, Simpson RK, Stacy M, Wooten GF; ICV GDNF Study Group. Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD. Neurology 2003; 60: 69–73. [DOI] [PubMed] [Google Scholar]
- 48.Backonja M, Williams L, Miao X, Katz N, Chen C. Safety and efficacy of neublastin in painful lumbosacral radiculopathy. Pain 2017; 158: 1802–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Turconi G, Kopra J, Võikar V, Kulesskaya N, Vilenius C, Piepponen TP, Andressoo J-O. Molecular therapy – methods & clinical development chronic two-fold elevation of endogenous GDNF levels is safe and enhances motor and dopaminergic function in aged mice. Mol Ther Methods Clin Dev 2020; 17: 831–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Usoskin D, Furlan A, Islam S, Abdo H, Lönnerberg P, Lou D, Hjerling-Leffler J, Haeggström J, Kharchenko O, Kharchenko PV, Linnarsson S, Ernfors P. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci 2015; 18: 145–153. [DOI] [PubMed] [Google Scholar]
- 51.Hirose M, Kuroda Y, Murata E. NGF/TrkA signaling as a therapeutic target for pain. Pain Pract 2016; 16: 175–182. [DOI] [PubMed] [Google Scholar]
- 52.Hou J-GG, Lin L-FH, Mytilineou C. Glial cell line-derived neurotrophic factor exerts neurotrophic effects on dopaminergic neurons in vitro and promotes their survival and regrowth after damage by 1-methyl-4-phenylpyridinium. J Neurochem 1996; 66: 74–82. [DOI] [PubMed] [Google Scholar]
- 53.Schlee S, Carmillo P, Whitty A. Quantitative analysis of the activation mechanism of the multicomponent growth-factor receptor Ret. Nat Chem Biol 2006; 2: 636–644. [DOI] [PubMed] [Google Scholar]
- 54.Yadav L, Pietilä E, Öhman T, Liu X, Mahato AK, Sidorova Y, Lehti K, Saarma M, Varjosalo M. PTPRA phosphatase regulates GDNF-dependent RET signaling and inhibits the RET mutant MEN2A oncogenic potential. iScience 2020; 23: 100871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beiswenger KK, Calcutt NA, Mizisin AP. Epidermal nerve fiber quantification in the assessment of diabetic neuropathy. Acta Histochem 2008; 110: 351–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gao F, Zheng ZM. Animal models of diabetic neuropathic pain. Exp Clin Endocrinol Diabetes 2014; 122: 100–106. [DOI] [PubMed] [Google Scholar]
- 57.Han X, Zhu S, Wang B, Chen L, Li R, Yao W, Qu Z. Antioxidant action of 7,8-dihydroxyflavone protects PC12 cells against 6-hydroxydopamine-induced cytotoxicity. Neurochem Int 2014; 64: 18–23. [DOI] [PubMed] [Google Scholar]
- 58.Bennett DL, Michael GJ, Ramachandran N, Munson JB, Averill S, Yan Q, McMahon SB, Priestley JV. A distinct subgroup of small DRG cells express GDNF receptor components and GDNF is protective for these neurons after nerve injury. J Neurosci 1998; 18: 3059–3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trevaskis JL, Sacramento CB, Jouihan H, Ali S, Le Lay J, Oldham S, Bhagroo N, Boland BB, Cann J, Chang Y, O'Day T, Howard V, Reers C, Winzell MS, Smith DM, Feigh M, Barkholt P, Schreiter K, Austen M, Andag U, Thompson S, Jermutus L, Coghlan MP, Grimsby J, Dohrmann C, Rhodes CJ, Rondinone CM, Sharma A. Neurturin and a GLP-1 analogue act synergistically to alleviate diabetes in Zucker diabetic fatty rats. Diabetes 2017; 66: 2007–2018. [DOI] [PubMed] [Google Scholar]
- 60.Ilieva M, Nielsen J, Korshunova I, Gotfryd K, Bock E, Pankratova S. Artemin and an artemin-derived peptide, artefin, induce neuronal survival, and differentiation through RET and NCAM. Front Mol Neurosci 2019; 12: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-mpx-10.1177_1744806920950866 for Novel RET agonist for the treatment of experimental neuropathies by Hanna Viisanen, Ulpukka Nuotio, Oleg Kambur, Arun Kumar Mahato, Viljami Jokinen, Tuomas Lilius, Wei Li, Hélder A Santos, Mati Karelson, Pekka Rauhala, Eija Kalso and Yulia A Sidorova in Molecular Pain
Supplemental material, sj-pdf-2-mpx-10.1177_1744806920950866 for Novel RET agonist for the treatment of experimental neuropathies by Hanna Viisanen, Ulpukka Nuotio, Oleg Kambur, Arun Kumar Mahato, Viljami Jokinen, Tuomas Lilius, Wei Li, Hélder A Santos, Mati Karelson, Pekka Rauhala, Eija Kalso and Yulia A Sidorova in Molecular Pain
Supplemental material, sj-pdf-3-mpx-10.1177_1744806920950866 for Novel RET agonist for the treatment of experimental neuropathies by Hanna Viisanen, Ulpukka Nuotio, Oleg Kambur, Arun Kumar Mahato, Viljami Jokinen, Tuomas Lilius, Wei Li, Hélder A Santos, Mati Karelson, Pekka Rauhala, Eija Kalso and Yulia A Sidorova in Molecular Pain